Synthesis, Characterization, Antibacterial, Antifungal and Anticorrosion Activities of 1,2,4-Triazolo[1,5-a]quinazolinone
Abstract
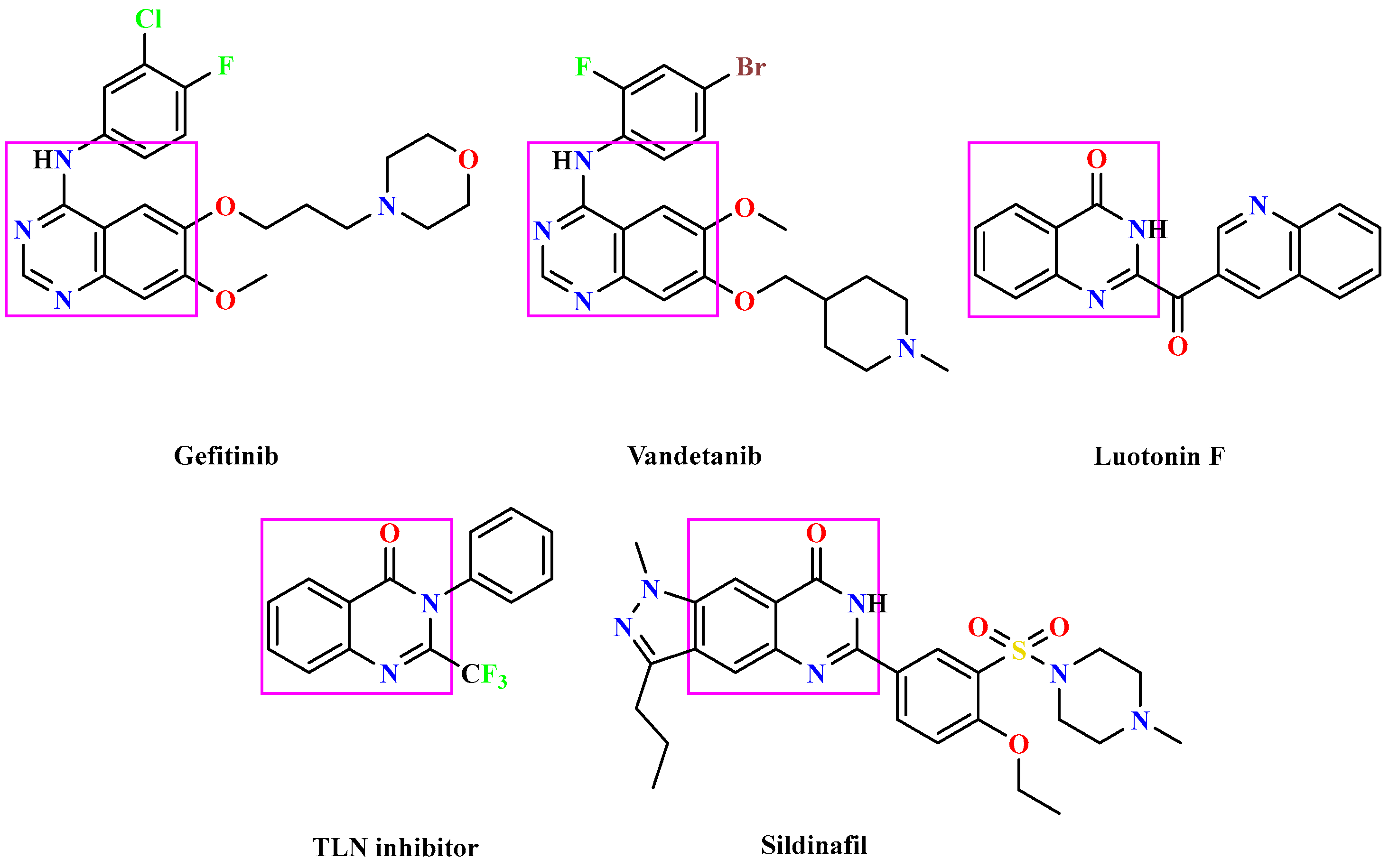
1. Introduction
2. Results and Discussion
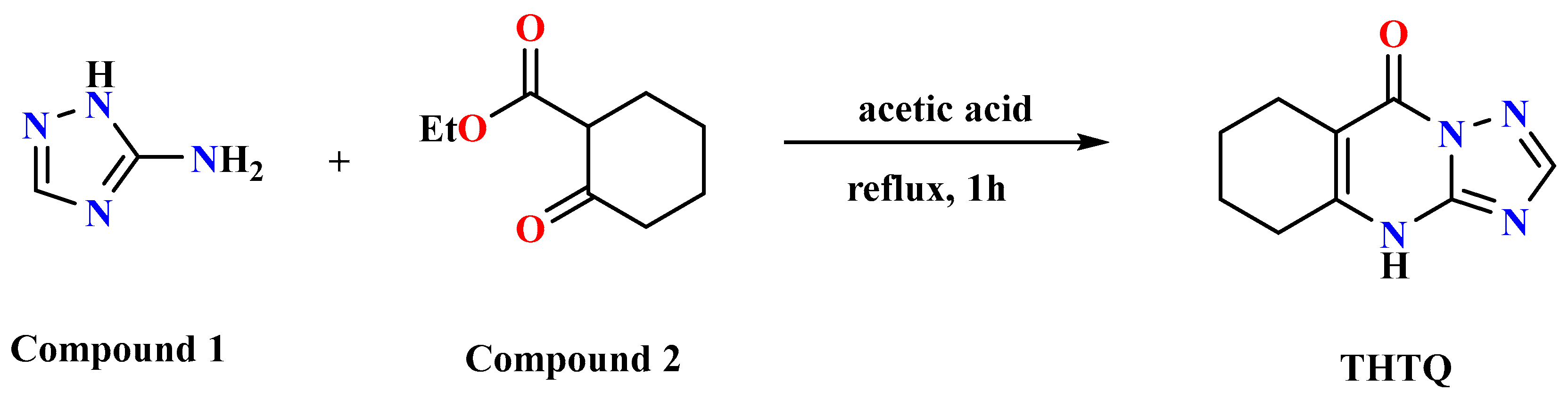
2.1. Chemistry
2.2. Biological Results
2.2.1. Antibacterial Activity Screening of Compound THTQ
2.2.2. Antifungal Activity of Compound THTQ
2.3. Molecular Docking
2.4. Corrosion Test
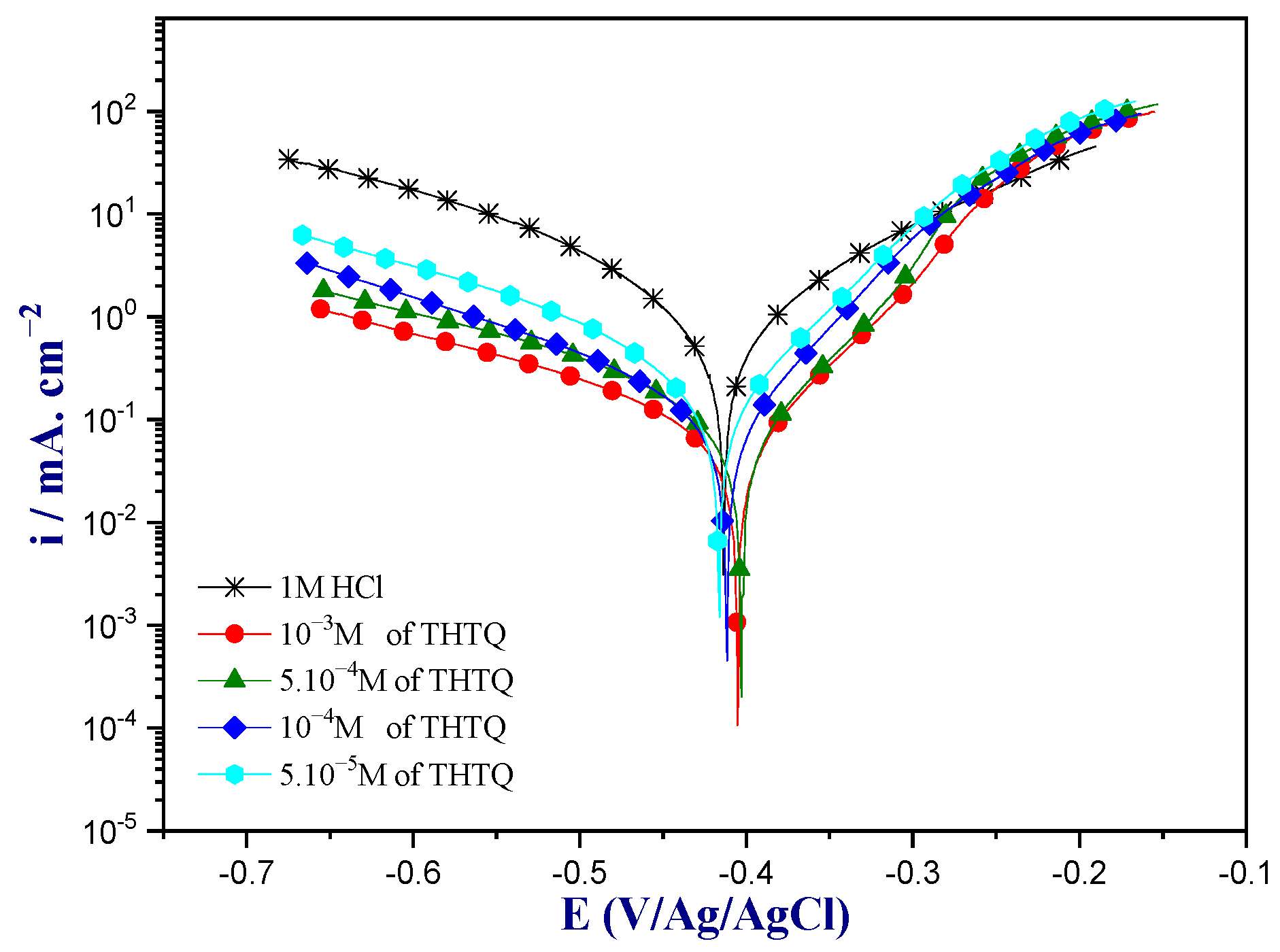
2.4.1. Potentiodynamic Polarization Analysis
Concentration Effect of the THTQ Inhibitor
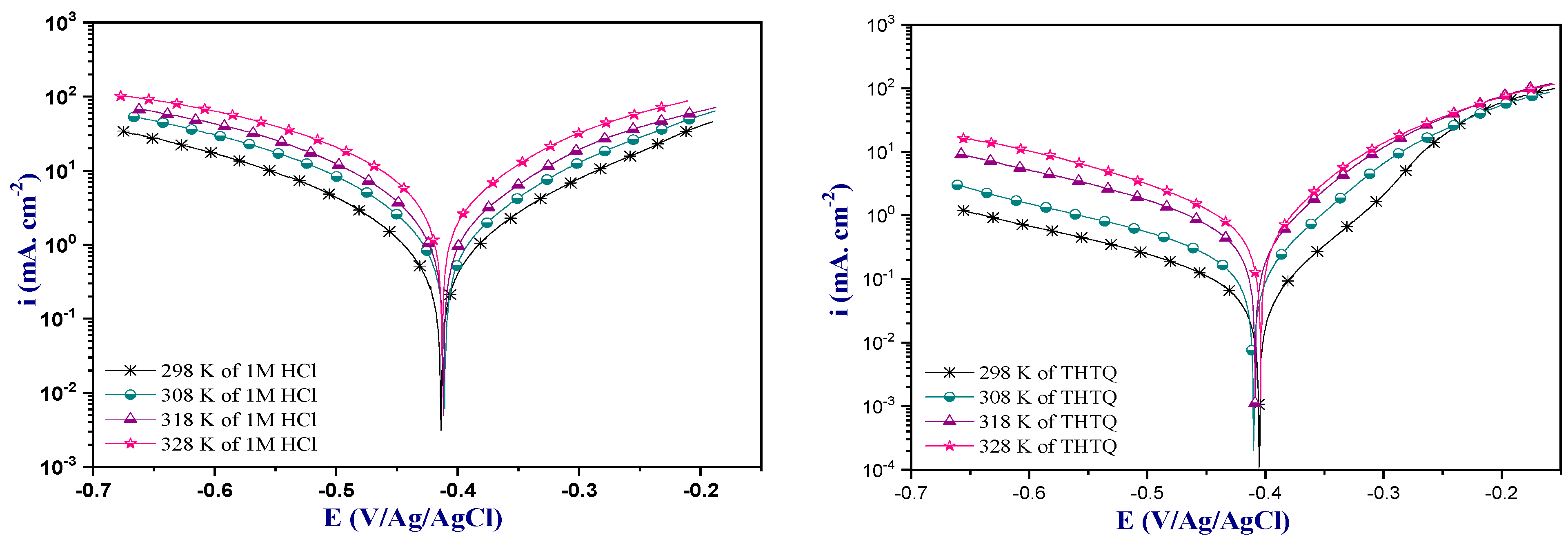
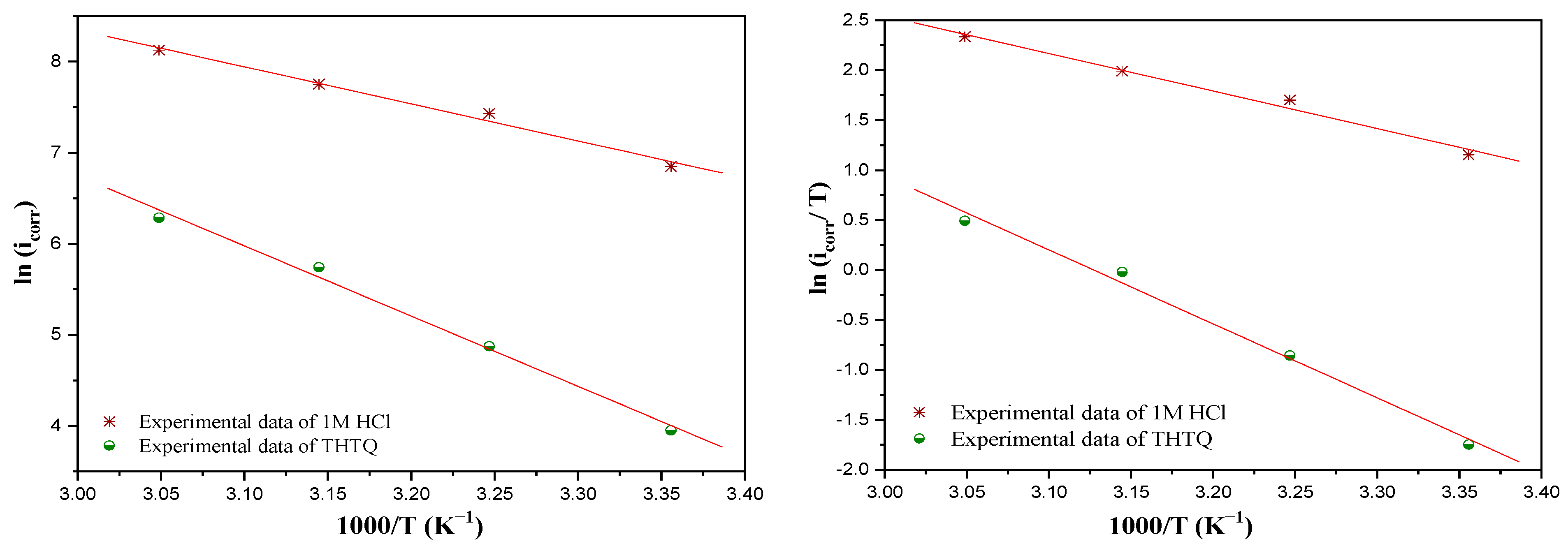
Effect of Temperature
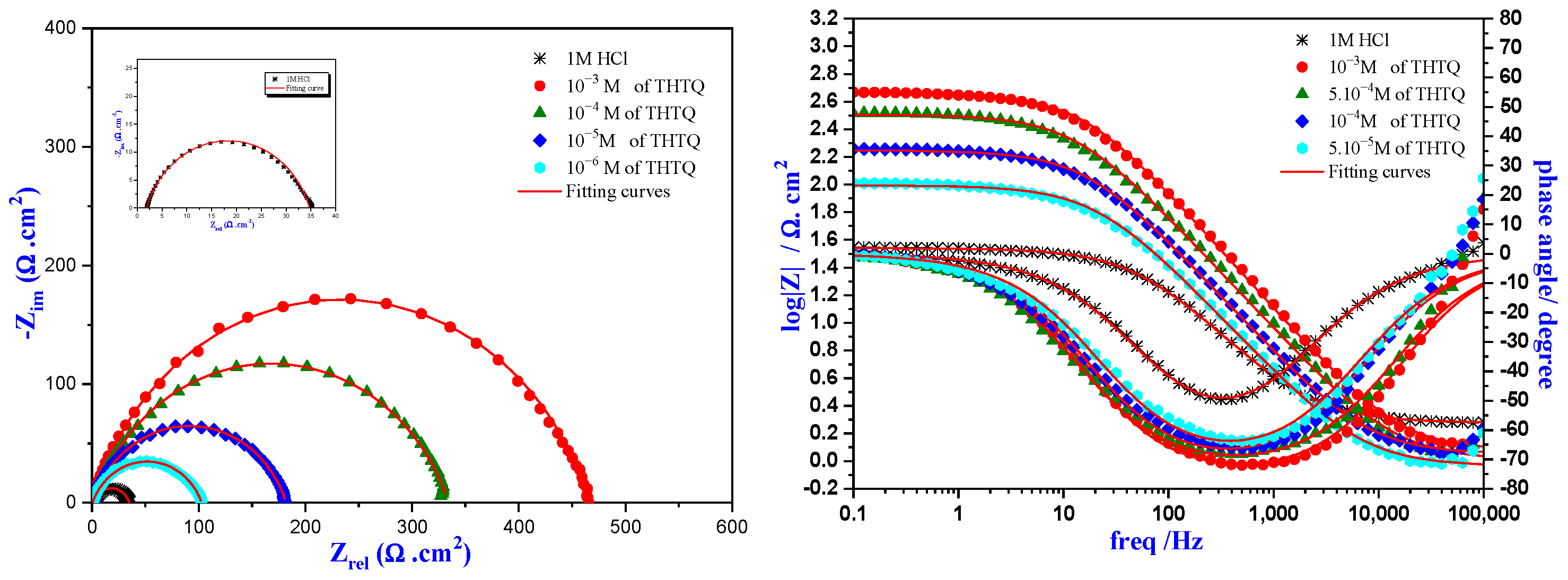
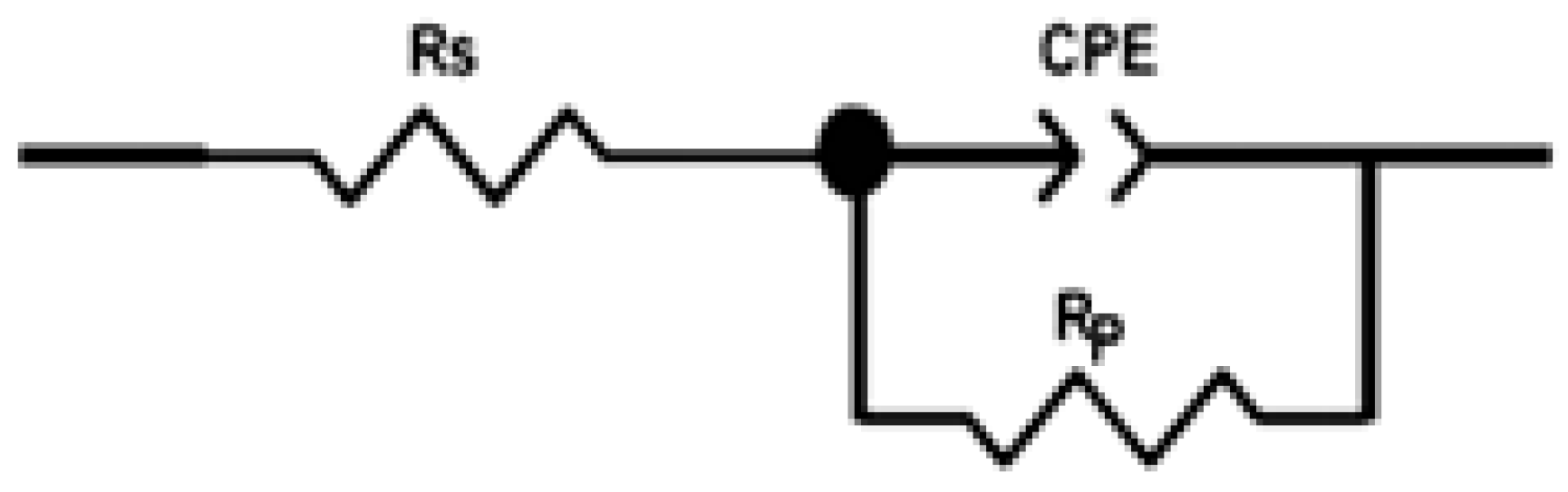
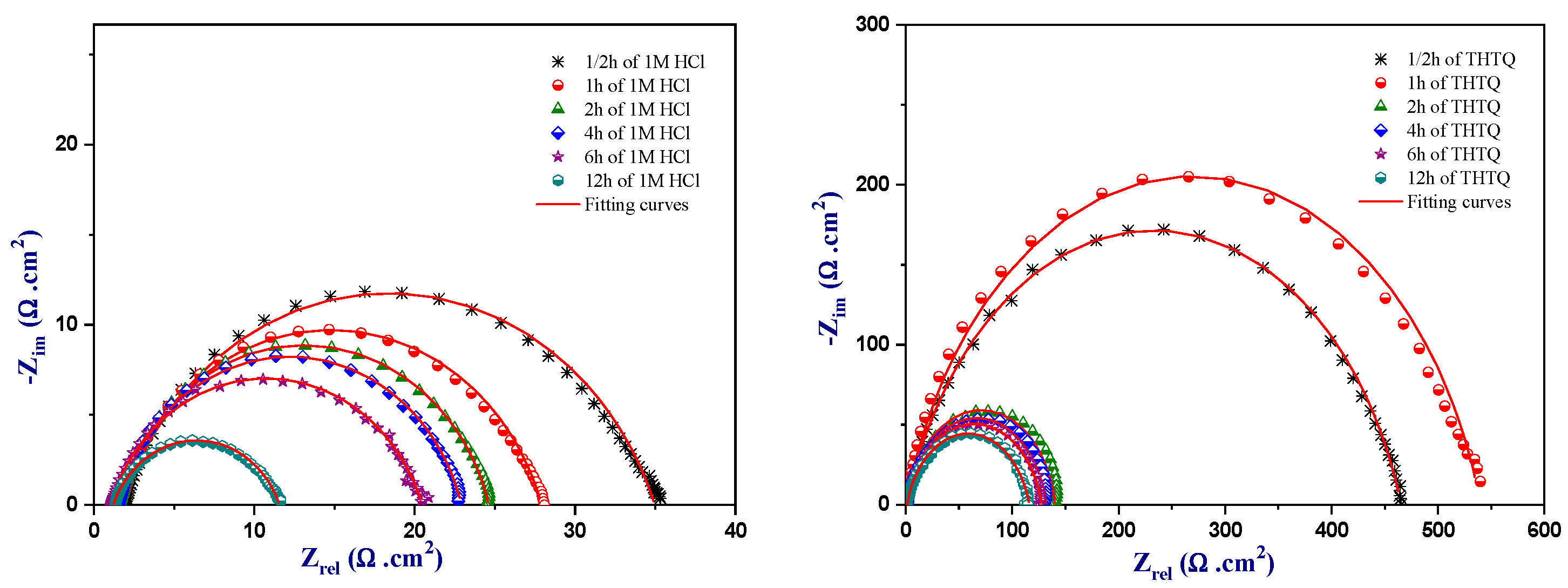
2.4.2. Electrochemical Impedance Spectroscopy of THTQ
Concentration Effect of THTQ
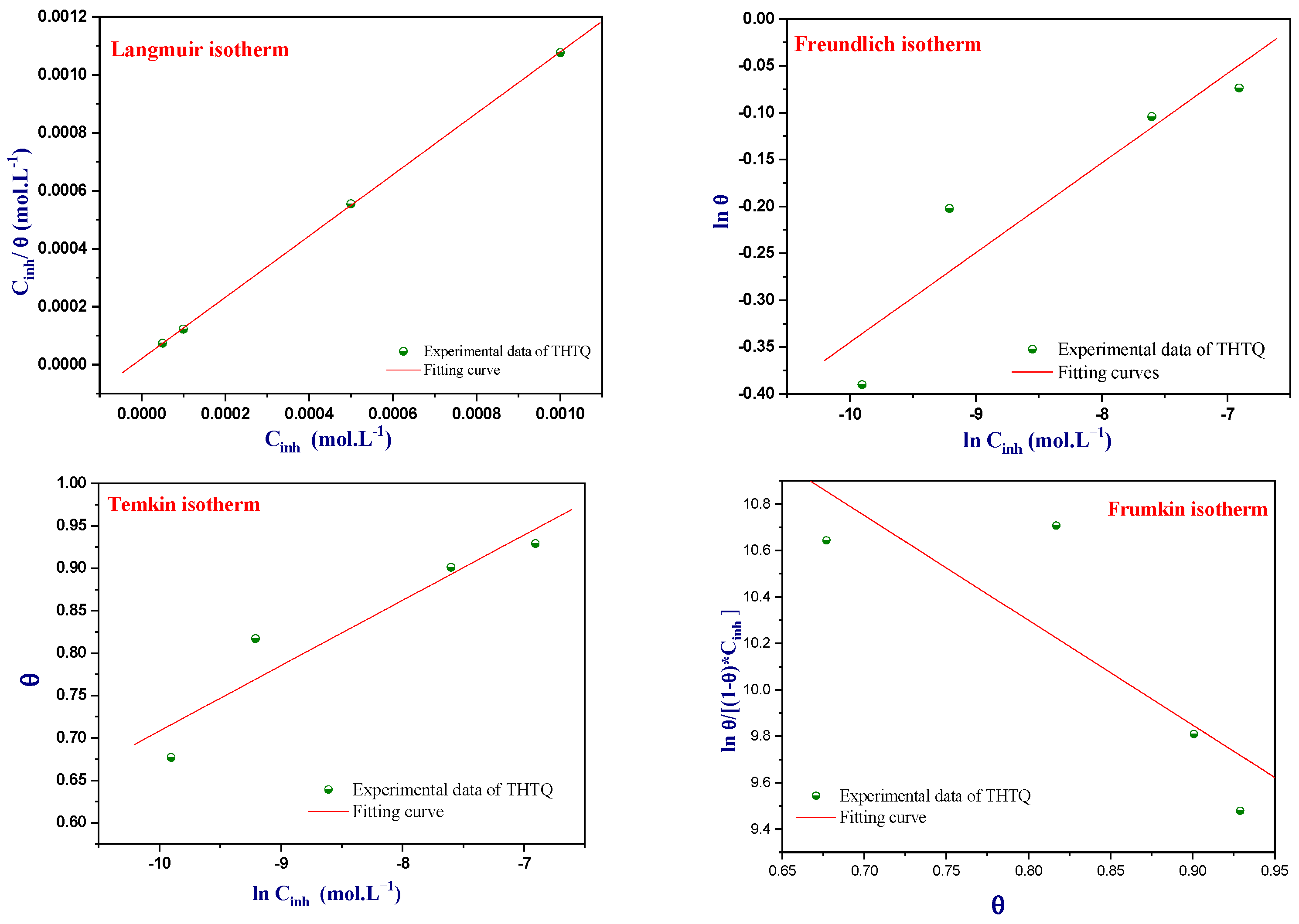
Adsorption Isotherms of THTQ
2.4.3. Immersed Time of THTQ
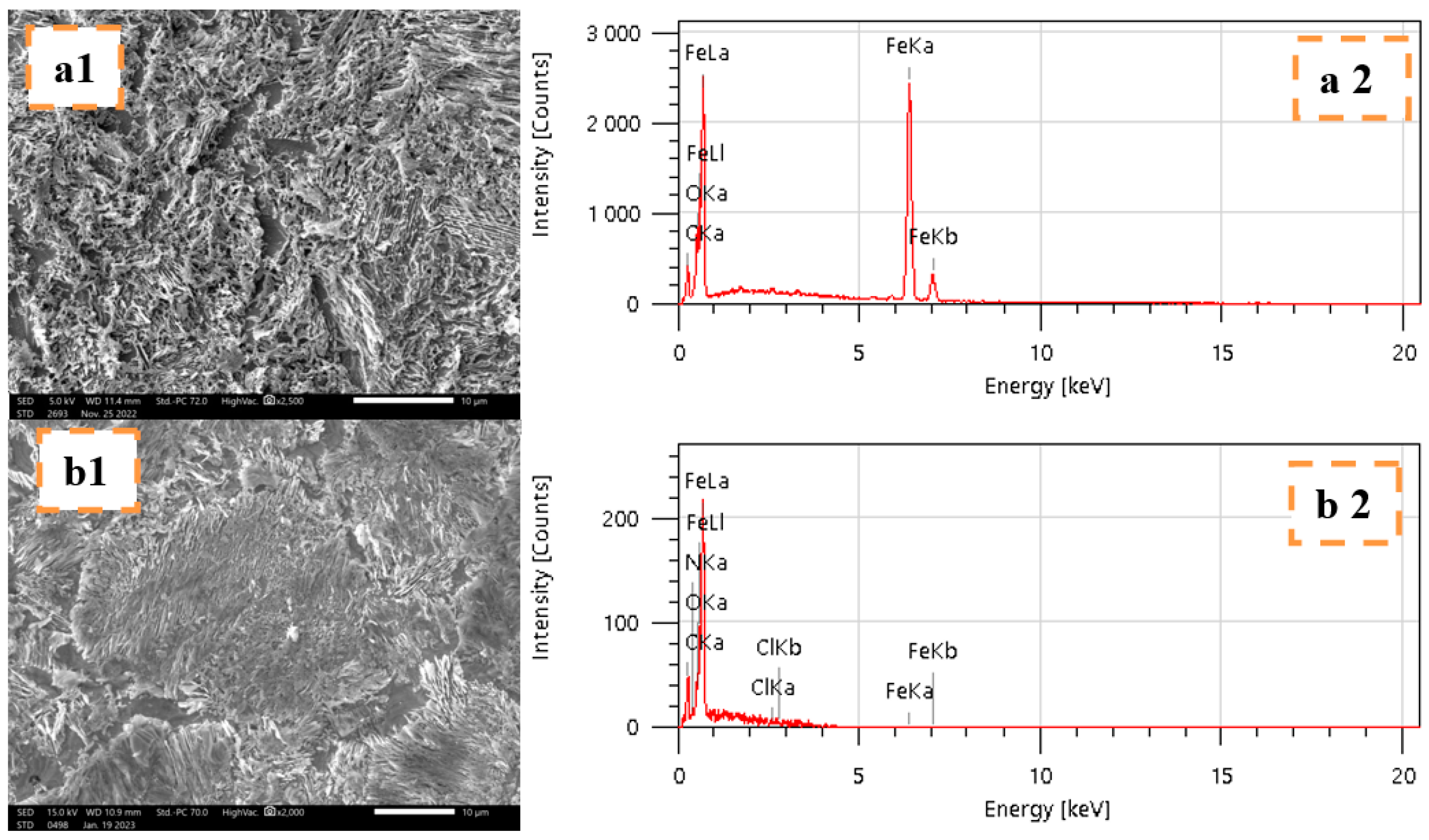
2.4.4. Surface Analyses MEB-EDX
2.4.5. In Silico Approach Study
2.4.6. Quantum Calculation
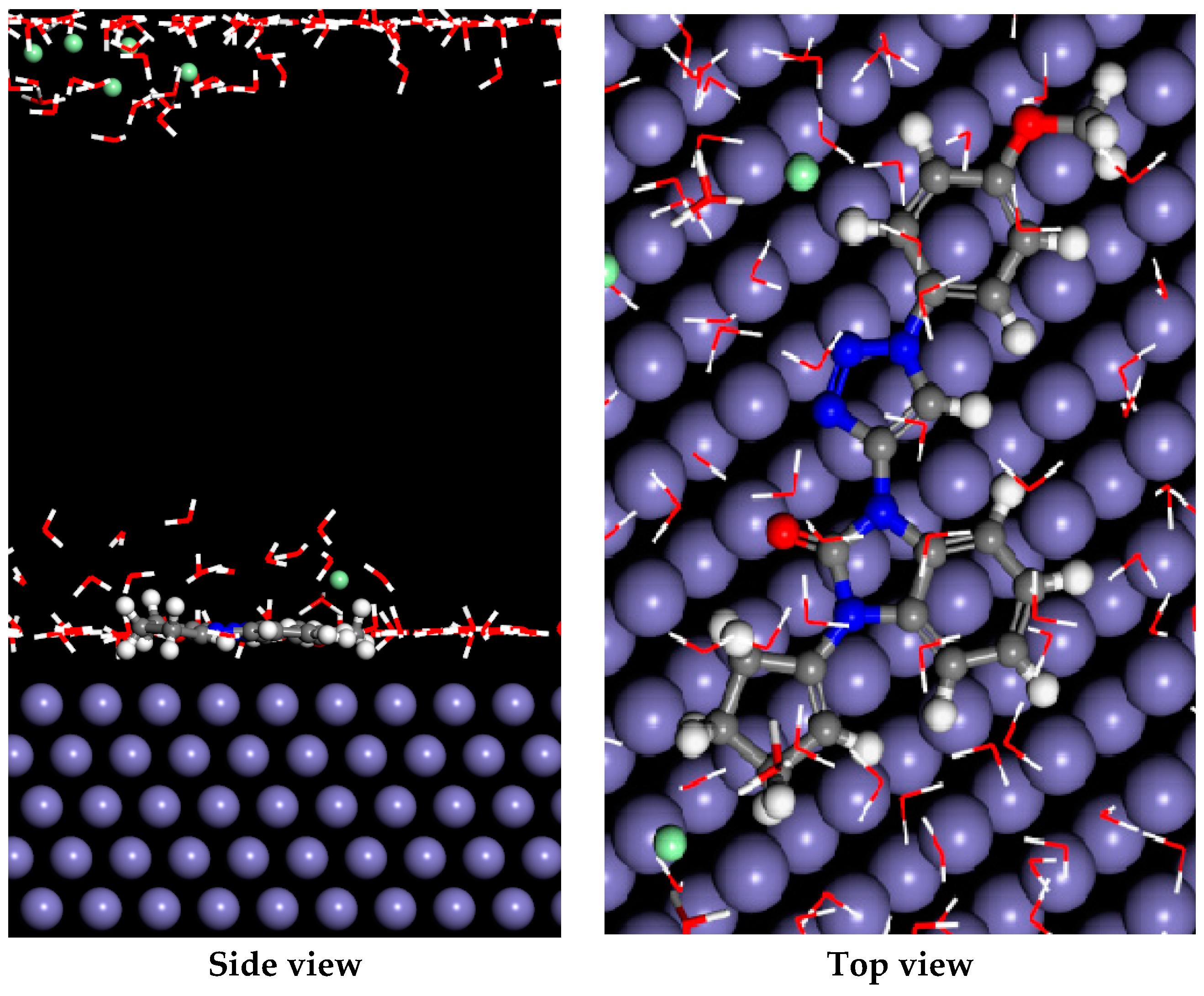
2.4.7. Monte Carlo Simulation Result
3. Materials and Methods
3.1. Chemistry
3.2. Biological Studies
3.2.1. Microbial Strains Tested
3.2.2. Diffusion Method
3.2.3. Determination of the Minimum Inhibitory Concentration
3.3. Molecular Docking
3.4. Corrosion Test
3.4.1. Materials and Solutions
3.4.2. Electrochemical Experiments
3.4.3. Theoretical Details
3.4.4. Monte Carlo Simulation Method
3.4.5. Surface Analyses
4. Conclusions
- THTQ demonstrated a significant antibacterial and antifungal activity;
- The electrochemical impedance spectroscopy (EIS) showed a good effectiveness with a maximum inhibition efficiency of 92.9% at the optimal concentration (10−3 M);
- The polarization curves confirmed THTQ’s inhibition efficiency, categorizing it as a mixed-type inhibitor;
- The Langmuir isotherm model was applicable to the THTQ inhibitor, indicating the formation of a protective layer on the steel surface, as confirmed by surface analysis using MEB-EDX;
- The theoretical approach combining DFT at B3LYP and Monte Carlo simulation provided insights into the inhibition potential of THTQ.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Brendel, M.; Sakhare, P.R.; Dahiya, G.; Subramanian, P.; Kaliappan, K.P. Serendipitous Synthesis of Pyridoquinazolinones via an Oxidative C–C Bond Cleavage. J. Org. Chem. 2020, 85, 8102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shao, Y.; Gong, J.; Zhu, J.; Cheng, T.; Chen, J. Selenium-Catalyzed Oxidative C–H Amination of (E)-3-(Arylamino)-2-styrylquinazolin-4(3H)-ones: A Metal-Free Synthesis of 1,2-Diarylpyrazolo[5,1-b]quinazolin-9(1H)-ones. J. Org. Chem. 2019, 84, 2798–2807. [Google Scholar] [CrossRef] [PubMed]
- Moussa, A.; Rahmati, A. Synthesis and characterization of silica-coated Fe3O4 nanoparticle@silylpropyl triethylammonium polyoxometalate as an organic–inorganic hybrid heterogeneous catalyst for the one-pot synthesis of tetrahydrobenzimidazo[2,1-b]quinazolin-1(2H)-ones. Appl. Organomet. Chem. 2020, 11, e5894. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, S.; Fan, L.; Yin, G. Cooperative Palladium and Copper Catalysis: One-pot Synthesis of Diamino-Substituted Naphthalenes from Aryl Halides, 1,4-Bis(trimethylsilyl)butadiyne and Amines. ChemCatChem 2020, 12, 1230–1235. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Z.; Fu, Q.; Yao, Q.; Huang, H.; Shen, J.; Cui, X. Ir-catalyzed regiospecific mono-sulfamidation of arylquinazolinones. Chin. Chem. Lett. 2020, 31, 58–60. [Google Scholar] [CrossRef]
