Managing Encapsulated Oil Extract of Date Seed Waste for High Hydroxyl Radical Scavenging Assayed via Hybrid Photo-Mediated/Spectrofluorimetric Probing
Abstract
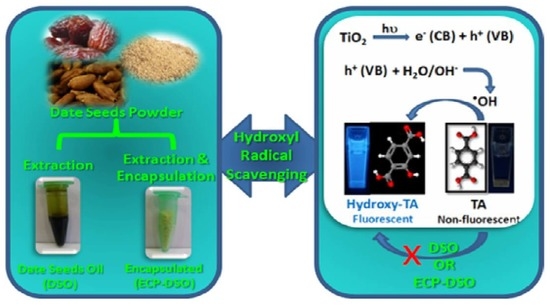
:1. Introduction
2. Results and Discussion
2.1. Characterization of DSO and ECP–DSO Powder
2.2. UV–Vis Electronic Spectroscopy of DSO
2.3. Characterization of the Nano-Titania
2.4. Nano-Titania and the Photocatalytic Productivity of •OH Radicals under Solar Irradiation
2.5. Phenolics and Flavonoids of DSO and ECP–DSO
2.6. Spectrofluorimetric Evaluation of the •OH Radical Scavenging Potentiality of DSO and ECP–DSO
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Dried Seed Powder
3.2.2. Date Seed Oil (DSO) Extraction
3.2.3. Gas Chromatography–Mass Spectrometry (GC/MS) of Date Seed Oil
3.2.4. Date Seed Oil Encapsulation
3.2.5. Measurement of Total Phenolics and Flavonoids
3.2.6. Addressing the Average Crystallite Size and Band Gap Characteristics of the Nano-Titania
3.2.7. Generating Hydroxyl Radicals (•OH) via Solar Irradiation of TiO2-NPs and Spectrofluorimetric Monitoring
3.2.8. The Activity of DSO and ECP–DSO in Scavenging •OH Radicals
3.3. Instruments
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Martindale, J.L.; Holbrook, N.J. Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell. Physiol. 2002, 192, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Atmani, D.; Chaher, N.; Berboucha, M.; Ayouni, K.; Lounis, H.; Boudaoud, H.; Debbache, N.; Atmani, D. Antioxidant capacity and phenol content of selected Algerian medicinal plants. Food Chem. 2009, 112, 303–309. [Google Scholar] [CrossRef]
- Al-Mamary, M.; Abdelwahab, S.I.; Al-Ghalibi, S.; Al-Ghasani, E. The antioxidant and tyrosinase inhibitory activities of some essential oils obtained from aromatic plants grown and used in Yemen. Sci. Res. Essays 2011, 6, 6840–6845. [Google Scholar]
- Borchani, C.; Besbes, S.; Blecker, C.; Attia, H. Chemical Characteristics and Oxidative Stability of Sesame Seed, Sesame Paste and Olive Oils. J. Agric. Sci. Tech. 2011, 12, 585–596. [Google Scholar]
- Wang, Z.; Yi, K.; Lin, Q.; Yang, L.; Chen, X.; Chen, H.; Liu, Y.; Wei, D. Free radical sensors based on inner-cuttingg raphene field-effect transistors. Nat. Commun. 2019, 10, 1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essawy, A.A.; Sayyah, S.M.; El-Nggar, A.M. Wastewater remediation by TiO2-impregnated chitosan nano-grafts exhibited dual functionality: High adsorptivity and solar-assisted self-cleaning. J. Photochem. Photobiol. B Biol. 2017, 173, 170–180. [Google Scholar] [CrossRef]
- Essawy, A.A.; Nassar, A.M.; Arafa, W.A.A. A novel photocatalytic system consists of Co(II) complex@ZnO exhibits potent antimicrobial activity and efficient solar-induced wastewater remediation. Sol. Energy 2018, 17, 388–397. [Google Scholar] [CrossRef]
- Essawy, A.A. Silver imprinted ZnO nanoparticles: Green synthetic approach, characterization and efficient sunlight-induced photocatalytic water detoxification. J. Clean. Prod. 2018, 183, 1011–1020. [Google Scholar] [CrossRef]
- Paolini, V.; Bergeaud, J.P.; Grisez, C.; Prevot, F.; Dorchies, P.; Hoste, H. Effects of condensed tannins on goats experimentally infected with Haemonchus contortus. Vet. Parasitol. 2003, 113, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Bjelakovic, G.; Nikolova, D.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of gastrointestinal cancers: A systematic review and meta-analysis Goran. Lancet 2004, 364, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Vivekananthan, D.P.; Penn, M.S.; Sapp, S.K.; Hsu, A.; Topol, E.J. Use of antioxidant vitamins for the prevention of cardiovascular disease: Meta-analysis of randomised trials. Lancet 2003, 361, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Shyamala, B.N.; Gupta, S.; Jyothi Lakshmi, A.; Prakash, J. Leafy vegetable extracts—Antioxidant activity and effect on storage stability of heated oils. Innov. Food Sci. Emerg. Technol. 2005, 6, 239–245. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [Green Version]
