Synergistic Promotion of Photocatalytic Degradation of Methyl Orange by Fluorine- and Silicon-Doped TiO2/AC Composite Material
Abstract
:1. Introduction
2. Results and Discussion
2.1. SEM Analysis
2.2. XRD Analysis
2.3. Nitrogen Physical Adsorption
2.4. FTIR Spectroscopy
2.5. UV–vis Absorption Spectra and Photoluminescence Analysis
2.6. Photocatalytic Studies
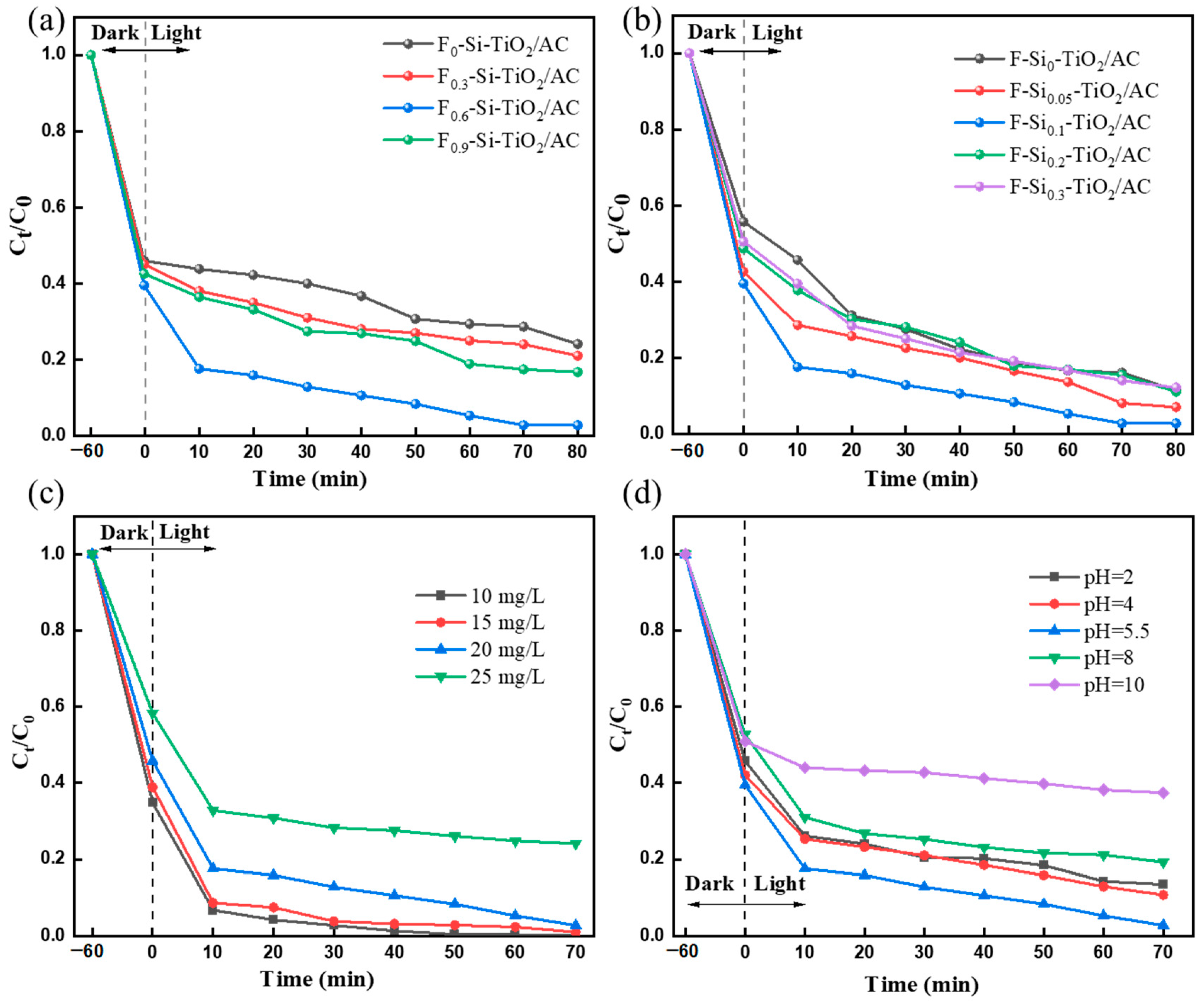
2.6.1. The Influence of Different Doping Elements
2.6.2. Effect of the Amount of F and Si Ion Doping
2.6.3. The Effect of the Initial Concentration of MO Solution
2.6.4. The Effect of Initial pH Conditions
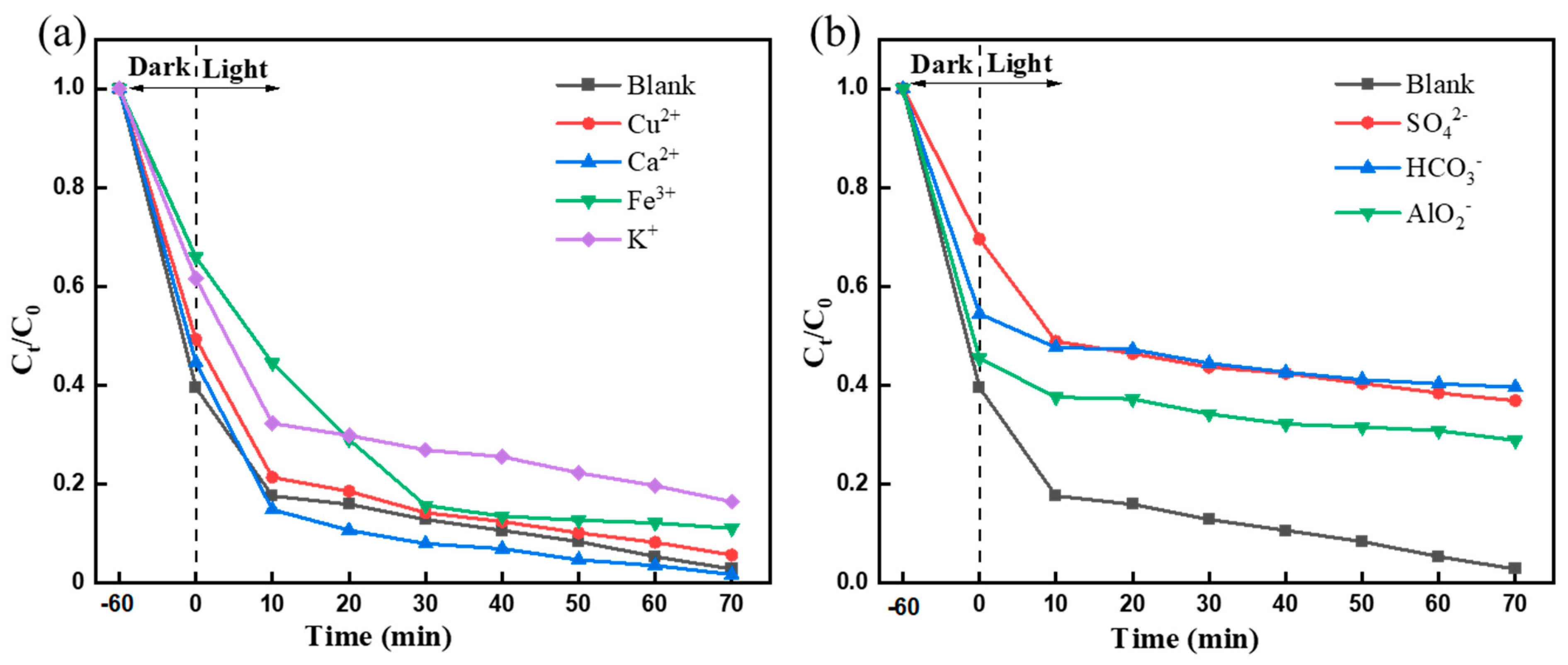
2.6.5. The Effect of Water Quality Parameters
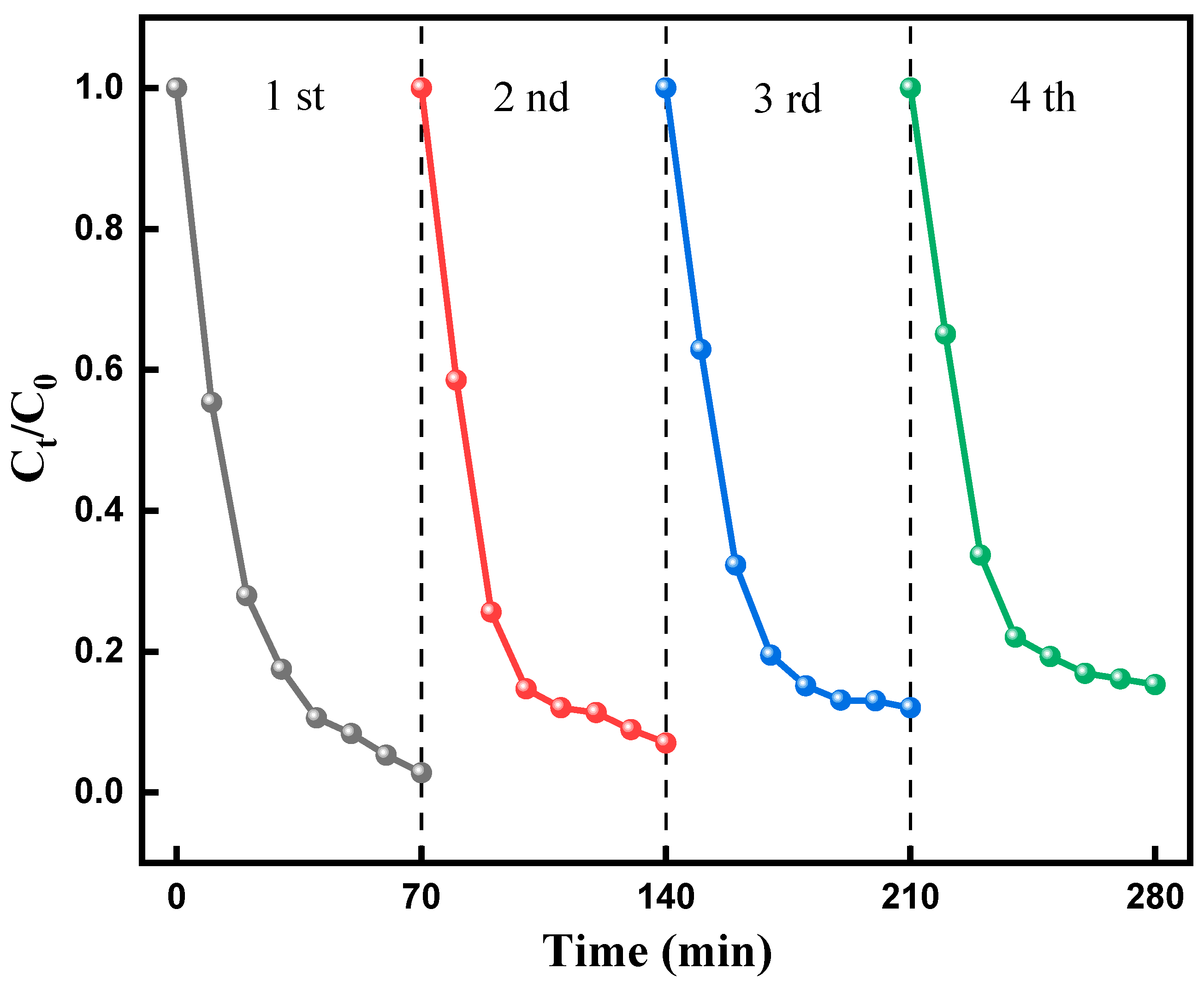
2.6.6. Reuse of F-Si-TiO2/AC
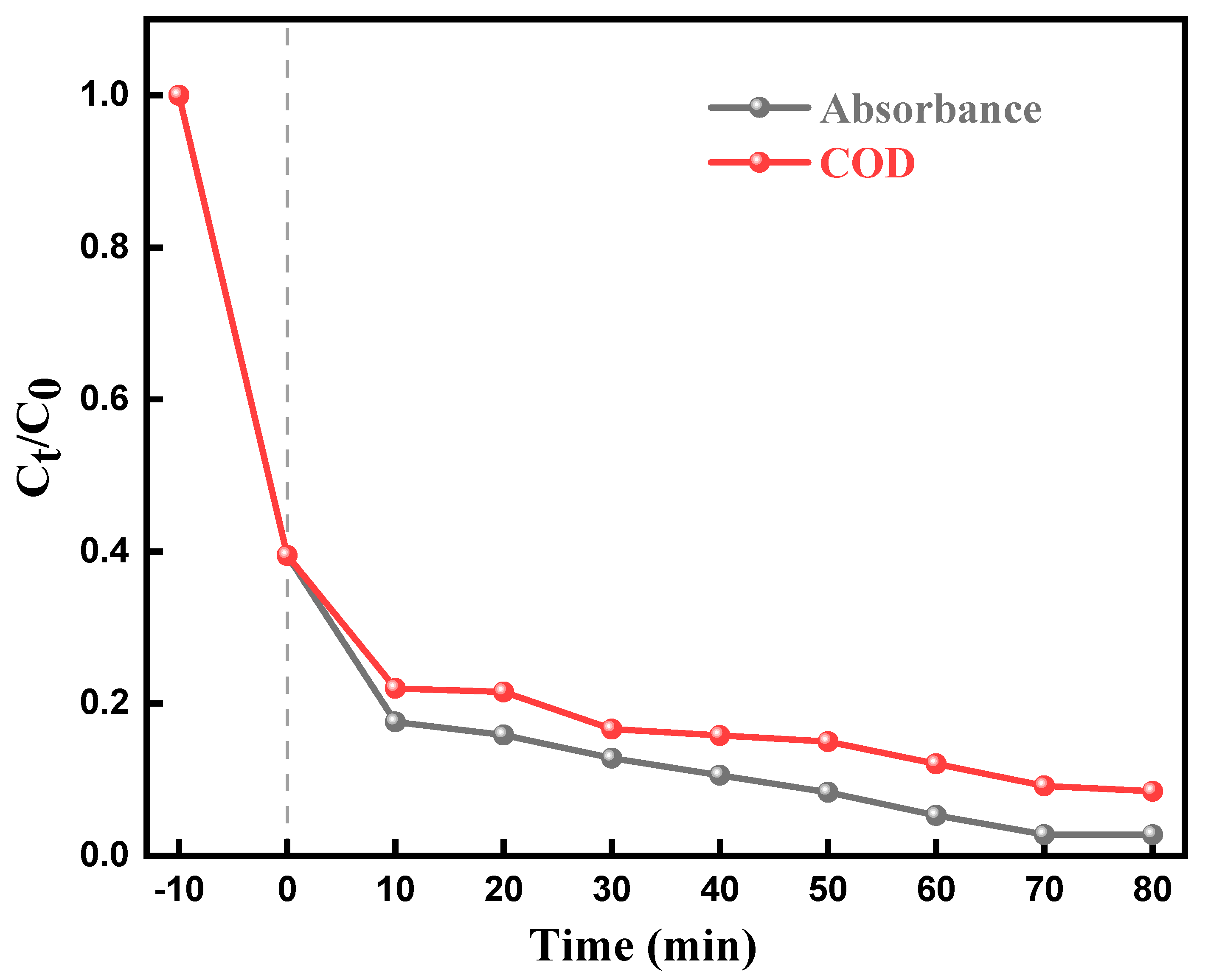
2.7. Mineralization of Dyes
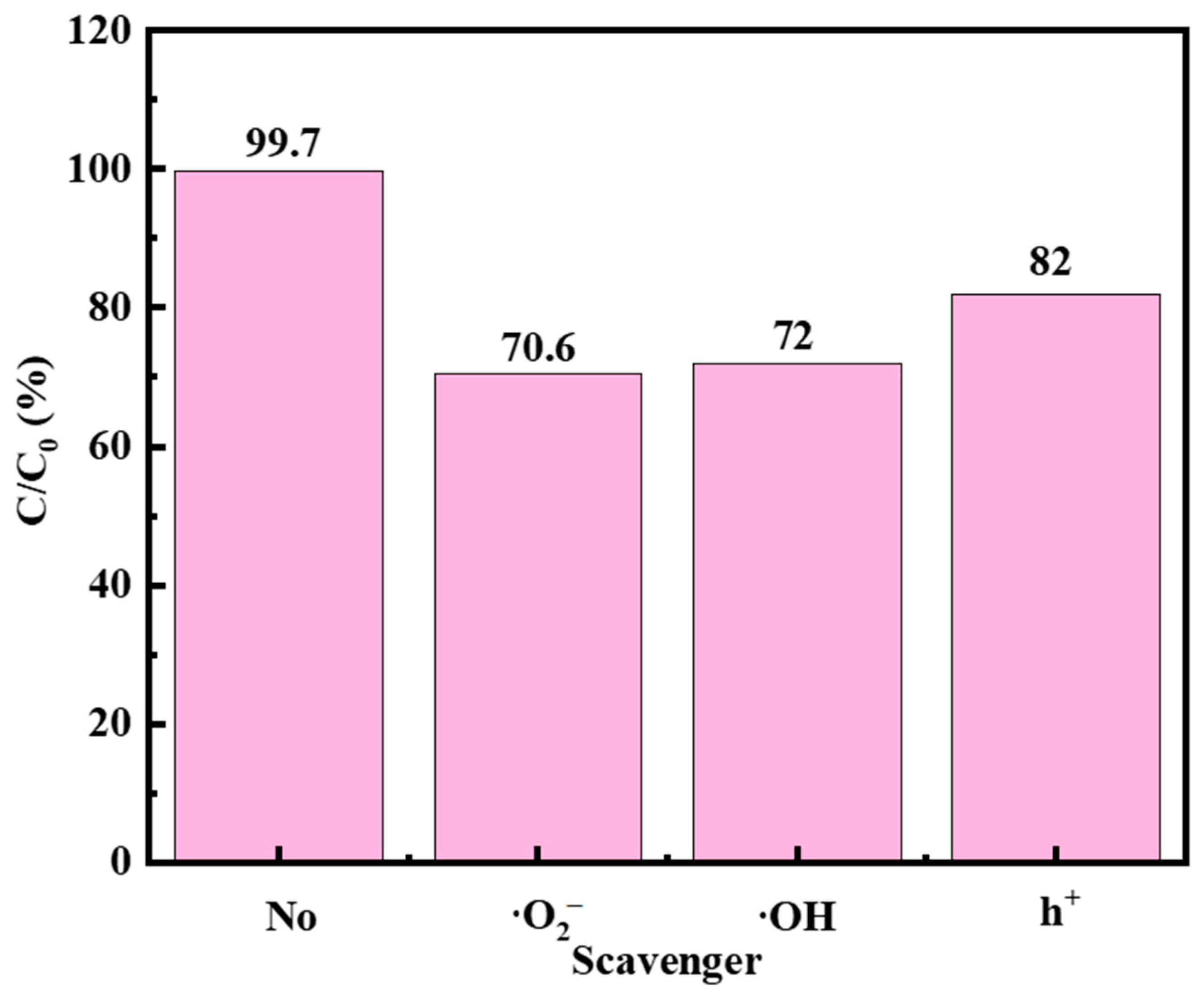
2.8. Free Radical Capture Experiments of F-Si-TiO2/AC
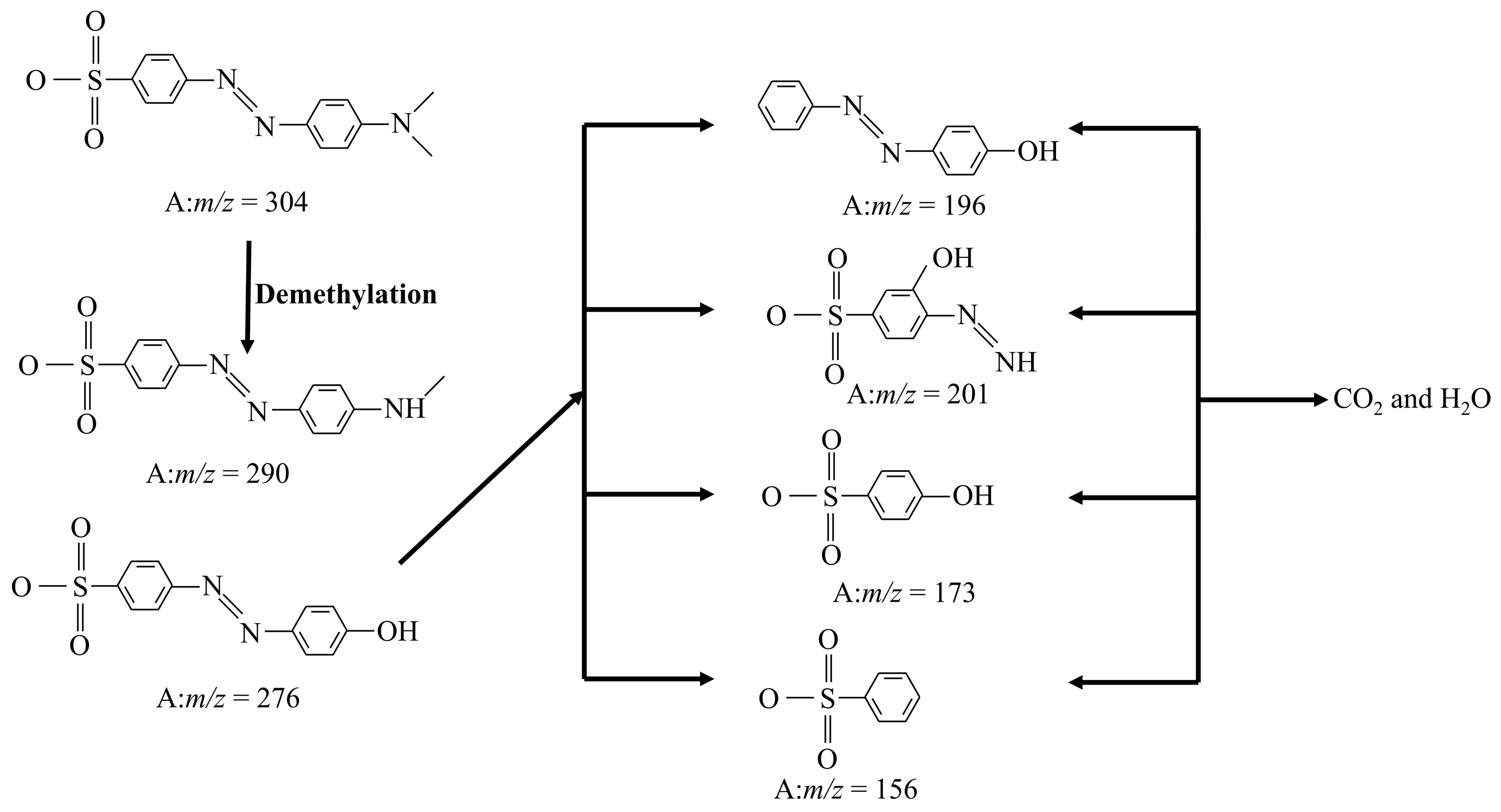
2.9. Reaction Intermediates Research of F-Si-TiO2/AC
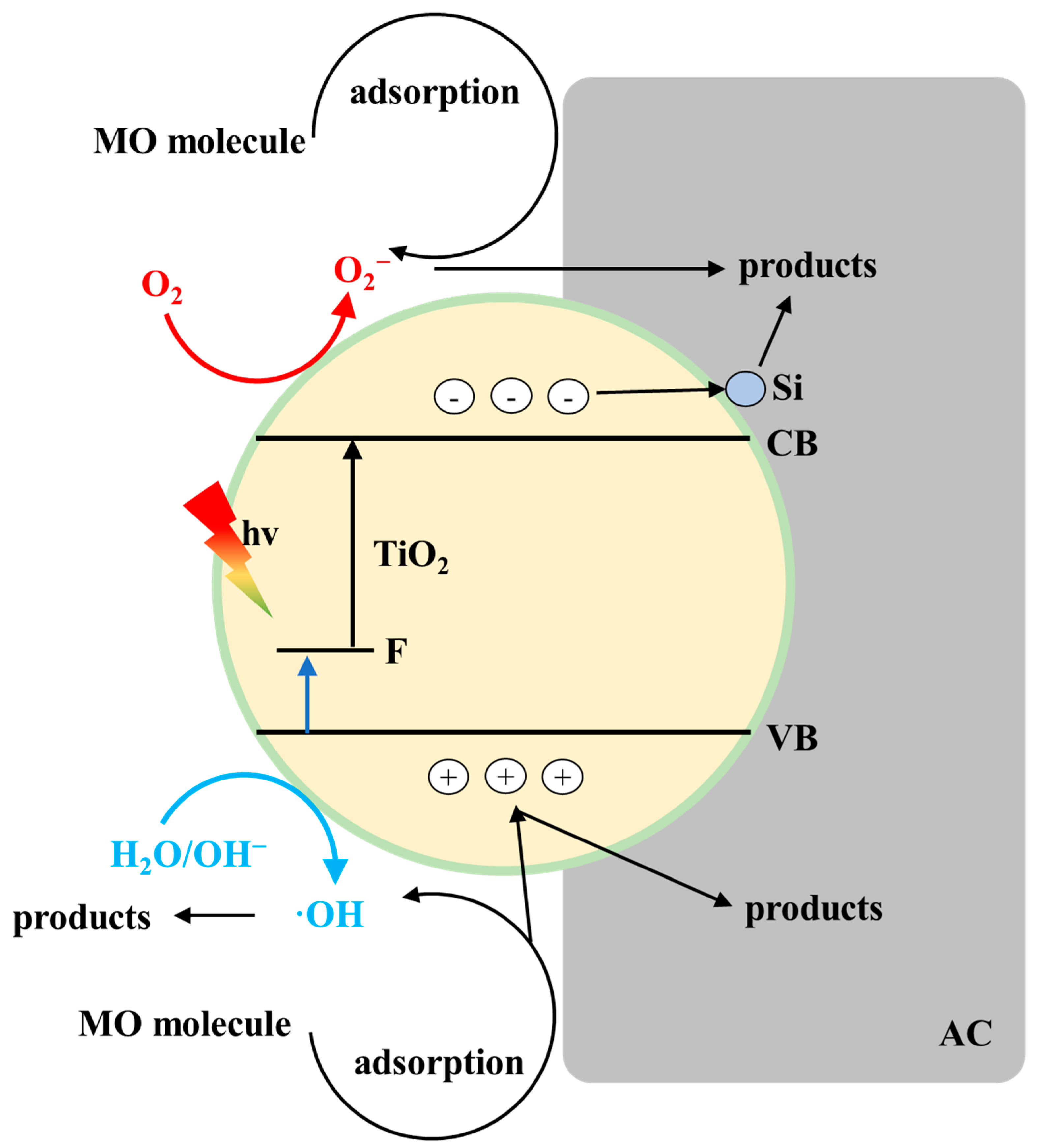
2.10. Synergistic Photodegradation Mechanism of F-Si-TiO2/AC
3. Experimental Procedure
3.1. Materials and Chemicals
3.2. Preparation of AC
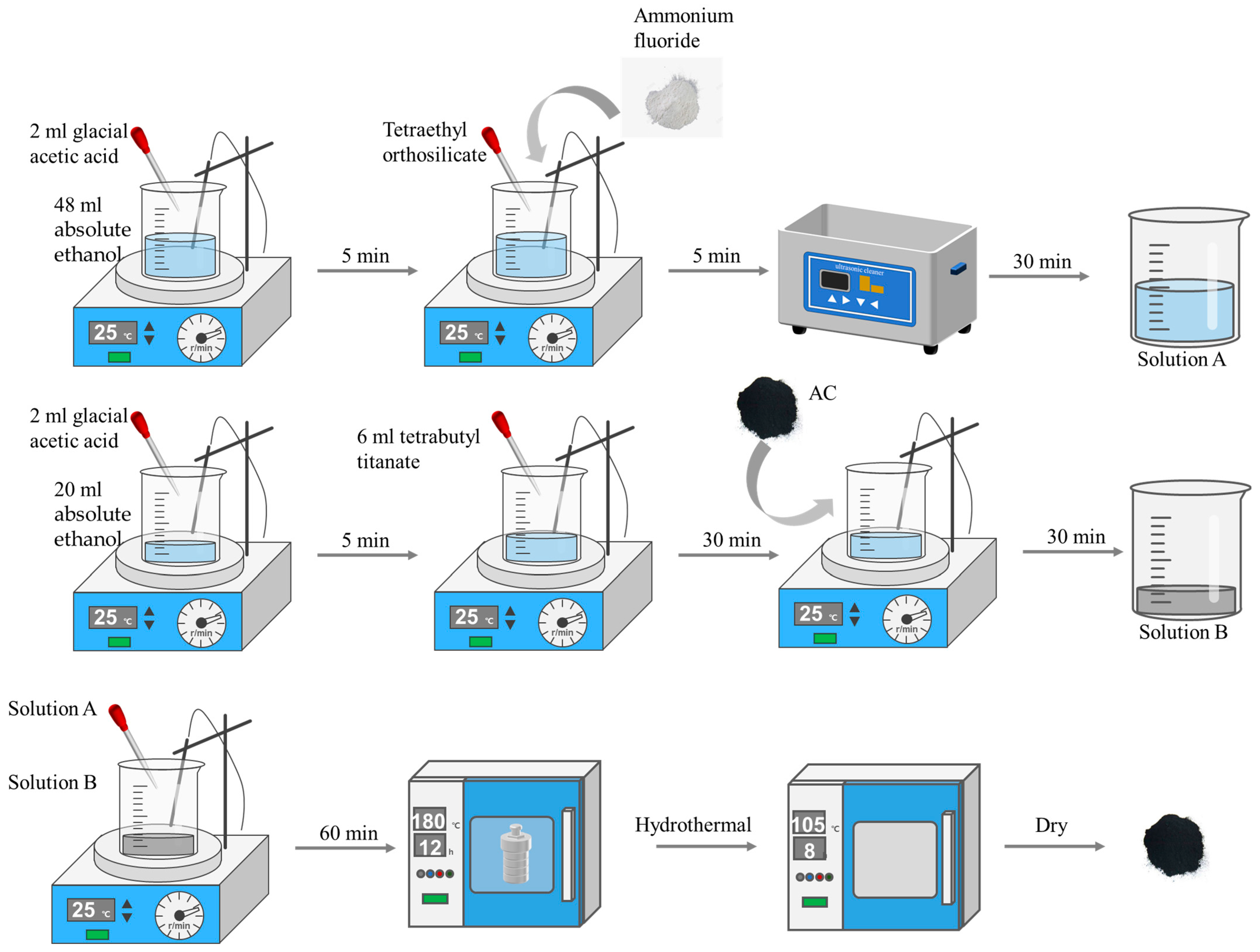
3.3. Preparation of TiO2/AC and F-Si-TiO2/AC
3.4. Material Characterization
3.5. Adsorption Experiments
3.6. Photodegradation Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mishra, A.; Kumari, M.; Swati; Kumar, R.; Iqbal, K.; Thakur, I.S. Persistent organic pollutants in the environment: Risk assessment, hazards, and mitigation strategies. Bioresour. Technol. Rep. 2022, 19, 101143. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef]
- Liu, H.; Ren, X.; Chen, L. Synthesis and characterization of magnetic metal–organic framework for the adsorptive removal of Rhodamine B from aqueous solution. J. Ind. Eng. Chem. 2016, 34, 278–285. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, D.; Liu, W.; Zheng, L.; Wang, Y.; Liu, X.; Fan, M.; Gong, Z. Rapid screening of rhodamine B in food by hydrogel solid-phase extraction coupled with direct fluorescence detection. Food Chem. 2020, 316, 126378. [Google Scholar] [CrossRef]
- Elamin, M.R.; Abdulkhair, B.Y.; Elamin, N.Y.; Ibnaouf, K.H.; Idriss, H.; Bakheit, R.; Modwi, A. Application of Synthesized Vanadium–Titanium Oxide Nanocomposite to Eliminate Rhodamine-B Dye from Aqueous Medium. Molecules 2023, 28, 176. [Google Scholar] [CrossRef]
