Abstract
In this research, a metal-free diastereoselective formal 1,3-dipolar cycloaddition of N-2,2,2-trifluoroethylisatin ketimines and cyclopentene-1,3-diones which can efficiently lead to the desymmetrization of cyclopentene-1,3-diones is developed. With the developed protocol, a series of tetracyclic spirooxindoles containing pyrrolidine and cyclopentane subunits can be smoothly obtained with good results (up to 99% yield and 91:9 dr). Furthermore, the methodology can be extended to trifluoromethyl-substituted iminomalonate, and the corresponding formal [3+2] cycloaddition reaction affords bicyclic heterocycles containing fused pyrrolidine and cyclopentane moieties in moderate yields with >20:1 dr. The synthetic potential of the methodology is demonstrated by the scale-up experiment and by versatile transformations of the products.
1. Introduction
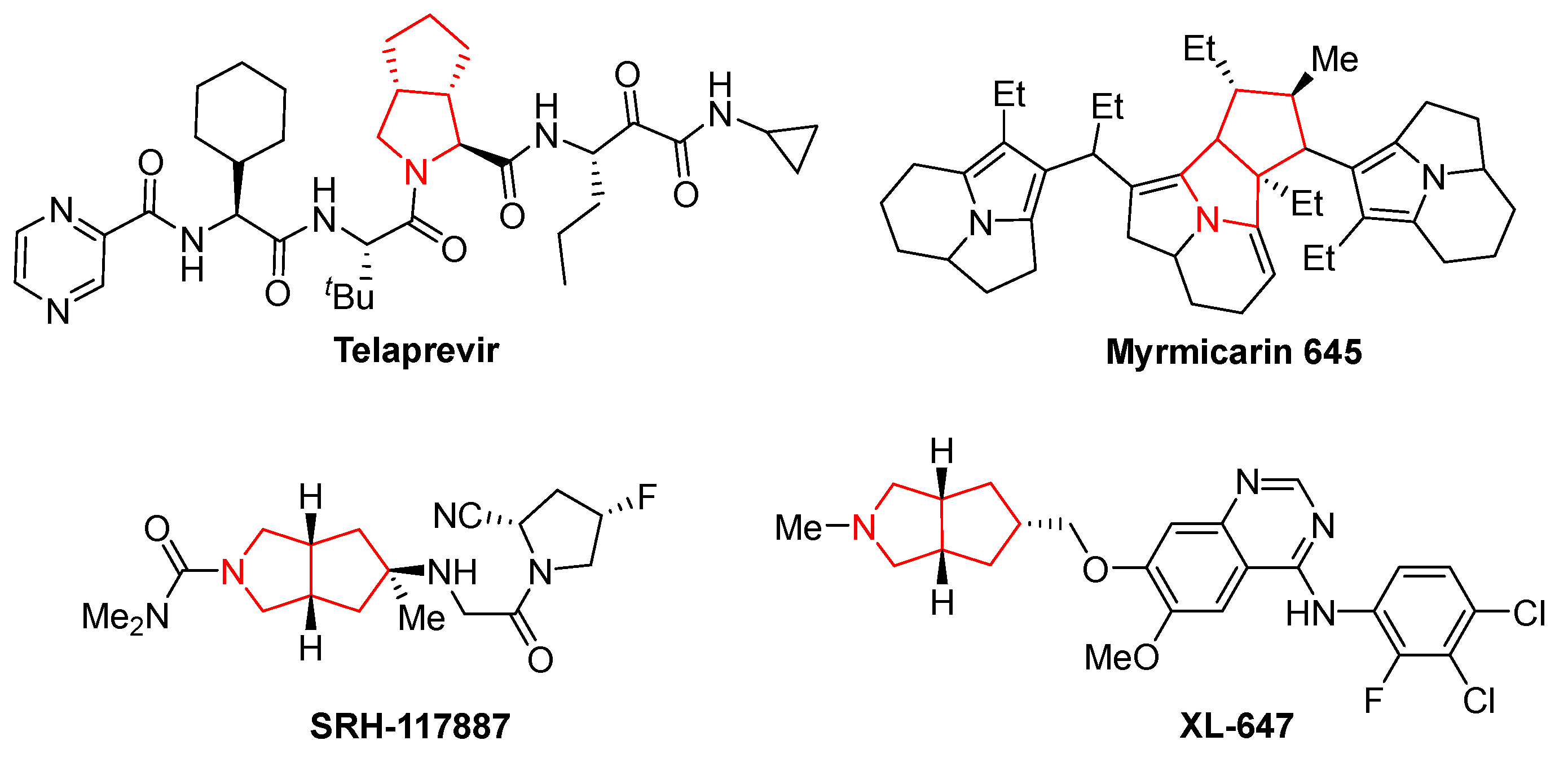
Desymmetric transformation of symmetrical molecules has long been considered one of the most powerful strategies for the construction of structurally diverse complex molecules with multiple stereogenic centers in organic synthesis, such as enzymatic desymmetrizations [1], organocatalytic desymmetrizations [2], and transition metal catalyzed desymmetrizations [3,4]. In addition, reviews on desymmetrization reactions about meso-diols [5], cyclohexanones [6,7], and cyclobutanones [8] are available. As a consequence, a wide variety of molecules possessing symmetries have been exploited for diverse desymmetrization reactions during the past several decades. For example, the desymmetrization of meso-cyclic ketones and kinetic resolution of racemic 2-arylcyclohexanones though Baeyer–Villiger oxidation was reported by the Feng group in 2012 [9]. The desymmetrization of cyclohexanones and their derivatives has been reported by several groups [10,11,12,13]. In 2013, Cu(I)-catalyzed azide–alkyne cycloaddition via asymmetric desymmetrization of oxindole-based 1,6-heptadiynes was reported by Zhou and coworkers [14]. The Cai group described copper-catalyzed intramolecular desymmetric aryl C-O coupling for the enantioselective construction of chiral dihydrobenzofurans and dihydrobenzopyrans [15]. The NHC-catalyzed desymmetrization of bisphenols to P-stereogenic phosphinates was reported by Chi and coworkers in 2015 [16]. Later, an iridium-catalyzed desymmetrization reaction involving allylic dearomatization of indoles was developed [17]. The desymmetrization of a centrosymmetric pseudo-para-diformyl[2.2]paracyclophane