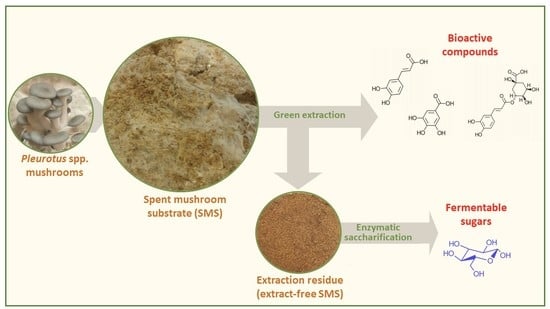
Evaluation of the Extraction of Bioactive Compounds and the Saccharification of Cellulose as a Route for the Valorization of Spent Mushroom Substrate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Spent Mushroom Substrate
2.2. Extraction of Bioactive Compounds from SMS Using Conventional Methods
2.2.1. Soxhlet Extraction
2.2.2. Reflux Extraction
2.3. Extraction of Bioactive Compounds from SMS Using Green Techniques
2.3.1. Ultrasound-Assisted Extraction
2.3.2. Subcritical-Water Extraction
2.4. Extraction of Bioactive Compounds from SMS of the Wild Strain of P. ostreatus
2.5. Phenolic Acids in the Extracts and Their Correlation with the Antioxidant Activity
2.6. Enzymatic Saccharification of SMS
3. Materials and Methods
3.1. Materials
3.2. Characterization of Biomass Materials
3.3. Extractions
3.3.1. Soxhlet Extraction
3.3.2. Reflux Extraction
3.3.3. Ultrasound-Assisted Extraction
3.3.4. Subcritical Water Extraction
3.4. Analytical Enzymatic Saccharification (AES)
3.5. Analytical Methods
3.5.1. Total Carbohydrates
3.5.2. Total Phenolics
3.5.3. Antioxidant Activity
3.5.4. Ergosterol Determination
3.5.5. HPLC Determination of Sugars and Phenolic Acids
3.6. Statistical Analysis
3.7. Formatting of Chemical Structures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Ramady, H.; Abdalla, N.; Badgar, K.; Llanaj, X.; Törős, G.; Hajdú, P.; Eid, Y.; Prokisch, J. Edible Mushrooms for Sustainable and Healthy Human Food: Nutritional and Medicinal Attributes. Sustainability 2022, 14, 4941. [Google Scholar] [CrossRef]
- Royse, D.J.; Baars, J.; Tan, Q. Current Overview of Mushroom Production in the World. In Edible and Medicinal Mushrooms; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2017; pp. 5–13. ISBN 978-1-119-14944-6. [Google Scholar]
- Kumla, J.; Suwannarach, N.; Sujarit, K.; Penkhrue, W.; Kakumyan, P.; Jatuwong, K.; Vadthanarat, S.; Lumyong, S. Cultivation of Mushrooms and Their Lignocellulolytic Enzyme Production through the Utilization of Agro-Industrial Waste. Molecules 2020, 25, 2811. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, L.A.; Prashar, A. Agricultural Crop Residue Burning and Its Environmental Impacts and Potential Causes—Case of Northwest India. J. Environ. Plan. Manag. 2021, 64, 464–484. [Google Scholar] [CrossRef]
- Martín, C. Pretreatment of Crop Residues for Bioconversion. Agronomy 2021, 11, 924. [Google Scholar] [CrossRef]
- Zisopoulos, F.K.; Ramírez, H.A.B.; van der Goot, A.J.; Boom, R.M. A Resource Efficiency Assessment of the Industrial Mushroom Production Chain: The Influence of Data Variability. J. Clean. Prod. 2016, 126, 394–408. [Google Scholar] [CrossRef]
- Atallah, E.; Zeaiter, J.; Ahmad, M.N.; Leahy, J.J.; Kwapinski, W. Hydrothermal Carbonization of Spent Mushroom Compost Waste Compared against Torrefaction and Pyrolysis. Fuel Process. Technol. 2021, 216, 106795. [Google Scholar] [CrossRef]
- Beyer, D.M. Impact of the Mushroom Industry on the Environment. Pennsylvania State University 2011. Available online: https://extension.psu.edu/impact-of-the-mushroom-industry-on-the-environment (accessed on 30 March 2023).
