Abstract
Quinone methides are a class of biologically active compounds that can be used in medicine as antibacterial, antifungal, antiviral, antioxidant, and anti-inflammatory agents. In addition, quinone methides have the potential to be used as pesticides, dyes, and additives for rubber and plastics. In this paper, we discuss a subclass of quinone methides: methylenequinone oximes. Although the first representatives of the subgroup were synthesized in the distant past, they still need to be additionally studied, while their chemistry, biological properties, and perspective of practical applications require to be comprehensively summarised. Based on the analysis of the literature, it can be concluded that methylenequinone oximes exhibit a diversified profile of properties and outstanding potential as new drug candidates and reagents in organic synthesis, both of electrophilic and nucleophilic nature, worthy of wide-ranging further research.
1. Introduction
Quinones, and especially benzoquinones, play an important role in the metabolism of all living cells, since they have the ability to act as mediators for both electron [1] and proton transfers [2,3]. Worth noting is also the fact that redox reactions mediated by benzoquinones may lead to the creation of reactive oxygen species, causing cellular damage [4,5]. At the same time, particularly substituted quinones can behave as antioxidants, protecting healthy cells [6]. Such a widespread pattern of activity of quinones makes some of them efficient antibiotics [7,8] and vitamins [9]. The shortcomings of currently used drugs dictate the urgent and growing need to discover the new classes of medicines and some quinonemethide derivatives may be useful to fill that gap.
Quinone methides are the derivatives of quinones in which one of the oxygen atoms is replaced with carbon. The chemistry of quinone methides is less developed and their biological properties are less explored. These compounds have a conjugated system, making them favourable for nucleophilic addition via an exocyclic double bond. It is worth noting that quinone methides are of more polar nature, compared to quinones; therefore, they are considered to have higher reactivity [10]. Most quinone methides have pronounced biological activity. Among biologically active substances containing quinone methide backbone, many can be found with antitumor [11,12,13], antibacterial [14,15,16], antifungal [17], DNA alkylating and cross-linking agent [18], antioxidant [19,20], and anti-inflammatory [19,21] properties. Moreover, the main pharmacological mechanism of action of some other anticancer phenolic drugs is based on active quinone methides that are metabolically formed in living cells [22].
A subgroup of quinone methides are their oximes. They represent a rather attractive group of organic compounds, since their natural and synthetic derivatives are characterized by a diverse range of biological properties, therefore making them an interesting object for scientists working in the fields of organic, pharmaceutical, and medicinal chemistry, as well as chemistry of natural compounds. Many quinone methide oximes have already found their application in industry, including pharmaceutical and medical sectors: namely, as components of photoresists [23,24], colour filters [25], additives for rubber [26,27] and plastic products [28,29,30,31], and as herbicides [32]. The quinone methide oximes were found to be intermediates in the organic synthesis of already known biologically active substances [33,34] and other important products [35,36]. It has also been reported that oximes of quinone methides can be used as antidotes [37,38], disinfectants [39], or to obtain prodrugs [40]. What is more interesting is that various quinone methide oxime derivatives reportedly possess antifungal [41,42] and antitumor [39,43] activities. The presence of a conjugated system in the molecules of quinone methide oximes allows for them to be used in the manufacturing of various dyes as well [44].
Based on the analysis of the literature, it can be concluded that synthetic and pharmaceutical chemistry, as well as the biological properties of quinone methide oxime derivatives are fairly diverse. The objective of this review is to shed some light on a subgroup of quinone methide oximes derivatives, namely, arylcyanomethylenequinone oximes (Figure 1). Throughout the review, the synthesis, methods of chemical modification, and biological activity of the subgroup are highlighted.
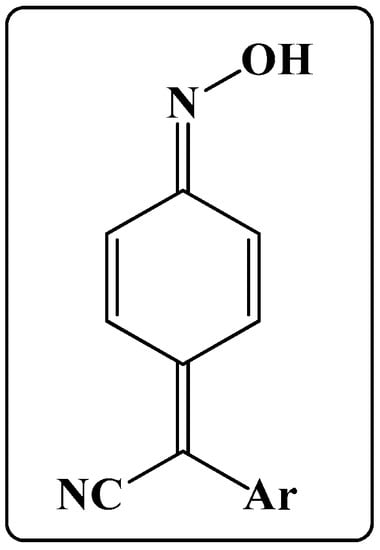
Figure 1.
The structure of arylcyanomethylenequinone oximes studied in this work.
Although the first representatives of these compounds were synthesized in the distant past [45], they are still insufficiently studied. To the best knowledge of the authors of this review, there is an absence of a professional literature review discussing arylcyanomethylenequinone oximes in detail. Several papers dedicated to the specific types of quinone methides put their focus on the generation of quinone methide core and its reactivity [46], on the application of quinone methides in asymmetric organocatalysis [47], on their biological properties [48,49], and potential application, e.g., in the fluorescent sensing of enzymatic activity [50]. The polycyclic friedelane-type triterpenoid quinone methides [51] and pyridinium quinone methide derivatives [52] are also known to be biologically active.
Due to the previously specified deficits in the literature review of the topic, we hereby decided to systemize the current knowledge of arylcyanomethylenequinone oximes covering the period between the earliest publications from 1960 until the contemporary publication from 2021. The availability and easiness of the synthesis make the discussed compounds even more favourable for potential usage in industry, medicine, and as precursors for organic synthesis. What is more important, the presence of two valuable moieties, namely, cyano- and oxime groups as well as dual electrophilic–nucleophilic nature of the arylcyanomethylenequinone oximes, render them very attractive objects for further transformations [24,25,32,42,53,54,55,56,57,58,59]. However, what makes (hetero) arylcyanomethylenequinone oximes, and their derivatives, interesting research subjects, especially in the field of medicine, is their biological activity. Among others, the most promising is potential usage of arylcyanomethylenequinone oximes as antitumor [60], antiparasitic [61], antifungal [41,62], anti-allergic [63], and anti-inflammatory [64] agents. In some works, there is also information that arylcyanomethylenequinone oximes can be used as plant protection herbicides [32,65], fungicides [66] and pesticides [67]. In conjunction with the previous information, we hope that the summarized description of the synthesis, chemical transformations and biological properties of arylcyanomethylenequinone oximes will turn out to be useful to the chemists researching new aspects of the medicinal chemistry.
2. Synthesis of Arylcyanomethylenequinone Oximes and Some of Their Derivatives
2.1. The Condensation of (Hetero) Arylacetonitriles with Nitro (Hetero) Arenes
There are not many methods applicable to the synthesis of both arylcyanomethylenequinone oximes and other quinone methide oxime derivatives. The analysis of the literature data showed that one of the best approaches for the construction of their backbone is the condensation of benzyl cyanides with 4-unsubstituted nitroarenes or nitroheteroarenes [60], which is a simple way of synthesis of various derivatives of such oximes.
The synthesis of phenylcyanomethylenequinone oxime was first described by Davis et al. [45] in 1960. The authors stated that when benzyl cyanide 1a and nitrobenzene 2a are added to a warm alcoholic solution of potassium hydroxide, the reaction mixture becomes dark red and a like-coloured solid soon precipitates. The solid dissolves in water, and upon acidification with acetic acid solution, a new yellow–orange solid precipitates with a yield of 77%; the latter solid is 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a (Scheme 1).
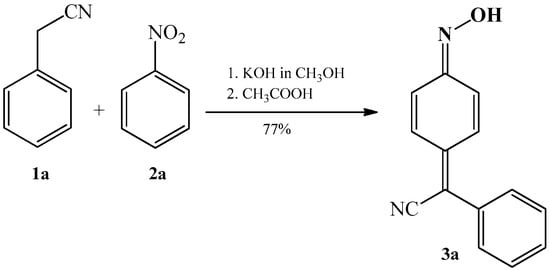
Scheme 1.
Condensation reaction of benzyl cyanide 1a with nitrobenzene 2a that produces 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a.
Simultaneously, in that work, the authors also reported that no evidence for the other possible reaction products, except for product 3a, were obtained. Attempts to isolate possible structures 4a, 5a, 6a were unsuccessful (Figure 2).
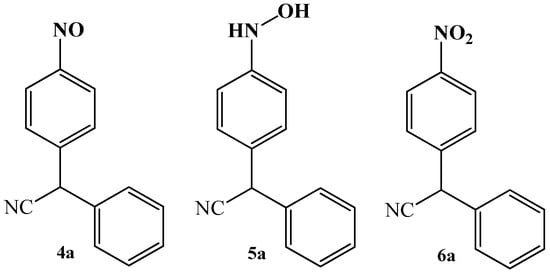
Figure 2.
Structures of other potential reaction products of condensation reaction between benzyl cyanide 1a and nitrobenzene 2a.
On the other hand, they have noted that in the reaction between benzyl cyanide 1a and nitrobenzene 2a, it is possible to obtain other products when the methanol solvent was replaced with pyridine: the mixture of products 5a and 6a (Scheme 2) was obtained then.

Scheme 2.
Condensation reaction of benzyl cyanide 1a with nitrobenzene 2a to produce analogues 5a and 6a of oxime 3a.
In the same paper the authors have also shown, for the first time, that nitrobenzene 2a can react with 4-substituted benzyl cyanides, such as 4-chlorobenzyl cyanide 1b and 4-methoxybenzyl cyanide 1c, as well as with α-naphthylacetonitrile 7a. Reactions took place in a methanolic solution of potassium hydroxide, forming corresponding quinone oximes 3ba, 3ca (Scheme 3), and 8a (Scheme 4).

Scheme 3.
Condensation reaction of 4-substituted benzyl cyanides 1b–c with nitrobenzene 2a to produce quinone oximes 3ba and 3ca.

