Nonaqueous Capillary Electrophoretic Separation of Analogs of (24R)-1,24-Dihydroxyvitamin D3 Derivative as Predicted by Quantum Chemical Calculations
Abstract
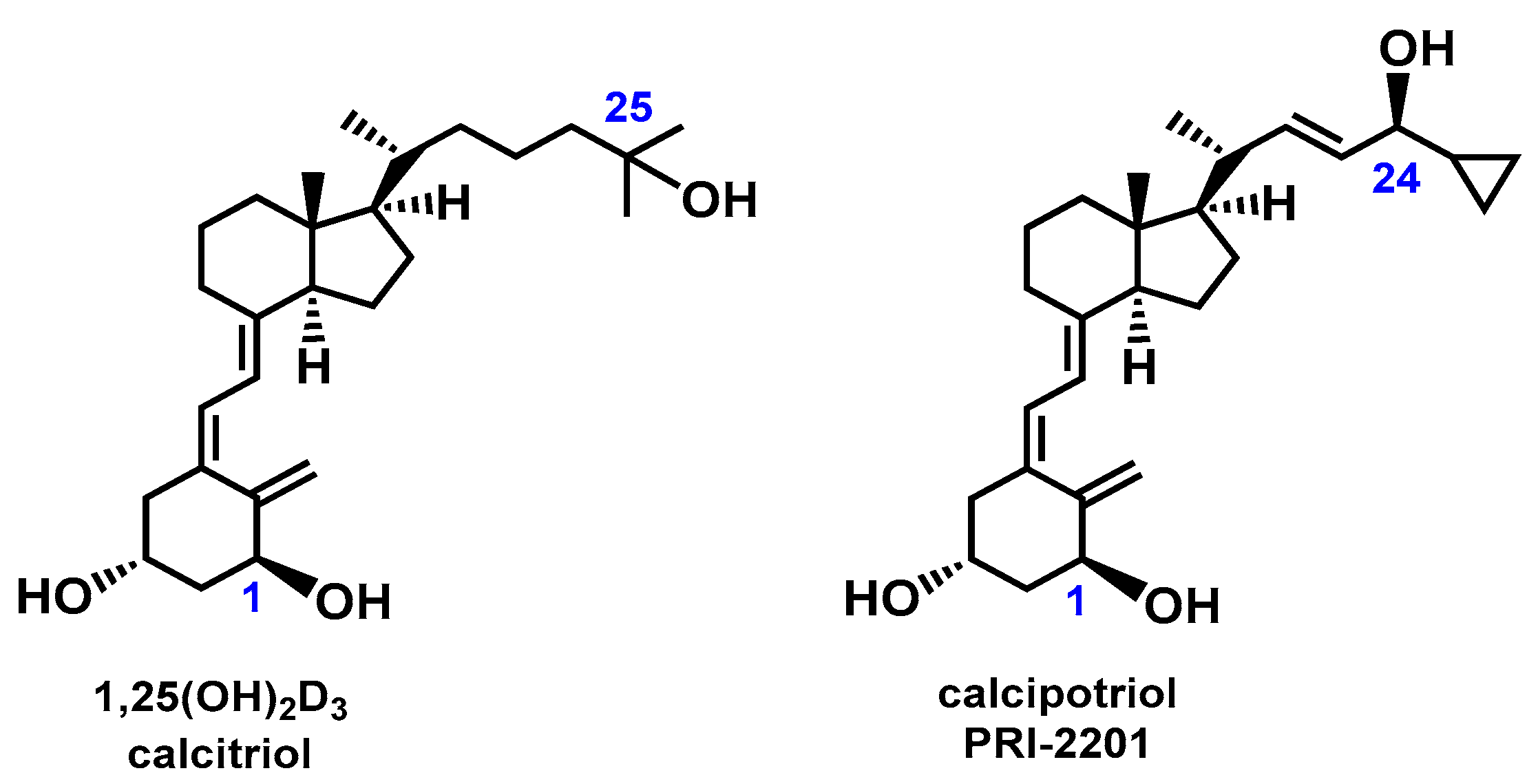
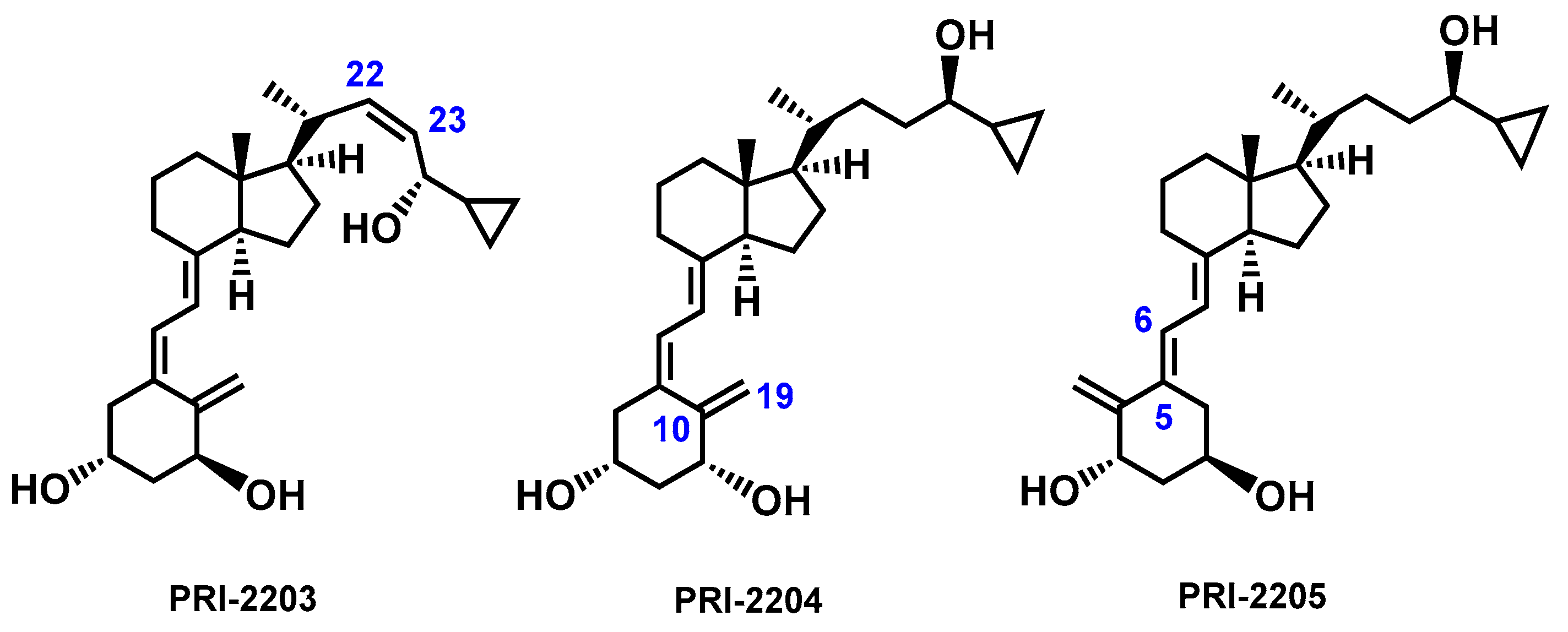
1. Introduction
2. Results and Discussion
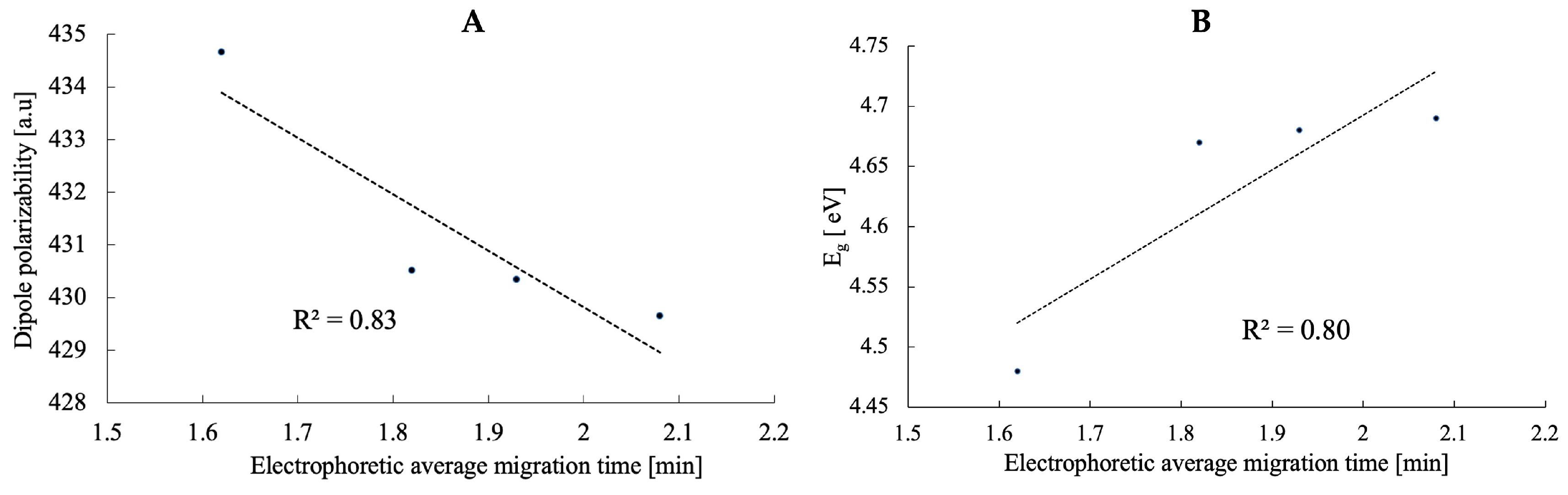
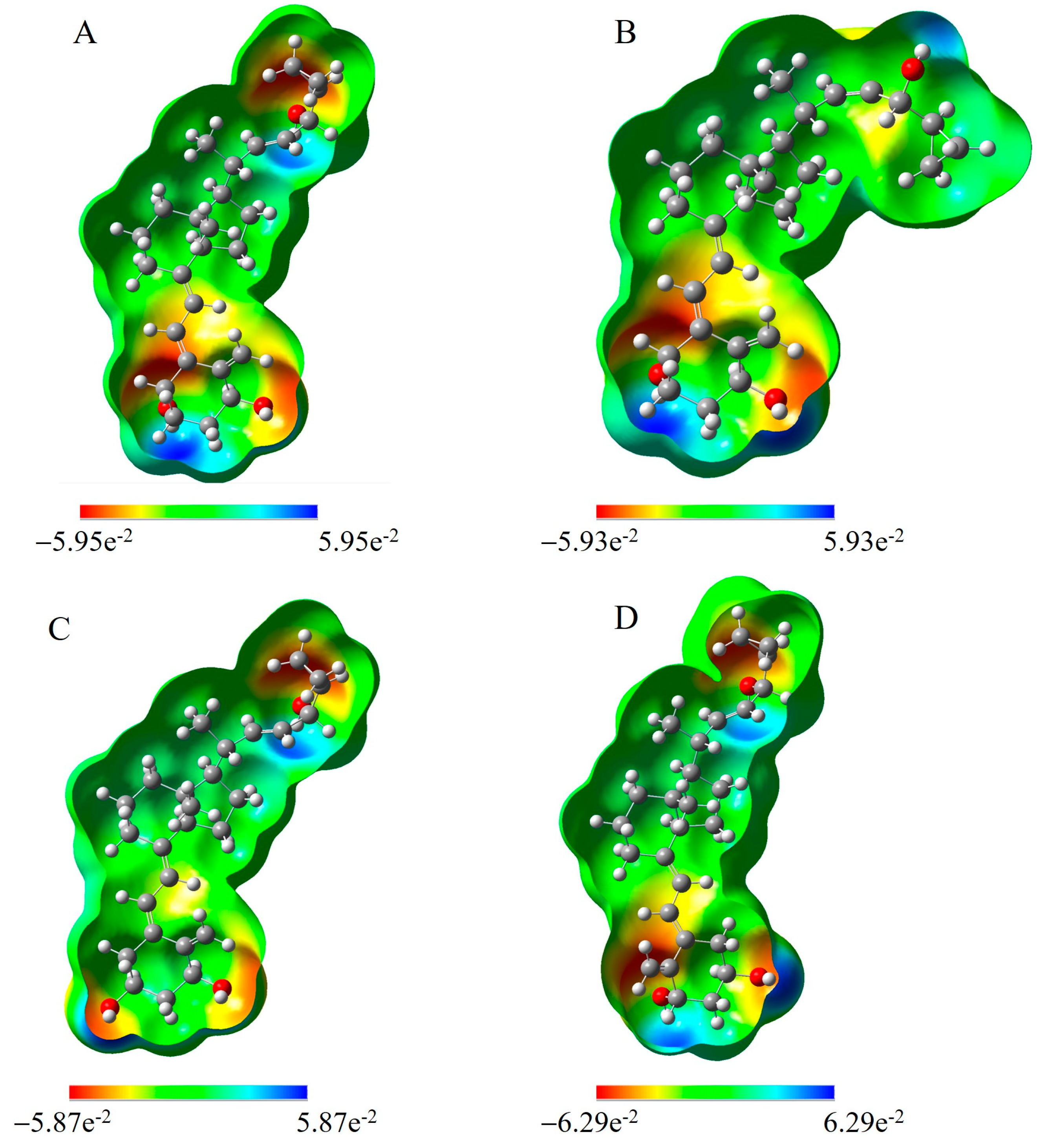
2.1. Electronic Properties of Vitamin D3 Analogs
2.2. Development of Capillary Electrophoresis
3. Materials and Methods
3.1. Quantum Chemical Calculations
3.2. Chemicals and Reagents
3.3. Instrumentation
3.4. CE Conditions
3.5. Preparation of Stock and Working Standard Solutions
3.6. Method Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lehmann, B. The vitamin D3 pathway in human skin and its role for regulation of biological processes. Photochem. Photobiol. 2005, 81, 1246–1251. [Google Scholar] [CrossRef]
- Sanchez, B.; Lopez-Martin, E.; Segura, C.; Labandeira-Garcia, J.L.; Perez-Fernandez, R. 1,25-Dihydroxyvitamin D(3) increases striatal GDNF mRNA and protein expression in adult rats. Brain Res. Mol. Brain Res. 2002, 108, 143–146. [Google Scholar] [CrossRef]
- Kamei, Y.; Kawada, T.; Fukuwatari, T.; Ono, T.; Kato, S.; Sugimoto, E. Cloning and sequencing of the gene encoding the mouse vitamin D receptor. Gene 1995, 152, 281–282. [Google Scholar] [CrossRef]
- Losel, R.; Wehling, M. Nongenomic actions of steroid hormones. Nat. Rev. Mol. Cell Biol. 2003, 4, 46–56. [Google Scholar] [CrossRef]
- Norman, A.W.; Ishizuka, S.; Okamura, W.H. Ligands for the vitamin D endocrine system: Different shapes function as agonists and antagonists for genomic and rapid response receptors or as a ligand for the plasma vitamin D binding protein. J. Steroid Biochem. Mol. Biol. 2001, 76, 49–59. [Google Scholar] [CrossRef]
- Brown, A.J.; Dusso, A.; Slatopolsky, E. Vitamin D. Am. J. Physiol. 1999, 277, F157–F175. [Google Scholar] [CrossRef]