- Martin, C.; Zervakis, G.I.; Xiong, S.; Koutrotsios, G.; Strætkvern, K.O. Spent Substrate from Mushroom Cultivation: Exploitation Potential towards Various Applications and Value-Added Products. Bioengineered 2023, in press. [Google Scholar]
- Kumar, K.; Mehra, R.; Guiné, R.P.F.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible Mushrooms: A Comprehensive Review on Bioactive Compounds with Health Benefits and Processing Aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef]
- Sanz, V.; Torres, M.D.; Lopez Vilarino, J.M.; Dominguez, H. Green Extraction of Phenolic Compounds from Perle Hallertau and Nuggets Hop Pellets. Food Biosci. 2022, 50, 102044. [Google Scholar] [CrossRef]
- Xiong, S.; Martín, C.; Eilertsen, L.; Wei, M.; Myronycheva, O.; Larsson, S.H.; Lestander, T.A.; Atterhem, L.; Jönsson, L.J. Energy-Efficient Substrate Pasteurisation for Combined Production of Shiitake Mushroom (Lentinula edodes) and Bioethanol. Bioresour. Technol. 2019, 274, 65–72. [Google Scholar] [CrossRef]
- Chen, F.; Xiong, S.; Gandla, M.L.; Stagge, S.; Martín, C. Spent Mushroom Substrates for Ethanol Production—Effect of Chemical and Structural Factors on Enzymatic Saccharification and Ethanolic Fermentation of Lentinula edodes-Pretreated Hardwood. Bioresour. Technol. 2022, 347, 126381. [Google Scholar] [CrossRef]
- Li, T.; Cui, L.; Song, X.; Cui, X.; Wei, Y.; Tang, L.; Mu, Y.; Xu, Z. Wood Decay Fungi: An Analysis of Worldwide Research. J. Soils Sediments 2022, 22, 1688–1702. [Google Scholar] [CrossRef]
- Bak, W.C.; Park, J.H.; Park, Y.A.; Ka, K.H. Determination of Glucan Contents in the Fruiting Bodies and Mycelia of Lentinula edodes Cultivars. Mycobiology 2014, 42, 301–304. [Google Scholar] [CrossRef] [Green Version]
- He, P.; Li, F.; Huang, L.; Xue, D.; Liu, W.; Xu, C. Chemical Characterization and Antioxidant Activity of Polysaccharide Extract from Spent Mushroom Substrate of Pleurotus eryngii. J. Taiwan. Inst. Chem. Eng. 2016, 69, 48–53. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Flores, H.E.; Contreras-Chávez, R.; Garnica-Romo, M.G. Effect of Extraction Processes on Bioactive Compounds from Pleurotus ostreatus and Pleurotus djamor: Their Applications in the Synthesis of Silver Nanoparticles. J. Inorg. Organomet. Polym. 2021, 31, 1406–1418. [Google Scholar] [CrossRef]
- Espinosa, E.; Rincón, E.; Morcillo-Martín, R.; Rabasco-Vílchez, L.; Rodríguez, A. Orange Peel Waste Biorefinery in Multi-Component Cascade Approach: Polyphenolic Compounds and Nanocellulose for Food Packaging. Ind. Crops Prod. 2022, 187, 115413. [Google Scholar] [CrossRef]
- Balto, A.S.; Lapis, T.J.; Silver, R.K.; Ferreira, A.J.; Beaudry, C.M.; Lim, J.; Penner, M.H. On the Use of Differential Solubility in Aqueous Ethanol Solutions to Narrow the DP Range of Food-Grade Starch Hydrolysis Products. Food Chem. 2016, 197, 872–880. [Google Scholar] [CrossRef] [Green Version]