- Khalil, M.I.; Sulaiman, S.A.; Boukraa, L. Antioxidant Properties of Honey and Its Role in Preventing Health Disorder. Open Nutraceuticals J. 2010, 3, 6–16. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T. Antioxidant properties of different fruit seeds and peels. Acta Sci. Pol. Technol. Aliment. 2007, 6, 29–36. [Google Scholar]
- Saryono, S.; Taufik, A.; Proverawati, A.; Efendi, F. Dietary supplementation of Phoenix dactylifera L. seeds decreases pro-inflammatory mediators in CCl4- induced rats. J. Herbmed Pharmacol. 2019, 8, 212–217. [Google Scholar] [CrossRef] [Green Version]
- El-Massry, K.; El-Ghorab, A.; Farouk, A.; Hamed, S.; Elgebaly, H.; Mosa, N.; Alsohaimi, I.; Mahmoud, A. Microencapsulation of Date Seed Oil by Spray-drying for Stabilization of Olive Oil as a Functional Food. Asian J. Sci. Res. 2019, 12, 516–523. [Google Scholar] [CrossRef] [Green Version]
- Ghafoor, K.; Sarker, Z.I.; Al-Juhaimi, F.Y.; Babiker, E.E.; Alkaltham, M.S.; Almubarak, A.K. Extraction and Evaluation of Bioactive Compounds from Date (Phoenix dactylifera) Seed Using Supercritical and Subcritical CO2 Techniques. Foods 2022, 11, 1806. [Google Scholar] [CrossRef]
- Rahman, M.; Kasapis, S.; Al-Kharusi, N.; Al-Marhubi, I.; Khan, A. Composition characterisation and thermal transition of date pits powders. J. Food Eng. 2007, 80, 1–10. [Google Scholar] [CrossRef]
- Habib, H.; Ibrahim, W. Nutritional quality evaluation of eighteen date pit varieties. Int. J. Food Sci. Nutr. 2009, 60, 99–111. [Google Scholar] [CrossRef]
- Herchi, W.; Kallel, H.; Boukhchina, S. Physicochemical properties and antioxidant activity of Tunisian date palm (Phoenix dactylifera L.) oil as affected by different extraction methods. Food Sci. Technol. Camp. 2014, 34, 464–470. [Google Scholar] [CrossRef] [Green Version]
- Nehdi, I.; Omri, S.; Khalil, M.; Al-Resayes, S. Characteristics and chemical composition of date palm (Phoenix canariensis) seeds and seed oil. Ind. Crop. Prod. 2010, 32, 360–365. [Google Scholar] [CrossRef]
- Carneiro, H.; Tonon, R.; Grosso, C.; Hubinger, M. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, B.; Borges, V.; Botrel, A. Gum Arabic/starch/ maltodextrin/inulin as wall materials on the microencapsulation of rosemary essential oil. Carbohydr. Polym. 2014, 101, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Keshani, S.; Daud, W.; Nourouzi, M.; Namvar, F.; Ghasemi, M. Spray drying, An overview on wall deposition, process and modeling. J. Food Eng. 2015, 146, 152–162. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.A.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC)) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Automatic method for determination of total antioxidant capacity using 2.2-diphenyl-1-picrylhydrazyl assay. Anal. Chim. Acta 2006, 558, 310–318. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Özyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [Green Version]
- Ozyurt, D.; Demirata, B.; Apak, R. Determination of Total Antioxidant Capacity by a New Spectrofluorometric Method Based on Ce(IV) Reduction: Ce(III) Fluorescence Probe for CERAC Assay. J. Fluoresc. 2011, 21, 2069–2076. [Google Scholar] [CrossRef]