Scheme 4.
Condensation reaction of α-naphthylacetonitrile 7a with nitrobenzene 2a to produce quinone oximes 8a.
The mechanism of condensation reaction between benzyl cyanide 1a and nitrobenzene 2a was described on Scheme 5. In the first step, nitrobenzene 2a undergoes nucleophilic attack of the benzyl cyanide anion 1’a at the 4-position, with subsequent addition of a proton to the oxygen of the nitro group to form intermediate 9a, which can also exist as a potassium salt. Further elimination of a water molecule leads to the formation of the nitroso derivative 4a. In turn, compound 4a rapidly transforms into an anion, possessing two alternative forms 10a and 10’a. Finally, acidification of the potassium salt 10’a leads to the formation of phenylcyanomethylenequinone oxime 3a.
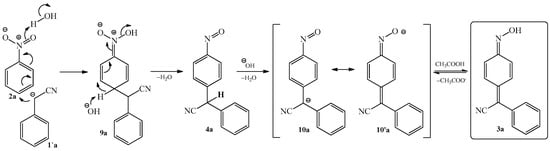
Scheme 5.
Mechanism of condensation reaction of nitrobenzene 1a with benzyl cyanide 2a to produce 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a.
In 1962, Lichtenberger and Weiss [68] reproduced the condensation reaction between nitrobenzene 1a and benzyl cyanide 2a, proposed by Davis et al. [45]. The authors reported that they had obtained 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a as the only reaction product, thus confirming previous studies.
In 1961, Davis et al., continuing the study of the course of the condensation of various 4-substituted benzyl cyanides 1a–c with 4-unsubstituted nitroarenes 11a–n (Scheme 6), published the work [69] in which detailed preparations, by the previously shown condensation method, were described. As a result, 34 new arylcyanomethylenequinone oxime analogues 12aa–cn were synthesized (Table 1). The reactions were realized in warm alcoholic solution of potassium hydroxide. This work showed what a large collection of compounds can be synthesized by varying virtually only four substituents (-H, -Cl, -OCH3 and -CH3).

Scheme 6.
Condensation reaction of 4-substituted benzyl cyanides 1a–c with 4-unsubstituted analogues of nitrobenzene 11a–n to produce quinone oximes 12aa–cn.

Table 1.
Analogues of arylcyanomethylenequinone oximes 12aa–cn.
In 1964, Davis [70] patented another example of condensation of benzyl cyanide 1a with 4-chloronitrobenzene 2b. The author described that in a course of reaction, the expected arylcyanomethylenequinone oxime is not created but an analogue of isoxazole 13 is formed as the only product (Scheme 7). The reaction was carried out in warm alcoholic solution of potassium hydroxide.

Scheme 7.
Condensation reaction of benzyl cyanide 1a with 4-chloronitrobenzene 2b to produce 3-phenyl-5-chloroanthranil 13.
In the same patent, Davis reported that in the reaction between benzyl cyanide 1a and 4-chloronitrobenzene 2b, when the solvent is changed from methanol to pyridine, the 4-chloro substituent is not retained in the product. In turn, 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a is formed (Scheme 8). This variation of the reaction, in contrast to the previous examples, allows to use 4-substituted nitroarenes in the synthesis of arylcyanomethylenequinone oximes.

Scheme 8.
Condensation reaction of benzyl cyanide 1a with 4-chloronitrobenzene 2b to produce 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a.
In 1965, Marey et al. [71] proposed the use of heteroaromatic analogues of benzyl cyanide 2a such as 3-pyridylacetonitrile 14 and 2-thienylacetonitrile 15 and (hetero)aromatic nitro compounds like 2-substituted nitrobenzene 16a–c and 2-nitrothiophene 17 in the condensation reaction. Reactions took place in warm methanolic solution of potassium hydroxide, forming corresponding heterocyclic analogues of arylcyanomethylenequinone oximes 18–21 (Scheme 9). The authors mentioned that yields of all reactions were >10%.

Scheme 9.
Condensation reaction of 3-pyridylacetonitrile 14 or 2-thienylacetonitrile 15 with 2-substituted nitrobenzene analogues 16a–c and 2-nitrothiophene 17 to produce oximes 18–21.
Continuing the study of synthesis of such heterocyclic oximes, in 1968, Fournari and Marey [72] reported on the study of reactions between heteroarylacetonitriles 14 and 15 with (hetero)aromatic nitro compounds 17 and 22–24. Reactions took place in a way analogous to the previous one, namely, deploying methanolic solution of potassium hydroxide, forming corresponding oximes 20 and 25–27 (Scheme 10a–d), thereby increasing the range of known oxime derivatives. The compounds were formed in low to moderate yields.

Scheme 10.
Condensation reaction between nitriles 14 and 15 and (hetero)aromatic nitro compounds 17, 22–24 forming heterocyclic analogues 20, 25 (a,b), heteroatom containing 26 (c), and bi(carbo)cyclic 27 (d) heteroarylcyanomethylenequinone oximes.
In the same year, Mitchell in two patents [73,74] described synthesis of derivatives of bis (phenylacetonitrile) oxime 29 and 31a–b in a reaction of condensation of benzyl cyanide 1a with 2,2′-dinitrobibenzyl 28 (Scheme 11a) as well as 1,4-bis (cyanomethyl) benzene 30a with nitrobenzene 2a (Scheme 11b) and 1,4-bis (cyanomethyl) diphenyl ether 30b, also, with nitrobenzene 2a (Scheme 11b). All reactions were realized in warm alcoholic solution of potassium hydroxide.
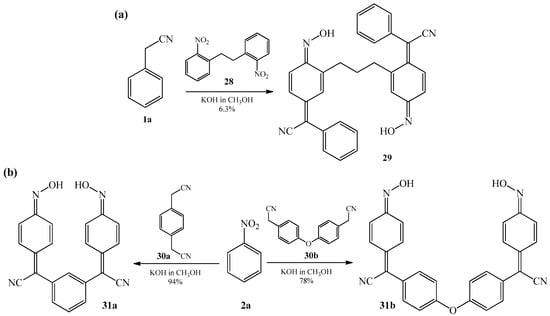
Scheme 11.
(a) Condensation reaction of benzyl cyanide 1a with 2,2′-dinitrobibenzyl 28; (b) Condensation reaction of nitrobenzene 2a with 1,4-bis(cyanomethyl)benzene 30a and 1,4-bis (cyanomethyl) diphenyl ether 30b.
In the 1970s, Takahashi et al. began studying the possibility of deploying catalysts and their effect on the course of the condensation reactions. Their research led to forming 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a in a reaction of benzyl cyanide 1a with nitrobenzene 2a mediated by base catalyst [75]. In 1984, Arseniyadis et al. [76] described the mechanisms of addition and substitution reactions of nitrile-stabilized carbanions, including the mechanism of the formation of 4-quinone methide oximes. Later, Freyne and Raeymaekers in 1991 [77], Freyne et al. in 1996 [78], Makosza and Wróbel in 1996 [79], Wróbel in 2000 [80], Yamato et al. in 2000 [24], Suwiński et al. in 2003 [81], Orlov et al. in 2007 [82], Konovalova et al. in 2008 [83], Orlov et al. in 2009 [84], Buehler et al. in 2010 [85], Orlov et al. in 2010 [86], and Hong et al. in 2016 [60] mentioned the synthesis of 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a by the already presented condensation of benzyl cyanide 1a with 4-unsubstituted nitrobenzenes 11a–n with attempts to optimize the conditions of these reactions via the use of catalysts, and changes of solvents, reaction times and temperature regimes, among other modifications.
In 2015, Kouakou et al. [59] used the already known approach of regioselective nucleophilic substitution of various 4-substituted benzyl cyanides 1a–d with N-alkyl-7-nitroindazoles 32a–b (Scheme 12). Reactions took place in methanolic solution of potassium hydroxide, forming corresponding 2-(7-hydroxyimino-1-alkyl-1,7-dihydroindazol-4-ylidene)-2-arylacetonitriles 33aa–db (Table 2).

Scheme 12.
Condensation reaction of various 4-substituted benzyl cyanides 1a–d with N-alkyl-7-nitroindazoles 32a–b to produce quinone oximes 33aa–db.

Table 2.
Analogues of 2-(7-hydroxyimino-1-alkyl-1,7-dihydroindazol-4-ylidene)-2-arylacetonitriles 33aa–db.
Alternative approaches to the synthesis of quinone methide oxime derivatives are severely underrepresented in the literature.
2.2. The Conversion of Quinone Methide Derivatives Using Hydroxylamine
As an alternative approach to the synthesis of quinone methide derivatives of oximes, the reaction of the corresponding quinone methide derivatives with the addition of hydroxylamine should be considered. This method has been known for a long time and has become more common in recent years for the conversion of a keto group into an oxime [87,88,89,90]. As an alternative transformation, which was not used for the synthesis of phenylcarboxymethylomethylenecyclohexa-2,5-dien-1-one oxime core, was presented by Wróbel in 2000 [80]. In that approach, the reaction between the nitroarene and phenylacetic ester furnished the nitroso compound which was in equilibrium with the oxime form.
In 2017, Piazza et al. [40] patented the synthesis of quinone methide oximes 36a–d. The processes were two-stepped. The first stage consisted of conversion of the corresponding hydroxyl derivatives 34a–d to quinone methides 35a–d. The reactions were realized using 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ). Dichloromethane (DCM) was used as a solvent. The reactions took place at 0 °C. As the last step, the quinone methides 35a–d were converted to quinone methide oximes 36a–d by the Gilman reagent ((CH3)2CuLi) and hydroxylamine (NH2OH), under reflux, with methanol as a solvent (Scheme 13).

Scheme 13.
Two-stepped transformation reaction of hydroxylamine 34a–d to produce quinone methide oximes 36a–d. The structures of substituents R represent drawings (a–d).
Methods applying quinone methides in obtaining arylcyanomethylenequinone oximes are virtually absent. This is due to the multitude of synthesis steps required in those type of processes leading to arylcyanomethylenequinone [91,92] (Scheme 14). In the presence of one-step condensation reaction of benzyl cyanide derivatives with 4-unsubstituted nitroarenes or their analogues, which is easier to adapt, the absence of processes deploying quinone methides is understandable.