- Losel, R.; Feuring, M.; Wehling, M. Non-genomic aldosterone action: From the cell membrane to human physiology. J. Steroid Biochem. Mol. Biol. 2002, 83, 167–171. [Google Scholar] [CrossRef]
- Wali, R.K.; Kong, J.; Sitrin, M.D.; Bissonnette, M.; Li, Y.C. Vitamin D receptor is not required for the rapid actions of 1,25-dihydroxyvitamin D3 to increase intracellular calcium and activate protein kinase C in mouse osteoblasts. J. Cell Biochem. 2003, 88, 794–801. [Google Scholar] [CrossRef]
- Boyan, B.D.; Bonewald, L.F.; Sylvia, V.L.; Nemere, I.; Larsson, D.; Norman, A.W.; Rosser, J.; Dean, D.D.; Schwartz, Z. Evidence for distinct membrane receptors for 1 alpha,25-(OH)(2)D(3) and 24R,25-(OH)(2)D(3) in osteoblasts. Steroids 2002, 67, 235–246. [Google Scholar] [CrossRef]
- Çaykara, B.; Öztürk, G.; Mutlu, H.H.; Arslan, E. Relationship Between Vitamin D, Calcium, and Phosphorus Levels. J. Acad. Res. Med. 2020, 10, 252–257. [Google Scholar]
- Khammissa, R.A.G.; Fourie, J.; Motswaledi, M.H.; Ballyram, R.; Lemmer, J.; Feller, L. The Biological Activities of Vitamin D and Its Receptor in Relation to Calcium and Bone Homeostasis, Cancer, Immune and Cardiovascular Systems, Skin Biology, and Oral Health. Biomed. Res. Int. 2018, 2018, 9276380. [Google Scholar] [CrossRef]
- Garcion, E.; Sindji, L.; Nataf, S.; Brachet, P.; Darcy, F.; Montero-Menei, C.N. Treatment of experimental autoimmune encephalomyelitis in rat by 1,25-dihydroxyvitamin D3 leads to early effects within the central nervous system. Acta Neuropathol. 2003, 105, 438–448. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Hayes, C.E.; DeLuca, H.F. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc. Natl. Acad. Sci. USA 1996, 93, 7861–7864. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Woodward, W.D.; Hayes, C.E.; DeLuca, H.F. 1,25-dihydroxyvitamin D3 is a positive regulator for the two anti-encephalitogenic cytokines TGF-beta 1 and IL-4. J. Immunol. 