A Review of Biologically Active Oxime Ethers
Abstract
:1. Introduction
2. Oxime Ethers with an Antifungal Activity
3. The Oxime Ethers with an Antibacterial Activity
4. The Oxime Ethers with Antiviral Activity
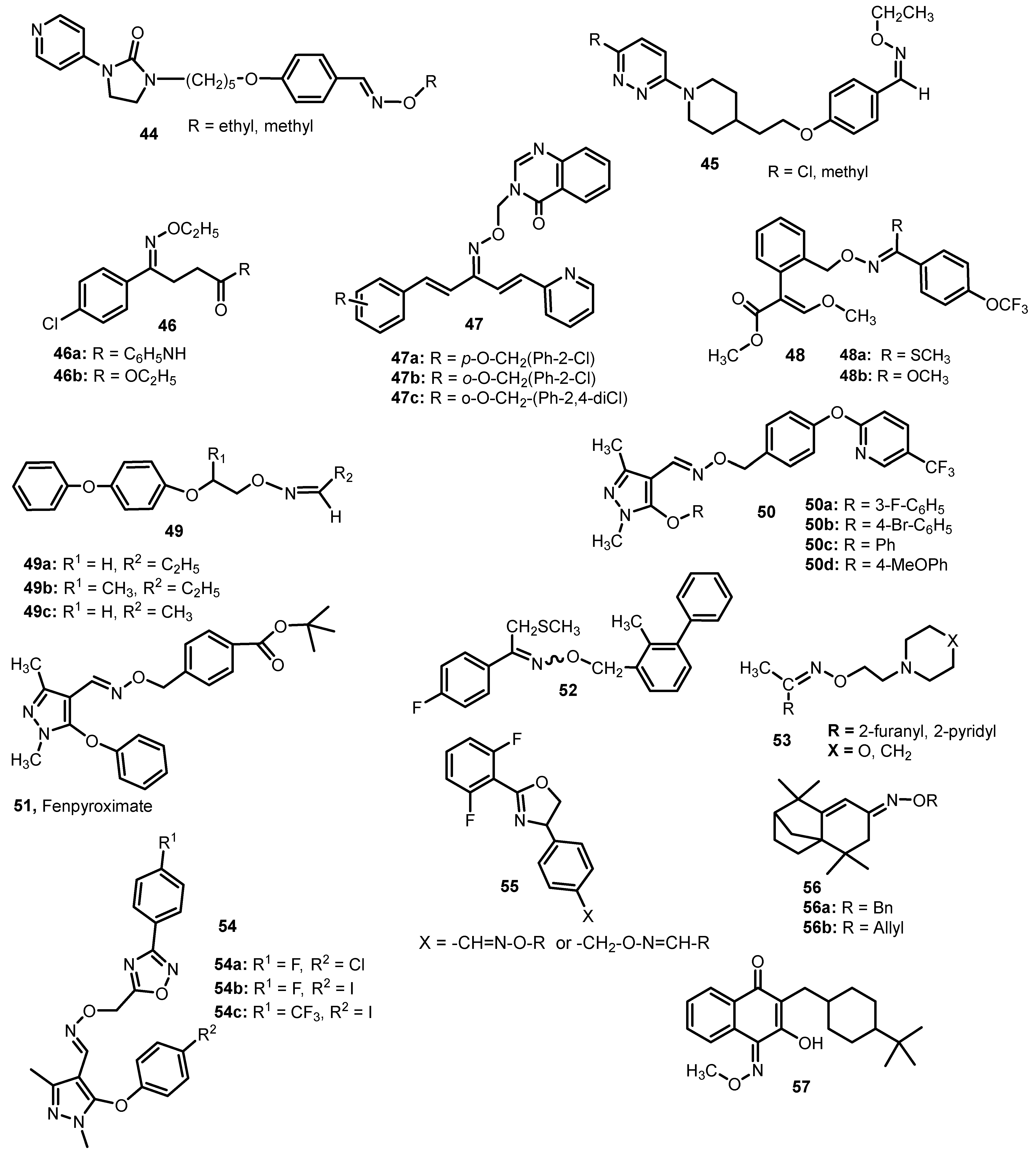
5. Oxime Ethers with Insecticidal, Acaricidal and Antiprotozoal Activity
6. The Oxime Ethers with Antidepressive Activity
7. The Oxime Ethers with an Anticonvulsant Activity
8. The Oxime Ethers with an Inhibition of the β-Receptors
9. The Oxime Ethers with an Anti-Inflammatory Activity
10. The Oxime Ethers as the PPAR Agonists
11. The Oxime Ethers with an Anticancer Activity
12. Oxime Ethers with Other Biological Activities
12.1. The Oxime Ethers with an Antioxidant Activity
12.2. The Oxime Ethers with an Antiulcer Activity
12.3. The Oxime Ethers with an Antiaggregation Activity
12.4. Compounds with Monoamine Oxidase (MAO) and Acetylcholinesterase (AChE) Inhibitory Activity
12.5. The Oxime Ethers with an Herbicidal Activity
13. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, A.K.; Mays, R.R.; Foley, K.A. Comprehensive Dermatologic Drug Therapy, 4th ed.; 42–Topical Antifungal Agents; Elsevier: Amsterdam, The Netherlands, 2021; pp. 480–492.e5. [Google Scholar] [CrossRef]
- Dell’Osso, B.; Allen, A.; Hollander, E. Fluvoxamine: A selective serotonin re-uptake inhibitor for the treatment of obsessive-compulsive disorder. Expert Opin. Pharmacother. 2005, 6, 2727–2740. [Google Scholar] [CrossRef]
- Wu, H.; Hu, T.; Hao, H.; Hill, M.; Xu, C.; Liu, Z. Inflammatory bowel disease and cardiovascular diseases: A concise review. EHJ Open 2022, 2, oeab029. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J. Siponimod: A Review in Secondary Progressive Multiple Sclerosis. CNS Drugs 2020, 34, 1191–1200. [Google Scholar] [CrossRef]
- Gupta, A. Oxiconazole in the treatment of superficial fungal infections. J. Am. Acad. Dermatol. 2007, 58 (Suppl. S2), AB91. [Google Scholar] [CrossRef]
- Gebhart, R.J.; Espinel-Ingroff, A.; Shadomy, S. In vitro Susceptibility Studies with Oxiconazole (Ro 13–8996). Chemotherapy 1984, 30, 244–247. [Google Scholar] [CrossRef]
- Rossello, A.; Bertini, S.; Lapucci, A.; Macchia, M.; Martinelli, A.; Rapposelli, S.; Herreros, E.; Macchia, B. Synthesis, antifungal activity, and molecular modeling studies of new inverted oxime ethers of oxiconazole. J. Med. Chem. 2002, 45, 4903–4912. [Google Scholar] [CrossRef]
- Emami, S.; Falahati, M.; Banifatemi, A.; Amanlou, M.; Shafiee, A. (E)- and (Z)-1,2,4-Triazolylchromanone oxime ethers as conformationally constrained antifungals. Bioorganic Med. Chem. 2004, 12, 3971–3976. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.; Falahati, M.; Banifatemi, A.; Shafiee, A. Stereoselective synthesis and antifungal activity of (Z)-trans-3-azolyl-2-methylchromanone oxime ethers. Bioorganic Med. Chem. 2004, 12, 5881–5889. [Google Scholar] [CrossRef]