Scheme 14.
A concept of multistep transformation of 4-alkoxybenzaldehydes to produce arylcyanomethylene quinone oximes.
3. Chemical Transformation of Arylcyanomethylenequinone Oximes
In the literature, chemical transformations of arylcyanomethylenequinone oximes are mostly represented by the conversion of oxime moiety and/or cyano group. From an oxime fragment, it is possible to obtain (a) the nitroso group by the isomerisation of quinone moiety; (b) amine by a reduction reaction; and (c) nitro compounds in an oxidation reaction. Under more “aggressive” reaction conditions, the conversion of the cyano group to (d) amine in a reduction reaction, and (e) eliminating of the cyano group in oxidative condition to form ketone, can also occur.
3.1. The Oxime–Nitroso Tautomerism Equilibrium in Arylcyanomethylenequinone Oxime
The first aspect worth noting of the chemical transformations is the analysis of the arylcyanomethylenequinone oxime’s structure. During the analysis of the literature data, the question naturally arose regarding the possibility of oxime–nitroso tautomerism of these compounds. The opinions of various scientific researchers engaged in the study of the field turned out to be ambiguous. Thus, in works of different times, such as Davis et al. in 1960 [45], Zsako et al. in 1971 [93], Adam et al. in 2000 [94], Suwiński et al. in 2003 [81], Long et al. in 2013 [95], Hong et al. in 2015 [33], Kouakou et al. in 2015 [59], and Eddahmi et al. in 2019 [96], the authors exclude the possibility of existence of oxime-nitroso tautomerism in the arylcyanomethylenequinone oximes. On the other hand, some authors of recent decades allow the existence of oxime–nitroso tautomerism in these compounds. In 2010, Orlov et al. [86] showed a shift in the equilibrium of nitroso–oxime tautomerism towards the formation of the 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a form (Scheme 15).

Scheme 15.
Oxime-nitroso tautomerism equilibrium between 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a and 4-nitrosodiphenylacetonitrile 4a.
In turn, in 2000, Wróbel [80] noted the possibility of the existence of tautomeric nitroso-derivatives. In 2016, Hong et al. [60] studied the structure of 4-(phenylcyanomethylene)-2-methylcyclohexa-2,5-dien-1-one oxime 12ak (Figure 3). In their work, they showed that the compound 12ak can isomerize from the oxime to the corresponding nitroso-tautomer 12′ak in solution, at different temperatures. Simultaneously, Hong et al. noted that the oxime form is the more stable one. Some other bicyclic particularly ortho-quinone metides derivatives, e.g., 33aa proved to be existing in both forms and more stable, the nitroso 33′aa form is formed during the heating of oxime [44].
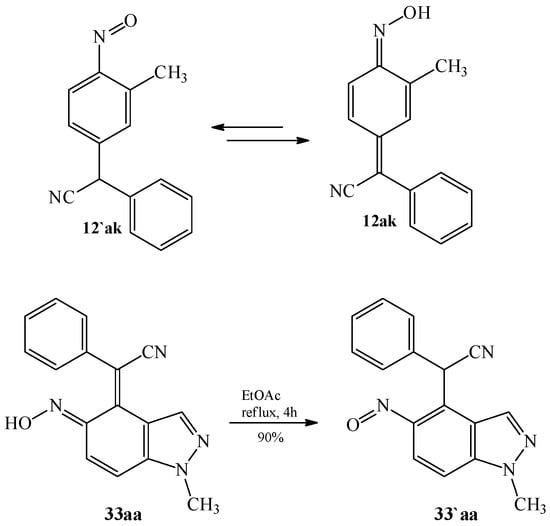
Figure 3.
The structures of oximes 12ak, 33aa, and nitroso compound 33′aa.
The questions of the existence of oxime–nitroso tautomerism can be resolved by X-ray structural analysis. At the moment, among the arylcyanomethylenequinone oximes, four structures have been found using X-ray structural analysis (Figure 4). The structure proved high stability of para-quinone methide moiety in differently substituted and poly(hetero)cyclic arylcyanomethylenequinone oximes [33,59,60,81].
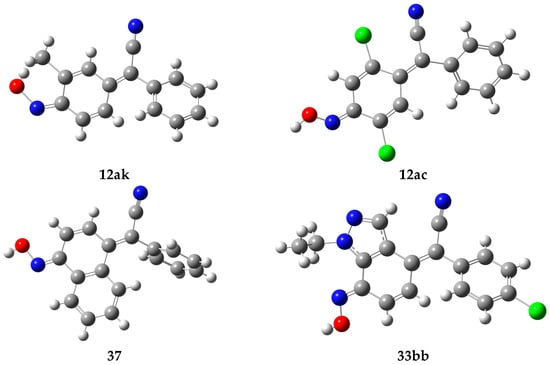
Figure 4.
Structures of arylcyanomethylenequinone oximes confirmed by X-ray structural analysis. X-ray structures were extracted from: [60] for 12ak, [33] for 12ac, [81] for 37, [59] for 33bb.
3.2. The Reduction Reactions of Arylcyanomethylenequinone Oxime
Mild chemical and catalytic reduction conversion of arylcyanomethylenequinone oximes to 4-aminoarylacetonitrile analogues has been the subject of several studies. An example of this reduction process is the reaction of 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a and its analogues 12ad–12ak and 38ba–da. In the effect, the 4-(arylcyanomethyl)-arylamines 39a and 39ad–39da were synthesized [54,55,56,80,97]. The reactions were realized using zinc and acetic acid in methanolic solution (Scheme 16).

Scheme 16.
Reduction reaction of 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a and its analogues 12ad–12ak and 38ba–38da to 4-(arylcyanomethyl)-arylamine 39a and its analogues 39ad–39da.
A more violent catalytic reduction of arylcyanomethylenequinone oximes converted both oxime moiety and cyano group. As a result, the corresponding 4-arylaminoarylethylamines are produced. Thanks to this the conversion of 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a and its analogues 12ad–ak and 38ba–da to 1-amino-2-(4-aminoaryl)-2-aryl-ethanes 40a, and 40ba–da occurred [55,97]. The presented reduction reactions employ hydrogen gas and Raney nickel in methanolic solution (Scheme 17).

Scheme 17.
Reduction reaction of 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a and its analogues 12ad–12ak and 38ba–38da to produce 1-amino-2-(4-aminophenyl)-2-phenyl-ethane 40a and its analogues 40ad–40da.
3.3. The Oxidation Reactions of Arylcyanomethylenequinone Oxime
The oxidation process of arylcyanomethylenequinone oximes, also depending on the conditions of the reaction, can take place either only by the oxime group with the formation of corresponding nitro compounds or affecting both oxime and cyano moieties [57,58,85,94]. An example of the first oxidation process is the reaction of 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a and its analogues 12aa–12bn, 38ba and 41ba–bb producing 4-(phenylcyanomethyl)-nitrobenzene 6a and its analogues 42aa–bn [58,94]. The reactions were realized using dimethyldioxirane (DMD) as an oxidant and water as a solvent (Scheme 18). The products were obtained in low yields typically in a range of 32–38%.
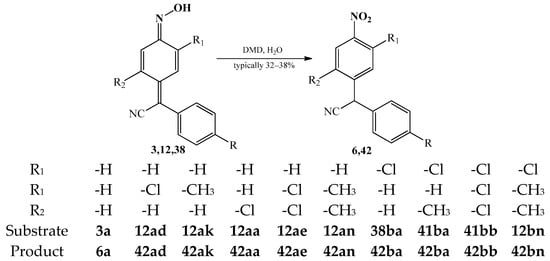
Scheme 18.
Oxidation reaction of 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a and its analogues 12aa–12bn, 38ba, and 41ba–bb to produce 4-(phenylcyanomethyl)-nitrobenzene 6a and its analogues 42aa–bn.
Simultaneous oxidative conversion of oxime moiety and the cyano group is possible using stronger oxidating agents. As a result, the corresponding (4-nitroaryl) (aryl) methanones are obtained, so that the conversion of 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a and its analogues 12ad–ak and 38ba–da leads to (4-nitrophenyl)(phenyl)methanone derivatives 43a–bn [57,85]. The oxidation processes are realized by treating the starting oximes 3a, 12ad–ak, and 38ba–da with hydrogen peroxide in a boiling potassium hydroxide solution in a methanol/water mixture (Scheme 19).

Scheme 19.
Oxidation reaction of 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a and its analogues 12ad–ak and 38ba–da to produce (4-nitrophenyl)(phenyl)methanone derivatives 43a–bn.
3.4. The Substitution of a Hydrogen Atom at the Oxime Group in Arylcyanomethylenequinone Oxime
The chemical transformations of arylcyanomethylenequinone oximes by substitution of a hydrogen atom at the oxime group are represented by: acylations, alkylations or interaction of hydrogen with other groups, e.g., using sulfonyl chlorides or phosphoryl chlorides [23,24,25,32,42,59,97].
3.4.1. The Acylation of a Hydrogen Atom at the Oxime Group
Several examples of acylation of 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a and its analogues 12aa–an are known [24,32,97]. Among others, acetyl chloride 44a, trichloroacetyl chloride 44b, 2-(dimethylamino)acetyl chloride 44c, benzoyl chloride 44d and pentafluorobenzoyl chloride 44e are described, being used as acylation agents. The processes could be carried out in tetrahydrofuran (THF) with the addition of triethylamine (TEA) under cooling (Scheme 20). As a result of the reactions, nitroso derivatives 45a_a to 45an_d were obtained.

Scheme 20.
Acylation reaction of 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a and its analogues 12aa–an.
3.4.2. The Alkylation of a Hydrogen Atom at the Oxime Group
In a similar way to the acylation processes, the alkylation reactions of 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a and its analogues 12aa–ak are described [42]. Among others, allyl bromide 46a, benzyl bromide 46b, N,N-dimethylaminopropyl chloride 46c, as well as ethyl bromoacetate 46d are used as alkylating agents. The reactions were carried out in dimethylformamide (DMF) with the addition of a methanolic solution of potassium hydroxide at the room temperature (Scheme 21). As a result of the reactions, O-alkylated oximes 47a_a–47ak_d were obtained in typically high yields.