1998, 160, 5314–5319. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre d’Hellencourt, C.; Montero-Menei, C.N.; Bernard, R.; Couez, D. Vitamin D3 inhibits proinflammatory cytokines and nitric oxide production by the EOC13 microglial cell line. J.Neurosci. Res. 2003, 71, 575–582. [Google Scholar] [CrossRef]
- Garcion, E.; Wion-Barbot, N.; Montero-Menei, C.N.; Berger, F.; Wion, D. New clues about vitamin D functions in the nervous system. Trends Endocrinol. Metab. 2002, 13, 100–105. [Google Scholar] [CrossRef]
- Regulska, M.; Leskiewicz, M.; Budziszewska, B.; Kutner, A.; Basta-Kaim, A.; Kubera, M.; Jaworska-Feil, L.; Lason, W. Involvement of PI3-K in neuroprotective effects of the 1,25-dihydroxyvitamin D3 analogue-PRI-2191. Pharmacol. Rep. 2006, 58, 900–907. [Google Scholar] [PubMed]
- Regulska, M.; Leskiewicz, M.; Budziszewska, B.; Kutner, A.; Jantas, D.; Basta-Kaim, A.; Kubera, M.; Jaworska-Feil, L.; Lason, W. Inhibitory effects of 1,25-dihydroxyvitamin D3 and its low-calcemic analogues on staurosporine-induced apoptosis. Pharmacol. Rep. 2007, 59, 393–401. [Google Scholar]
- Kajta, M.; Makarewicz, D.; Zieminska, E.; Jantas, D.; Domin, H.; Lason, W.; Kutner, A.; Lazarewicz, J.W. Neuroprotection by co-treatment and post-treating with calcitriol following the ischemic and excitotoxic insult in vivo and in vitro. Neurochem. Int. 2009, 55, 265–274. [Google Scholar] [CrossRef]
- Brewer, L.D.; Thibault, V.; Chen, K.C.; Langub, M.C.; Landfield, P.W.; Porter, N.M. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J.Neurosci. 2001, 21, 98–108. [Google Scholar] [CrossRef]
- Ibi, M.; Sawada, H.; Nakanishi, M.; Kume, T.; Katsuki, H.; Kaneko, S.; Shimohama, S.; Akaike, A. Protective effects of 1 alpha,25-(OH)(2)D(3) against the neurotoxicity of glutamate and reactive oxygen species in mesencephalic culture. Neuropharmacology 2001, 40, 761–771. [Google Scholar] [CrossRef]