- Mojzych, M. 12.19—Three Heterocyclic Rings Fused (6-6-6). Three Heterocycl. Rings Fused 2020, 12, 597–620. [Google Scholar] [CrossRef]
- Nikoofar, K.; Peyrovebaghi, S.S. 1-Butyl-2-methylpipyridinium iodide ([BMPPY]I): Novel ionic liquid for the synthesis of 6-hydroxy-6-(1H-indol-3-yl)indolo[2,1-b]quinazolin-12(6H)-ones under green solvent-free conditions. Res. Chem. Intermed. 2019, 45, 4287–4298. [Google Scholar] [CrossRef]
- Miao, J.; Sang, X.; Wang, Y.; Deng, S.; Hao, W. Synthesis of thiazolo[2,3-b]quinazoline derivatives via base-promoted cascade bicyclization of o-alkenylphenyl isothiocyanates with propargylamines. Org. Biomol. Chem. 2019, 17, 6994–6997. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, J.; Zhou, C.; Zheng, M.; Feng, D.; Liang, G.; She, Y. Extended π-conjugated quinazolinone derivatives with enhanced third-order nonlinear optical response. Dye. Pigment. 2019, 166, 314–322. [Google Scholar] [CrossRef]
- Xie, L.; Lu, C.; Jing, D.; Ou, X.; Zheng, K. Metal-Free Synthesis of Polycyclic Quinazolinones Enabled by a (NH4)2S2O8-Promoted Intramolecular Oxidative Cyclization. Eur. J. Org. Chem. 2019, 22, 3649–3653. [Google Scholar] [CrossRef]
- Al-Salahi, R.; Anouar, E.H.; Marzouk, M.; Taie, H.A.A.; Abuelizz, H.A. Screening and evaluation of antioxidant activity of some 1,2,4-triazolo[1,5-a] quinazoline derivatives. Future Med. Chem. 2018, 10, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Palaniraja, J.; Kumar, S.S.; Ramki, S.; Arunachalam, P.; Roopan, S.M. Conventional spectroscopic identification of biologically active imidazo-pyrimido fused acridines: In vitro anti-bacterial and anti-feedant activity. J. Mol. Liq. 2017, 578, 634–640. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Luo, X.; Chen, Y. Recent research progress and outlook in agricultural chemical discovery based on quinazoline scaffold. Pestic. Biochem. Physiol. 2022, 184, 105122. [Google Scholar] [CrossRef] [PubMed]
- Karan, R.; Agarwal, P.; Sinha, M.; Mahato, N. Recent Advances on Quinazoline Derivatives: A Potential Bioactive Scaffold in Medicinal Chemistry. ChemEngineering 2021, 5, 73. [Google Scholar] [CrossRef]
- Muhammad, Z.A.; Farghaly, T.A.; Althagafi, I.; Al-Hussain, S.A.; Zaki, M.E.; Harras, M.F. Synthesis of antimicrobial azoloazines and molecular docking for inhibiting COVID-19. J. Heterocycl. Chem. 2021, 58, 1286–1301. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Wang, M.; Liu, Q.; Lei, X.; Wu, M.; Guo, S.; Yi, D.; Li, Q.; Ma, L.; et al. Quinoline and quinazoline derivatives inhibit viral RNA synthesis by SARS-CoV-2 RdRp. ACS Infect. Dis. 2021, 7, 1535–1544. [Google Scholar] [CrossRef]
- Ewes, W.A.; Elmorsy, M.A.; El-Messery, S.M.; Nasr, M.N. Synthesis, biological evaluation and molecular modeling study of [1,2,4]-Triazolo[4,3-c] quinazolines: New class of EGFR-TK inhibitors. Bioorg. Med. Chem. 2020, 28, 115373. [Google Scholar] [CrossRef]
- Azab, A.E.; Alesawy, M.S.; Eldehna, W.M.; Elwan, A.; Eissa, I.H. New [1,2,4]triazolo[4,3-c] quinazoline derivatives as vascular endothelial growth factor receptor-2 inhibitors and apoptosis inducers: Design, synthesis, docking, and antiproliferative evaluation. Arch. Pharm. 2022, 355, e2200133. [Google Scholar] [CrossRef]
- El-Shershaby, M.H.; Ghiaty, A.; Bayoumi, A.H.; Ahmed, H.E.; El-Zoghbi, M.S.; El-Adl, K.; Abulkhair, H.S. 1,2,4-triazolo[4,3-c] quinazolines: A bioisosterism-guided approach towards the development of novel PCAF inhibitors with potential anticancer activity. New J. Chem. 2021, 45, 11136–11152. [Google Scholar] [CrossRef]