was realized by the group of Micouin [18]. In 2021, an unprecedented desymmetrization of meso-1,3-diones by enantioselective intermolecular condensation was presented by Zhao and coworkers [19]. Recently, the desymmetrization of oxetanes and silanediols have been respectively reported in [20,21]. Prochiral cyclopentene-1,3-diones, as readily available, inexpensive, and air-stable compounds, have proven to be ideal electrophiles in reactions with various nucleophiles via a desymmetrization process for the synthesis of highly functionalized 1,3-cyclopentanedione via the Michael addition process [22,23,24,25]. As an alternative, desymmetrization of cyclopentenediones via [3+2] or [4+2] cycloaddition has been reported for the synthesis of 1,3-cyclopentanediones [26,27,28,29]. However, an extensive literature search revealed that the related 1,3-dipolar cycloaddition reaction with cyclopentene-1,3-diones as electrophilic dipolarophiles has been little explored. In 2015, the Wang and Singh groups independently reported Ag(I)-catalyzed desymmetrization of prochiral cyclopentenediones via formal 1,3-dipolar cycloaddition with azomethine-ylide for the synthesis of highly functionalized 5,5-fused bicyclic pyrrolidine derivatives [30,31]. Later, using DIPEA as the base, the diastereoselective 1,3-dipolar cycloaddition reaction of cyclopentene-1,3-diones and N-phenacylbenzothiazolium bromides for the synthesis of tetracyclic products bearing five stereogenic centers was described by the group of Pan [32]. In 2018, an efficient kinetic resolution of readily available racemic cyclopentene-1,3-diones was developed via Ag(I)-catalyzed asymmetric 1,3-dipolar cycloaddition of azomethine ylides [33]. Notably, these 1,3-dipolar cycloaddition reactions of cyclopentenediones and azomethine-ylides could provide an efficient approach to accessing bicyclic heterocycles containing fused pyrrolidine and cyclopentane moieties. These are well-known structural cores of many biologically active natural products and pharmaceuticals, such as the HCV NS3-4A protease inhibitors Telaprevir [34] and SRH-117887 [35,36], the alkaloid Myrmicarin 645 [37], and the tyrosine kinase inhibitor XL-647 [38] (Figure 1). Considering the outstanding potential of such bicyclic heterocycles for drug research and development, it remains attractive to explore efficient and convenient means of synthesizing structurally diverse fused pyrrolidine–cyclopentane skeletons from readily available starting materials.