- García-Rosero, H.; Romero-Cano, L.A.; Aguilar-Aguilar, A.; Bailón-García, E.; Carvalho, A.P.; Pérez-Cadenas, A.F.; Carrasco-Marín, F. Adsorption and thermal degradation of Atenolol using carbon materials: Towards an advanced and sustainable drinking water treatment. J. Water Process. Eng. 2022, 49, 102987. [Google Scholar] [CrossRef]
- Zheng, Y.; Cheng, B.; Fan, J.; Yu, J.; Ho, W. Review on nickel-based adsorption materials for Congo red. J. Hazard. Mater. 2021, 403, 123559. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, X.; Zhuo, N.; Zhu, G.; Cai, Z. Interaction-sedimentation strategy for highly efficient removal of refractory humic substances in biologically treated wastewater effluent: From mechanistic investigation to full-scale application. J. Hazard. Mater. 2021, 418, 126145. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, S.; Liu, H.; Li, L.; Guo, R. Fe-N-C-based cathode catalyst enhances redox reaction performance of microbial fuel cells: Azo dyes degradation accompanied by electricity generation. J. Environ. Chem. Eng. 2023, 11, 109264. [Google Scholar] [CrossRef]
- Varjani, S.; Rakholiya, P.; Ng, H.Y.; You, S.; Teixeira, J.A. Microbial degradation of dyes: An overview. Bioresour. Technol. 2020, 314, 123728. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, Y.; Zhu, J.; Li, C.; Chen, G. A Comprehensive Review on Wastewater Nitrogen Removal and Its Recovery Processes. Int. J. Environ. Res. Public Health 2023, 20, 3429. [Google Scholar] [CrossRef]
- Filho, C.M.T.; Silva, R.D.S.; Cavalcanti, C.D.K.; Granato, M.A.; Machado, R.A.F.; Marangoni, C. Membrane distillation for the recovery textile wastewater: Influence of dye concentration. J. Water Process. Eng. 2022, 46, 102611. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, Y.; Chen, Z.; Wu, S.; Fang, X.; Yao, Y. Progress in the Preparation and Modification of Zinc Ferrites Used for the Photocatalytic Degradation of Organic Pollutants. Int. J. Environ. Res. Public Health 2022, 19, 10710. [Google Scholar] [CrossRef]
- Zhai, G.; Zhou, J.; Xie, M.; Jia, C.; Hu, Z.; Xiang, H.; Zhu, M. Improved photocatalytic property of lignin-derived carbon nanofibers through catalyst synergy. Int. J. Biol. Macromol. 2023, 233, 123588. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Miino, M.C.; Damiani, S. Treatments for color removal from wastewater: State of the art. J. Environ. Manag. 2019, 236, 727–745. [Google Scholar] [CrossRef]