- Babazadeh-Qazijahani, M.; Badali, H.; Irannejad, H.; Hosein Afsarian, M.; Emami, S. Imidazolylchromanones containing non-benzylic oxime ethers: Synthesis and molecular modeling study of new azole antifungals selective against Cryptococcus gattii. Eur. J. Med. Chem. 2014, 76, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Demirayak, S.; Uçucu, U.; Benkli, K.; Gündoğdu-Karaburun, N.; Karaburun, A.C.; Akar, D.; Karabacak, M.; Kiraz, N. Synthesis and antifungal activities of some aryl (benzofuran-2-yl)ketoximes. Il Farmaco 2002, 57, 609–612. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, C.; Wang, W.; Che, X.; Cao, Y.; Dong, G.; Wang, S.; Ji, H.; Miao, Z.; Yao, J.; et al. Structure-based rational design, synthesis and antifungal activity of oxime-containing azole derivatives. Bioorganic Med. Chem. Lett. 2010, 20, 2942–2945. [Google Scholar] [CrossRef] [PubMed]
- Parthiban, P.; Kabilan, S.; Ramkumar, V.; Jeong, Y.T. Stereocontrolled facile synthesis and antimicrobial activity of oximes and oxime ethers of diversely substituted bispidines. Bioorganic Med. Chem. Lett. 2010, 20, 6452–6458. [Google Scholar] [CrossRef] [PubMed]
- Parthiban, P.; Aridoss, G.; Rathika, P.; Ramkumar, V.; Kabilan, S. Synthesis, spectral, crystal and antimicrobial studies of biologi-cally potent oxime ethers of nitrogen, oxygen and sulfur heterocycles. Bioorganic Med. Chem. Lett. 2009, 19, 2981–2985. [Google Scholar] [CrossRef]
- Ramalingan, C.; Park, Y.T.; Kabilan, S. Synthesis, stereochemistry, and antimicrobial evaluation of substituted piperidin-4-one oxime ethers. Eur. J. Med. Chem. 2006, 41, 683–696. [Google Scholar] [CrossRef]
- Karakurt, A.; Dalkara, S.; Ozalp, M.; Ozbey, S.; Kendi, E.; Stables, J.P. Synthesis of some 1-(2-naphthyl)-2-(imidazole-1-yl)ethanone oxime and oxime ether derivatives and their anticonvulsant and antimicrobial activities. Eur. J. Med. Chem. 2001, 36, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Takagaki, M.; Ozaki, M.; Fujimoto, S.; Fukumoto, S. Development of a novel fungicide, pyribencarb. J. Pestic. Sci. 2014, 39, 177–178. [Google Scholar] [CrossRef] [Green Version]
- Gullino, M.L.; Mesclachin, E.; Mezzalama, M. Sensivity to cymoxanil in populations of Plasmopara viticola in northern Italy. Plant Pathol. 1997, 46, 729–736. [Google Scholar] [CrossRef]
- Xie, Y.-Q.; Huang, Z.-L.; Yan, H.-D.; Li, J.; Ye, L.-Y.; Che, L.-M.; Tu, S. Design, synthesis, and biological activity of oxime ether strobilurin derivatives containing indole moiety as novel fungicide. Chem. Biol. Drug Des. 2015, 85, 743–755. [Google Scholar] [CrossRef]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Enoxastrobin (accessed on 3 January 2023).