- Bouchard, A.; Hofland, G.W.; Witkamp, G.-J. Properties of Sugar, Polyol, and Polysaccharide Water−Ethanol Solutions. J. Chem. Eng. Data 2007, 52, 1838–1842. [Google Scholar] [CrossRef]
- Rodríguez García, S.L.; Raghavan, V. Green Extraction Techniques from Fruit and Vegetable Waste to Obtain Bioactive Compounds—A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6446–6466. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound Assisted Extraction (UAE) of Bioactive Compounds from Fruit and Vegetable Processing by-Products: A Review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Lustres, R.; Sanz, V.; Domínguez, H.; Torres, M.D. Ultrasound-Assisted Extraction of High-Value Fractions from Fruit Industrial Processing Waste. Foods 2022, 11, 2089. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Patiño, J.C.; Gómez-Cruz, I.; Romero, I.; Gullón, B.; Ruiz, E.; Brnčić, M.; Castro, E. Ultrasound-Assisted Extraction as a First Step in a Biorefinery Strategy for Valorisation of Extracted Olive Pomace. Energies 2019, 12, 2679. [Google Scholar] [CrossRef] [Green Version]
- Cabeza, A.; Piqueras, C.M.; Sobrón, F.; García-Serna, J. Modeling of Biomass Fractionation in a Lab-Scale Biorefinery: Solubilization of Hemicellulose and Cellulose from Holm Oak Wood Using Subcritical Water. Bioresour. Technol. 2016, 200, 90–102. [Google Scholar] [CrossRef]
- Rodríguez-Seoane, P.; Díaz-Reinoso, B.; Domínguez, H. Pressurized Solvent Extraction of Paulownia Bark Phenolics. Molecules 2022, 27, 254. [Google Scholar] [CrossRef]
- Daneshfar, A.; Ghaziaskar, H.S.; Homayoun, N. Solubility of Gallic Acid in Methanol, Ethanol, Water, and Ethyl Acetate. J. Chem. Eng. Data 2008, 53, 776–778. [Google Scholar] [CrossRef]
- Vilas-Boas, S.M.; Vieira, V.; Brandão, P.; Alves, R.S.; Coutinho, J.A.P.; Pinho, S.P.; Ferreira, O. Solvent and Temperature Effects on the Solubility of Syringic, Vanillic or Veratric Acids: Experimental, Modeling and Solid Phase Studies. J. Mol. Liq. 2019, 289, 111089. [Google Scholar] [CrossRef] [Green Version]
- Qiao, L.; Ye, X.; Sun, Y.; Ying, J.; Shen, Y.; Chen, J. Sonochemical Effects on Free Phenolic Acids under Ultrasound Treatment in a Model System. Ultrason. Sonochem. 2013, 20, 1017–1025. [Google Scholar] [CrossRef]
- Valanciene, E.; Jonuskiene, I.; Syrpas, M.; Augustiniene, E.; Matulis, P.; Simonavicius, A.; Malys, N. Advances and Prospects of Phenolic Acids Production, Biorefinery and Analysis. Biomolecules 2020, 10, 874. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Kalogeropoulos, N.; Stathopoulos, P.; Kaliora, A.C.; Zervakis, G.I. Bioactive Compounds and Antioxidant Activity Exhibit High Intraspecific Variability in Pleurotus ostreatus Mushrooms and Correlate Well with Cultivation Performance Parameters. World J. Microbiol. Biotechnol. 2017, 33, 98. [Google Scholar] [CrossRef]