Scheme 21.
Alkylation reaction of 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a and its analogues 12aa–ak.
3.4.3. Other Substitution Reactions at Oxygen Atom of the Oxime Group
In the literature, several examples of substitution reaction of the hydrogen atom of 4 arylcyanomethylenequinone oxime for phosphorus containing moiety are also reported. The first example includes a reaction series of 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a with chlorides such as sulfonyl or phosphoryl chlorides as well as chlorosilane [24,25]. The reactions took place in the presence of triethylamine (TEA) at the room temperature and in dimethylformamide (DMF) as a solvent. Scheme 22 summarizes substitution agents, as well as possible products 49aa–af, for these transformations.
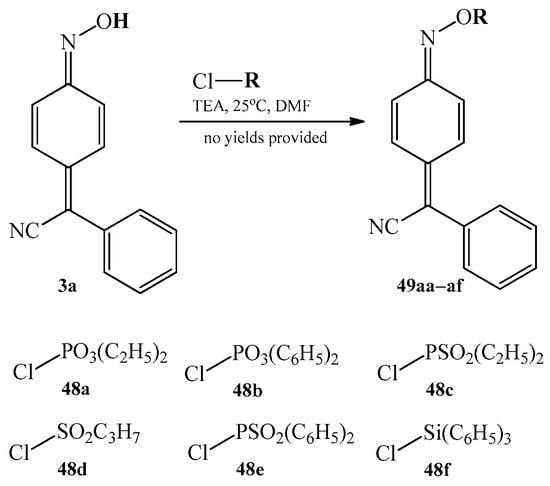
Scheme 22.
Summary of the substitution reactions of 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a for phosphorus containing moiety.
Another example of substitution of the hydrogen atom in an oxime group is a series of reactions of 2-(7-hydroxyimino-1-alkyl-1,7-dihydroindazol-4-ylidene)-2-arylacetonitriles 33ba, 33ca and 33cb with 4-methylbenzylsulfonyl chloride 50a or 4-methoxybenzylsulfonyl chloride 50b [23,59]. The reactions could be realized in the presence of triethylamine (TEA) at the room temperature and in pyridine solution. Scheme 23 summarizes possible products 51ba, 51ca, and 51cb for these transformations [59].
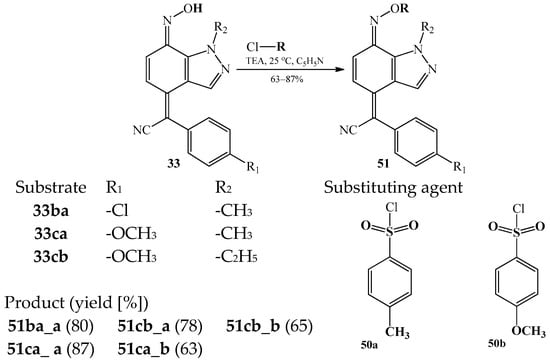
Scheme 23.
Summary of the substitution reactions of 2-(7-hydroxyimino-1-alkyl-1,7-dihydroindazol-4-ylidene)-2-arylacetonitriles 33aa–db with 4-methylbenzylsulfonyl chloride 50a or 4-methoxybenzylsulfonyl chloride 50b.
4. Biological Properties of Arylcyanomethylenequinone Oximes
The creation of pharmaceuticals based on newly synthesized biologically active substances, in order to improve the medical provision of the population, is one of the main tasks of the healthcare system [98]. Thanks to the achievements of bioorganic chemistry, molecular biology (especially the determination of the structures of some receptors and enzymes by X-ray structural analysis), the structures of biological targets were identified, which led to the design of powerful and selective ligands or inhibitors. The synthesis of molecules acting on various biological targets and capable of modulating them has become an innovative approach to the design of drugs. An example of such an approach to designing new drugs can be the creation of “binary” structures containing several functionally significant parts of the molecule [99]. One of the promising classes of such compounds for the search for new biologically active substances could be arylcyanomethylenequinone oxime derivatives, which, on the one hand, contain several pharmacologically significant structure fragments in their structure, like oxime moiety, cyano group, quinonemethide fragment, and which still are small molecules, but on the other hand, which may not induct a “structural alerts” as less selective quinones [100]. It is not surprising that there are already numerous publications reporting on the various biological activities of arylcyanomethylenequinone oxime derivatives.
The first research references regarding biological activity of arylcyanomethylenequinone oximes were described in the middle of 1960s. At that time, Seidl [42] reported that the oximes proved to exhibit analgesic and antiallergic properties as well as these compounds can be useful against many strains of fungi, such as skin fungi and rotting fungi, and against parasites, such as trichomonas. In turn, in 1969 Zuccarello et al. [101] described potential of adrenal-inhibitory effects of arylcyanomethylenequinone oximes by inhibition of aldosterone biosynthesis. Some other types of biological activities of arylcyanomethylenequinone oximes were discovered in the end of the 1990s and more widely studied since then.
4.1. Antiallergic Activity
According to the literature, the arylcyanomethylenequinone oxime can be considered a promising antiallergic biologically active substance. In 1995, Gleason et al. [63] presented a study about the biological activity of arylcyanomethylenequinone oximes as a lipoxygenase inhibitor. The results show that oximes can inhibit both interleukin-4 (IL-4)-treated human monocyte 15-lipoxygenase (15-LO) and isolated soybean 15-lipoxygenase (15-LO) activity. In turn, in 1996, Freyne et al. [78] reported that arylcyanomethylenequinone oximes can also be potent inhibitors of both phosphodiesterase III and IV through downregulation of cells such as basophils and mast cells, which are useful not only in the treatment of allergic disorders, but also atopic diseases and related afflictions.
4.2. Antifungal Activity
In the two articles, Martyna et al. [62] in 2020 and Masłyk et al. [102] in 2019 reported biological activity of the simplest phenylcyanomethylenequinone oxime 3a. The authors described that the 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a acts as strongly antifungal against Candida, by inhibiting the formation of hyphae. Importantly, exploiting the path of reduction of the levels of cellular phosphoproteome, the compound 3a did not show toxicity to human erythrocytes and zebrafish embryos. Additionally, the oxime 3a effectively destroyed clinical isolates of Candida, affecting the mature biofilm, moderately disrupting membrane permeability and disrupting the growth of clinical isolates of fungi with a resistance to two systemic antifungal drugs—ketoconazole and caspofungin. Based on this, the authors proposed the mechanism of action of the agent 4-AN in C. albicans cells, which consists of the downregulation of cellular phosphorylation due to the inhibition of the activity of some protein kinases, which leads to the death of C. albicans cells mainly by necrosis.
4.3. Anti-Inflammatory Activity
In 1984, Michel et al. [64] reported on the anti-inflammatory activity of arylcyanomethylene quinone oximes. That is related with the fact that these compounds can inhibit prostaglandin synthetase (PGS). This activity was tested for 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a, 4-(4-chlorophenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 38ba and 4-(4-methylphenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 38da (Figure 5).
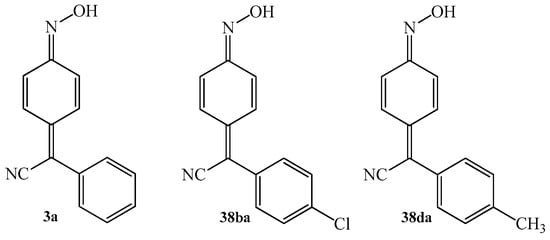
Figure 5.
The chemical structure of 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a and its derivatives 38ba, 38da with potential anti- inflammatory activity.
4.4. Anticancer Activity
Many articles [39,40,43,60] describe the anticancer activity of arylcyanomethylene quinone oximes and their derivatives. According to the literature, the first information about this kind of research was published in 1975 by Dore and Viel [43]. In 1990, Freyne et al. [39] tested and described 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime analogues. The authors noted that the studied compounds inhibit the elimination of retinoic acids from blood plasma. Those compounds can be used in the treatment of disorders characterized by increased proliferation and/or abnormal differentiation of epithelial cells. In turn, Hong et al. [60] synthesized and tested several 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a and their derivatives 52a–k (Figure 6). The authors reported that compounds 3a and 52a–k displayed strong antitumor activity against Caki-1 and 769-P.
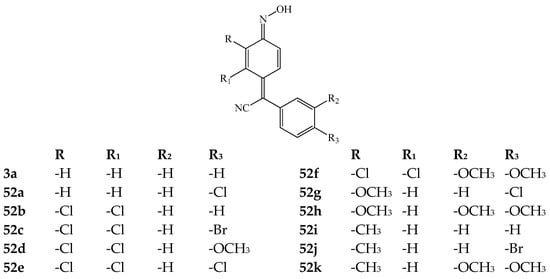
Figure 6.
The chemical structure of 4-(phenylcyanomethylene)-cyclohexa-2,5-dien-1-one oxime 3a and its derivatives 52a–k with potential anticancer activity.
In 2017, Piazza et al. [40] carried out biological studies of patented oximes 36a–d (Figure 7). The aim of these studies was to establish an orthotopic mouse model of lung cancer using A549 human lung adenocarcinoma cells, and to investigate the therapeutic efficacy of oximes 36a–d. Based on obtained results, the authors stated that compounds 36a–d are promising anticancer agents as RAS inhibitors. The human cancer cells used in this research included A-549, HT-29, MDA-MB-231, Colo-205, Caco2, HCT-116, SW-480, and DLD-1.
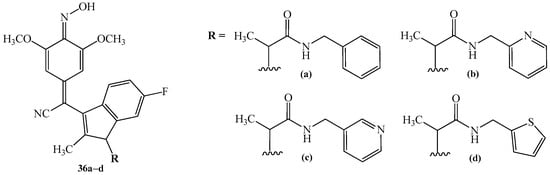
Figure 7.
The chemical structure of oximes 36a–d with potential anticancer activity (a–d).
4.5. Positive Inotropic and Lusitropic Effects