- Sen, S.K.; Raut, S.; Bandyopadhyay, P.; Raut, S. Fungal decolouration and degradation of azo dyes: A review. Fungal Biol. Rev. 2016, 30, 112–133. [Google Scholar] [CrossRef]
- Zheng, A.L.T.; Ohno, T.; Andou, Y. Recent Progress in Photocatalytic Efficiency of Hybrid Three-Dimensional (3D) Graphene Architectures for Pollution Remediation. Top. Catal. 2022, 65, 1634–1647. [Google Scholar] [CrossRef]
- Zheng, A.L.T.; Sabidi, S.; Ohno, T.; Maeda, T.; Andou, Y. Cu2O/TiO2 decorated on cellulose nanofiber/reduced graphene hydrogel for enhanced photocatalytic activity and its antibacterial applications. Chemosphere 2022, 286, 131731. [Google Scholar] [CrossRef]
- Hussain, A.; Rauf, A.; Ahmed, E.; Khan, M.S.; Mian, S.A.; Jang, J. Modulating Optoelectronic and Elastic Properties of Anatase TiO2 for Photoelectrochemical Water Splitting. Molecules 2023, 28, 3252. [Google Scholar] [CrossRef]
- Mao, B.; Liu, C.; Cui, X.; Li, Y.; Duan, Q. Thermo-Responsive ZnPc-g-TiO2-g-PNIPAM Photocatalysts Sensitized with Phthalocyanines for Water Purification under Visible Light. Molecules 2022, 27, 3330. [Google Scholar] [CrossRef]
- Chen, C.; Liu, P.; Xia, H.; Zhou, M.; Zhao, J.; Sharma, B.K.; Jiang, J. Photocatalytic Cleavage of β-O-4 Ether Bonds in Lignin over Ni/TiO2. Molecules 2020, 25, 2109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, Y.; Yang, X.; Chen, J.; Zhong, J.; Li, J.; Li, M.; Wan, Z. Enhanced photocatalytic detoxication properties of OVs-rich Pd/N–TiO2 heterojunctions: Excellent charge separation and mechanism insight. Mater. Today Chem. 2023, 28, 101358. [Google Scholar] [CrossRef]
- Song, Q.; Sun, C.; Wang, Z.; Bai, X.; Wu, K.; Li, Q.; Zhang, H.; Zhou, L.; Pang, H.; Liang, Y.; et al. Directed charge transfer in all solid state heterojunction of Fe doped MoS2 and C–TiO2 nanosheet for enhanced nitrogen photofixation. Mater. Today Phys. 2021, 21, 100563. [Google Scholar] [CrossRef]
- Biswal, L.; Nayak, S.; Parida, K. Rationally designed Ti3C2/N, S-TiO2/g-C3N4 ternary heterostructure with spatial charge separation for enhanced photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2022, 621, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, T.; Dai, K.; Zhang, J. Construction of TiO2 nanosheets with exposed {001} facets/Zn0.2Cd0.8S-DETA heterostructure with enhanced visible light hydrogen production. Appl. Surf. Sci. 2020, 516, 146141. [Google Scholar] [CrossRef]
