Multi-Resonant Indolo[3,2,1-jk]carbazole-Based Host for Blue Phosphorescent Organic Light-Emitting Diodes
Highlights
- Multi-resonant indolo[3,2,1-jk]carbazole (ICz) moiety has high triplet-state energy with lower energy levels, which are used to construct a new host of blue phosphorescent OLEDs.
- The function of the two hosts centers on ICz moiety, and is balanced in hole and electron transport.
- The ICz-based multi-resonant has a bipolar nature with superior thermal stability, and suits a basic structure for a blue phosphorescent host.
- The less conjugated host with ICz moiety has a larger energy gap and better charge transport capability, affording better electro-luminescent performance in the device.
Abstract
1. Introduction
2. Results and Discussion
2.1. Theoretical Calculation
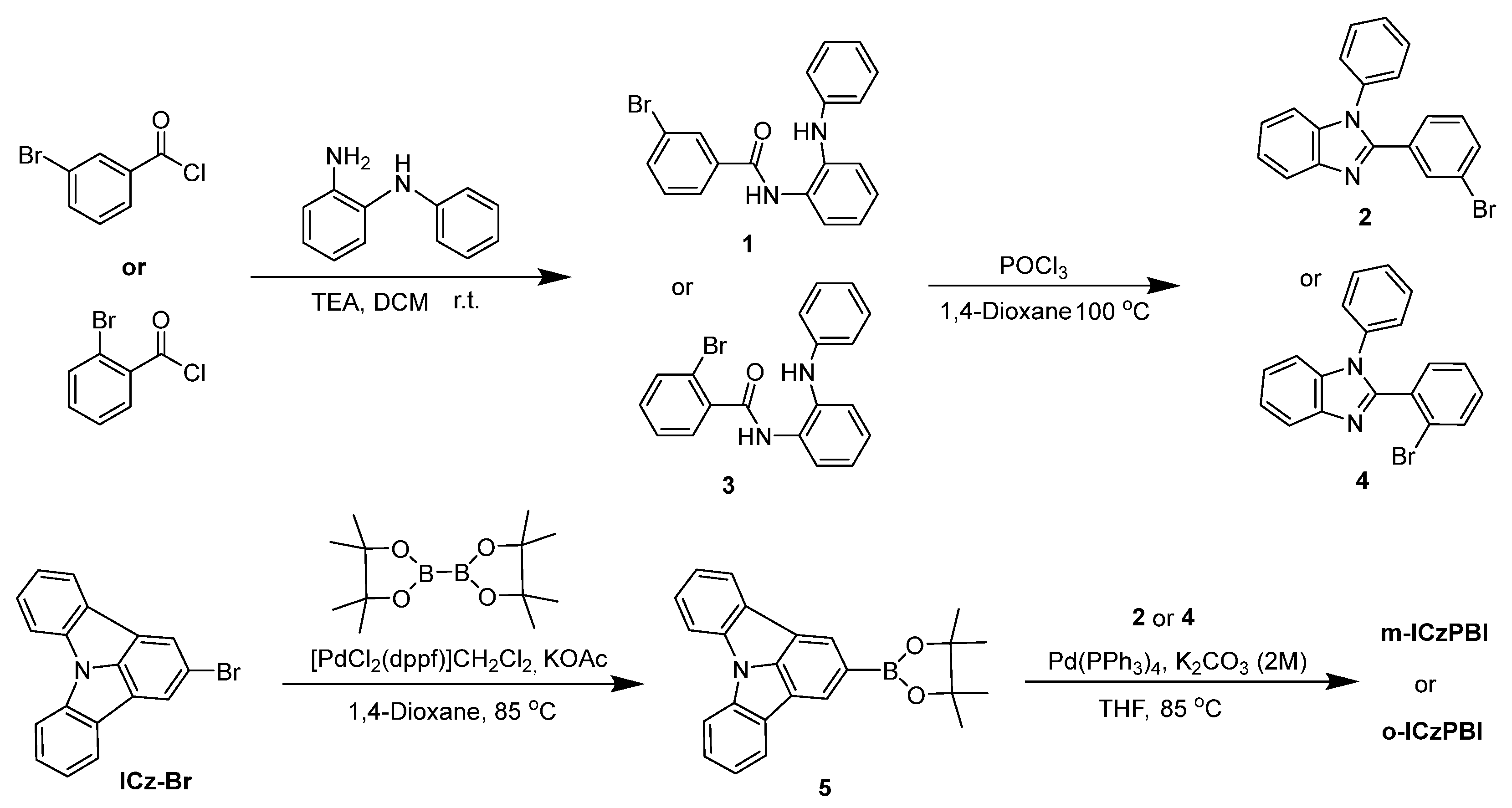
2.2. Synthesis and Structure Characterization
2.3. Thermal Properties
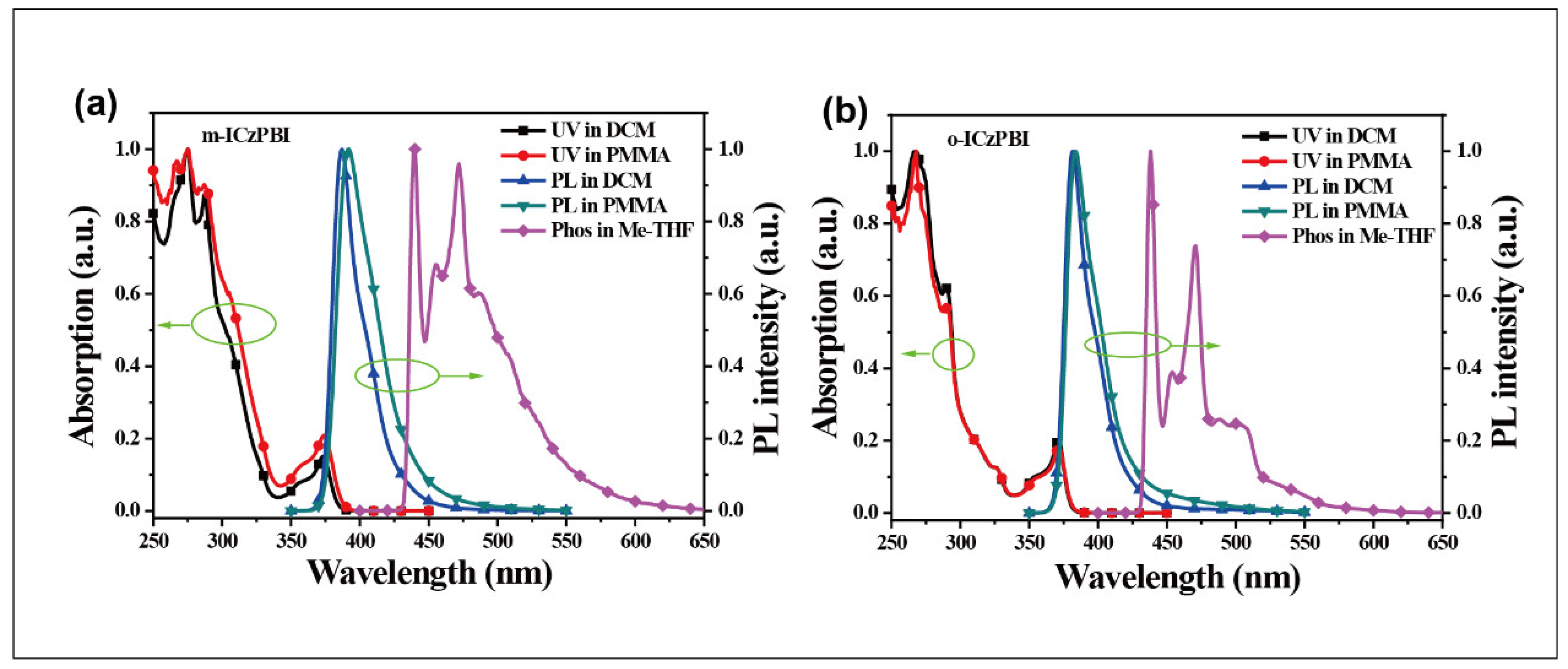
2.4. Photophysical Properties
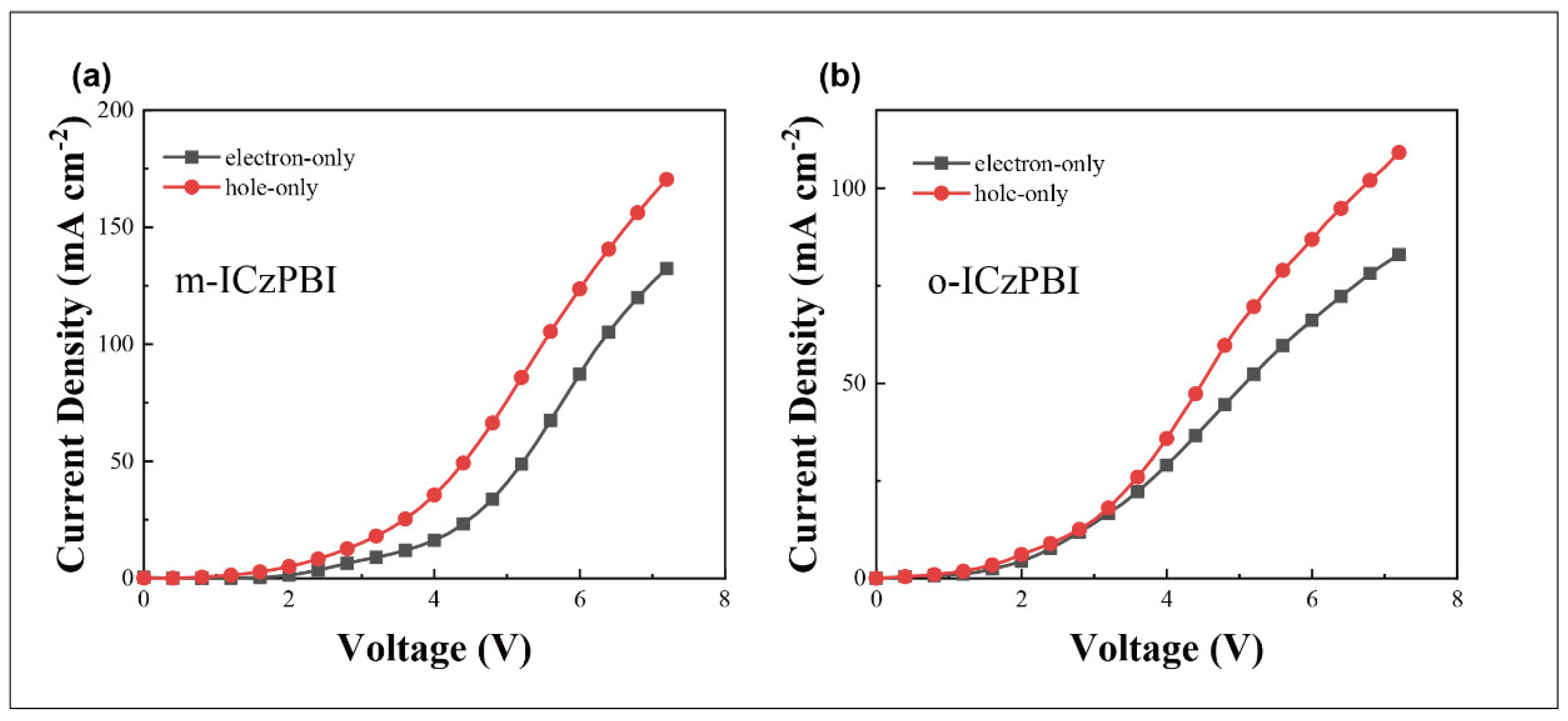
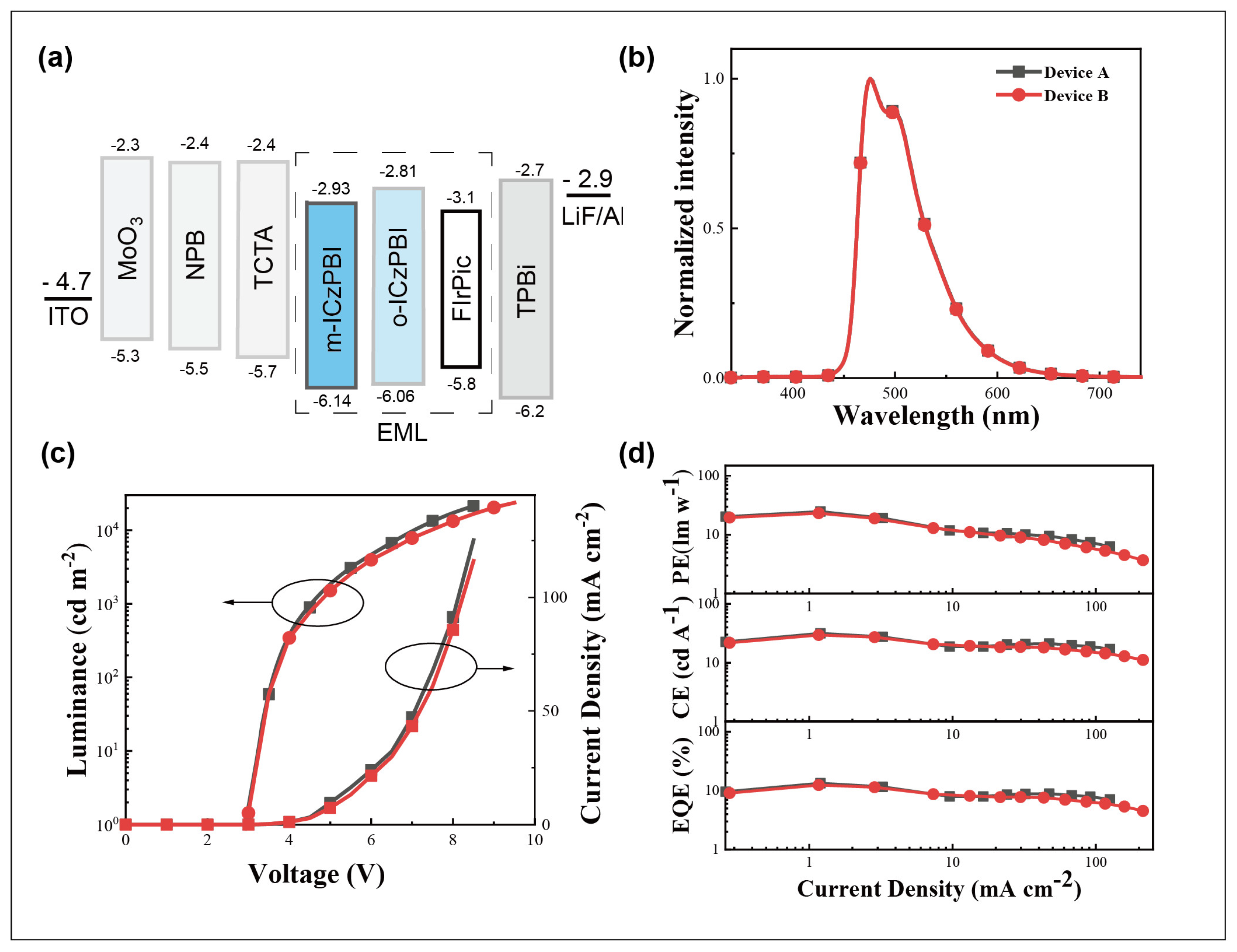
2.5. Electroluminescent Properties
3. Materials and Methods
3.1. Structure Characterization
3.2. Electrochemical Analysis
3.3. Thermal Property Analysis
3.4. Absorption and Emission
3.5. Computational Analysis
3.6. OLED Fabrication and Characterizations
3.7. Synthesis and Structure Characterization
3.7.1. Synthesis of m-ICzPBI
3.7.2. Synthesis of o-ICzPBI