- Xie, D.; Shi, J.; Zhang, A.; Lei, Z.; Zu, G.; Fu, Y.; Gan, X.; Yin, L.; Song, B.; Hu, D. Syntheses, antiviral activities and induced resistance mechanisms of novel quinazoline derivatives containing a dithioacetal moiety. Bioorg. Chem. 2018, 80, 433–443. [Google Scholar] [CrossRef]
- Fernández, G.A.; Castro, E.F.; Rosas, R.A.; Fidalgo, D.M.; Adler, N.S.; Battini, L.; Espana de Marco, M.J.; Fabiani, M.; Bruno, A.M.; Bollini, M.; et al. Design and Optimization of Quinazoline Derivatives: New Non-nucleoside Inhibitors of Bovine Viral Diarrhea Virus. Front. Chem. 2020, 8, 590235. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, M.; Zhao, J.; Wang, Y.; Zhu, M.; Wang, J.; Cen, S.; Wang, Y. Design, synthesis and in vitro anti-influenza A virus evaluation of novel quinazoline derivatives containing S-acetamide and NH-acetamide moieties at C-4. Eur. J. Med. Chem. 2020, 206, 112706. [Google Scholar] [CrossRef]
- Fan, Z.; Shi, J.; Luo, N.; Ding, M.; Bao, X. Synthesis, crystal structure, and agricultural antimicrobial evaluation of novel quinazoline thioether derivatives incorporating the 1,2,4-triazolo[4,3-a]pyridine moiety. J. Agric. Food Chem. 2019, 67, 11598–11606. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Ding, M.; Luo, N.; Shi, J.; Huang, J.; Bao, X. Design, synthesis, crystal structure and in vitro antimicrobial activity of novel 1,2,4-triazolo[1,5-a]pyrimidine-containing quinazolinone derivatives. Mol. Divers. 2021, 25, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Krasovska, N.; Stavytskyi, V.; Nosulenko, I.; Karpenko, O.; Voskoboinik, O.; Kovalenko, S.I. Quinazoline-containing Hydrazydes of Dicarboxylic Acids and Products of Their Structural Modification—A Novel Class of Anti-inflammatory Agents. Acta Chim. Slov. 2021, 68, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.N.; Liu, C.Y.; Wang, T.; Li, Y.L.; Xu, K.; Lou, H.X. Two New Quinazoline Derivatives from the Moss Endophytic Fungus Aspergillus sp. and Their Anti-inflammatory Activity. Nat. Prod. Bioprospect. 2021, 11, 105–110. [Google Scholar] [CrossRef]
- Ibrahim, O.F.; Bakhite, E.A.; Metwally, S.A.; El-Ossaily, Y.A.; Abdu-Allah, H.H.; Al-Taifi, E.A.; Kandel, M. Synthesis, Characterization, and Antifungal Activity of Some New Thieno[2,3-b]pyridines Incorporating Quinazoline or Benzimidazole Moiety. Russ. J. Bioorg. Chem. 2021, 47, 918–928. [Google Scholar] [CrossRef]
- Devipriya, D.; Roopan, S.M. UV-light intervened synthesis of imidazo fused quinazoline and its solvatochromism, antioxidant, antifungal and luminescence properties. J. Photochem. Photobiol. B Biol. 2019, 190, 42–49. [Google Scholar] [CrossRef]
- Ibrahim, Z.Y.U.; Uzairu, A.; Shallangwa, G.A.; Abechi, S.E.; Isyaku, S. Virtual screening and molecular dynamic simulations of the antimalarial derivatives of 2-anilino 4-amino substituted quinazolines docked against a Pf-DHODH protein target. Egypt. J. Med. Hum. Genet. 2022, 23, 119. [Google Scholar] [CrossRef]
- Mizukawa, Y.; Ikegami-Kawai, M.; Horiuchi, M.; Kaiser, M.; Kojima, M.; Sakanoue, S.; Miyagi, S.; Chick, C.N.; Togashi, H.; Tsubuki, M.; et al. Quest for a potent antimalarial drug lead: Synthesis and evaluation of 6,7-dimethoxyquinazoline-2,4-diamines. Bioorg. Med. Chem. 2021, 33, 116018. [Google Scholar] [CrossRef]
- Ghaleb, A.; Aouidate, A.; Bouachrine, M.; Lakhlifi, T.; Sbai, A. In silico exploration of aryl halides analogues as CheckpointKinase 1 inhibitors by using 3D QSAR, molecular docking study, and ADMET screening. Adv. Pharm. Bull. 2019, 9, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Nahlé, A.; Salim, R.; El Hajjaji, F.; Aouad, M.R.; Messali, M.; Ech-Chihbi, E.; Hammouti, B.; Taleb, M. Novel triazole derivatives as ecological corrosion inhibitors for mild steel in 1.0 M HCl: Experimental & theoretical approach. RSC Adv. 2021, 11, 4147–4162. [Google Scholar] [CrossRef] [PubMed]