- Ishihara, A.; Goto, N.; Kikkawa, M.; Ube, N.; Ushijima, S.; Ueno, M.; Ueno, K.; Osaki-Oka, K. Identification of Antifungal Compounds in the Spent Mushroom Substrate of Lentinula edodes. J. Pestic. Sci. 2018, 43, 108–113. [Google Scholar] [CrossRef] [Green Version]
- Yim, H.S.; Chye, F.Y.; Tan, C.T.; Ng, Y.C.; Ho, C.W. Antioxidant Activities and Total Phenolic Content of Aqueous Extract of Pleurotus ostreatus (Cultivated Oyster Mushroom). Malays. J. Nutr. 2010, 16, 281–291. [Google Scholar]
- Showkat, M.M.; Falck-Ytter, A.B.; Strætkvern, K.O. Phenolic Acids in Jerusalem Artichoke (Helianthus tuberosus L.): Plant Organ Dependent Antioxidant Activity and Optimized Extraction from Leaves. Molecules 2019, 24, 3296. [Google Scholar] [CrossRef] [Green Version]
- Genaro-Mattos, T.C.; Maurício, Â.Q.; Rettori, D.; Alonso, A.; Hermes-Lima, M. Antioxidant Activity of Caffeic Acid against Iron-Induced Free Radical Generation—A Chemical Approach. PLoS ONE 2015, 10, e0129963. [Google Scholar] [CrossRef] [Green Version]
- Nasir, A.M.; Ruslan, N.R.N.; Zakaria, Z.; Hassan, S.A.M.; Ishak, N.; Rohaizad, N.M.; Gunny, A.A.N. β-Glucan Extraction from Mycelium in Spent Mushroom Substrate of Pleurotus ostreatus and Schizophyllum commune. IOP Conf. Ser. Earth Environ. Sci. 2021, 765, 012012. [Google Scholar] [CrossRef]
- Synytsya, A.; Míčková, K.; Synytsya, A.; Jablonský, I.; Spěváček, J.; Erban, V.; Kováříková, E.; Čopíková, J. Glucans from Fruit Bodies of Cultivated Mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and Potential Prebiotic Activity. Carbohydr. Polym. 2009, 76, 548–556. [Google Scholar] [CrossRef]
- Gandla, M.L.; Martín, C.; Jönsson, L.J. Analytical Enzymatic Saccharification of Lignocellulosic Biomass for Conversion to Biofuels and Bio-Based Chemicals. Energies 2018, 11, 2936. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Martín, C.; Lestander, T.A.; Grimm, A.; Xiong, S. Shiitake Cultivation as Biological Preprocessing of Lignocellulosic Feedstocks—Substrate Changes in Crystallinity, Syringyl/Guaiacyl Lignin and Degradation-Derived by-Products. Bioresour. Technol. 2022, 344, 126256. [Google Scholar] [CrossRef]
- Zadrazil, F. Changes in In Vitro Digestibility of Wheat Straw During Fungal Growth and after Harvest of Oyster Mushrooms (Pleurotus spp.) on Laboratory and Industrial Scale. J. Appl. Anim. Res. 1997, 11, 37–48. [Google Scholar] [CrossRef]
- Chen, F.; Xiong, S.; Sundelin, J.; Martín, C.; Hultberg, M. Potential for Combined Production of Food and Biofuel: Cultivation of Pleurotus pulmonarius on Soft- and Hardwood Sawdusts. J. Clean. Prod. 2020, 266, 122011. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of Lignocellulose: Formation of Inhibitory by-Products and Strategies for Minimizing Their Effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Lan, Y.; Wu, Z.; Peng, Y.; Chen, S.; Huang, Z.; Xu, L.; Gelbič, I.; Guan, X.; Zhang, L.; et al. Pretreatment of Spent Mushroom Substrate for Enhancing the Conversion of Fermentable Sugar. Bioresour. Technol. 