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chen, Y.M.; Qian, C.Y.; Qin, K.; Li, H.B.; Shi, X.B.; Lu, Z.Z.; Ma, H.L.; Qin, T.S.; Hang, X.C.; Huang, W. Ultrapure Blue Phosphorescent Organic Light-Emitting Diodes Employing a Twisted Pt(II) Complex. ACS Appl. Mater. Interfaces 2021, 13, 52833–52839. [Google Scholar] [CrossRef] [PubMed]
- Baldo, M.A.; O’Brien, D.F.; You, Y.; Shoustikov, A.; Sibley, S.; Thompson, M.E.; Forrest, S.R. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 1998, 395, 151–154. [Google Scholar] [CrossRef]
- Hang, X.-C.; Fleetham, T.; Turner, E.; Brooks, J.; Li, J. Highly Efficient Blue-Emitting Cyclometalated Platinum(II) Complexes by Judicious Molecular Design. Angew. Chem. Int. Ed. 2013, 52, 6753–6756. [Google Scholar] [CrossRef]
- Baldo, M.A.; Adachi, C.; Forrest, S.R. Transient analysis of organic electrophosphorescence. II. Transient analysis of triplet-triplet annihilation. Phys. Rev. B 2000, 62, 10967–10977. [Google Scholar] [CrossRef]
- Liu, X.K.; Zheng, C.J.; Xiao, J.; Ye, J.; Liu, C.L.; Wang, S.D.; Zhao, W.M.; Zhang, X.H. Novel bipolar host materials based on 1,3,5-triazine derivatives for highly efficient phosphorescent OLEDs with extremely low efficiency roll-off. Phys. Chem. Chem. Phys. 2012, 14, 14255–14261. [Google Scholar] [CrossRef]
- Zhu, M.; Ye, T.; He, X.; Cao, X.; Zhong, C.; Ma, D.; Qin, J.; Yang, C. Highly efficient solution-processed green and red electrophosphorescent devices enabled by small-molecule bipolar host material. J. Mater. Chem. 2011, 21, 9326–9331. [Google Scholar] [CrossRef]
- Jung, M.; Lee, K.H.; Lee, J.Y.; Kim, T. A bipolar host based high triplet energy electroplex for an over 10,000 h lifetime in pure blue phosphorescent organic light-emitting diodes. Mater. Horiz. 2020, 7, 559–565. [Google Scholar] [CrossRef]