- Pang, D.; Wang, Y.; Ma, X.; Ouyang, F. Fluorine promoted and silica supported TiO2 for photocatalytic decomposition of acrylonitrile under simulant solar light irradiation. Chem. Eng. J. 2014, 258, 43–50. [Google Scholar] [CrossRef]
- Chen, R.; Chen, J.; Gao, X.; Ao, Y.; Wang, P. Probing the role of surface acid sites on the photocatalytic degradation of tetracycline hydrochloride over cerium doped CdS via experiments and theoretical calculations. Dalton Trans. 2021, 50, 16620–16630. [Google Scholar] [CrossRef]
- Shifu, C.; Yunguang, Y.; Wei, L. Preparation, characterization and activity evaluation of TiN/F-TiO2 photocatalyst. J. Hazard. Mater. 2011, 186, 1560–1567. [Google Scholar] [CrossRef]
- Shu, Y.; Ji, J.; Zhou, M.; Liang, S.; Xie, Q.; Li, S.; Liu, B.; Deng, J.; Cao, J.; Liu, S.; et al. Selective photocatalytic oxidation of gaseous ammonia at ppb level over Pt and F modified TiO2. Appl. Catal. B Environ. 2022, 300, 120688. [Google Scholar] [CrossRef]
- Ta, Q.T.H.; Tran, N.M.; Tri, N.N.; Sreedhar, A.; Noh, J.-S. Highly surface-active Si-doped TiO2/Ti3C2Tx heterostructure for gas sensing and photodegradation of toxic matters. Chem. Eng. J. 2021, 425, 131437. [Google Scholar] [CrossRef]
- Long, R.; English, N.J. Band gap engineering of (N, Si)-codoped TiO2 from hybrid density functional theory calculations. New J. Phys. 2012, 14, 053007. [Google Scholar] [CrossRef]
- Zeng, G.; You, H.; Du, M.; Zhang, Y.; Ding, Y.; Xu, C.; Liu, B.; Chen, B.; Pan, X. Enhancement of photocatalytic activity of TiO2 by immobilization on activated carbon for degradation of aquatic naphthalene under sunlight irradiation. Chem. Eng. J. 2021, 412, 128498. [Google Scholar] [CrossRef]
- Samsudin, E.M.; Hamid, S.B.A.; Juan, J.C.; Basirun, W.J.; Centi, G. Enhancement of the intrinsic photocatalytic activity of TiO2 in the degradation of 1,3,5-triazine herbicides by doping with N,F. Chem. Eng. J. 2015, 280, 330–343. [Google Scholar] [CrossRef]
- Uvarov, V.; Popov, I. Metrological characterization of X-ray diffraction methods for determination of crystallite size in nano-scale materials. Mater. Charact. 2007, 58, 883–891. [Google Scholar] [CrossRef]