In their reports, Freyne and Raeymaekers [77,103] described positive inotropic and lusitropic effects of arylcyanomethylenequinone oximes. The authors showed that these compounds can be potent inhibitors of the phosphodiesterase type IIIc (cardiotonic-sensitive PDE III). Inhibition of PDE IIIc leads to an elevation of cAMP in cardiac muscle, which in turn enhances sarcolemmal entry of Ca2⁺ into the cell, increases the release and reuptake of Ca2⁺ by the sarcoplasmic reticulum, and probably also increases the sensitivity of contractile proteins to Ca2⁺. As a result, an increased contractile force of the heart ensues (positive inotropy) as well as a faster relaxation of the heart (positive lusitropy). Particularly important is the observation that the positive inotropic and lusitropic effects generally do not coincide with a simultaneous increase of other haemodynamic variables such as heart rate and blood pressure. Concomitant increases of heart rate and/or blood pressure would indeed put an extra strain on the heart and cancel the beneficial positive cardiac inotropy and lusitropy.
In vivo experiments with these oximes show moderate systemic vasodilation and hence, a decrease in blood pressure. The heart rate generally only increases at high doses. Overall, arylcyanomethylenequinone oximes caused a dramatic increase in cardiac output by cardiac positive inotropy and lusitropy and without a major influence on heart rate and/or blood pressure. Consequently, the oximes can be considered to be therapeutical drugs for the treatment of those suffering from Congestive Heart Failure. Said condition may result from a heart attack, infection of the heart, chronic hypertension, deficiencies in the operation of the heart valves and other disorders of the heart, leading to congestive heart failure (CHF).
4.6. Potential Application of Arylcyanomethylenequinone Oximes in Veterinary Medicine
The application of arylcyanomethylenequinone oximes in veterinary medicine have also been described several times in the literature. These compounds can be successfully applied as anthelmintics and coccidiostats. The most important compounds that show these properties include: 4-(4-cholophenylcyanomethylene)-3-chlorocyclohexa-2,5-dien-1-one oxime 12ba, 4-(2,4-dicholophenylcyanomethylene)-3-chlorocyclohexa-2,5-dien-1-one oxime 53a, and 4-(3-trifluoromethylphenylcyanomethylene)-2,5-dichlorocyclohexa-2,5-dien-1-one oxime 53b (Figure 8). The compounds 12ba, 53a–b were the subject of tests on animals. In 1973, Anssen et al. [61] reported about the application of oximes 12ba, 53a–b as feed additives for chickens against Eimeria. According to the reports, simultaneously, mortality decreases and the recovery of animals can be observed. In turn, in 1976, Janssen and Sipido [104] presented very high potency of oximes 12ba, 53a–b against the large American liver fluke in sheep.
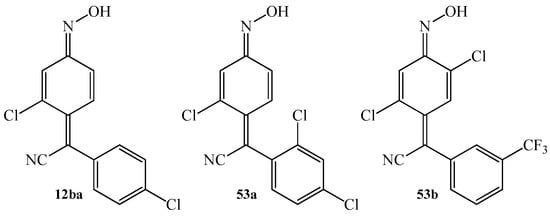
Figure 8.
The chemical structure of oximes 12ba and 53a–b with potential anthelmintic and coccidiostatic activity.
Another interesting compound related with veterinary medicine is 4-(4-cholophenylcyanomethylene)-2-methyl-5-chlorocyclohexa-2,5-dien-1-one oxime 54 (Figure 9) [56,105,106,107,108]. The modification of oxime 54 allows for the synthesis of the drug Closantel 55 (Figure 9) which is a halogenated salicylanilide with potent anti-parasitic activity. It is widely used in the management of parasitic infestation in animals but is contraindicated in humans. In 2016, Tabatabaei et al. [109] reported a patient example of unintentional ingestion of Closantel (55). After application of the drug, the symptoms such as the progressive loss of vision in both eyes and mild headache were observed. The research results showed destruction in the outer retina according to Optical Coherence Tomography (OTC) and possibly, visual pathways according to visual evoked potential (VEP) and electroretinogram (ERG), and confirmed toxic optic neuropathy. The brain imaging was normal. In turn, mild anaemia and liver transaminase enzymes elevation were the only systemic complications.
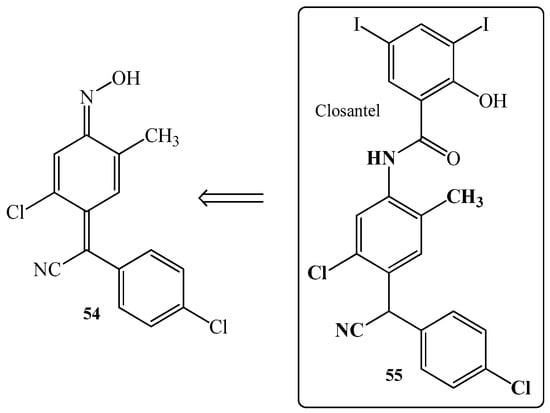
Figure 9.
The chemical structure of 4-(4-cholophenylcyanomethylene)-2-methyl-5-chlorocyclohexa-2,5-dien-1-one oxime 54 and medicine Closantel 55.
4.7. Potential Application of Arylcyanomethylenequinone Oximes as Plant Protection Products
In several documents, it is mentioned that arylcyanomethylenequinone oximes can be used as plant protection products, e.g., herbicides [32,65] and fungicides [66]. One of the most important and studied representatives of herbicides is 4-(α-cyanobenzylidene)-2,5-cyclohexadiene-4-one O-(methylcarbamoyl) oximes 56 (Figure 10). The compound 56 was tested in 1973 by Dixon [32]. The author reported that oxime 56 next to the destroying properties of selective plants can also stimulate plant growth as well as serve as inhibitor of the enzymatic activity of certain insects.
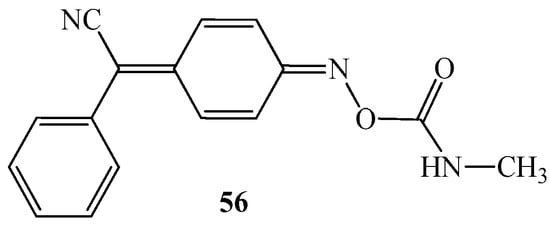
Figure 10.
The chemical structure of 4-(α-cyanobenzylidene)-2,5-cyclohexadiene-4-one O-(methylcarbamoyl) oximes 56 as potential herbicide agent.
In 1986, D’Silva [67] reported about a series of ureas (57) derived from the corresponding arylcyanomethylenequinone oximes. D’Silva claimed that the ureas can be successfully used as pesticide agents (Figure 11).
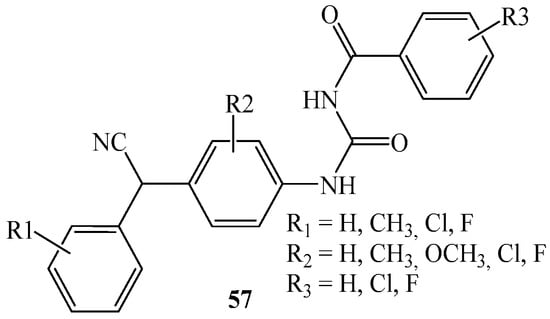
Figure 11.
The general structure of 57.
According to the author, compounds 57 prevent attack by insects and they have relatively high residual toxicity. In turn, with respect to plants, they have a high margin of safety in that when used in a sufficient amount to kill or repel the pests, they do not burn or injure the plant, and they resist weathering that includes wash-off caused by rain, decomposition by ultraviolet light, oxidation, or hydrolysis in the presence of moisture. In addition, these oximes may be used in the soil, upon the seeds, or the roots of plants without injuring either the seeds or roots of plants. In the patent [67], the tests on larvae of the southern armyworm, the Mexican bean beetle, the tobacco budworm, and the cotton bollworm were described. In all cases, the mortality of larvae was recorded, caused by arylcyanomethylenequinone oximes urea.
5. Conclusions and Future Outlook
As can be deduced from the above analysis of the literature, the presented approach to the synthesis of arylcyanomethylenequinone oximes is characterized by relative easiness of preparation (it is not multi-staged, nor does it require the use of oxidizing agents that can lead to the formation of by-products and isomeric mixtures, and the use of catalysts and especially prepared solvents is also unnecessary). Condensation reactions of (hetero) arylacetonitriles with (hetero)nitroarenes are characterized by an employment of easily available starting reagents, regioselectivity, and simplicity. They present a facile way to synthesize not only mono-, but also bis-derivatives of arylcyanomethylenequinone oximes or their heterocyclic analogues.
Analysis of the literature also clearly showed that arylcyanomethylenequinone oxime derivatives are versatile feedstock to production of their nitro, keto, and amino derivatives. What is even more important, the chemical transformations of said oximes occur in mild conditions, simplifying preparation of various systems containing aryl-groups.
The wide palette of biological activity, e.g., cardiotonic-sensitive PDE III inhibitory, kinase inhibitory, RAS inhibitory, anticancer, anti-inflammatory, antiallergic, antifungal, anti-parasitic, as well as plant protection activity, e.g., herbicidic, fungicidic, and pesticidic activity, are reported to be found in arylcyanomethylenequinone oximes. Further studies of the bioactivity of the discussed group of compounds lead to uncovering of unexpected diversity of various biological activities, useful from the standpoint of industrial and medical applications.
Despite certain progress made in chemical and biological studies of arylcyanomethylenequinone oximes, the application potential of them is still underestimated. This could be rationalized by taking into consideration the fact that the mode of their action and biochemical mechanisms are still waiting to be discovered to give a rise to the new drugs based on their core.