- Shin, M.H.; Holmes, M.D.; Hankinson, S.E.; Wu, K.; Colditz, G.A.; Willett, W.C. Intake of dairy products, calcium, and vitamin D and risk of breast cancer. J. Natl. Cancer Inst. 2002, 94, 1301–1311. [Google Scholar] [CrossRef]
- Grant, W.B.; Garland, C.F. Evidence supporting the role of vitamin D in reducing the risk of cancer. J. Intern. Med. 2002, 252, 178–179, author reply 179–180. [Google Scholar] [CrossRef]
- Guyton, K.Z.; Kensler, T.W.; Posner, G.H. Vitamin D and vitamin D analogs as cancer chemopreventive agents. Nutr. Rev. 2003, 61, 227–238. [Google Scholar] [CrossRef]
- Grant, W.B. Ecologic studies of solar UV-B radiation and cancer mortality rates. Recent Results Cancer Res. 2003, 164, 371–377. [Google Scholar] [CrossRef]
- Pietraszek, A.; Malinska, M.; Chodynski, M.; Krupa, M.; Krajewski, K.; Cmoch, P.; Wozniak, K.; Kutner, A. Synthesis and crystallographic study of 1,25-dihydroxyergocalciferol analogs. Steroids 2013, 78, 1003–1014. [Google Scholar] [CrossRef]
- Wietrzyk, J.; Chodynski, M.; Fitak, H.; Wojdat, E.; Kutner, A.; Opolski, A. Antitumor properties of diastereomeric and geometric analogs of vitamin D3. Anticancer Drugs 2007, 18, 447–457. [Google Scholar] [CrossRef]
- Milczarek, M.; Chodynski, M.; Filip-Psurska, B.; Martowicz, A.; Krupa, M.; Krajewski, K.; Kutner, A.; Wietrzyk, J. Synthesis and Biological Activity of Diastereomeric and Geometric Analogs of Calcipotriol, PRI-2202 and PRI-2205, Against Human HL-60 Leukemia and MCF-7 Breast Cancer Cells. Cancers 2013, 5, 1355–1378. [Google Scholar] [CrossRef]
- Milczarek, M.; Psurski, M.; Kutner, A.; Wietrzyk, J. Vitamin D analogs enhance the anticancer activity of 5-fluorouracil in an in vivo mouse colon cancer model. BMC Cancer 2013, 13, 294. [Google Scholar] [CrossRef]
- Brunetto, M.R.; Obando, M.A.; Gallignani, M.; Alarcon, O.M.; Nieto, E.; Salinas, R.; Burguera, J.L.; Burguera, M. HPLC determination of Vitamin D(3) and its metabolite in human plasma with on-line sample cleanup. Talanta 2004, 64, 1364–1370. [Google Scholar] [CrossRef]