- Bouoidina, A.; Ech-Chihbi, E.; El-Hajjaji, F.; El Ibrahimi, B.; Kaya, S.; Taleb, M. Anisole derivatives as sustainable-green inhibitors for mild steel corrosion in 1 M HCl: DFT and molecular dynamic simulations approach. J. Mol. Liq. 2021, 324, 115088. [Google Scholar] [CrossRef]
- Arrousse, N.; Salim, R.; Abdellaoui, A.; El Hajjaji, F.; Hammouti, B.; Mabrouk, E.H.; Diño, W.A.; Taleb, M. Synthesis, characterization, and evaluation of xanthene derivative as highly effective, nontoxic corrosion inhibitor for mild steel immersed in 1 M HCl solution. J. Taiwan Inst. Chem. Eng. 2021, 120, 344–359. [Google Scholar] [CrossRef]
- Bouoidina, A.; Haldhar, R.; Salim, R.; Ech-chihbi, E.; Ichou, H.; El-Hajjaji, F.; Kim, S.C.; El Ibrahimi, B.; Kaya, S.; Taleb, M. An effective and smart corrosion inhibitor in acidic environment: Experimental & theoretical studies. Korean J. Chem. Eng. 2023, 40, 235–247. [Google Scholar] [CrossRef]
- El Arrouji, S.; Karrouchi, K.; Berisha, A.; Alaoui, K.I.; Warad, I.; Rais, Z.; Radi, S.; Taleb, M.; Ansar, M.; Zarrouk, A. New pyrazole derivatives as effective corrosion inhibitors on steel-electrolyte interface in 1 M HCl: Electrochemical, surface morphological (SEM) and computational analysis. Colloids Surf. A Physicochem. Eng. Asp. 2020, 604, 125325. [Google Scholar] [CrossRef]
- Ech-chihbi, E.; Nahlé, A.; Salim, R.; Oudda, H.; El Hajjaji, F.; El Kalai, F.; El Aatiaoui, A.; Taleb, M. An investigation into quantum chemistry and experimental evaluation of imidazopyridine derivatives as corrosion inhibitors for C-steel in acidic media. J. Bio-Tribo-Corros. 2019, 5, 24. [Google Scholar] [CrossRef]
- Rahmani, H.; Alaoui, K.I.; Azzouzi, M.E.; Benhiba, F.; El Hallaoui, A.; Rais, Z.; Taleb, M.; Saady, A.; Labriti, B.; Aouniti, A.; et al. Corrosion assessement of mild steel in acid environment using novel triazole derivative as a anti-corrosion agent: A combined experimental and quantum chemical study. Chem. Data Collect. 2019, 24, 100302. [Google Scholar] [CrossRef]
- El Hajjaji, F.; Salim, R.; Taleb, M.; Benhiba, F.; Rezki, N.; Chauhan, D.S.; Quraishi, M.A. Pyridinium-based ionic liquids as novel eco-friendly corrosion inhibitors for mild steel in molar hydrochloric acid: Experimental & computational approach. Surf. Interfaces 2021, 22, 100881. [Google Scholar] [CrossRef]
- Berecz, G.; Reiter, J.; Csaszar, J. On triazoles. XL [1]. Non catalytic dehalogenation of some 5-chloro-1,2,4-triazolo[1,5-a]pyrimidine derivatives. J. Heterocycl. Chem. 1999, 36, 1199–1212. [Google Scholar] [CrossRef]
- Merzouki, O.; Arrousse, N.; El Barnossi, A.; Ech-chihbi, E.; Fernine, Y.; Iraqi Housseini, A.; Rais, Z.; Taleb, M. Eco-friendly synthesis, characterization, in-silico ADMET and molecular docking analysis of novel carbazole derivatives as antibacterial and anti-fungal agents. J. Mol. Struct. 2022, 1271, 133966. [Google Scholar] [CrossRef]
- Alaoui Mrani, S.; Ech-chihbi, E.; Arrousse, N.; Rais, Z.; El Hajjaji, F.; ElAbiad, C.; Radi, S.; Mabrouki, J.; Taleb, M.; Jodeh, S. DFT and electrochemical investigations on the corrosion inhibition of mild steel by novel Schif’s base derivatives in 1M HCl solution. Arab. J. Sci. Eng. 2021, 46, 5691–6570. [Google Scholar] [CrossRef]
- Ardakani, E.K.; Kowsari, E.; Ehsani, A. Imidazolium-derived polymeric ionic liquid as a green inhibitor for corrosion inhibition of mild steel in 1.0 M HCl: Experimental and computational study. Colloids Surf A Physicochem. Eng. Asp. 2020, 586, 124195. [Google Scholar] [CrossRef]