- Maheshwaran, A.; Sree, V.G.; Park, H.-Y.; Kim, H.; Han, S.H.; Lee, J.Y.; Jin, S.-H. High Efficiency Deep-Blue Phosphorescent Organic Light-Emitting Diodes with CIE x, y (<= 0.15) and Low Efficiency Roll-Off by Employing a High Triplet Energy Bipolar Host Material. Adv. Funct. Mater. 2018, 28, 1802945. [Google Scholar]
- Bader, D.; Froehlich, J.; Kautny, P. Thienopyrrolo 3,2,1-jk carbazoles: Building Blocks for Functional Organic Materials. J. Org. Chem. 2020, 85, 3865–3871. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.V.; Lim, J.; Lee, J.Y. Strategic Synchronization of 7,7-Dimethyl-5,7-dihydroindeno 2,1-b carbazole for Narrow-Band, Pure Violet Organic Light-Emitting Diodes with an Efficiency of > 5% and a CIE y Coordinate of < 0.03. ACS Appl. Mater. Interfaces 2021, 13, 14453–14459. [Google Scholar]
- Liao, X.-J.; Zhu, J.-J.; Yuan, L.; Yan, Z.-P.; Luo, X.-F.; Zhang, Y.-P.; Lu, J.-J.; Zheng, Y.-X. Efficient organic light-emitting diodes based on iridium(Ⅲ) complexes containing indolo 3,2,1-jk carbazole derivatives with narrow emission bandwidths and low efficiency roll-offs. J. Mater. Chem. C 2021, 9, 8226–8232. [Google Scholar] [CrossRef]
- Patil, V.V.; Lee, K.H.; Lee, J.Y. Isomeric fused benzocarbazole as a chromophore for blue fluorescent organic light-emitting diodes. J. Mater. Chem. C 2020, 8, 8320–8327. [Google Scholar] [CrossRef]
- Patil, V.V.; Lee, K.H.; Lee, J.Y. Dibenzo c,g indolo 3,2,1-jk carbazole as a new chromophore for blue organic light-emitting diodes. J. Mater. Chem. C 2019, 7, 14301–14305. [Google Scholar] [CrossRef]