- Temova, Z.; Roskar, R. Stability-Indicating HPLC-UV Method for Vitamin D3 Determination in Solutions, Nutritional Supplements and Pharmaceuticals. J. Chromatogr. Sci. 2016, 54, 1180–1186. [Google Scholar] [CrossRef]
- Rashidi, L.; Rashidi Nodeh, H.; Shahabuddin, S. Determination of Vitamin D3 in the Fortified Sunflower Oil: Comparison of Two Developed Methods. Food Anal. Methods 2022, 15, 330–337. [Google Scholar] [CrossRef]
- Craige Trenerry, V.; Plozza, T.; Caridi, D.; Murphy, S. The determination of vitamin D3 in bovine milk by liquid chromatography mass spectrometry. Food Chem. 2011, 125, 1314–1319. [Google Scholar] [CrossRef]
- Pedersen-Bjergaard, S.; Einar Rasmussen, K.; Tilander, T. Separation of fat-soluble vitamins by hydrophobic interaction electrokinetic chromatography with tetradecylammonium ions as pseudostationary phase. J. Chromatogr. A 1998, 807, 285–295. [Google Scholar] [CrossRef]
- Odrzywolska, M.; Chodynski, M.; Halkes, S.J.; van de Velde, J.P.; Fitak, H.; Kutner, A. Convergent synthesis, chiral HPLC, and vitamin D receptor affinity of analogs of 1,25-dihydroxycholecalciferol. Chirality 1999, 11, 249–255. [Google Scholar] [CrossRef]
- Odrzywolska, M.; Chodynski, M.; Zorgdrager, J.; Van De Velde, J.P.; Kutner, A. Diastereoselective synthesis, binding affinity for vitamin D receptor, and chiral stationary phase chromatography of hydroxy analogs of 1,25-dihydroxycholecalciferol and 25-hydroxycholecalciferol. Chirality 1999, 11, 701–706. [Google Scholar] [CrossRef]
- Kenndler, E. A critical overview of non-aqueous capillary electrophoresis. Part I: Mobility and separation selectivity. J. Chromatogr. A 2014, 1335, 16–30. [Google Scholar] [CrossRef]
- Zolek, T.; Yasuda, K.; Brown, G.; Sakaki, T.; Kutner, A. In Silico Prediction of the Metabolic Resistance of Vitamin D Analogs against CYP3A4 Metabolizing Enzyme. Int. J. Mol. Sci. 2022, 23, 7845. [Google Scholar] [CrossRef]