- Oka, T.; Yamashita, S.; Midorikawa, M.; Saiki, S.; Muroya, Y.; Kamibayashi, M.; Katsumura, Y. Spin-trapping reactions of a novel gauchetype radical trapper G-CYPMPO. Anal. Chem. 2011, 83, 9600–9604. [Google Scholar] [CrossRef]
- Xue, Y.; Luan, Q.; Yang, D.; Yao, X.; Zhou, K. Direct evidence for hydroxyl radical scavenging activity of cerium oxide nanoparticles. J. Phys. Chem. C 2011, 115, 4433–4438. [Google Scholar] [CrossRef]
- Zhu, A.; Liu, Y.; Rui, Q.; Tian, Y. Selective and sensitive determination of hydroxyl radicals generated from living cells through an electrochemical impedance method. Chem. Commun. 2011, 47, 4279–4281. [Google Scholar] [CrossRef]
- Linxiang, L.; Abe, Y.; Nagasawa, Y.; Kudo, R.; Usui, N.; Imai, K.; Miyata, N. An HPLC assay of hydroxyl radicals by the hydroxylation reaction of terephthalic acid. Biomed. Chromatogr. 2004, 18, 470–474. [Google Scholar] [CrossRef]
- Zhu, B.Z.; Mao, L.; Huang, C.H.; Qin, H.; Fan, R.M.; Kalyanaraman, B.; Zhu, J.G. Unprecedented hydroxyl radical-dependent two-step chemiluminescence production by polyhalogenated quinoid carcinogens and H2O2. Proc. Natl. Acad. Sci. USA 2012, 109, 16046–16051. [Google Scholar] [CrossRef]
- Huang, W.T.; Xie, W.Y.; Shi, Y.; Luo, H.Q.; Li, N.B. A simple and facile strategy based on Fenton-induced DNA cleavage for fluorescent turn-on detection of hydroxyl radicals and Fe2+. J. Mat. Chem. 2012, 22, 1477–1481. [Google Scholar] [CrossRef]
- Duanghathaipornsuk, S.; Farrell, E.J.; Alba-Rubio, A.C.; Zelenay, P.; Kim, D.-S. Detection Technologies for Reactive Oxygen Species: Fluorescence and Electrochemical Methods and Their Applications. Biosensors 2021, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Zhang, L.; Geng, Y. Determination of the antioxidant capacity of different food natural products with a new developed flow injection spectrofluorimetry detecting hydroxyl radicals. Talanta 2005, 65, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dai, M.; Yuan, Z. Methods for Detection of Reactive Oxygen Species. Anal. Methods 2018, 10, 4625. [Google Scholar] [CrossRef]
- Essawy, A.A.; Ibrahim, B.; Abdel-Farid, I.B. Hybrid solvothermal/sonochemical-mediated synthesis of ZnO NPs generative of •OH radicals: Photoluminescent approach to evaluate •OH scavenging activity of Egyptian and Yemeni Punica granatum arils extract. Ultrason. Sonochem. 2022, 89, 106152. [Google Scholar] [CrossRef] [PubMed]
- Tirzitis, G.; Bartosz, G. Determination of antiradical and antioxidant activity: Basic principles and new insights. Acta Biochim. Pol. 2010, 57, 139–142. [Google Scholar] [CrossRef]
- Essawy, A.A.; Sayyah, S.M.; El-Nggar, A.M. Ultrasonic-mediated synthesis and characterization of TiO2-loaded chitosan-grafted polymethylaniline nanoparticles of potent efficiency in dye uptake and sunlight driven self cleaning applications. RSC Adv. 2016, 6, 2279. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Sun, B.; Qiao, P.; Ren, L.; Li, M.; Jiang, B.; Pan, K.; Zhou, W. Surface oxygen vacancy defect-promoted electron hole separation for porous defective ZnO hexagonal plates and enhanced solar driven photocatalytic performance. Chem. Eng. J. 2020, 379, 122295. [Google Scholar] [CrossRef]
- Žerjav, G.; Albreht, A.; Vovk, I.; Pintar, A. Revisiting terephthalic acid and coumarin as probes for photoluminescent determination of hydroxyl radical formation rate in heterogeneous photocatalysis. Appl. Catal. A Gen. 2020, 598, 117566. [Google Scholar] [CrossRef]