2013, 148, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.; Kapoor, S.; Thakur, R.; Sharma, E.; Tiwari, R.K.; Joshi, S.J. Lignocellulolytic Enzymes and Bioethanol Production from Spent Biomass of Edible Mushrooms Using Saccharomyces cerevisiae and Pachysolen tannophilus. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Martin, C.; Lopez, Y.; Plasencia, Y.; Hernandez, E. Characterisation of Agricultural and Agro-Industrial Residues as Raw Materials for Ethanol Production. Chem. Biochem. Eng. Q. 2006, 20, 443–447. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass. Laboratory Analytical Procedure (LAP); Technical Report; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass. Laboratory Analytical Procedure (LAP); Technical Report; National Renewable Energy Laboratory: Golden, CO, USA, 2005. [Google Scholar]
- Sluiter, A. Determination of Structural Carbohydrates and Lignin in Biomass. Laboratory Analytical Procedure (LAP); Version 7 August 2011; Technical Report; National Renewable Energy Laboratory: Golden, CO, USA, 2008; p. 18. [Google Scholar]
- Albalasmeh, A.A.; Berhe, A.A.; Ghezzehei, T.A. A New Method for Rapid Determination of Carbohydrate and Total Carbon Concentrations Using UV Spectrophotometry. Carbohydr. Polym. 2013, 97, 253–261. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu Assay Revisited: Improvement of Its Specificity for Total Phenolic Content Determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Guo, C.; Yang, J.; Wei, J.; Li, Y.; Xu, J.; Jiang, Y. Antioxidant Activities of Peel, Pulp and Seed Fractions of Common Fruits as Determined by FRAP Assay. Nutr. Res. 2003, 23, 1719–1726. [Google Scholar] [CrossRef]










| BPKO | NO | |
|---|---|---|
| Cellulose | 38.3 (0.6) | 37.5 (0.6) |
| Hemicelluloses | 22.1 | 20.4 |
| Xylan | 18.5 (0.6) | 16.6 (0.6) |
| Anhydroarabinose | 3.6 (0.1) | 3.8 (0.3) |
| Klason lignin | 16.0 (0.6) | 18.4 (0.9) |
| Water extractives | 12.0 | 13.7 |
| Total carbohydrates | 10.7(1.0) | 9.8 (0.3) |
| Total phenolics | 1.0 (0.2) | 1.0 (<0.1) |
| Ethanol extractives | 1.5 | 1.3 |
| Total phenolics | 0.3 (<0.1) | 0.2 (<0.1) |
| Total extractives | 13.5 | 15.0 |
| Ergosterol | <0.1 | <0.1 |
| Ash | 2.0 (0.3) | 2.7 (0.5) |
| No. | Temperature, °C | Time, Min | Extraction Efficiency 1, g/100 g Biomass |
|---|---|---|---|
| 1 | 35 | 30 | 10.9 (4.7) |
| 2 | 35 | 45 | 20.1 (8.0) |
| 3 | 35 | 60 | 12.4 (1.4) |
| 4 | 50 | 30 | 61.6 (2.6) |
| 5 | 50 | 45 | 60.7 (3.9) |
| 6 | 50 | 60 | 67.5 (1.1) |
| 7 | 65 | 30 | 69.4 (6.0) |
| 8 | 65 | 45 | 80.8 (3.1) |
| 9 | 65 | 60 | 81.7 (0.5) |
| Parameter/Extraction Method | BPKO SMS | NO SMS |
|---|---|---|
| Extraction yield, % (w/w) | ||
| Soxhlet extraction | 9.5 (0.3) | 10.7 (0.2) |
| Reflux extraction | 8.5 (0.6) | 12.6 (0.1) |
| UAE | 11.1 (0.1) | 12.3 (0.3) |