- Chen, J.; Qin, Y.; Chen, Z.; Yang, Z.; Yang, W.; Wang, Y. Gas circulating fluidized beds photocatalytic regeneration of I-TiO2 modified activated carbons saturated with toluene. Chem. Eng. J. 2016, 293, 281–290. [Google Scholar] [CrossRef]
- Chen, M.; Ma, J.; Zhang, B.; He, G.; Li, Y.; Zhang, C.; He, H. Remarkable synergistic effect between {001} facets and surface F ions promoting hole migration on anatase TiO2. Appl. Catal. B Environ. 2017, 207, 397–403. [Google Scholar] [CrossRef]
- Wang, J.; Liu, B.; Nakata, K. Effects of crystallinity, {001}/{101} ratio, and Au decoration on the photocatalytic activity of anatase TiO2 crystals. Chin. J. Catal. 2019, 40, 403–412. [Google Scholar] [CrossRef]
- Yilmaz, M.; Cirak, B.B.; Aydogan, S.; Grilli, M.L.; Biber, M. Facile electrochemical-assisted synthesis of TiO2 nanotubes and their role in Schottky barrier diode applications. Superlattices Microstruct. 2018, 113, 310–318. [Google Scholar] [CrossRef]
- Asencios, Y.J.; Lourenço, V.S.; Carvalho, W.A. Removal of phenol in seawater by heterogeneous photocatalysis using activated carbon materials modified with TiO2. Catal. Today 2020, 388–389, 247–258. [Google Scholar] [CrossRef]
- Ao, W.; Qu, J.; Yu, H.; Liu, Y.; Liu, C.; Fu, J.; Dai, J.; Bi, X.; Yuan, Y.; Jin, Y. TiO2/activated carbon synthesized by microwave-assisted heating for tetracycline photodegradation. Environ. Res. 2022, 214, 113837. [Google Scholar] [CrossRef]
- Xie, G.; Wang, L.; Zhu, Q.; Xu, L.; Song, K.; Yu, Z. Modification of SiO2 Nanoparticle-Decorated TiO2 Nanocomposites with Silane Coupling Agents for Enhanced Opacity in Blue Light-Curable Ink. ACS Appl. Nano Mater. 2022, 5, 9678–9687. [Google Scholar] [CrossRef]
- Komatsuda, S.; Asakura, Y.; Vequizo, J.J.M.; Yamakata, A.; Yin, S. Enhanced photocatalytic NO decomposition of visible-light responsive F-TiO2/(N,C)-TiO2 by charge transfer between F-TiO2 and (N,C)-TiO2 through their doping levels. Appl. Catal. B Environ. 2018, 238, 358–364. [Google Scholar] [CrossRef]
- Zhao, J.; Li, W.; Li, X.; Zhang, X. Low temperature synthesis of water dispersible F-doped TiO2 nanorods with enhanced photocatalytic activity. RSC Adv. 2017, 7, 21547–21555. [Google Scholar] [CrossRef] [Green Version]
- Guayaquil-Sosa, J.; Serrano-Rosales, B.; Valadés-Pelayo, P.; de Lasa, H. Photocatalytic hydrogen production using mesoporous TiO2 doped with Pt. Appl. Catal. B Environ. 2017, 211, 337–348. [Google Scholar] [CrossRef]