- Zarrouk, A.; Hammouti, B.; Lakhlif, T.; Bentiss, F. New 1 H-pyrrole-2:5-dione derivatives as efcient organic inhibitors of carbon steel corrosion in hydrochloric acid medium: Electrochemical XPS and DFT Studies. Corros. Sci. 2015, 90, 572–584. [Google Scholar] [CrossRef]
- Yadav, D.K.; Quraishi, M.A. Electrochemical investigation of substituted pyranopyrazoles adsorption on mild steel in acid solution. Ind. Eng. Chem. Res. 2012, 51, 8194–8210. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Ahmadi-Ashtiani, A. Corrosion inhibition efciency of some phosphoramide derivatives: DFT computations and MD simulations. J. Mol. Liq. 2019, 298, 111–409. [Google Scholar] [CrossRef]
- Lazrak, J.; Salim, R.; Arrousse, N.; Ech-chihbi, E.; El-Hajjaji, F.; Taleb, M.; Farah, A.; Ramzi, A. Mentha viridis oil as a green efective corrosion inhibitor for mild steel in 1 M HCl medium. Int. J. Corros. Scale Inhib. 2020, 9, 1580–1606. [Google Scholar] [CrossRef]
- Saady, A.; Ech-chihbi, E.; El-Hajjaji, F.; Benhiba, F.; Zarrouk, A.; KandriRodi, Y.; Taleb, M.; El Biache, A.; Rais, Z. Molecular dynamics, DFT and electrochemical to study the interfacial adsorption behavior of new imidazo[4,5-b] pyridine derivative as corrosion inhibitor in acid medium. J. Appl. Electrochem. 2021, 51, 245–265. [Google Scholar] [CrossRef]
- Tan, B.; Zhang, S.; Liu, H.; Guo, Y.; Qiang, Y.; Li, W.; Guo, L.; Xu, C.; Chen, S. Corrosion inhibition of X65 steel in sulfuric acid by two food favorants 2-isobutylthiazole and 1-(1.3-Thiazol-2-yl) ethanone as the green environmental corrosion inhibitors: Combination of experimental and theoretical researches. J. Colloid Interface Sci. 2018, 538, 519–529. [Google Scholar] [CrossRef]
- Salim, R.; Nahlé, A.; El-Hajjaji, F.; Ech-chihbi, E.; Benhiba, F.; El Kalai, F.; Benchat, N.; Oudda, H.; Guenbour, A.; Taleb, M.; et al. Experimental, Density Functional Theory, and Dynamic Molecular Studies of Imidazopyridine Derivatives as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid. Surf. Eng. Appl. Electrochem. 2021, 57, 233–254. [Google Scholar] [CrossRef]
- Ech-chihbi, E.; Nahlé, A.; Salim, R.; Benhiba, F.; Moussaif, A.; El-Hajjaji, F.; Oudda, H.; Guenbour, A.; Taleb, M.; Warad, I.; et al. Zarrouk. Computational, MD simulation, SEM/EDX and experimental studies for understanding adsorption of benzimidazole derivatives as corrosion inhibitors in 1.0 M HCl solution. J. Alloys Compd. 2020, 20, 155842. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Potential role of a novel green eco-friendly inhibitor in corrosion inhibition of mild steel in HCl solution: Detailed macro/micro-scale experimental and computational explorations, Constr. Build. Mater. 2020, 245, 118464. [Google Scholar] [CrossRef]
- Alaoui, K.; Touir, R.; Galai, M.; Serrar, H.; Ouakki, M.; Kaya, S.; Tüzün, B.; Boukhris, S.; EbnTouhami, M.; El Kacimi, Y. Electrochemical and Computational Studies of Some Triazepine Carboxylate Compounds as Acid Corrosion Inhibitors for Mild Steel. J. Bio Tribo-Corros. 2018, 4, 37. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Haque, J.; Dohare, P.; Lgaz, H.; Salghi, R.; Quraishi, M.A. Effect of electron donating functional groups on corrosion inhibition of mild steel in hydrochloric acid: Experimental and quantum chemical study. J. Taiwan Inst. Chem. Eng. 2018, 82, 233–251. [Google Scholar] [CrossRef]
- Arrousse, N.; Salim, R.; Kaddouri, Y.; Zarrouk, A.; Zahri, D.; El Hajjaji, F.; Touzani, R.; Taleb, M.; Jodeh, S. The inhibition behavior of two pyrimidine-pyrazole derivatives against corrosion in hydrochloric solution: Experimental, surface analysis and in silico approach studies. Arab. J. Chem. 2020, 13, 5949–5965. [Google Scholar] [CrossRef]