| Extraction efficiency, % (w/w) | ||
| Soxhlet extraction | 69.9 (1.9) | 71.1 (1.5) |
| Reflux extraction | 62.9 (4.6) | 83.7 (0.5) |
| UAE | 81.7 (0.5) | 82.0 (2.2) |
| Total phenolics, mg GAE/g biomass | ||
| Soxhlet extraction | 9.1 (0.3) | 8.3 (0.2) |
| Reflux extraction | 8.6 (0.2) | 9.5 (0.2) |
| UAE | 5.6 (0.5) | 7.4 (0.3) |
| Total carbohydrates, g/L | ||
| Soxhlet extraction | 7.8 (0.5) | 6.1 (0.5) |
| Reflux extraction | 8.1 (0.5) | 7.9 (0.9) |
| UAE | 7.3 (0.5) | 7.4 (0.6) |
| Antioxidant activity, mM GAE | ||
| Soxhlet extraction | 0.4 (<0.1) | 0.5 (<0.1) |
| Reflux extraction | 0.4 (<0.1) | 0.5 (<0.1) |
| UAE | 0.3 (<0.1) | 0.4 (<0.1) |
| Extract Sample 2 | Chlorogenic Acid | Caffeic Acid | p-Coumaric Acid | Ferulic Acid | Gallic Acid | Vanillic Acid |
|---|---|---|---|---|---|---|
| SoE-80 | 6.0 (0.2) | 0.7 (0.2) | 2.3 (0.1) | 0.9 (0.1) | 5.5 (0.4) | 12.4 (1.0) |
| SoE-40 | 7.3 (0.3) | 1.1 (0.2) | 2.3 (0.1) | 1.1 (0.1) | 3.6 (0.4) | 8.2 (2.5) |
| SoE-20 | 4.6 (0.3) | 0.6 (<0.1) | 1.6 (<0,1) | 0.8 (<0.1) | 3.0 (0.6) | 6.9 (1.3) |
| ReE-60 | 4.8 (0.3) | 0.7 (0.1) | 1.7 (0.1) | 0.7 (<0.1) | 2.8 (0.3) | 3.8 (0.4) |
| ReE-120 | 4.2 (0.2) | 0.7 (0.1) | 1.1 (0.1) | 0.6 (<0.1) | 2.8 (0.4) | 4.5 (0.4) |
| ReE-240 | 4.8 (<0.1) | 0.7 (0.1) | 1.5 (0.1) | 0.7 (0.1) | 2.5 (0.3) | 5.8 (0.6) |
| UAE 35–30 | 3.1 (<0.1) | 0.2 (0.1) | 1.3 (0.1) | 0.5 (<0.1) | 2.6 (0.1) | 2.9 (0.3) |
| UAE 50–45 | 3.6 (<0.1) | 0.3 (<0.1) | 1.3 (0.1) | 0.6 (<0.1) | 3.2 (0.2) | 1.8 (0.4) |
| UAE 65–60 | 4.6 (0.1) | 0.3 (<0.1) | 1.7 (<0.1) | 0.8 (0.1) | 3.0 (0.3) | 3.0 (0.2) |
| SWE 10 | 5.4 (<0.1) | 1.7 (0.4) | 1.2 (0.1) | 0.7 (0.1) | 11.1 (0.5) | 16.4 (0.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klausen, S.J.; Falck-Ytter, A.B.; Strætkvern, K.O.; Martin, C. Evaluation of the Extraction of Bioactive Compounds and the Saccharification of Cellulose as a Route for the Valorization of Spent Mushroom Substrate. Molecules 2023, 28, 5140. https://doi.org/10.3390/molecules28135140
Klausen SJ, Falck-Ytter AB, Strætkvern KO, Martin C. Evaluation of the Extraction of Bioactive Compounds and the Saccharification of Cellulose as a Route for the Valorization of Spent Mushroom Substrate. Molecules. 2023; 28(13):5140. https://doi.org/10.3390/molecules28135140
Chicago/Turabian StyleKlausen, Sarah J., Anne Bergljot Falck-Ytter, Knut Olav Strætkvern, and Carlos Martin. 2023. "Evaluation of the Extraction of Bioactive Compounds and the Saccharification of Cellulose as a Route for the Valorization of Spent Mushroom Substrate" Molecules 28, no. 13: 5140. https://doi.org/10.3390/molecules28135140
APA StyleKlausen, S. J., Falck-Ytter, A. B., Strætkvern, K. O., & Martin, C. (2023). Evaluation of the Extraction of Bioactive Compounds and the Saccharification of Cellulose as a Route for the Valorization of Spent Mushroom Substrate. Molecules, 28(13), 5140. https://doi.org/10.3390/molecules28135140