- Zhao, C.; Schwartz, T.; Stoeger, B.; White, F.J.; Chen, J.; Ma, D.; Froehlich, J.; Kautny, P. Controlling excimer formation in indolo 3,2,1-jk carbazole/9H-carbazole based host materials for RGB PhOLEDs. J. Mater. Chem. C 2018, 6, 9914–9924. [Google Scholar] [CrossRef] [PubMed]
- Kautny, P.; Wu, Z.; Eichelter, J.; Horkel, E.; Stoeger, B.; Chen, J.; Ma, D.; Froehlich, J.; Lumpi, D. Indolo 3,2,1-jk carbazole based planarized CBP derivatives as host materials for PhOLEDs with low efficiency roll-off. Org. Electron. 2016, 34, 245–253. [Google Scholar] [CrossRef]
- Patil, V.V.; Park, Y.H.; Lee, K.H.; Lee, J.Y. Donor and acceptor interlock by a planar indolo 3,2,1-jk carbazole for a suppressed non-radiative mechanism in thermally activated delayed fluorescent emitters. J. Mater. Chem. C 2020, 8, 14490–14498. [Google Scholar] [CrossRef]
- Luo, X.-F.; Ni, H.-X.; Shen, L.; Wang, L.; Xiao, X.; Zheng, Y.-X. An indolo 3,2,1-jk carbazole-fused multiple resonance-induced thermally activated delayed fluorescence emitter for an efficient narrowband OLED. Chem. Commun. 2023, 59, 2489–2492. [Google Scholar] [CrossRef]
- Hiraga, Y.; Kuwahara, R.; Hatta, T. Novel indolo 3,2,1-jk carbazole-based bipolar host material for highly efficient thermally activated delayed-fluorescence organic light-emitting diodes. Tetrahedron 2021, 94, 132317. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, L.; Dai, H.; Huang, T.; Du, M.; Wang, D.; Zhang, D.; Duan, L. Orbital Symmetry Engineering in Fused Polycyclic Heteroaromatics toward Extremely Narrowband Green Emissions with an FWHM of 13nm. Adv. Mater. 2023, 35, e2211316. [Google Scholar] [CrossRef]