- Kaya, S.; Kaya, C. A new equation for calculation of chemical hardness of groups and molecules. Mol. Phys. 2015, 113, 1311–1319. [Google Scholar] [CrossRef]
- Savaş, K.; Kaya, C. A simple method for the calculation of lattice energies of inorganic ionic crystals based on the chemical hardness. Inorg. Chem. 2015, 54, 8207–8213. [Google Scholar] [CrossRef]
- Chattaraj, P.K.; Fuentealba, P.; Jaque, P.; Toro-Labbé, A. Validity of the minimum polarizability principle in molecular vibrations and internal rotations: An ab initio SCF study. J. Phys. Chem. A 1999, 103, 9307–9312. [Google Scholar] [CrossRef]
- Kaya, S.; Kaya, C. A new equation based on ionization energies and electron affinities of atoms for calculating of group electronegativity. Comput. Theor. Chem. 2015, 1052, 42–46. [Google Scholar] [CrossRef]
- Agour, A.; Mssillou, I.; Mechchate, H.; Es-safi, I.; Allali, A.; El Barnossi, A.; Kamaly, O.; Alshawwa, S.Z.; Moussaoui, A.; Bari, A.; et al. Brocchia cinerea (Delile) Vis. Essential Oil Antimicrobial Activity and Crop Protection against Cowpea Weevil Callosobruchus maculatus (Fab.). Plants 2022, 11, 583. [Google Scholar] [CrossRef]
- Lafraxo, S.; Barnossi, A.; El Moussaoui, A.; Bourhia, M.; Salamatullah, A.M.; Alzahrani, A.; Akka, A.A.; Choubbane, A.; Akhazzane, M.; Aboul-soud, M.A.M.; et al. Essential Oils from Leaves of Juniperus thurifera L.; Exhibiting Antioxidant, Antifungal and Antibacterial Activities against Antibiotic-Resistant Microbes. Horticulturae 2022, 8, 321. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Chebbac, K.; Ghneim, H.K.; Moussaoui, A.; El Bourhia, M.; El Barnossi, A.; Ouaritini, Z.B.; Salamatullah, A.M.; Alzahrani, A.; Aboul-soud, M.A.M.; Giesy, J.P.; et al. Antioxidant and Antimicrobial Activities of Chemically-Characterized Essential Oil from Artemisia aragonensis Lam. against Drug-Resistant Microbes. Molecules 2022, 27, 1136. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDockVina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef]
- Shaw, D.E.; Irwin, J. Anton 2: Raising the bar for performance and programmability in a special-purpose molecular dynamics supercomputer. In Proceedings of the International Conference for High Performance Computing, Networking, Storage and Analysis, New Orleans, LA, USA, 16–21 November 2014; pp. 41–53. [Google Scholar] [CrossRef]
- Eeckhoudt, J.; Bettens, T.; Geerlings, P.; Cammi, R.; Chen, B.; Alonso, M.; De Proft, F. Conceptual density functional theory under pressure: Part I. XP-PCM method applied to atoms. Chem. Sci. 2022, 13, 9329–9350. [Google Scholar] [CrossRef]
- Al-Otaibi, J.S.; Mary, Y.S.; Mary, Y.S.; Kaya, S.; Erkan, S. Spectral analysis and DFT investigation of some benzopyran analogues and their self-assemblies with graphene. J. Mol. Liq. 2020, 317, 113924. [Google Scholar] [CrossRef]
- Koopmans, T. Über die Zuordnung von Wellenfunktionen und Eigenwertenzu den einzelnenElektroneneines Atoms. Physica 1934, 1, 104–113. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpály, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Gazquez, J.L.; Cedillo, A.; Vela, A. Electrodonating and electroaccepting powers. J. Phys. Chem. A 2007, 111, 1966–1970. [Google Scholar] [CrossRef]
- Serdaroğlu, G.; Kaya, S.; Touir, R. Eco-friendly sodium gluconate and trisodium citrate inhibitors for low carbon steel in simulated cooling water system: Theoretical study and molecular dynamic simulations. J. Mol. Liq. 2020, 319, 114108. [Google Scholar] [CrossRef]