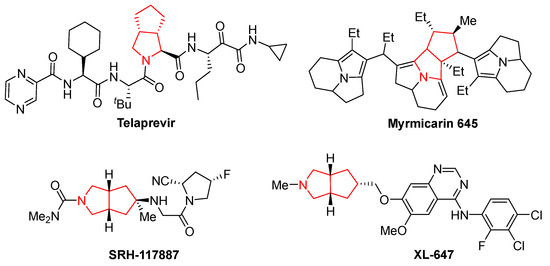
Figure 1.
Representative bioactive molecules containing fused pyrrolidine and cyclopentane moieties.
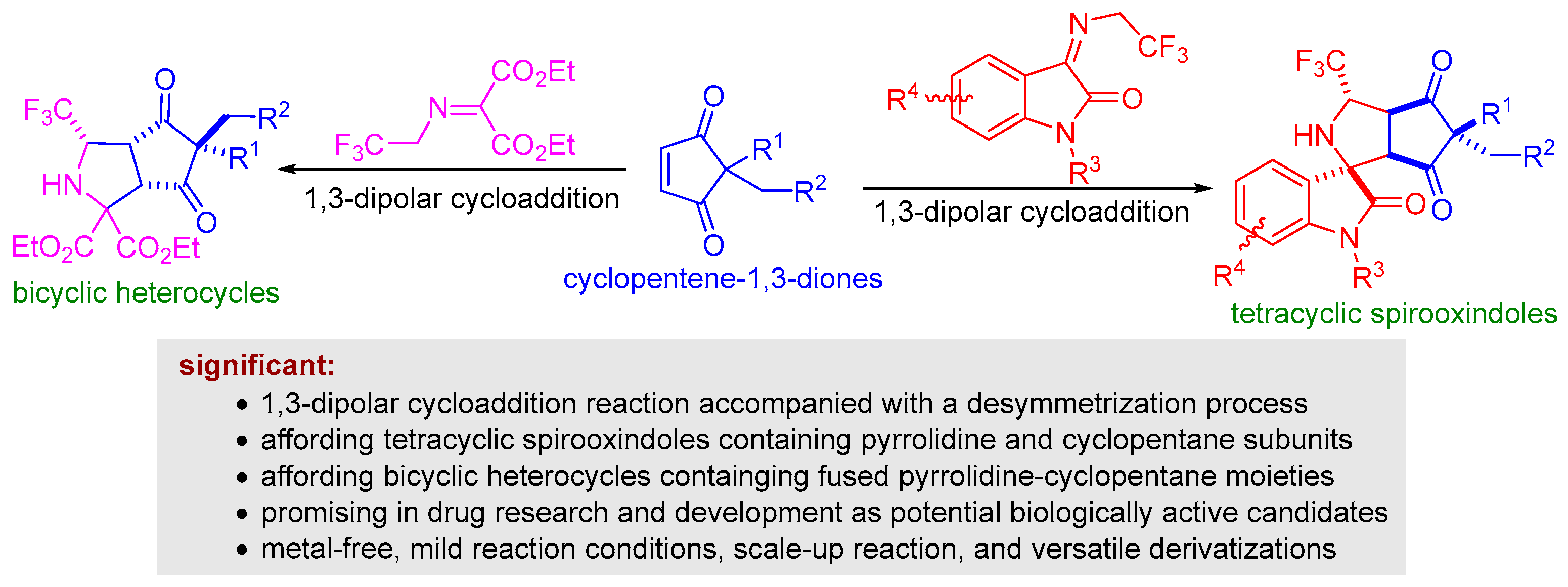
N-2,2,2-trifluoroethylisatin ketimines are one class of active azomethine-ylide precursors that are readily prepared from isatins and trifluoroethylamine by condensation reaction [39]. In recent years, a wide variety of 1,3-dipolar cycloadditions have been developed by using N-2,2,2-trifluoroethylisatin ketimines reacted with various dipolarophiles to synthesize CF3-containing spirocyclic oxindoles bearing multiple stereogenic centers [40,41]. As a continuation of our research interest in 1,3-dipolar cycloaddition for synthesis of polyheterocycles [42,43,44], we envisaged that a series of highly functional polyheterocycles fused by CF3-containing spirocyclic oxindole, pyrrolidine, and cyclopentane substructures could be assembled via the formal 1,3-dipolar cycloaddition reaction of N-2,2,2-trifluoroethylisatin ketimines and cyclopentene-1,3-diones under suitable conditions, and that this reaction would be capable of causing the desymmetrization transformation of cyclopentene-1,3-diones. (Scheme 1). Furthermore, we found that the 1,3-dipolar cycloaddition of trifluoromethyl-substituted iminomalonate and cyclopentene-1,3-diones is feasible through a desymmetrization process, leading to the formation of bicyclic heterocycles containing fused pyrrolidine-cyclopentane moieties (Scheme 1). The resulting novel tetracyclic spirooxindoles and bicyclic heterocycles were characterized by melting point, NMR, and HRMS. Herein, we report our latest results on this subject.