- Tu, S.; Xie, Y.-Q.; Gui, S.-Z.; Ye, L.-Y.; Huang, Z.-L.; Huang, Y.-B.; Che, L.-M. Synthesis and fungicidal activities of novel benzothiophene-substituted oxime ether strobilurins. Bioorganic Med. Chem. Lett. 2014, 24, 2173–2176. [Google Scholar] [CrossRef]
- Zhang, T.; Xie, R.; Zhang, T.; Mei, X.; Yang, J.; Ning, J. Design, synthesis and bioactivities of novel oxime ether derivatives. J. Pestic. Sci. 2013, 38, 88–90. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.-B.; Luo, H.-A.; Wang, X.-G.; Huang, M.-Z.; Huang, L.; Pang, H.-L.; Mao, C.-H.; Pei, H.; Huang, C.-Q.; Sun, J.; et al. Synthesis and evaluation O-benzyl oxime-ether derivatives containing β-methoxyacrylate moiety for insecticidal and fungicidal activities. Bull. Korean Chem. Soc. 2014, 35, 1073–1076. [Google Scholar] [CrossRef] [Green Version]
- Wenderoth, B.; Anke, T.; Rentzea, C.; Ammermann, E.; Pommer, E.-H.; Steglich, W. Oxime Ethers and Fungicides Containing These Compounds. U.S. Patent 4,829,085, 9 May 1989. [Google Scholar]
- Sun, S.; Yan, J.; Tai, L.; Chai, J.; Hu, H.; Han, L.; Lu, A.; Yang, C.; Chen, M. Novel (Z)/(E)-1,2,4-triazole derivatives containing oxime ethers moiety as potential ergosterol biosynthesis inhibitors: Design, preparation, antifungal evaluation, and molecular docking. Mol. Divers. 2022, 27, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-Y.; Kang, G.-Q.; Huang, M.; Duan, W.-G.; Lin, G.-S.; Huang, M.; Wang, X. Synthesis, bioactivity and computational simulation study of novel (Z)-3-caren-5-one oxime ethers as potential antifungal agents. Res. Chem. Intermed. 2022, 48, 2135–2153. [Google Scholar] [CrossRef]
- Liu, S.; Qian, P.; Wan, F.-X.; Shi, Y.-H.; Jiang, L. Design, synthesis, and biological activity of novel 2-(pyridin-3-yl)etan-1-one oxime ethers bearing adamantane moiety. J. Chin. Chem. Soc. 2019, 66, 330–334. [Google Scholar] [CrossRef]
- Cortés, I.; di Liberto, M.G.; Kaufman, T.; Derita, M.G.; Bracca, A.B.J. Synthesis and evaluation of aromatic methoxime deriva-tives against five postharvest phytopathogenic fungi of fruits. Main structure-activity relationships. Food Chem. 2020, 321, 126701. [Google Scholar] [CrossRef] [PubMed]
- Dellamonica, P. Cefuroxime axetil. Int. J. Antimicrob. Agents 1994, 4, 23–36. [Google Scholar] [CrossRef]
- Walstad, R.A.; Nilsen, O.G.; Berg, K.J. Pharmacokinetics and clinical effects of cefuroxime in patients with severe renal insufficiency. Eur. J. Clin. Pharmacol. 1983, 24, 391–398. [Google Scholar] [CrossRef]
- Young, R.A.; Gonzalez, J.P.; Sorkin, E.M. Roxithromycin. A review of its antibacterial activity, pharmacokinetic properties and clinical efficacy. Drugs 1989, 37, 8–41. [Google Scholar] [CrossRef]
- Mirjafary, Z.; Abdoli, M.; Saeidian, H.; Kakanejadifard, A.; Morteza, S.; Farnia, F. Review on synthesis of acyclic and cyclic oxime ethers. RSC Adv. 2016, 6, 17740–17758. [Google Scholar] [CrossRef]
- Blondeau, J.M.; Missaghi, B. Gemifloxacin: A new fluoroquinolone. Expert Opin. Pharmacother. 2004, 5, 1117–1152. [Google Scholar] [CrossRef]
- Feng, L.; Lv, K.; Liu, M.; Wang, S.; Zhao, J.; You, X.; Li, S.; Cao, J.; Guo, H. Synthesis and in vitro antibacterial activity of gemifloxacin derivatives containing a substituted benzyloxime moiety. Eur. J. Med. Chem. 2012, 55, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Letafat, B.; Emami, S.; Mohammadhosseini, N.; Faramarzi, M.A.; Samadi, N.; Shafiee, A.; Foroumadi, A. Synthesis and antibacterial activity of new N-[2-(thiophen-3-yl)ethyl] piperazinyl quinolones. Chem. Pharm. Bull. 2007, 55, 894–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhandari, K.; Srinivas, N.; Keshava, G.B.S.; Shukla, P.K. Tetrahydronaphthyl azole oxime ethers: The conformationally rigid analogues of oxiconazole as antibacterials. Eur. J. Med. Chem. 2009, 44, 437–447. [Google Scholar] [CrossRef]