- Jyoti; Dmitrieva, E.; Zlolek, T.; Maciejewska, D.; Rybakiewicz-Sekita, R.; Kutner, W.; Noworyta, K.R. An insight into the polymerization process of the selected carbazole derivatives—Why does it not always lead to a polymer formation? Electrochim. Acta 2022, 429, 140948. [Google Scholar] [CrossRef]
- Karakas, A.; Karakaya, M.; Ceylan, Y.; El Kouari, Y.; Taboukhat, S.; Boughaleb, Y.; Sofiani, Z. Ab-initio and DFT methodologies for computing hyperpolarizabilities and susceptibilities of highly conjugated organic compounds for nonlinear optical applications. Opt. Mater. 2016, 56, 8–17. [Google Scholar] [CrossRef]
- Khajehzadeh, M.; Sadeghi, N. Molecular structure, X-ray crystallography, spectroscopic characterization, solvent effect, NLO, NBO, FMO analysis of [Cu(bpabza)] complexe. J. Mol. Liq. 2018, 249, 281–293. [Google Scholar] [CrossRef]
- Hinchliffe, A.; Perez, J.J.; Machado, H.J.S. Density functional studies of molecular polarizabilities. Part 4: The C10H8 molecules azulene, fulvalene and naphthalene. Electron. J. Theor. Chem. 1997, 2, 325–336. [Google Scholar] [CrossRef]
- Chattaraj, P.K.; Sengupta, S. Popular electronic structure principles in a dynamical context. J. Phys. Chem. 1996, 100, 16126–16130. [Google Scholar] [CrossRef]
- Kurtz, H.A.; Dudis, D.S. Quantum Mechanical Methods for Predicting Nonlinear Optical Properties. Rev. Comput. Chem. 1998, 12, 241–280. [Google Scholar]
- Fleming, I. Molecular Orbitals and Organic Chemical Reactions; Reference Edition; Wiley: Hoboken, NJ, USA, 2010; pp. 5–27. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional theory vs density-functional fits: The best of both. J. Chem. Phys. 2022, 157, 234102. [Google Scholar] [CrossRef]
- Pople, J.A.; Gordon, M. Molecular orbital theory of the electronic structure of organic compounds. I. Substituent effects and dipole moments. J. Am. Chem. Soc. 1967, 89, 4253–4261. [Google Scholar] [CrossRef]