- Park, H.; Choi, W. Effects of TiO2 Surface Fluorination on Photocatalytic Reactions and Photoelectrochemical Behaviors. J. Phys. Chem. B 2004, 108, 4086–4093. [Google Scholar] [CrossRef]
- Liu, W.; Yun, Y.; Li, M.; Mao, J.; Li, C.; Li, C.; Hu, L. Preparation of hollow ceramic photocatalytic membrane grafted with silicon-doped TiO2 nanorods and conversion of high-concentration NO. Chem. Eng. J. 2022, 437, 135261. [Google Scholar] [CrossRef]
- Jiang, G.; Lin, Z.; Chen, C.; Zhu, L.; Chang, Q.; Wang, N.; Wei, W.; Tang, H. TiO2 nanoparticles assembled on graphene oxide nanosheets with high photocatalytic activity for removal of pollutants. Carbon 2011, 49, 2693–2701. [Google Scholar] [CrossRef]
- Augugliaro, V.; Baiocchi, C.; Prevot, A.B.; García-López, E.; Loddo, V.; Malato, S.; Marcí, G.; Palmisano, L.; Pazzi, M.; Pramauro, E. Azo-dyes photocatalytic degradation in aqueous suspension of TiO2 under solar irradiation. Chemosphere 2002, 49, 1223–1230. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Fu, C.-C.; Juang, R.-S. Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways. J. Clean. Prod. 2018, 202, 413–427. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Xu, H.; Xin, Y.; Wang, A.; Liu, K. Schottky heterogeneous interface design of Ti3C2/Bi4O5I2 to enhance the photocatalytic performance. Crystengcomm 2023. [Google Scholar] [CrossRef]
- Chen, P.; Liang, Y.; Xu, Y.; Zhao, Y.; Song, S. Synchronous photosensitized degradation of methyl orange and methylene blue in water by visible-light irradiation. J. Mol. Liq. 2021, 334, 116159. [Google Scholar] [CrossRef]
- Tseng, S.-J.; Huang, Y.-S.; Wu, Q.-Y.; Wang, W.-L.; Wu, J.J. Hollow-structured Pd/TiO2 as a dual functional photocatalyst for methyl orange oxidation and selective reduction of nitrate into nitrogen. J. Ind. Eng. Chem. 2023, 119, 386–394. [Google Scholar] [CrossRef]
- Liu, Y.; Xiang, Y.; Xu, H.; Li, H. The reuse of nano-TiO2 under different concentration of CO32- using coagulation process and its photocatalytic ability in treatment of methyl orange. Sep. Purif. Technol. 2022, 282, 120152. [Google Scholar] [CrossRef]