- Khan, S.A.; Asiri, A.M.; Saleem, K. Synthesis and biological evaluation of new oxime-ether derivatives of steroid as anti-bacterial agents. J. Saudi Chem. Soc. 2012, 16, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.; Lee, D.U. Eco-friendly synthesis, physicochemical studies, biological assay and molecular docking of steroidal oxime-ethers. EXCLI J. 2015, 14, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Kosmalski, T.; Kutkowska, J.; Gzella, A.K.; Nowakiewicz, A. New heterocyclic oxime ethers of 1-(benzofuran-2-yl)ethan-1-one and their antimicrobial activity. Acta Pol. Pharm. 2015, 72, 289–295. [Google Scholar] [PubMed]
- Kirilmis, C.; Koca, M.; Cukurovali, A.; Ahmedzade, M.; Kazaz, C. Synthesis, reactivity and biological activity of novel bisbenzofuran-2-yl-methanone derivatives. Molecules 2005, 10, 1399–1408. [Google Scholar] [CrossRef] [Green Version]
- Zarenezhad, E.; Sadeghian, S.; Shekoohi, K.; Emami, I.; Ghasemian, A.M.; Zarenezhad, A. Synthesis, Biological Evaluation and In Silico Studies of Oxime Ether Derivatives Containing a Quinoxaline Moiety. Russ. J. Bioorganic Chem. 2023, 49, 101–113. [Google Scholar] [CrossRef]
- Akunuri, R.; Veerareddy, V.; Kaul, G.; Akhir, A.; Unnissa, T.; Parupalli, R.; Madhavi, Y.V.; Chopra, S.; Nanduri, S. Synthesis and antibacterial evaluation of (E)-1-(1H-indol-3-yl) ethanone O-benzyl oxime derivatives against MRSA and VRSA strains. Bioorganic Chem. 2021, 116, 105288. [Google Scholar] [CrossRef]
- Swetha, Y.; Reddy, E.R.; Kumar, J.R.; Trivedi, R.; Giribabu, L.; Sridhar, B.; Rathodf, B.; Prakasham, R.S. Synthesis, characterization and antimicrobial evaluation of ferrocene–oxime ether benzyl 1H-1,2,3-triazole hybrids. New J. Chem. 2019, 43, 8341–8351. [Google Scholar] [CrossRef]
- Parthiban, P.; Rathika, P.; Ramkumar, V.; Son, S.M.; Jeong, Y.T. Stereospecific synthesis of oximes and oxime ethers of 3-azabicycles: A SAR study towards antimicrobial agents. Bioorganic Med. Chem. Lett. 2010, 20, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- Soltani Rad, M.N.; Behrouz, S.; Zarenezhad, E.; Moslemin, M.H.; Zarenezhad, A.; Mardkhoshnood, M.; Behrouz, M.; Rostami, S. Synthesis of fluorene and/or benzophenone O-oxime ethers containing amino acid residues and study of their cardiovascular and antibacterial effects. Med. Chem. Res. 2014, 23, 3810–3822. [Google Scholar] [CrossRef]
- Reddy, D.S.; Kongot, M.; Netalkar, S.P.; Kurjogi, M.M.; Kumar, R.; Avecilla, F.; Kumar, A. Synthesis and evaluation of novel coumarin-oxime ethers as potential anti-tubercular agents: Their DNA cleavage ability and BSA interaction study. Eur. J. Med. Chem. 2018, 150, 864–875. [Google Scholar] [CrossRef] [PubMed]
- Chern, J.-H.; Lee, C.-C.; Chang, C.-S.; Lee, Y.-C.; Tai, C.-L.; Lin, Y.-T.; Shia, K.-S.; Lee, C.-Y.; Shih, S.-R. Synthesis and antienteroviral activity of a series of novel, oxime ether-containing pyridyl imidazolidinones. Bioorganic Med. Chem. Lett. 2004, 14, 5051–5056. [Google Scholar] [CrossRef] [PubMed]