- He, X.; Lou, J.; Li, B.; Wang, H.; Peng, X.; Li, G.; Liu, L.; Huang, Y.; Zheng, N.; Xing, L.; et al. An Ultraviolet Fluorophore with Narrowed Emission via Coplanar Molecular Strategy. Angew. Chem. Int. Ed. 2022, 61, e202209425. [Google Scholar] [CrossRef]
- Luo, X.-F.; Song, S.-Q.; Ni, H.-X.; Ma, H.; Yang, D.; Ma, D.; Zheng, Y.-X.; Zuo, J.-L. Multiple-Resonance-Induced Thermally Activated Delayed Fluorescence Materials Based on Indolo 3,2,1-jk carbazole with an Efficient Narrowband Pure-Green Electroluminescence. Angew. Chem. Int. Ed. 2022, 61, e202209984. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.T.; Balasubramaniam, E.; Danel, A.; Tomasik, P. Dipyrazolopyridine derivatives as bright blue electroluminescent materials. Appl. Phys. Lett. 2000, 77, 933–935. [Google Scholar] [CrossRef]
- Cheng, S.-H.; Hung, W.-Y.; Cheng, M.-H.; Chen, H.-F.; Chaskar, A.; Lee, G.-H.; Chou, S.-H.; Wong, K.-T. Highly twisted biphenyl-linked carbazole-benzimidazole hybrid bipolar host materials for efficient PhOLEDs. J. Mater. Chem. C 2014, 2, 8554–8563. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Shiren, K.; Nakajima, K.; Nomura, S.; Nakatsuka, S.; Kinoshita, K.; Ni, J.P.; Ono, Y.; Ikuta, T. Ultrapure Blue Thermally Activated Delayed Fluorescence Molecules: Efficient HOMO-LUMO Separation by the Multiple Resonance Effect. Adv. Mater. 2016, 28, 2777–2781. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-G.; Kim, B.S.; Lee, J.Y.; Hwang, S.