- Zhou, Y.; Cai, T.; Liu, S.; Liu, Y.; Chen, H.; Li, Z.; Du, J.; Lei, Z.; Peng, H. N-doped magnetic three-dimensional carbon microspheres@TiO2 with a porous architecture for enhanced degradation of tetracycline and methyl orange via adsorption/photocatalysis synergy. Chem. Eng. J. 2021, 411, 128615. [Google Scholar] [CrossRef]
- Gomez-Polo, C.; Larumbe, S.; Gil, A.; Muñoz, D.; Fernández, L.R.; Barquín, L.F.; García-Prieto, A.; Fdez-Gubieda, M.; Muela, A. Improved photocatalytic and antibacterial performance of Cr doped TiO2 nanoparticles. Surf. Interfaces 2021, 22, 100867. [Google Scholar] [CrossRef]
- Guo, Z.; Wu, H.; Li, M.; Tang, T.; Wen, J.; Li, X. Phosphorus-doped graphene quantum dots loaded on TiO2 for enhanced photodegradation. Appl. Surf. Sci. 2020, 526, 146724. [Google Scholar] [CrossRef]
- Zhang, S.; Lei, C.; Song, L. Chiral induction enhanced photocatalytic degradation of methyl orange by Ag@Ag3PO4 and the reaction mechanism. Ceram. Int. 2023, 49, 26548–26557. [Google Scholar] [CrossRef]
- Bi, T.; Du, Z.; Chen, S.; He, H.; Shen, X.; Fu, Y. Preparation of flower-like ZnO photocatalyst with oxygen vacancy to enhance the photocatalytic degradation of methyl orange. Appl. Surf. Sci. 2023, 614, 156240. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, G.; Guo, X.; Lu, Y.; Zhu, Y. Optimization of Preparation Conditions of F-N-TiO2/AC Composite Photocatalyst for Degradation of Printing and Dyeing Wastewater. Mater. Perform. Charact. 2022, 11, 256–266. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, L.; Liu, H.; Chang, L.; Lv, S.; Niu, B.; Zheng, J.; Liu, S.; Fu, J. Hollow polyphosphazene microcapsule with rigid-flexible coupling cationic skeletons for highly efficient and selective adsorption of anionic dyes from water. Appl. Surf. Sci. 2023, 626, 157234. [Google Scholar] [CrossRef]
- Hoang, N.T.-T.; Tran, A.T.-K.; Le, T.-A.; Nguyen, D.D. Enhancing efficiency and photocatalytic activity of TiO2-SiO2 by combination of glycerol for MO degradation in continuous reactor under solar irradiation. J. Environ. Chem. Eng. 2021, 9, 105789. [Google Scholar] [CrossRef]
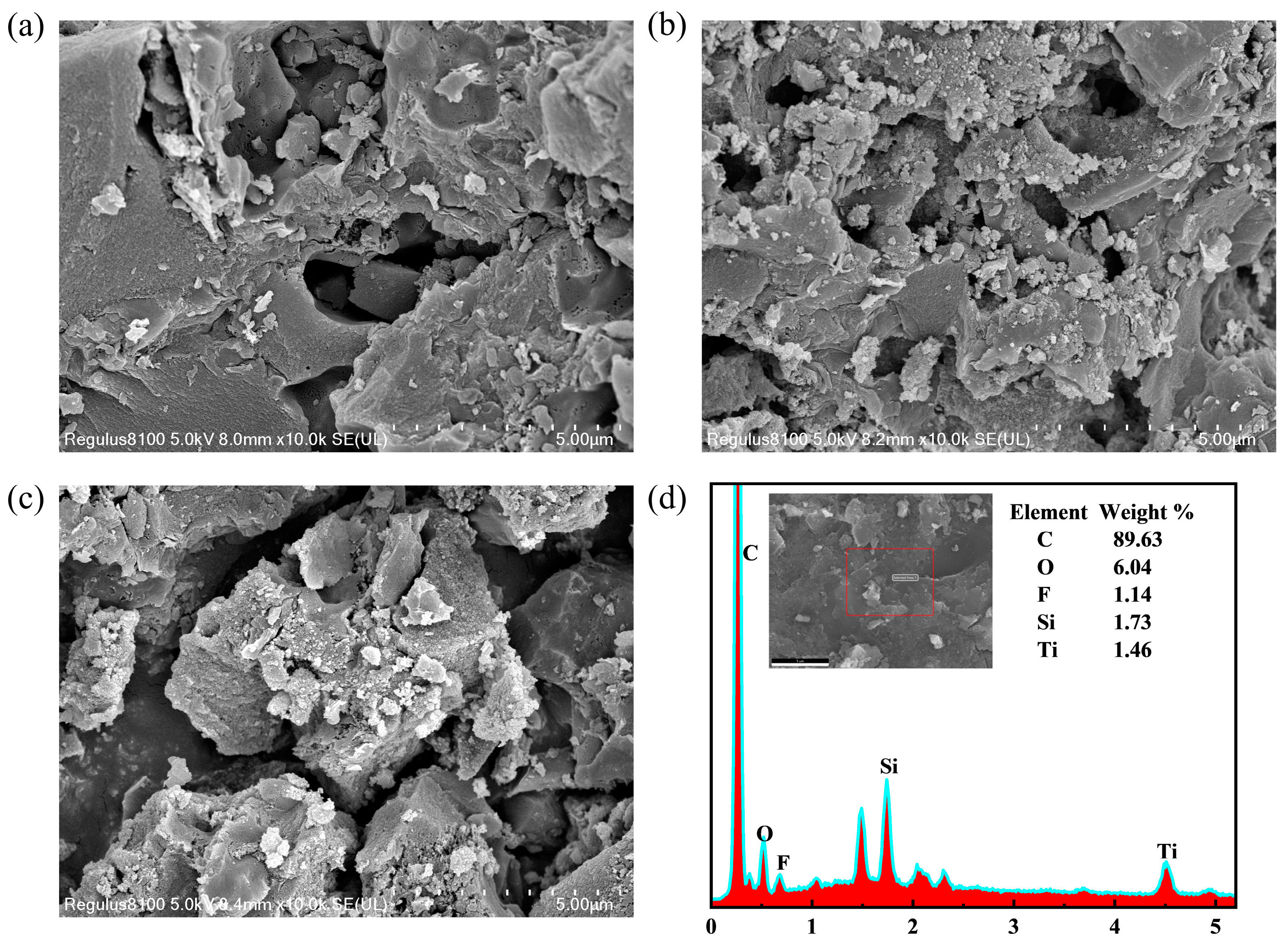

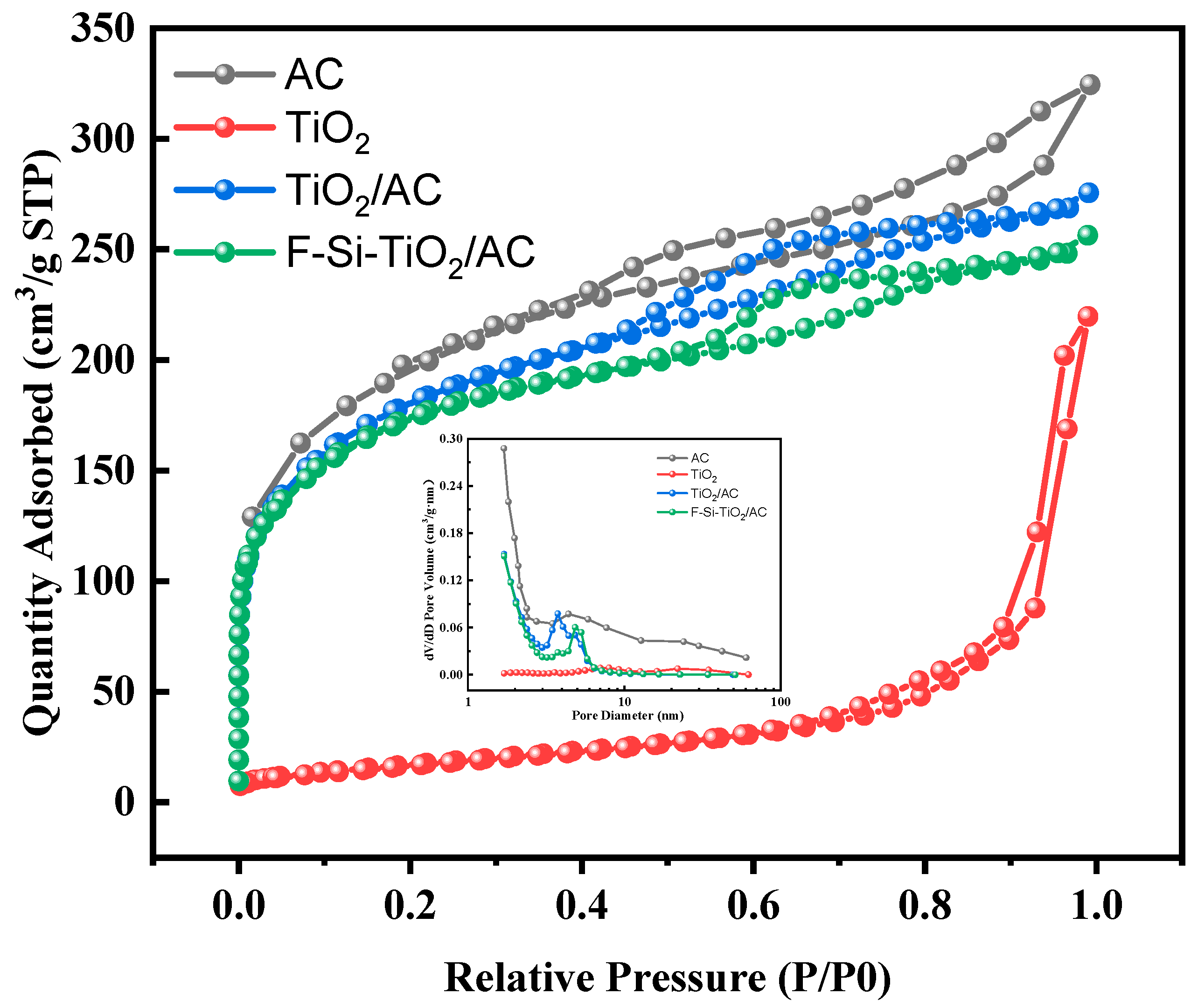

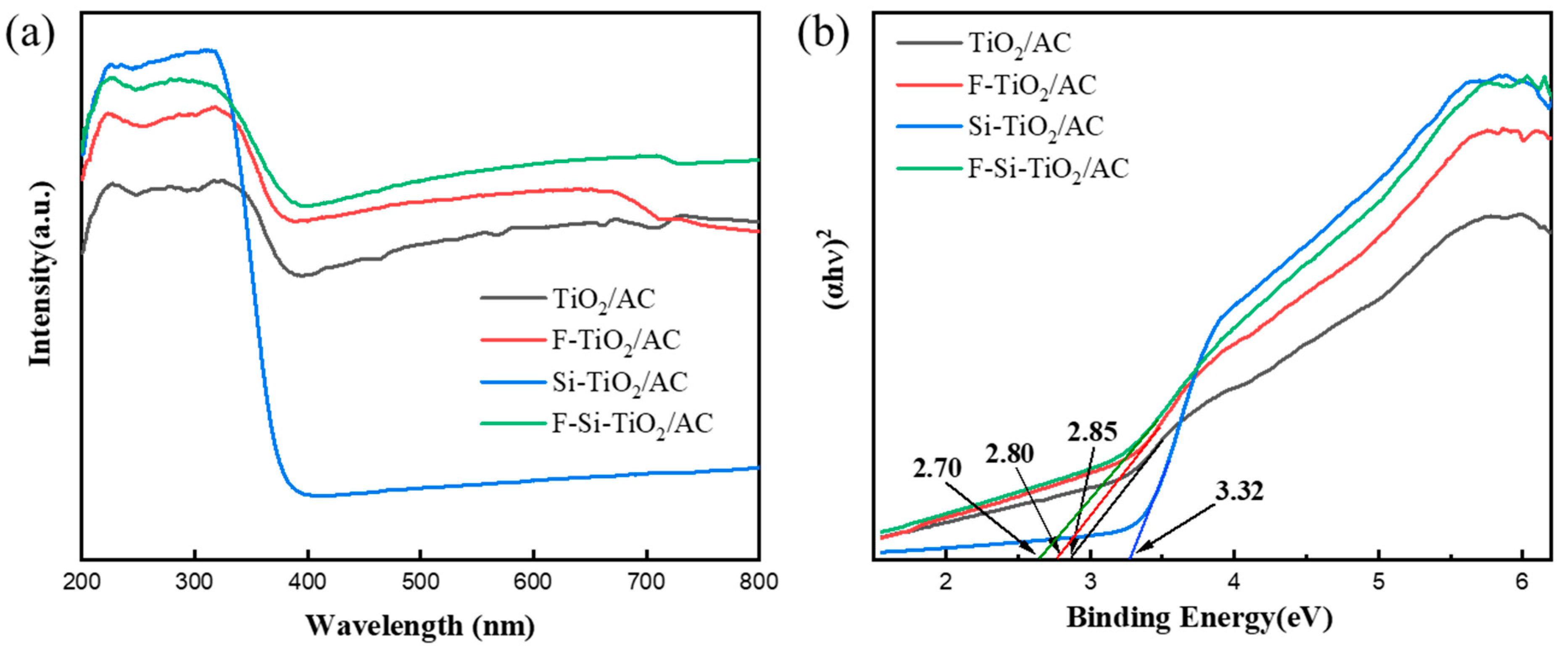


| Samples | Crystalline Size (nm) | A and B | C | 2Θ (101) | Predominant Facet (%) | FWHM (101) | Interplanar Distance |
|---|---|---|---|---|---|---|---|
| TiO2/AC | 10.2 | 3.818 | 9.467 | 25.201 | 58.8 | 1.487 | 3.536 |
| F-TiO2/AC | 9.4 | 3.812 | 9.459 | 25.098 | 63.5 | 1.502 | 3.550 |