- Barnard, D.L.; Hubbard, V.D.; Smee, D.F.; Sidwell, R.W.; Watson, K.G.W.; Tucker, S.P.T.; Reece, P.A.R. In vitro activity of expanded-spectrum pyridazinyl oxime ethers related to Pirodavir: Novel capsid-binding inhibitors with potent antipicornavirus activity. Antimicrob. Agents Chemother. 2004, 48, 1766–1772. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Zhou, M.; Cui, X.; Wei, Z.; Wei, W. Discovery of oxime ethers as Hepatitis B Virus (HBV) inhibitors by docking, screening and in vitro investigation. Molecules 2018, 23, 637. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Wang, X.; Tang, X.; Xia, R.; Guo, T.; Zhang, C.; Li, X.; Xue, W. Design, synthesis, antiviral bioactivities and interaction mechanisms of penta-1,4-diene-3-one oxime ether derivatives containing a quinazolin-4(3H)-one scaffold. BMC Chem. 2019, 13, 34. [Google Scholar] [CrossRef] [Green Version]
- Ohsumi, T.; Hatakoshi, M.; Kisida, H.; Matsuo, N.; Nakayama, I.; Itaya, N. Oxime ethers: New potent insect growth regulators. Agric. Biol. Chem. 1985, 49, 3197–3202. [Google Scholar] [CrossRef]
- Dai, H.; Chen, J.; Li, H.; Dai, B.; He, H.; Fang, Y.; Shi, Y. Synthesis and bioactivities of novel pyrazole oxime derivatives containing a 5-trifluoromethylpyridyl moiety. Molecules 2016, 21, 276. [Google Scholar] [CrossRef] [Green Version]
- Motoba, K.; Nishizawa, H.; Suzuki, T.; Hamaguchi, H.; Uchida, M.; Funayama, S. Species-specific detoxification metabolism of Fenpyroximate, a potent acaracide. Pestic. Biochem. Physiol. 2000, 67, 73–84. [Google Scholar] [CrossRef]
- Liu, A.; Ou, X.; Huang, M.; Wang, X.; Liu, X.; Wang, Y.; Chen, C.; Yao, J. Synthesis and insecticidal activities of novel oxime ether pyrethroids. Pest Manag. Sci. 2005, 61, 166–170. [Google Scholar] [CrossRef]
- Abid, M.; Husain, K.; Azam, A. Synthesis and antiamoebic activity of new oxime ether derivatives containing 2-acetylpyridine/2-acetylfuran. Bioorganic Med. Chem. Lett. 2005, 15, 4375–4379. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Chen, J.; Li, G.; Ge, S.; Shi, Y.; Fang, Y.; Ling, Y. Design, synthesis, and bioactivities of novel oxadiazole-substituted pyrazole oximes. Bioorganic Med. Chem. Lett. 2017, 27, 950–953. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Zheng, Y.; Wei, X.; Ma, Q.; Wei, P.; Liu, Y.; Qin, Y.; Yang, N.; Sun, Y.; et al. Design, Synthesis, Acaricidal Activity, and Mechanism of Oxazoline Derivatives Containing an Oxime Ether Moiety. J. Agric. Food Chem. 2014, 62, 3064–3072. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, A.; Xu, H.; Mo, Y.; Chen, J.; Shen, L. Design, Synthesis, and Bioassay of Novel Compounds of Isolongifolenone Oxime Derivatives. Helv. Chim. Acta 2016, 99, 696–703. [Google Scholar] [CrossRef]
- Mäntylä, A.; Rautio, J.; Navalainen, T.; Vepsälainen, J.; Juvonen, R.; Kendrick, H.; Garnier, T.; Croft, S.L.; Järvinen, T. Synthesis and antileishmenial activity of novel buparvaquone oxime derivatives. Bioorganic Med. Chem. 2004, 12, 3497–3502. [Google Scholar] [CrossRef] [PubMed]
- Sukhatme, V.P.; Reiersen, A.M.; Vayttaden, S.J.; Sukhatme, V.V. Fluvoxamine: A Review of Its Mechanism of Action and Its Role in COVID-19. Front. Pharmacol. 2021, 20, 652688. [Google Scholar] [CrossRef]
- Delgado, P.L.; Price, L.H.; Charney, D.S.; Heninger, G.R. Efficacy of fluvoxamine in treatment-refractory depression. J. Affect. Disord. 1988, 15, 55–60. [Google Scholar] [CrossRef]
- Facente, S.N.; Reiersen, A.M.; Lenze, E.J.; Boulware, D.R.; Klausner, J.D. Fluvoxamine for the Early Treatment of SARS-CoV-2 Infection: A Review of Current Evidence. Drugs 2021, 81, 2081–2089. [Google Scholar] [CrossRef]
- Mahdi, M.; Hermán, L.; Réthelyi, J.M.; Bálint, B.L. Potential Role of the Antidepressants Fluoxetine and Fluvoxamine in the Treatment of COVID-19. Int. J. Mol. Sci. 2022, 23, 3812. [Google Scholar] [CrossRef]