To summarize, arylcyanomethylenequinone oxime derivatives deserve further studies, both in the fields of their transformations and their potential application in medicine, agriculture, and industry, as they present a promising source of innovation in those fields.
Author Contributions
Conceptualization, Y.S. and O.M.D.; methodology, O.M.D.; validation, K.K.; formal analysis, R.N. and M.S.; investigation, Y.S.; writing—original draft preparation, K.K., Y.S.; writing—review and editing, K.K., O.M.D.; visualization, R.N., M.S., K.K.; supervision, O.M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the financial support from the Polish National Science Centre grant number 2019/33/B/NZ7/01608.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to express their gratitude to Nazar Rad from Ivan Franko National University of Lviv, Ukraine, for drawing their attention to this class of compounds.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare no financial interests/personal relationships which may be considered as potential competing interests.
Sample Availability
Not applicable.
References
- Peter, R.; Bendall, D.S. A mechanism for the reduction of cytochromes by quinols in solution and its relevance to biological electron transfer reactions. FEBS Lett. 1979, 105, 189–194. [Google Scholar] [CrossRef]
- Rich, P.R. Electron and proton transfers in chemical and biological quinone systems. Faraday Discuss. Chem. Soc. 1982, 74, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.; Wraight, C.A. A crucial role for AspL213 in the proton transfer pathway to the secondary quinone of reaction centers from Rhodobacter sphaeroides. Biochim. Biophys. Acta Bioenerg. 1990, 1020, 107–111. [Google Scholar] [CrossRef]
- Bolton, J.L.; Trush, M.A.; Penning, T.M. Role of quinones in toxicology. Chem. Res. Toxicol. 2000, 13, 135–160. [Google Scholar] [CrossRef]
- Monks, T.J.; Jones, D.C. The metabolism and toxicity of quinones, quinonimines, quinone methides, and quinone-thioethers. Curr. Drug. Metab. 2002, 3, 425–438. [Google Scholar] [CrossRef]
- Ralph, S.J.; Moreno-Sanchez, R.; Neuzil, J.; Rodriguez-Enriquez, S. Inhibitors of succinate: Quinone reductase/complex ii regulate production of mitochondrial reactive oxygen species and protect normal cells from ischemic damage but induce specific cancer cell death. Pharm. Res. 2011, 28, 2695–2730. [Google Scholar] [CrossRef]
- Janeczko, M.; Demchuk, O.M.; Strzelecka, D.; Kubiński, K.; Masłyk, M. New family of antimicrobial agents derived from 1,4-naphthoquinone. Eur. J. Med. Chem. 2016, 124, 1019–1025. [Google Scholar] [CrossRef]
- Campanini-Salinas, J.; Andrades-Lagos, J.; Hinojosa, N.; Moreno, F.; Alarcón, P.; González-Rocha, G.; Burbulis, I.E.; Vásquez-Velásquez, D. New quinone antibiotics against methicillin-resistant S. aureus. Antibiotics 2021, 10, 614. [Google Scholar] [CrossRef]
- Green, D.E.; Isler, C.M.; Morton, R.A. Nomenclature of quinones with isoprenoid side-chains recommendations (1973). Arch. Biochem. Biophys. 1974, 165, 1–5. [Google Scholar] [CrossRef]
- Cavitt, S.B.; Sarrafizadeh, H.; Gardner, P.D. The structure of o-quinone methide trimer. J. Org. Chem. 1962, 27, 1211–1216. [Google Scholar] [CrossRef]
- Lee, J.-H.; Choi, K.J.; Seo, W.D.; Jang, S.Y.; Kim, M.; Lee, B.W.; Kim, J.Y.; Kang, S.; Park, K.H.; Lee, Y.-S. Enhancement of radiation sensitivity in lung cancer cells by celastrol is mediated by inhibition of Hsp90. Int. J. Mol. Med. 2011, 27, 441–446. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, X.W.; Cai, T.Y.; Cao, J.; Tu, C.X.; Lu, W.; He, Q.J.; Yang, B. Celastrol acts as a potent antimetastatic agent targeting beta 1 integrin and inhibiting cell-extracellular matrix adhesion, in part via the p38 mitogen-activated protein kinase pathway. J. Pharmacol. Exp. Ther. 2010, 334, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, A.M.; Gohar, A.A.; Naiem, Z.; Bar, F.M.A. Zeitschrift für naturforschung. C. Taxodione, a DNA-binding compound from Taxodium distichum L. (Rich.). Z. Naturforsch. C 2008, 63, 355–360. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Kang, S.C. Antibacterial abietane-type diterpenoid, taxodone from Metasequoia glyptostroboides Miki ex Hu. J. Biosci. 2010, 35, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Tada, M.; Kurabe, J.; Yoshida, T.; Ohkanda, T.; Matsumoto, Y. Syntheses and antibacterial activities of diterpene catechol derivatives with abietane, totarane and podocarpane skeletons against methicillin-resistant Staphylococcus aureus and Propionibacterium acnes. Chem. Pharm. Bull. 2010, 58, 818–824. [Google Scholar] [CrossRef]
- Jansen, R.; Gerth, K.; Steinmetz, H.; Reinecke, S.; Kessler, W.; Kirschning, A.; Müller, R. Elansolid A3, a unique p-quinone methide antibiotic from Chitinophaga sancti. Chem. Eur. J. 2011, 17, 7739–7744. [Google Scholar] [CrossRef]
- Kusumoto, N.; Ashitani, T.; Murayama, T.; Ogiyama, K.; Takahashi, K. Antifungal abietane-type diterpenes from the cones of Taxodium distichum Rich. J. Chem. Ecol. 2010, 36, 1381–1386. [Google Scholar] [CrossRef]
- Wang, P.; Song, Y.; Zhang, L.; He, H.; Zhou, X. Quinone methide derivatives: Important intermediates to DNA alkylating and DNA cross-linking actions. Curr. Med. Chem. 2005, 12, 2893–2913. [Google Scholar] [CrossRef]
- Allison, A.C.; Cacabelos, R.; Lombardi, V.R.; Alvarez, X.A.; Vigo, C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer’s disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2001, 25, 1341–1357. [Google Scholar] [CrossRef] [PubMed]
- Kolak, U.; Kabouche, A.; Öztürk, M.; Kabouche, Z.; Topçu, G.; Ulubelen, A. Antioxidant diterpenoids from the roots of Salvia barrelieri. Phytochem. Anal. 2009, 20, 320–327. [Google Scholar] [CrossRef]
- Kim, D.H.; Shin, E.K.; Kim, Y.H.; Lee, B.W.; Jun, J.G.; Park, J.H.; Kim, J.K. Suppression of inflammatory responses by celastrol, a quinone methide triterpenoid isolated from Celastrus regelii. Eur. J. Clin. Investig. 2009, 39, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Messina, P.; Labbé, E.; Buriez, O.; Hillard, E.A.; Vessières, A.; Hamels, D.; Top, S.; Jaouen, G.; Frapart, Y.M.; Mansuy, D.; et al. Deciphering the activation sequence of ferrociphenol anticancer drug candidates. Chem. Eur. J. 2012, 18, 6581–6587. [Google Scholar] [CrossRef] [PubMed]
- Yamato, H.; Bleier, H.; Birbaum, J.L.; Kunz, M.; Dietliker, K.; De Leo, C.; Asakura, T. New Oxime Sulfonates and Use Thereof as Latent Sulfonic Acids for Photoresists. International Patent Publication No. WO9901429A1, 14 January 1999. [Google Scholar]
- Yamato, H.; Asakura, T.; Birbaum, J.L.; Kurt, D. Oximes and Use Thereof as Latent Acids for Photoresists. International Patent Publication No. WO2000026219A1, 11 May 2000. [Google Scholar]
- Suzuki, H.; Miyazawa, T.; Kikuchi, T. Dye-Containing Resist Composition Containing Photoacid Generator, and Cyclohexadiene-Type Oxime Sulfonate Compound as the Photoacid Generator for Forming Color Filter. International Patent Publication No. WO2,008,032,736, 20 March 2008. [Google Scholar]
- Arai, T.; Hirata, H. Fiber-Reinforced Rubber Laminated Materials. Japan Patent Publication No. JP44,026,549B, 6 November 1969. [Google Scholar]
- Takaba, S.; Takehara, T. Manufacture of Bonded Unit of Ethylene-α-olefin Rubber Compositions and Fibers and Their Transmission Belt. Japan Patent Publication No. JP2006124484A, 18 May 2006. [Google Scholar]
- Yamaguchi, Y.; Takagi, K. Polyolefin Foams. Japan Patent Publication No. JP51045170A, 17 April 1976. [Google Scholar]
- Foghel, S.; Banta, M.; Pirvulescu, S.; Nichitovici, D.; Dobrescu, D.; Pietris, V. Material for Anticorrosive Films. Romania Patent Publication No. RO59038A3, 15 January 1976. [Google Scholar]
- Yoshida, N.; Toko, M. Bonding Method of Outer Panel and Reinforcing Member in Assembling Vehicle by Using Mastic Adhesive. Japan Patent Publication No. JP2018135472A, 30 August 2018. [Google Scholar]
- Yuan, R. Method for Catalytic Synthesis of p-benzoquinone Dioxime. China Patent Publication No. CN101712635A, 26 May 2010. [Google Scholar]
- Dixon, W.D. 4-oxo-(α-cyanobenzylidene)-2,5-cyclohexadiene-4-one O-(methylcarbamoyl)oximes. Herbicidal and Plant Growth-Regulating Federal Republic of. Germany Patent Publication No. DE2257527A1, 30 May 1973. [Google Scholar]
- Hong, Z.; Chen, G.; Jiang, H.J.; Chen, R.E.; Su, W.K. Crystal structure of α-(4-(hydroxyimino)-2, 5-dichloro-2, 5-cyclohexadien-1-ylidene)-benzeneacetonitrile, C14H8Cl2N2O. Z. Kristallogr.-New Cryst. Struct. 2015, 230, 71–72. [Google Scholar] [CrossRef]