-H. Synthesis of dimesitylborane-substituted phenylcarbazoles as bipolar host materials and the variation of the green PHOLED performance with the substituent position of the boron atom. Dalton Trans. 2014, 43, 7712–7715. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yang, X.; Pan, B.; Wang, L.; Chen, J.; Ma, D.; Yang, C. Benzimidazole-carbazole-based bipolar hosts for high efficiency blue and white electrophosphorescence applications. J. Mater. Chem. 2012, 22, 13223–13230. [Google Scholar] [CrossRef]
- Tsai, M.H.; Hong, Y.H.; Chang, C.H.; Su, H.C.; Wu, C.C.; Matoliukstyte, A.; Simokaitiene, J.; Grigalevicius, S.; Grazulevicius, J.V.; Hsu, C.P. 3-(9-carbazolyl)carbazoles and 3,6-di(9-carbazolyl)carbazoles as effective host materials for efficient blue organic electrophosphorescence. Adv. Mater. 2007, 19, 862. [Google Scholar] [CrossRef]
- Wang, T.; Shi, M.; Fang, D.; He, J.; Zhang, M.; Zhang, S.; Wei, G.; Meng, H. Novel spiro fluorene-9,9′-xanthene -based hole transport layers for red and green PHOLED devices with high efficiency and low efficiency roll-off. J. Mater. Chem. C 2021, 9, 3247–3256. [Google Scholar] [CrossRef]
- Pan, J.; Wang, L.; Chen, W.; Sang, S.; Sun, H.; Wu, B.; Hang, X.-C.; Sun, Z.; Huang, W. Non-fullerene small molecule acceptors with three-dimensional thiophene/selenophene-annulated perylene diimides for efficient organic solar cells. J. Mater. Chem. C 2020, 8, 6749–6755. [Google Scholar] [CrossRef]