- Lingjærde, O.; Asker, T.; Bugge, A.; Engstrand, E.; Eide, Å.; Grinaker, H.; Herlofsen, H.; Ose, E.; Øfsti, E. Noxiptilin (Agedal)—A new tricyclic antidepressant with a faster onset of action? A double-blind, multicentre comparison with amitriptyline. Pharmacopsychiatry 1975, 8, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Merkaš, S.; Litvić, M.; Cepanec, I.; Vinković, V. Synthesis of Novel, Potentially Biologically Active Dibenzosubereonone Derivatives. Molecules 2005, 10, 1429–1437. [Google Scholar] [CrossRef] [Green Version]
- Schutz, S.; Behner, O.; Hoffmeister, F. Basic Oximes and Their Preparation. U.S. Patent 3,989,844, 2 November 1976. [Google Scholar]
- Bozdag, O.; Gümüse, B.; Demirdamar, R.; Büyükbingöl, E.; Rolland, Y.; Ertan, R. Synthesis of some novel oxime ether derivatives and their activity in the ‘behavioral despair test’. Eur. J. Med. Chem. 1998, 33, 133–141. [Google Scholar] [CrossRef]
- Welle, H.B.A.; Claassen, V. Oxime Ethers Having Anti-Depressive Activity. U.S. Patent 4,081,551, 28 March 1978. [Google Scholar]
- de Sousa, D.P.; Schefer, R.R.; Brocksom, U.; Brocksom, T.J. Synthesis and antidepressant evaluation of three para-benzoquinone mono-oximes and their oxy derivatives. Molecules 2006, 11, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Nencetti, S.; Mazzoni, M.R.; Ortore, G.; Lapucci, A.; Giuntini, J.; Orlandini, E.; Banti, I.; Nuti, E.; Lucacchini, A.; Giannaccini, G.; et al. Synthesis, molecular docking and binding studies of selective serotonin transporter inhibitors. Eur. J. Med. Chem. 2011, 46, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.; Kebriaeezadeh, A.; Ahangar, N.; Khorasani, R. Imidazolylchromanone oxime ethers as potential anticonvulsant agents: Anticonvulsive evaluation in PTZ-kindling model of epilepsy and SAR study. Bioorganic Med. Chem. Lett. 2011, 21, 655–659. [Google Scholar] [CrossRef]
- Knutsen, L.J.S.; Andersen, K.E.; Lau, J.; Lundt, B.F.; Henry, R.F.; Morton, H.E.; Nærum, L.; Petersen, H.; Stephensen, H.; Suzdak, P.D.; et al. Synthesis of novel GABA uptake inhibitors. 3. Diaryloxime and diarylvinyl ether derivatives of nipecotic acid and guvacine as anticonvulsant agents. J. Med. Chem. 1999, 42, 3447–3462. [Google Scholar] [CrossRef]
- Alexander, S.P.; Mathie, A.; Peters, J.A. Guide to receptors and channels, 1st edition. Br. J. Pharmacol. 2004, 141, S1–S3. [Google Scholar] [CrossRef]
- Himber, J.; Sallee, V.L.; Andermann, G.; Bouzoubaa, M.; Leclerc, G.; De Santis, L. Effects of topically apllied falintolol: A new beta-adrenergic antagonist for treatment of glaucoma. J. Ocul. Pharmacol. 1987, 3, 111–120. [Google Scholar] [CrossRef]
- Ghabbour, H.A.; El-Bendary, E.R.; El-Ashmawy, M.B.; El-Kerdawy, M.M. Synthesis, docking study and β-adrenoceptor activity of some new oxime ether derivatives. Molecules 2014, 19, 3417–3435. [Google Scholar] [CrossRef] [Green Version]
- Angelone, T.; Caruso, A.; Rochais, C.; Caputo, A.M.; Cerra, M.C.; Dallemagne, P.; Filice, E.; Genest, D.; Pasqua, T.; Puoci, F.; et al. Indenopyrazole oxime ethers: Synthesis and β1-adrenergic blocking activity. Eur. J. Med. Chem. 2015, 92, 672–681. [Google Scholar] [CrossRef]
- Bai, R.-R.; Xu, S.-T.; Liu, J.; Hong, W.; Tang, Y.-Q.; Wu, X.-M.; Xie, W.-J.; Yao, H.-Q.; Xu, J.-Y. Synthesis and β-adrenergic blocking activity of oxime ether hybrids derived from a natural isochroman-4-one. Chin. J. Nat. Med. 2013, 11, 538–545. [Google Scholar] [CrossRef]
- Tandon, V.K.; Kumar, M.; Awasthi, A.K.; Saxena, H.O.; Goswamy, G.K. Potential hypotensive agents: Synthesis and hypotensive activity of oxime ethers derived from 1-naphthoxepines and related compounds. Bioorganic Med. Chem. Lett. 2004, 14, 3177–3180. [Google Scholar] [CrossRef]