- Erdoğan, Ş.; Safi, Z.S.; Kaya, S.; Işın, D.Ö.; Guo, L.; Kaya, C. A computational study on corrosion inhibition performances of novel quinoline derivatives against the corrosion of iron. J. Mol. Struct. 2017, 1134, 751–761. [Google Scholar] [CrossRef]



| Staphylococcus aureus | Escherichia coli | Bacillus subtilis | Proteus mirabilis | |||||
|---|---|---|---|---|---|---|---|---|
| Antibacterial Activity (mm) | MIC (mg/mL) | Antibacterial Activity (mm) | MIC (mg/mL) | Antibacterial Activity (mm) | MIC (mg/mL) | Antibacterial Activity (mm) | MIC (mg/mL) | |
| THTQ | 15 | 7.5 | 16 | 3.75 | 9 | 7.5 | 17 | 3.75 |
| 15 | 7.5 | 15 | 3.75 | 9 | 7.5 | 17 | 1.875 | |
| 15 | 7.5 | 16 | 3.75 | 9 | 7.5 | 19 | 1.875 | |
| Streptomycin | 24 | 1.875 | 27 | 0.94 | 24 | 1.875 | 25 | 0.94 |
| 24 | 1.875 | 27 | 0.94 | 24 | 1.875 | 25 | 0.94 | |
| 24 | 1.875 | 27 | 0.94 | 24 | 1.875 | 25 | 0.94 | |
| DMSO | Rs | - | Rs | - | Rs | - | Rs | - |
| Candida albicans | Aspergillus niger | Aspergillus flavus | Fusarium oxysporum | |||||
|---|---|---|---|---|---|---|---|---|
| Antifungal Activity (mm) | MIC (mg/mL) | Antifungal Activity (%) | MIC (mg/mL) | Antifungal Activity (%) | MIC (mg/mL) | Antifungal Activity (%) | MIC (mg/mL) | |
| THTQ | 25 | 7.5 | 28.57 | 15 | Rs | - | 38.09 | 15 |
| 22 | 7.5 | 26.19 | 15 | 38.09 | 15 | |||
| 24 | 7.5 | 26.19 | 15 | 42.85 | 15 | |||
| Fluconazole | 20 | 7.5 | 28.57 | 15 | Rs | - | 45 | 7.5 |
| 20 | 7.5 | 28.57 | 15 | 45 | 7.5 | |||
| 20 | 7.5 | 28.57 | 15 | 45 | 7.5 | |||
| DMSO | Rs | - | Rs | - | Rs | - | Rs | - |
| Ligand | Biding Score (Kcal/mol) | Number Hydrogen Bond | |
|---|---|---|---|
| 3FV5 | THTQ | −6.1 | 1 |
| Streptomycin | −5.3 | 7 | |
| 1EA1 | THTQ | −7.1 | 1 |
| Fluconazole | −7.0 | 3 |
| Medium | Conc (M) | −Ecorr (mV/Ag/AgCl) | icorr (µA. cm−2) | −βc (mV. dec−1) | βa (mV. dec−1) | ȠPP % |
|---|---|---|---|---|---|---|
| 1M HCl | - | 413 | 944 | 139 | 128 | - |
| THTQ | 10−6 | 416 | 226 | 139 | 79 | 76.0 |
| 10−5 | 413 | 108 | 135 | 66 | 81.7 | |
| 10−4 | 403 | 80 | 132 | 60 | 91.5 | |
| 10−3 | 405 | 52 | 131 | 62 | 94.4 |
| Medium | Temp. (K) | −Ecorr (mV/Ag/AgCl) | icorr (µA. cm−2) | −βc (mV. dec−1) | βa (mV. dec−1) | ȠPP % |
|---|---|---|---|---|---|---|
| 1M HCl | 298 | 413 | 944 | 139 | 128 | - |
| 308 | 410 | 1690 | 137 | 129 | - | |
| 318 | 411 | 2328 | 126 | 125 | - | |
| 328 | 412 | 3387 | 120 | 133 | - | |
| THTQ | 298 | 405 | 52 | 131 | 62 | 94.4 |
| 308 | 410 | 131 | 137 | 64 | 92.2 | |
| 318 | 407 | 312 | 115 | 65 | 86.5 | |
| 328 | 405 | 537 | 117 | 70 | 84.1 |
| Activation Parameters | Ea (kJ/mol) | ∆H* kJ/mol | ∆S* (J/mol·K) |
|---|---|---|---|
| 1M HCl | 33.86 | 31.26 | −82.73 |
| THTQ | 64.17 | −61.58 | −5.11 |
| Medium | Conc (M) | Rs (Ω.cm2) | Rp (Ω.cm2) | CPE | Cdl (µF.cm−2) | Ɵ | ƞimp % | |
|---|---|---|---|---|---|---|---|---|
| Q (µF.S n−1) | ndl | |||||||
| HCl | 1 | 1.7 | 33.0 | 312.7 | 0.784 | 89.1 | - | - |
| THTQ | 10−6 | 0.40 | 102.3 | 256.8 | 0.759 | 80.9 | 0.677 | 67.7 |
| 10−5 | 0.73 | 181.2 | 148.5 | 0.790 | 56.9 | 0.817 | 81.7 | |
| 10−4 | 0.66 | 333.7 | 106.7 | 0.781 | 41.8 | 0.901 | 90.1 | |
| 10−3 | 0.41 | 465.0 | 58.8 | 0.809 | 25.2 | 0.929 | 92.9 | |
| Isotherms | Linear Equations | Descriptions |
|---|---|---|
| Langmuir | (4) | K is a coefficient that represents the strength of adsorption between the inhibitor and the metal surface. Cinh refers to the concentration of the inhibitor used in the experiment. ϴ is the inhibitor recovery rate, which is a measure of the efficiency of the inhibitor in protecting the metal surface from corrosion. |