- Kawamura, Y.; Yanagida, S.; Forrest, S.R. Energy transfer in polymer electrophosphorescent light emitting devices with single and multiple doped luminescent layers. J. Appl. Phys. 2002, 92, 87–93. [Google Scholar] [CrossRef]
- Hippola, C.; Danilovic, D.; Bhattacharjee, U.; Perez-Bolivar, C.; Sachinthani, K.A.N.; Nelson, T.L.; Anzenbacher, P.; Petrich, J.W.; Shinar, R.; Shinar, J. Bright Deep Blue TADF OLEDs: The Role of Triphenylphosphine Oxide in NPB/TPBi:PPh3O Exciplex Emission. Adv. Opt. Mater. 2020, 8, 0191282. [Google Scholar] [CrossRef]
- Dong, S.-C.; Xu, L.; Tang, C.W. Chemical degradation mechanism of TAPC as hole transport layer in blue phosphorescent OLED. Org. Electron. 2017, 42, 379–386. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Jiang, C.; Fang, Y.; Bai, P.; Wang, Y. Study on Electron Transport Characterization in TPBi Thin Films and OLED Application. J. Phys. Chem. C 2021, 125, 16753–16758. [Google Scholar] [CrossRef]
- Jeon, H.S.; Pyo, B.; Park, H.; Park, S.-R.; Suh, M.C. Improved out-coupling efficiency of organic light emitting diodes by manipulation of optical cavity length. Org. Electron. 2015, 20, 49–54. [Google Scholar] [CrossRef]

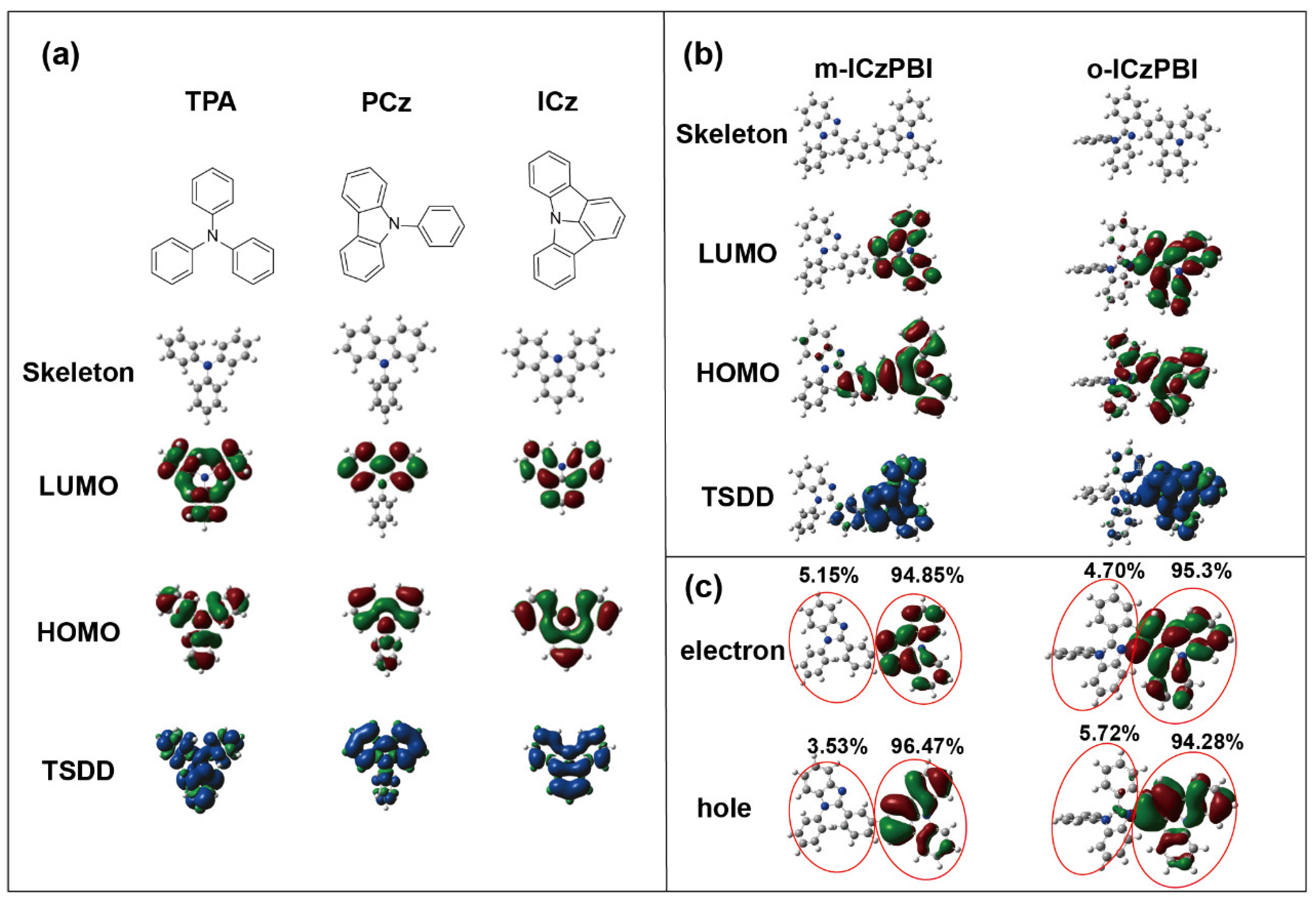
| Motif | HOMO (eV) | LUMO (eV) | Eg (eV) | ES1 (eV) | ET1 (eV) |
|---|---|---|---|---|---|
| TPA | −4.95 | −0.30 | 4.65 | 3.93 | 3.18 |
| PCz | −5.33 | −0.65 | 4.68 | 4.04 | 3.18 |
| ICz | −5.56 | −1.25 | 4.31 | 3.68 | 2.98 |
| Compound | λabs a (nm) | λPL a/b (nm) | ES1 (eV) | ET1 (eV) | EHOMO/ELUMO/Eg c (eV) | EIP d (eV) | Eopt e (eV) |
|---|---|---|---|---|---|---|---|
| m-ICzPBI | 275, 287, 374 | 387/392 | 3.32 | 2.83 | −5.81/−2.17/3.64 | −6.14 | 3.21 |