- Worek, F.; Wille, T.; Koller, M.; Thiermann, H. Structural requirements for effective oximes—Evaluation of kinetic in vitro data with phosphylated human AChE and structurally different oximes. Chem. Biol. Interact. 2013, 203, 125–128. [Google Scholar] [CrossRef]
- Buehler, S.; Ott, M.; Pfleiderer, W. Novel photolabile protective groups for improved processes to prepare oligonucleotide arrays. International Patent Publication No. WO2,004,074,300A2, 2 September 2004. [Google Scholar]
- Cao, X.; Song, M.; Sui, Z. Reaction mechanism of protonated benzidine oxidation with chlorine dioxide in water. J. Text. Res. 2013, 34, 106–112. [Google Scholar]
- Soukup, O.; Jun, D.; Tobin, G.; Kuca, K. The summary on non-reactivation cholinergic properties of oxime reactivators: The interaction with muscarinic and nicotinic receptors. Arch. Toxicol. 2013, 87, 711–719. [Google Scholar] [CrossRef]
- Malatesta, P.; Quaglia, G.; La Floresta, E. Acetylcholinesterase reactivators. I. Oxime of quinoliniumphenacyl chloride. Farmaco Sci. 1968, 23, 757–764. [Google Scholar]
- Freyne, E.J.E.; Venet, M.G.; Raeymaekers, A.H.M.; Sanz, G.C. Preparation of (1H-azol-1-ylmethyl)quinolines, -quinazolines, and -quinoxalines as Drugs. European Patent Publication No. EP371,564A2, 6 June 1990. [Google Scholar]
- Piazza, G.A.; Chen, X.; Keeton, A.B.; Boyd, M.R. Compounds, Compositions and Methods of Treating Cancer. International Patent Publication No. WO2017106520A1, 22 June 2017. [Google Scholar]
- Masłyk, M.; Janeczko, M.; Demchuk, O.M.; Boguszewska-Czubara, A.; Golczyk, H.; Sierosławska, A.; Rymuszka, A.; Martyna, A.; Kubiński, K. A representative of arylcyanomethylenequinone oximes effectively inhibits growth and formation of hyphae in Candida albicans and Influences the Activity of Protein Kinases in vitro. Saudi Pharm. J. 2018, 26, 244–252. [Google Scholar] [CrossRef]
- Seidl, G.; Nesemann, G. Phenylcyanomethylene Quinone Oxime Derivatives. Federal Republic of. Germany Patent Publication No. DE1194845B, 16 June 1965. [Google Scholar]
- Dore, C.J.; Viel, C. Cytotoxic activity of molecules possessing an ethylenic double bond substituted in α and β positions by an electron-attracting group. Eur. J. Med. Chem. 1975, 10, 47–54. [Google Scholar]
- Poorhaji, S.; Pordel, M.; Ramezani, S. New heterocyclic green, blue and orange dyes from indazole: Synthesis, tautomerism, alkylation studies, spectroscopic characterization and DFT/TD-DFT calculations. J. Mol. Struc. 2016, 1119, 151–156. [Google Scholar] [CrossRef]
- Davis, R.B.; Pizzini, L.C.; Benigni, J.D. The condensation of aromatic nitro compounds with arylacetonitriles. I. Nitrobenzene. J. Am. Chem. Soc. 1960, 82, 2913–2915. [Google Scholar] [CrossRef]
- Toteva, M.M.; Richard, J.P. The generation and reactions of quinone methides. Adv. Phys. Org. Chem. 2011, 45, 39–91. [Google Scholar] [CrossRef]
- Caruana, L.; Fochi, M.; Bernardi, L. The emergence of quinone methides in asymmetric organocatalysis. Molecules 2015, 20, 11733–11764. [Google Scholar] [CrossRef]
- Sugumaran, M. Reactivities of Quinone Methides versus o-Quinones in Catecholamine Metabolism and Eumelanin Biosynthesis. Int. J. Mol. Sci. 2016, 17, 1576. [Google Scholar] [CrossRef]
- Ito, S.; Sugumaran, M.; Wakamatsu, K. Chemical Reactivities of ortho-Quinones Produced in Living Organisms: Fate of Quinonoid Products Formed by Tyrosinase and Phenoloxidase Action on Phenols and Catechols. Int. J. Mol. Sci. 2020, 21, 6080. [Google Scholar] [CrossRef]
- Kern, D.; Kormos, A. Self-Immobilizing Quinone Methides for the Fluorescent Sensing of Enzyme Activity. Chemosensors 2023, 11, 155. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Leal, A.S.; Valdeira, A.S.; Goncalves, B.M.F.; Alho, D.P.S.; Figueiredo, S.A.C.; Silvestre, S.M.; Mendes, V.I.S. Oleanane-, ursane-, and quinone methide friedelane-type triterpenoid derivatives: Recent advances in cancer treatment. Eur. J. Med. Chem. 2017, 142, 95–130. [Google Scholar] [CrossRef] [PubMed]
- Dicken, R. Synthesis of Pyridine and Pyridinium Quinone Methide Precursors: Studies Towards the Realkylation of Aged Acetylcholinesterase. Available online: https://kb.osu.edu/handle/1811/69265 (accessed on 26 April 2023).
- Ma, D.; Lyu, X.I.; Yu, L.; Han, T.; Zhang, G.; Wu, C. Preparation of p-Benzoquinone Dioxime. China Patent Publication No. CN113735737A, 3 December 2021. [Google Scholar]
- Hong, Z.; Jiang, H.; Cui, H.; Zhang, B.; Lu, C.; Chen, X.; Yao, P.; Li, S.; Yao, Z. Ultrasonic-Accelerated Catalytic Transfer Hydrogenation Reduction of 4-chloro-α-(2-chloro-4-(hydroxyimino)-5-methyl-2,5-cyclo-hexadienylidene)phenylacetonitril. China Patent Publication No. CN104945282A, 30 September 2015. [Google Scholar]
- Marey, T.; Person, M. Mechanism of the polarographic reduction of the products of condensation of acetonitriles, substituted with an aromatic or heterocyclic group, with aromatic or heterocyclic nitro-derivatives. Bull. Soc. Chim. Fr. 1970, 7, 2756–2762. [Google Scholar]
- Zhao, G.; Yang, H.; Tan, W. Preparation Method of Sodium Closantel Injection. China Patent Publication No. CN109091454A, 28 December 2018. [Google Scholar]
- Davis, R.B.; Weber, J.D. Condensation of aromatic nitro compounds with arylacetonitriles. IV. Some reactions of the arylcyanomethylenequinone oximes. J. Org. Chem. 1962, 27, 1605–1608. [Google Scholar] [CrossRef]
- Yamada, K.; Shigehiro, K.; Tomizawa, T.; Iida, H. The effect of surfactants and β-cyclodextrin on the photooxidation of stable carbanions. Bull. Chem. Soc. Jpn. 1978, 51, 3302–3306. [Google Scholar] [CrossRef]
- Kouakou, A.; Abbassi, N.; Chicha, H.; Ammari, L.E.; Saadi, M.; Rakib, E.M. Synthesis of novel substituted indazoles via nucleophilic substitution of hydrogen (SNH). Heteroat. Chem. 2015, 26, 374–381. [Google Scholar] [CrossRef]
- Hong, Z.; Li, J.J.; Chen, G.; Jiang, H.J.; Yang, X.F.; Pan, H.; Su, W.K. Solvent-free mechanochemical synthesis of arylcyanomethylenequinone oximes from phenylacetonitriles and 4-unsubstituted nitroaromatic compounds using KF/nano-gamma-Al2O3 as catalyst. RSC Adv. 2016, 6, 13581–13588. [Google Scholar] [CrossRef]
- Anssen, M.A.C.; Offenwert, T.T.; Sanczuk, S. Anthelmintic and Coccidiostatic 4’-benzoyl- and 4’-benzylsalicylanilides. Federal Republic of. Germany Patent Publication No. DE2311229A1, 13 September 1973. [Google Scholar]
- Martyna, A.; Masłyk, M.; Janeczko, M.; Kochanowicz, E.; Gielniewski, B.; Świercz, A.; Demchuk, O.M.; Kubiński, K. Antifungal agent 4-an changes the genome-wide expression profile, downregulates virulence-associated genes and induces necrosis in Candida albicans cells. Molecules 2020, 25, 2928. [Google Scholar] [CrossRef] [PubMed]
- Gleason, M.M.; Rojas, C.J.; Learn, K.S.; Perrone, M.H.; Bilder, G.E. Characterization and Inhibition of 15-lipoxygenase in human monocytes: Comparison with soybean 15-lipoxygenase. Am. J. Physiol. Physiol. 1995, 268, C1301–C1307. [Google Scholar] [CrossRef]
- Michel, F.; Mercklein, L.; De Paulet, A.C.; Dore, J.C.; Gilbert, J.; Miquel, J.F. The effect of various acrylonitriles and related compounds on prostaglandin biosynthesis. Prostaglandins 1984, 27, 69–84. [Google Scholar] [CrossRef]
- Dixon, W.D. Herbicidal 4-oxo-α-phenyl-2,5-cyclohexadiene-Δ’, α-acetonitrile o-(alkylcarbamoyl)oximes. Africa Patent Publication No. ZA7208311A, 29 August 1973. [Google Scholar]
- Shimada, T.; Yoshida, K. Phytotoxicity test of futharide, a newly developed fungicide for rice-blast. Tokyo Agr. Chem. Insp. Sta. Bull. 1971, 11, 141–142. [Google Scholar]
- D’Silva, T.D. Pesticidal α-cyanobenzyl phenyl benzoyl Urea Compounds. U.S. Patent Publication No. US4578402A, 25 March 1986. [Google Scholar]
- Lichtenberger, J.; Weiss, F. Derivatives of phenylfiuoroform. I. Trifiuoromethylbanzophenones. Bull. Soc. Chim. Fr. 1962, 1, 587–593. [Google Scholar]
- Davis, R.B.; Pizzini, L.C.; Bara, E.J. The condensation of aromatic nitro compounds with arylacetonitriles. III. Some ortho- and meta-substituted nitrobenzenes. J. Org. Chem. 1961, 26, 4270–4274. [Google Scholar] [CrossRef]
- Davis, R.B. Arylcyanomethylenequinone Oximes. U.S. Patent Publication No. US3156704A, 10 November 1964. [Google Scholar]
- Marey, T.; Fournari, P.; Tirouflet, J. Condensation of nitriles with aromatic and heterocyclic nitro derivatives. Compt. Rend. 1965, 260, 4340–4342. [Google Scholar]