| Si-TiO2/AC | 8.2 | 3.802 | 9.482 | 25.158 | 63.3 | 1.500 | 3.541 |
| F-Si-TiO2/AC | 9.4 | 3.824 | 9.502 | 25.102 | 69.6 | 1.516 | 3.549 |
| Samples | SBET (m2·g−1) | Pore Volume (cm3·g−1) | Average Pore Size (nm) |
|---|---|---|---|
| AC | 672.623 | 0.486 | 2.846 |
| P25 | 62.639 | 0.341 | 2.177 |
| TiO2/AC | 618.518 | 0.428 | 2.765 |
| F-Si-TiO2/AC | 591.285 | 0.398 | 2.690 |
| Sample | K(×10–4/min) for MO Visible Light Irr. | Band Gap and Mid-Gap Level Energy (eV) | F: TiO2 | Si: TiO2 | Degradation (%) |
|---|---|---|---|---|---|
| TiO2 | 12.9 | 3.20 | 0 | 0 | 14.3 |
| TiO2/AC | 57.8 | 2.85 | 0 | 0 | 67.0 |
| Si-TiO2/AC | 30.5 | 3.32 | 0 | 0.1 | 58.2 |
| F-TiO2/AC | 191.4 | 2.80 | 0.6 | 0 | 89.8 |
| F-Si-TiO2/AC | 318.0 | 2.70 | 0.6 | 0.1 | 97.2 |
| Photocatalysts | Catalyst Dosage (mg/100 mL) | Initial Dye Concentration (mg/L) | Rection Time (min) | Efficiency (%) | Ref |
|---|---|---|---|---|---|
| Pd/TiO2 | 100 | 20 | 60 | 100 | [52] |
| TiO2 | 100 | 20 | 75 | 72.79 | [53] |
| C/TiO2 | 50 | 40 | 200 | 99.7 | [54] |
| Cr-TiO2 | 50 | 1.6 | 300 | 78 | [55] |
| P-C-TiO2 | 100 | 10 | 14 | 95 | [56] |
| Ag@AgP3O4 | 100 | 10 | 30 | 80 | [57] |
| ZnO | 30 | 10 | 30 | 94.5 | [58] |
| F-Si-TiO2/AC | 10 | 10 | 70 | 99.7 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Zhu, Y.; Zhou, Y.; Wu, C.; Chen, Z.; Chen, G. Synergistic Promotion of Photocatalytic Degradation of Methyl Orange by Fluorine- and Silicon-Doped TiO2/AC Composite Material. Molecules 2023, 28, 5170. https://doi.org/10.3390/molecules28135170
Zhu J, Zhu Y, Zhou Y, Wu C, Chen Z, Chen G. Synergistic Promotion of Photocatalytic Degradation of Methyl Orange by Fluorine- and Silicon-Doped TiO2/AC Composite Material. Molecules. 2023; 28(13):5170. https://doi.org/10.3390/molecules28135170
Chicago/Turabian StyleZhu, Jinyuan, Yingying Zhu, Yifan Zhou, Chen Wu, Zhen Chen, and Geng Chen. 2023. "Synergistic Promotion of Photocatalytic Degradation of Methyl Orange by Fluorine- and Silicon-Doped TiO2/AC Composite Material" Molecules 28, no. 13: 5170. https://doi.org/10.3390/molecules28135170
APA StyleZhu, J., Zhu, Y., Zhou, Y., Wu, C., Chen, Z., & Chen, G. (2023). Synergistic Promotion of Photocatalytic Degradation of Methyl Orange by Fluorine- and Silicon-Doped TiO2/AC Composite Material. Molecules, 28(13), 5170. https://doi.org/10.3390/molecules28135170