- Carty, E.; Macey, M.; McCartney, S.A.; Rampton, D.S. Ridogrel, a dual thromboxane synthase inhibitor and receptor antagonist: Anti-Inflammatory profile in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2000, 14, 807–817. [Google Scholar] [CrossRef]
- Gannarapu, M.R.; Vasamsetti, S.B.; Punna, N.; Royya, N.K.; Pamulaparthy, S.R.; Nanubolu, J.B.; Kotamraju, S.; Banda, N. Synthesis of novel 1,2-benzothiazine 1,1-dioxide-3-ethanone oxime N-aryl acetamide ether derivatives as potent anti-inflammatory agents and inhibitors of monocyte-to-macrophage transformation. Eur. J. Med. Chem. 2014, 75, 143–150. [Google Scholar] [CrossRef]
- El-Gamal, M.I.; Bayomi, S.M.; El-Ashry, S.M.; Said, S.A.; Abdel-Aziz, A.A.-M.; Abdel-Aziz, N.I. Synthesis and anti-inflammatory activity of novel (substituted)benzylidene acetone oxime ether derivatives: Molecular modeling study. Eur. J. Med. Chem. 2010, 45, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Han, H.O.; Kim, S.H.; Kim, K.-H.; Hur, G.-G.; Yim, H.J.; Chung, H.-K.; Woo, S.H.; Koo, K.D.; Lee, C.h.-S.; Koh, J.S.; et al. Design and synthesis of oxime ethers of α-acyl-β-phenylpropanoic acids as PPAR dual agonists. Bioorganic Med. Chem. Lett. 2007, 17, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Hurtevent, A.; Le Naour, M.; Leclerc, V.; Carato, P.; Melnyk, P.; Hennuyer, N.; Staels, B.; Beucher-Gaudin, M.; Caignard, D.-H.; Dacquet, C.; et al. Effect of 6-Benzoyl-benzothiazol-2-one scaffold on the pharmacological profile of α-alkoxyphenylproponic acid derived PPAR agonists. J. Enzym. Inhib. Med. Chem. 2020, 35, 524–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makadia, P.; Shah, S.R.; Pingali, H.; Zaware, P.; Patel, D.; Pola, S.; Thube, B.; Priyadarshini, P.; Suthar, D.; Shah, M.; et al. Effect of structurally constrained oxime-ether linker on PPAR subtype selectivity: Discovery of a novel and potent series of PPAR-pan agonists. Bioorganic Med. Chem. 2011, 19, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Surkau, G.; Böhm, K.J.; Müller, K.; Prinz, H. Synthesis, antiproliferative activity and inhibition of tubulin polymerization by anthracenone-based oxime derivatives. Eur. J. Med. Chem. 2010, 45, 3354–3364. [Google Scholar] [CrossRef]
- Park, H.-J.; Lee, K.; Park, S.-J.; Ahn, B.; Lee, J.-C.; Cho, H.; Lee, K.-I. Identification of antitumor activity of pyrazole oxime ethers. Bioorganic Med. Chem. Lett. 2005, 15, 3307–3312. [Google Scholar] [CrossRef]
- Vágvölgyi, M.; Martins, A.; Kulmány, Á.; Zupkó, I.; Gáti, T.; Simon, A.; Tóth, G.; Hunyadi, A. Nitrogen-containing ecdysteroid derivatives vs. multi-drug resistance in cancer: Preparation and antitumor activity of oximes, oxime ethers and a lactam. Eur. J. Med. Chem. 2018, 144, 730–739. [Google Scholar] [CrossRef] [Green Version]
- Jindal, D.P.; Chattopadhaya, R.; Guleria, S.; Gupta, R. Synthesis and antineoplastic activity of 2-alkylaminoethyl derivatives of various steroidal oximes. Eur. J. Med. Chem. 2003, 38, 1025–1034. [Google Scholar] [CrossRef]
- Ajduković, J.J.; Jakimov, D.S.; Rárova, L.; Strnad, M.; Dzichenka, Y.U.; Usanov, S.; Škorić, D.Đ.; Jovanović-Šanta, S.S.; Sakač, M.N. Novel alkylaminoethyl derivatives of adrostane 3-oximes as anticancer candidates: Synthesis and evaluation of cytotoxic effects. RSC Adv. 2021, 11, 37449–37461. [Google Scholar] [CrossRef] [PubMed]
- Berényi, Á.; Minorics, R.; Iványi, Z.; Ocsovszki, I.; Ducza, E.; Thole, H.; Messinger, J.; Wölfling, J.; Mótyán, G.; Mernyák, E.; et al. Synthesis and investigation of the anticancer effects of estrone-16-oxime ethers in vitro. Steroids 2013, 78, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Díaz, J.E.; Martinez, D.C.; López, L.V.; Mendez, G.M.; Vera, R.; Loaiza, A.E. Synthesis and in vitro antiproliferative activity of flavone and 6-hydroxyflavone oxime ethers derivatives. J. Braz. Chem. Soc. 2018, 29, 177–184. [Google Scholar] [CrossRef]