- Fournari, P.; Marey, T. Synthesis and spectroscopic results of the products resulting from the condensation of aromatic or heterocyclic nitro-derivatives with acetonitrile substituted with an aromatic or a heterocyclic group. Bull. Soc. Chim. Fr. 1968, 8, 3223–3233. [Google Scholar]
- Mitchell, C.D. Phenylacetonitrile Oximes. U.S. Patent Publication No. US3374248A, 19 March 1968. [Google Scholar]
- Mitchell, C.D. Bis(phenylacetonitrile oximes) as Ultraviolet Light Absorbers in Plastics. U.S. Patent Publication No. US3374249A, 19 March 1968. [Google Scholar]
- Takahashi, K.; Tsuboi, T.; Kazutoshi, Y.; Hirotada, I. Studies on the base-catalyzed reaction. VIII. Reactions of cyanophenylmethanide ion with nitrobenzene. Nippon Kagaku Kaishi 1976, 1, 144–147. [Google Scholar] [CrossRef]
- Arseniyadis, S.; Kyler, K.S.; Watt, D.S. Addition and substitution reactions of nitrile-stabilized carbanions. Org. React. 1984, 31, 1–364. [Google Scholar] [CrossRef]
- Freyne, E.J.E.; Raeymaekers, A.H.M. Positive Inotropic and Lusitropic 3,5-dihydroimidazo[2,1-b]quinazolin-2(1H)-one Derivatives, Compositions and Use. U.S. Patent Publication No. US5043327A, 27 August 1991. [Google Scholar]
- Freyne, E.J.E.; Raeymaekers, A.H.M.; De Chaffoy, D.C.D. 1,3-Dihydro-2H-imidazo[4,5-b]quinolin-2-one Derivatives as Inhibitors of Phosphodiesterase III and IV. U.S. Patent Publication No. US5521187A, 28 May 1996. [Google Scholar]
- Makosza, M.; Wróbel, Z. Conversion of 1-(o-nitroaryl) methyl p-tolyl sulfones into anthranilic ester analogues. Acta Chem. Scand. 1996, 50, 646–648. [Google Scholar] [CrossRef]
- Wróbel, Z. Synthesis of 4-arylsulfonylquinolines by double condensation of allyl aryl sulfones with nitroarenes. Eur. J. Org. Chem. 2000, 3, 521–525. [Google Scholar] [CrossRef]
- Suwiński, J.; Świerczek, K.; Wagner, P.; Kubicki, M.; Borowiak, T.; Stowikowska, J. Oximes as intermediates or final products in reactions of nitroheteroarenes with nucleophiles in the presence of sodium methoxide-methanol system. J. Heterocycl. Chem. 2003, 40, 523–528. [Google Scholar] [CrossRef]
- Orlov, V.Y.; Bazlov, D.A.; Ganzha, V.V.; Kotov, A.D.; Rusakov, A.I. Direction of the nucleophilic substitution reaction of hydrogen in nitrobenzene by arylacetonitrile carbanion. Bashk. Khim. Zh. 2007, 14, 22–25. [Google Scholar]
- Konovalova, N.V. Aromatic nucleophilic substitution of hydrogen in nitroarenes by phenylacetonitrile carbanion. Bashk. Khim. Zh. 2008, 15, 103–104. [Google Scholar]
- Orlov, V.Y. Selection of optimal conditions for synthesis of 2[4-(hydroxyimino)-cyclohexa-2,5-dien-1-ylidene]arylacetonitriles. Khim. Tekhnolog. 2009, 10, 393–395. [Google Scholar]
- Buehler, S.; Ott, M.; Pfleiderer, W. Photolabile Protective Groups for Improved Processes to Prepare Oligonucleotide Arrays. U.S. Patent Publication No. US7759513B2, 20 July 2010. [Google Scholar]
- Orlov, V.Y.; Kotov, A.D.; Bazlov, D.A.; Ganzha, V.V.; Konovalova, N.V. Theoretical calculation of the equilibrium constant of nitroso-oxime tautomerization of phenylcyanomethylene-2,5-cyclohexadien-1-one monooxime. Izv. Vyss. Uchebnykh Zaved. 2010, 53, 124–125. [Google Scholar]
- Li, X.Z.; Jiang, S.Y.; Li, G.Q.; Jiang, Q.R.; Li, J.W.; Li, C.C.; Han, Y.Q.; Song, B.L.; Ma, X.R.; Qi, W.; et al. Synthesis of heterocyclic ring-fused analogs of HMG499 as novel degraders of HMG-CoA reductase that lower cholesterol. Eur. J. Med. Chem. 2022, 236, e114323. [Google Scholar] [CrossRef]
- Qiu, W.; Song, B.; Jiang, Q.; Jiang, S.; Shi, X.; Li, C. Cholic Acid Derivatives as HMG-CoA Reductase Inhibitors and Their Preparation, Pharmaceutical Compositions and Use in the Reducing Cholesterol. China Patent Publication No. CN113,563,405A, 29 October 2021. [Google Scholar]
- Zheng, X.; Zhang, Y.; Fu, C. Preparation of Antiviral Drug Molnupiravir Using Microchannel Reactor. China Patent Publication No. CN112608357A, 6 April 2021. [Google Scholar]
- Zhao, P.; Guo, Y.; Luan, X. Total synthesis of dalesconol a by Pd (0)/norbornene-catalyzed three-fold domino reaction and Pd (II)-catalyzed trihydroxylation. J. Am. Chem. Soc. 2021, 143, 21270–21274. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Qi, Y.; Sun, S.; Liu, X.; Li, W.; Liu, L. Synthesis of sterically hindered α-aminonitriles through 1,6-aza-conjugate addition of anilines to δ-cyano substituted para-quinone methides. Chin. J. Org. Chem. 2020, 40, 3934–3943. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, H.; Wang, G.; Wang, Z.; Liu, Z.; Liu, L. Enantioselective construction of single and vicinal all-carbon quaternary stereocenters through ion-pair-catalyzed 1,6-conjugate addition. Org. Lett. 2021, 23, 7248–7253. [Google Scholar] [CrossRef] [PubMed]
- Zsako, I.; Anghel, C.; Cohn, R. Acid-base equilibrium of the phenylcyanomethylene-p-quinone oxime. Stud. UBB Chem. 1971, 16, 5–8. [Google Scholar]
- Adam, W.; Makosza, M.; Zhao, C.G.; Surowiec, M. On the mechanism of the dimethyldioxirane oxidation of σH adducts (Meisenheimer complexes) generated from nitroarenes and carbanions. J. Org. Chem. 2000, 65, 1099–1101. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Panunzio, M.; Qin, W.; Bongini, A.; Monari, M. Efficient aldol-type reaction of o-protected α-hydroxy aldehydes and n-trimethylsilyl ketene imines: Synthesis of β,γ-dihydroxy-nitriles. Eur. J. Org. Chem. 2013, 23, 5127–5142. [Google Scholar] [CrossRef]
- Eddahmi, M.; Moura, N.M.M.; Bouissane, L.; Gamouh, A.; Faustino, M.A.F.; Cavaleiro, J.A.S.; Paz, F.A.A.; Mendes, R.F.; Lodeiro, C.; Santos, S.M.; et al. New nitroindazolylacetonitriles: Efficient synthetic access via vicarious nucleophilic substitution and tautomeric switching mediated by anions. New J. Chem. 2019, 43, 14355–14367. [Google Scholar] [CrossRef]
- Davis, R.B.; Weber, J.D. Condensation of aromatic nitro compounds with arylacetonitriles. V–VI. Some reactions of the arylcyanomethylenequinone oximes. J. Chem. Eng. Data 1963, 8, 578–581. [Google Scholar] [CrossRef]
- Welsch, M.E.; Snyder, S.A.; Stockwell, B.R. Privileged scaffolds for library design and drug discovery. Curr. Opin. Chem. Biol. 2001, 14, 347–361. [Google Scholar] [CrossRef]
- Contreras, J.-M.; Bourguignon, J.-J. Identical and Non-Identical Twin Drugs. In The Practice of Medicinal Chemistry, 1st ed.; Wermouth, C.G., Ed.; Academic: San Diego, CA, USA, 2003; pp. 251–273. [Google Scholar]
- Stepan, A.F.; Walker, D.P.; Bauman, J.; Price, D.A.; Baillie, T.A.; Kalgutkar, A.S.; Aleo, M.D. Structural alert/reactive metabolite concept as applied in medicinal chemistry to mitigate the risk of idiosyncratic drug toxicity: A perspective based on the critical examination of trends in the top 200 drugs marketed in the United States. Chem. Res. Toxicol. 2011, 24, 1345–1410. [Google Scholar] [CrossRef]
- Zuccarello, W.A.; Blank, B.; Frishmuth, G.J.; Cohen, S.R.; Scaricaciottoli, D.; Owings, F.F. Synthesis and adrenocortical-inhibiting activity of substituted 2, 2-diphenethylamines. J. Med. Chem. 1969, 12, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Masłyk, M.; Janeczko, M.; Martyna, A.; Czernik, S.; Tokarska-Rodak, M.; Chwedczuk, M.; Foll-Josselin, B.; Ruchaud, S.; Bach, S. The anti-Candida albicans agent 4-AN inhibits multiple protein kinases. Molecules 2019, 24, e153. [Google Scholar] [CrossRef]
- Freyne, E.J.E.; Raeymaekers, A.H.M. Positive Inotropic and Lusitropic 3,5-dihydro-imidazo[2,1-b]quinazolin-2(1H)-one Derivatives. European Patent Publication No. EP406958A2, 9 January 1991. [Google Scholar]
- Janssen, M.A.C.; Sipido, V.K. Salicylanilide Derivatives. Federal Republic of. Germany Patent Publication No. DE2,610,837A1, 30 September 1976. [Google Scholar]
- Fang, B.; Xu, M.; Ke, J. Method for Preparing Closantel. China Patent Publication No. CN109,851,526A, 7 June 2019. [Google Scholar]
- Casanova, C.D. 2-(4-Amino-2-chloro-5-alkylphenyl)-2-(4-chlorophenyl)acetonitrile. Belgium Patent Publication No. BE895742A1, 16 May 1983. [Google Scholar]
- Foguet, A.R. Process for the Preparation of N-[5-chloro-4-{(4-chlorophenyl)cyanomethyl]-2-methylphenyl]-2-hydroxy-3,5-diiodobenzamide. Spain Patent Publication No. ES524551A1, 16 December 1984. [Google Scholar]
- Lei, S.; Meiju, X. Study on synthesis of closantel sodium. Guangdong Chem. Ind. 2013, 40, 14−15. [Google Scholar]
- Tabatabaei, S.A.; Soleimani, M.; Mansouri, M.R.; Mirshahi, A.; Inanlou, B.; Abrishami, M.; Pakrah, A.R.; Masarat, H. Closantel: A veterinary drug with potential severe morbidity in humans. BMC Ophthalmol. 2016, 16, e207. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).