| Freundlich | (5) | 0 < Z < 1: the adsorption of inhibitor on the surface of the metal is favorable and easy. Z = 1: moderate adsorption of inhibitor on the metal surface. Z > 1: the adsorption behavior of inhibitor is difficult and less favorable. |
| Frumkin | (6) | d represents the interaction between adsorbed molecules. |
| Temkin | (7) | a is the repulsion or attraction interaction coefficient among adsorbed compounds. |
| Isotherms | R2 | Parameters | K | ∆G°ads (kJ/mol) | |
|---|---|---|---|---|---|
| Langmuir | 0.999 | slope | 1.05 | 4.94 × 104 | −43.9 |
| Freundlich | 0.930 | n | 10.46 | 1.84 × 100 | −18.6 |
| Temkin | 0.944 | a | −6.49 | 2.18 × 108 | −64.7 |
| Frumkin | 0.836 | a | 2.25 | 9.10 × 10−7 | 17.3 |
| Medium | Time (h) | Rs (Ω.cm2) | Rp (Ω.cm2) | Q (µF.S n−1) | ndl | Cdl (µF.cm−2) | ƞimp % |
|---|---|---|---|---|---|---|---|
| 1M HCl | 1/2 | 1.7 | 33.0 | 312.70 | 0.784 | 89.1 | - |
| 1 | 1.6 | 26.4 | 364.90 | 0.810 | 122.7 | - | |
| 2 | 1.5 | 23.0 | 433.0 | 0.835 | 174.3 | - | |
| 4 | 1.5 | 21.4 | 627.00 | 0.834 | 267.0 | - | |
| 6 | 1.0 | 19.4 | 963.80 | 0.796 | 349.4 | - | |
| 12 | 1.2 | 10.4 | 949.2 | 0.764 | 419.5 | - | |
| THTQ | 1/2 | 0.41 | 465.0 | 58.8 | 0.809 | 25.2 | 92.9 |
| 1 | 0.29 | 540.0 | 35.48 | 0.829 | 15.58 | 93.9 | |
| 2 | 1.52 | 138.7 | 81.52 | 0.899 | 49.38 | 76.2 | |
| 4 | 1.35 | 130.4 | 103.9 | 0.883 | 58.9 | 74.7 | |
| 6 | 1.21 | 126.7 | 137.9 | 0.857 | 70.35 | 73.9 | |
| 12 | 1.11 | 115.0 | 207.3 | 0.837 | 101.0 | 71.3 |
| Specimens | Fe | C | O | N | Cl |
|---|---|---|---|---|---|
| Mild steel in 1 M HCl | 90.39 | 5.87 | 3.74 | - | - |
| Mild steel in THTQ | 86.13 | 9.50 | 2.29 | 1.38 | 0.70 |
| Descriptors | EHOMO (eV) | ELUMO (eV) | ΔEgap (eV) | Ƞ (eV) | σ (eV−1) | χ (eV) | ΔEback-donation | ΔNFe/110 |
|---|---|---|---|---|---|---|---|---|
| THTQ | −6.5444 | −1.2754 | 5.2690 | 2.6345 | 0.3795 | 3.9099 | −0.6586 | 0.1727 |
| System | Eads | Inhibitor: dEad/dNi | Rigid Adsorption Energy | Deformation Energy | H2O: dEad/dNi | H3O+: dEad/dNi | Cl−: dEad/dNi |
|---|---|---|---|---|---|---|---|
| Fe110/THTQ | −7698.63 | −276.32 | −4068.72 | −3629.91 | −31.58 | −172.83 | −149.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ettahiri, W.; Salim, R.; Adardour, M.; Ech-chihbi, E.; Yunusa, I.; Alanazi, M.M.; Lahmidi, S.; Barnossi, A.E.; Merzouki, O.; Iraqi Housseini, A.; et al. Synthesis, Characterization, Antibacterial, Antifungal and Anticorrosion Activities of 1,2,4-Triazolo[1,5-a]quinazolinone. Molecules 2023, 28, 5340. https://doi.org/10.3390/molecules28145340
Ettahiri W, Salim R, Adardour M, Ech-chihbi E, Yunusa I, Alanazi MM, Lahmidi S, Barnossi AE, Merzouki O, Iraqi Housseini A, et al. Synthesis, Characterization, Antibacterial, Antifungal and Anticorrosion Activities of 1,2,4-Triazolo[1,5-a]quinazolinone. Molecules. 2023; 28(14):5340. https://doi.org/10.3390/molecules28145340
Chicago/Turabian StyleEttahiri, Walid, Rajae Salim, Mohamed Adardour, Elhachmia Ech-chihbi, Ismaeel Yunusa, Mohammed M. Alanazi, Sanae Lahmidi, Azeddin El Barnossi, Oussama Merzouki, Abdelilah Iraqi Housseini, and et al. 2023. "Synthesis, Characterization, Antibacterial, Antifungal and Anticorrosion Activities of 1,2,4-Triazolo[1,5-a]quinazolinone" Molecules 28, no. 14: 5340. https://doi.org/10.3390/molecules28145340
APA StyleEttahiri, W., Salim, R., Adardour, M., Ech-chihbi, E., Yunusa, I., Alanazi, M. M., Lahmidi, S., Barnossi, A. E., Merzouki, O., Iraqi Housseini, A., Rais, Z., Baouid, A., & Taleb, M. (2023). Synthesis, Characterization, Antibacterial, Antifungal and Anticorrosion Activities of 1,2,4-Triazolo[1,5-a]quinazolinone. Molecules, 28(14), 5340. https://doi.org/10.3390/molecules28145340