- Breneman, C.M.; Wiberg, K.B. Determing atom-centered monopoles from molecular electrostatic potentials. The need for high sampling in formamide conformational analysis. J. Comput. Chem. 1990, 11, 361–373. [Google Scholar] [CrossRef]


| Analogs | Hybrid Functional B3LYP 6-311 (d,p) in Methanol | Average Electrophoretic Migration Time a [min] | ||||
|---|---|---|---|---|---|---|
| μtotal | αtotal | ELUMO | EHOMO | Eg | ||
| [Debye] | [a.u.] | [eV] | [eV] | [eV] | ||
| PRI-2201 | 4.23 | 429.65 | −0.97 | −5.66 | −4.69 | 2.08 ± 0.07 |
| PRI-2203 | 3.14 | 430.34 | −0.97 | −5.66 | −4.68 | 1.93 ± 0.07 |
| PRI-2204 | 3.09 | 430.51 | −1.04 | −5.71 | −4.67 | 1.82 ± 0.05 |
| PRI-2205 | 3.49 | 434.66 | −1.19 | −5.68 | −4.48 | 1.62 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grodner, B.; Żołek, T.; Kutner, A. Nonaqueous Capillary Electrophoretic Separation of Analogs of (24R)-1,24-Dihydroxyvitamin D3 Derivative as Predicted by Quantum Chemical Calculations. Molecules 2023, 28, 5055. https://doi.org/10.3390/molecules28135055
Grodner B, Żołek T, Kutner A. Nonaqueous Capillary Electrophoretic Separation of Analogs of (24R)-1,24-Dihydroxyvitamin D3 Derivative as Predicted by Quantum Chemical Calculations. Molecules. 2023; 28(13):5055. https://doi.org/10.3390/molecules28135055
Chicago/Turabian StyleGrodner, Błażej, Teresa Żołek, and Andrzej Kutner. 2023. "Nonaqueous Capillary Electrophoretic Separation of Analogs of (24R)-1,24-Dihydroxyvitamin D3 Derivative as Predicted by Quantum Chemical Calculations" Molecules 28, no. 13: 5055. https://doi.org/10.3390/molecules28135055
APA StyleGrodner, B., Żołek, T., & Kutner, A. (2023). Nonaqueous Capillary Electrophoretic Separation of Analogs of (24R)-1,24-Dihydroxyvitamin D3 Derivative as Predicted by Quantum Chemical Calculations. Molecules, 28(13), 5055. https://doi.org/10.3390/molecules28135055