- Latif, A.D.; Gonda, T.; Vágvölgyi, M.; Kúsz, N.; Kulmány, Á.; Ocsovszki, I.; Zomborszki, Z.P.; Zupkó, I.; Hunyadi, A. Synthesis and in vitro antitumor activity of naringenin oxime and oxime ether derivatives. Int. J. Mol. Sci. 2019, 20, 2184. [Google Scholar] [CrossRef] [Green Version]
- Chakravarti, B.; Akhtar, T.; Rai, B.; Yadav, M.; Siddiqui, J.A.; Dwivedi, S.K.D.; Thakur, R.; Singh, A.K.; Kumar, H.; Khan, K.; et al. Thioaryl naphthylmethanone oxime ether analogs as novel anticancer agents. J. Med. Chem. 2014, 57, 8010–8025. [Google Scholar] [CrossRef]
- Su, S.; Chen, M.; Li, Q.; Wang, Y.; Chen, S.; Sun, N.; Xie, C.; Huai, Z.; Huang, Y.; Xue, W. Novel penta-1,4-diene-3-one derivatives containing quinazoline and oxime ether fragments: Design, synthesis and bioactivity. Bioorganic Med. Chem. 2021, 32, 115999. [Google Scholar] [CrossRef]
- Kim, T.; Kim, H.-I.; An, J.-Y.; Lee, J.; Lee, N.-R.; Heo, J.; Kim, J.-E.; Yu, J.; Lee, Y.S.; Inn, K.-S.; et al. Identification of novel estrogen receptor (ER) agonists that have additional and complementary anti-cancer activities via ER-independent mechanism. Bioorganic Med. Chem. Lett. 2016, 26, 1844–1848. [Google Scholar] [CrossRef]
- Kosmalski, T.; Hetmann, A.; Studzińska, R.; Baumgart, S.; Kupczyk, D.; Roszek, K. The Oxime Ethers with Heterocyclic, Alicyclic and Aromatic moiety as Potentail Anti-Cancer Agents. Molecules 2022, 27, 1374. [Google Scholar] [CrossRef]
- Kim, G.H. Proton Pump Inhibitor-Related Gastric Mucosal Changes. Gut Liver. 2021, 15, 646–652. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Song, Y.; Zhang, J.; Chen, L.; Wang, J. Synthesis and in vitro anti-ulcer effect of bisabolangelone oxime ether derivatives. Adv. Mater. Res. 2013, 781–784, 1122–1125. [Google Scholar] [CrossRef]
- Varache-Lembège, M.; Nuhrich, A.; Renard, P.; Duboudin, F.; Vercauteren, J.; Devaux, G. Platelet antiaggregant methoxyphenylthienyl ketoxime ethers: Synthesis and structure-activity relationships. Arch. Pharm. 1995, 328, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.M.; Rangarajan, T.M.; Chaudhary, R.; Singh, R.P.; Singh, M.; Singh, R.P.; Tondo, A.R.; Gambacorta, N.; Nicolotti, O.; Mathew, B.; et al. Novel class of chalcone oxime ethers as potent monoamine oxidase-B and acetylcholinesterase inhibitors. Molecules 2020, 25, 2356. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ma, M.; Sun, L.; Zeng, Z.; Jiang, H. Synthesis, herbicidal evaluation, and structure-activity relationship of benzophenone oxime ether derivatives. J. Chem. 2015, 2015, 435219. [Google Scholar] [CrossRef] [Green Version]














Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosmalski, T.; Kupczyk, D.; Baumgart, S.; Paprocka, R.; Studzińska, R. A Review of Biologically Active Oxime Ethers. Molecules 2023, 28, 5041. https://doi.org/10.3390/molecules28135041
Kosmalski T, Kupczyk D, Baumgart S, Paprocka R, Studzińska R. A Review of Biologically Active Oxime Ethers. Molecules. 2023; 28(13):5041. https://doi.org/10.3390/molecules28135041
Chicago/Turabian StyleKosmalski, Tomasz, Daria Kupczyk, Szymon Baumgart, Renata Paprocka, and Renata Studzińska. 2023. "A Review of Biologically Active Oxime Ethers" Molecules 28, no. 13: 5041. https://doi.org/10.3390/molecules28135041
APA StyleKosmalski, T., Kupczyk, D., Baumgart, S., Paprocka, R., & Studzińska, R. (2023). A Review of Biologically Active Oxime Ethers. Molecules, 28(13), 5041. https://doi.org/10.3390/molecules28135041