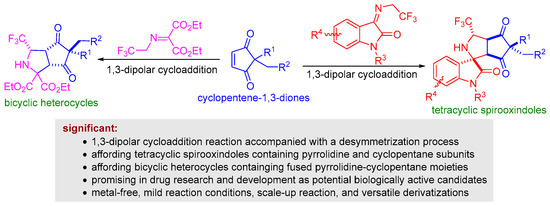
Scheme 1.
1,3-Dipolar cycloaddition reaction of trifluoroethyl amine-derived ketimines enabling the desymmetrization of cyclopentene-1,3-diones.
2. Results and Discussion
2.1. Optimization Studies
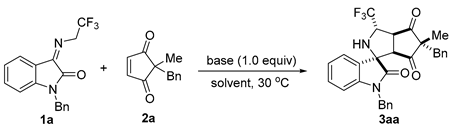
Initially, we selected the N-2,2,2-trifluoroethylisatin ketimine 1a and cyclopentene-1,3-dione 2a as model substrates for the optimization of reaction conditions. As shown in Table 1, to our delight, when using 1,1,3,3-tetramethylguanidine (TMG) as the base the reaction could proceed well in 1,2-dicholorethane (DCE) at 30 °C, providing the tetracyclic spirooxindole containing pyrrolidine and cyclopentane subunits 3aa in 73% yield with 81:19 dr (Table 1, entry 1). By screening different organic bases (Table 1, entry 2–4) such as 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), 1,5-diazabicyclo[4.3.0]non-5-ene (DBN), and 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD), we found that DBU was the best candidate and that the corresponding cycloaddition product 3aa could be obtained in 87% yield with good diastereoselectivity (Table 1, entry 2). In the presence of the inorganic base CS2CO3, the reaction took place but yielded the cycloaddition product 3aa with poor results (only 30% yield and 71:29 dr) after 96 h (Table 1, entry 5). Next, different solvents, including toluene, THF, MeCN, and MeOH, were investigated, and it was found that DCE was the optimal solvent in terms of yield and diastereoselectivity (Table 1, entry 2 vs. entries 6–10). We were pleased to find that changing the base from DBU to 1,4-diazabicyclo[2.2.2]octane (DABCO) in DCE could lead to better results (90% yield and 84:16 dr), despite increasing the reaction time to 48 h (Table 1, entry 12). Raising the reaction temperature to 50 °C delivered product 3aa in 90% yield and 84:16 dr with a reduction of the reaction time to 24 h (Table 1, entry 13). By altering the ratio of substrates 1a:2a from 1.2:1 to 1.5:1, the isolated yield of product 3aa could be slightly improved to 92% (Table 1, entry 14). Finally, the product 3aa was obtained in 96% yield with 85:15 dr at 50 °C for 24 h by increasing the amount of DABCO from 1.0 equivalent to 1.5 equivalents (Table 1, entry 15).

Table 1.
Optimization of reaction conditions [a].