| o-ICzPBI | 267, 290, 371 | 382/383 | 3.35 | 2.83 | −5.68/−2.18/3.50 | −6.06 | 3.25 |
| Device | Von (V) | @ max/1000/5000 cd m−2 | λmax (nm) | CIE (x, y) | ||
|---|---|---|---|---|---|---|
| EQE (%) | PE (lm W−1) | CE (cd A−1) | ||||
| A | 3.0 | 13.4/11.2/8.6 | 24.8/18.3/10.6 | 31.6/26.3/20.3 | 475 | 0.17, 0.40 |
| B | 3.0 | 12.5/10.6/7.8 | 23.2/10.5/8.8 | 29.5/25.4/18.5 | 475 | 0.17, 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Yuan, P.; Song, J.; Chang, Y.; Jiao, X.; Zhao, J.; Zhang, C.; Li, W.; Hang, X.-C. Multi-Resonant Indolo[3,2,1-jk]carbazole-Based Host for Blue Phosphorescent Organic Light-Emitting Diodes. Molecules 2023, 28, 5118. https://doi.org/10.3390/molecules28135118
Li X, Yuan P, Song J, Chang Y, Jiao X, Zhao J, Zhang C, Li W, Hang X-C. Multi-Resonant Indolo[3,2,1-jk]carbazole-Based Host for Blue Phosphorescent Organic Light-Emitting Diodes. Molecules. 2023; 28(13):5118. https://doi.org/10.3390/molecules28135118
Chicago/Turabian StyleLi, Xiang, Peng Yuan, Jinyu Song, Yu Chang, Xueting Jiao, Jianfeng Zhao, Cong Zhang, Wenjuan Li, and Xiao-Chun Hang. 2023. "Multi-Resonant Indolo[3,2,1-jk]carbazole-Based Host for Blue Phosphorescent Organic Light-Emitting Diodes" Molecules 28, no. 13: 5118. https://doi.org/10.3390/molecules28135118
APA StyleLi, X., Yuan, P., Song, J., Chang, Y., Jiao, X., Zhao, J., Zhang, C., Li, W., & Hang, X.-C. (2023). Multi-Resonant Indolo[3,2,1-jk]carbazole-Based Host for Blue Phosphorescent Organic Light-Emitting Diodes. Molecules, 28(13), 5118. https://doi.org/10.3390/molecules28135118