- Iqbal, E.; Salim, K.A.; Lim, L.B.L. Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. J. King Saud Univ.-Sci. 2015, 27, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Edris, A.E.; Kalemba, D.; Adamiec, J.; Piątkowski, M. Microencapsulation of Nigella sativa oleoresin by spray drying for food and nutraceutical applications. Food Chem. 2016, 204, 326–333. [Google Scholar] [CrossRef]
- Dutta, S.; Bhattacharjee, P. Microencapsulation of enzyme-assisted supercritical carbon dioxide extract of small cardamom by spray drying. J. Food Meas. Charact. 2017, 11, 310–319. [Google Scholar] [CrossRef]
- McNamee, B.F.; O’Riordan, E.D.; O’Sullivan, M. Effect of partial replacement of gum arabic with carbohydrates on its microencapsulation properties. J. Agric. Food Chem. 2001, 49, 3385–3388. [Google Scholar] [CrossRef]
- Syll, O.; Khalloufi, S.; Mèjean, S.; Schuck, P. The effects of total protein/total solid ratio and pH on the spray drying process and rehydration properties of soy powder. Powder Technol. 2016, 289, 60–64. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Kahkonen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem 1999, 47, 3954–3962. [Google Scholar] [CrossRef]








| Sample | Concentration (μg/mL) | •OH Radical Scavenging (%) ± SD | Rate of •OH Radical Productivity (min−1) ± SD | R2 |
|---|---|---|---|---|
| DSO * | 80 | 25.12 ± 0.28 | -- | -- |
| 140 | 34.83 ± 0.35 | -- | -- | |
| 200 | 68.65 ± 0.22 | 0.041± 0.003 | 0.91 | |
| DSO-ECP * | 80 | 63.39 ± 0.46 | -- | -- |
| 140 | 80.11 ± 0.19 | -- | -- | |
| 200 | 92.71 ± 0.25 | 0.031 ± 0.002 | 0.98 | |
| TBHQ | 200 | 94.60 ± 0.29 | 0.028 ± 0.001 | 0.93 |
| Date Seed Oil Content (% DW *) = 5.78 | ||
|---|---|---|
| Fatty Acids | (%) | |
| Lauric | C12:0 | 15.17 ± 0.19 |
| Myristic | C14:0 | 9.21 ± 0.11 |
| Palmitic acid | C16:0 | 7.44 ± 0.20 |
| Stearic acid | C18:0 | 6.36 ± 0.24 |
| Arachidic (Eicosanoic) | C20:0 | 1.3 ± 0.12 |
| Oleic acid | C18:1 | 46.32 ± 0.38 |
| Linoleic acid | C18:2 | 13.03 ± 0.18 |
| α-Linolenic acid | C18:3 | 1.15 ± 0.10 |
| Total saturated FAs | SFA ** | 39.48 ± 0.32 |
| Total Polyunsaturated FAs | PUFA *** | 57.62 ± 0.41 |
| Acid Value (AV) mg KOH/g DSO | 0.59 | |
| Peroxide index (meq/kg DSO) | 2.18 | |
| Total phenolic content (mg GAE/g) | 26.85 ± 0.50 | |
| Flavonoids (mg QE/g) | 11.41 ± 0.17 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Essawy, A.A.; El-Massry, K.F.; Alsohaimi, I.H.; El-Ghorab, A. Managing Encapsulated Oil Extract of Date Seed Waste for High Hydroxyl Radical Scavenging Assayed via Hybrid Photo-Mediated/Spectrofluorimetric Probing. Molecules 2023, 28, 5160. https://doi.org/10.3390/molecules28135160
Essawy AA, El-Massry KF, Alsohaimi IH, El-Ghorab A. Managing Encapsulated Oil Extract of Date Seed Waste for High Hydroxyl Radical Scavenging Assayed via Hybrid Photo-Mediated/Spectrofluorimetric Probing. Molecules. 2023; 28(13):5160. https://doi.org/10.3390/molecules28135160
Chicago/Turabian StyleEssawy, Amr A., Khaled F. El-Massry, Ibrahim Hotan Alsohaimi, and A. El-Ghorab. 2023. "Managing Encapsulated Oil Extract of Date Seed Waste for High Hydroxyl Radical Scavenging Assayed via Hybrid Photo-Mediated/Spectrofluorimetric Probing" Molecules 28, no. 13: 5160. https://doi.org/10.3390/molecules28135160
APA StyleEssawy, A. A., El-Massry, K. F., Alsohaimi, I. H., & El-Ghorab, A. (2023). Managing Encapsulated Oil Extract of Date Seed Waste for High Hydroxyl Radical Scavenging Assayed via Hybrid Photo-Mediated/Spectrofluorimetric Probing. Molecules, 28(13), 5160. https://doi.org/10.3390/molecules28135160