2.2. Substrate Scope Studies
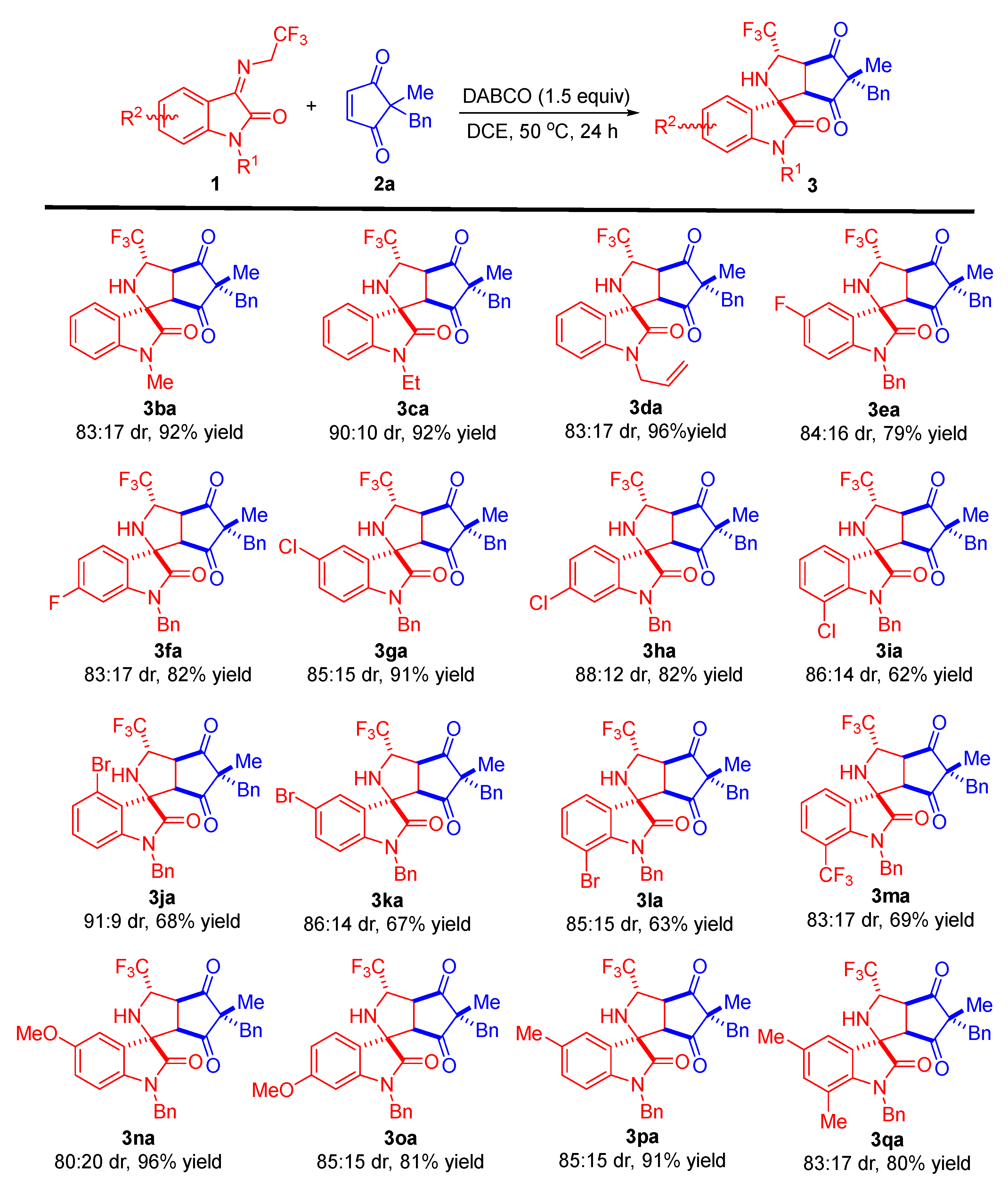
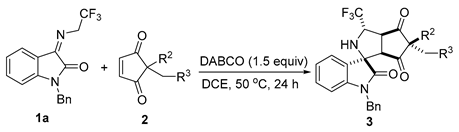
With the optimized reaction conditions in hand, we set out to investigate the substrate scope and generality of the metal-free diastereoselective formal 1,3-dipolar cycloaddition of different N-2,2,2-trifluoroethylisatin ketimines 1 and various cyclopentene-1,3-diones 2. First, the substrate scope of N-2,2,2-trifluoroethylisatin ketimines was explored; the results are shown in Scheme 2. The reaction system was applicable to different substituents (Me-, Et-, and allyl-) at the N1 position of the isatin-derived ketimines. These substrates were able to react smoothly with cyclopentene-1,3-dione 2a to afford the corresponding tetracyclic spirooxindoles 3ba–da in high yields and good diastereoselectivities (up to 96% yield and 90:10 dr). Furthermore, the introduction of an electron-withdrawing group, such as F-, Cl-, or Br-, into the oxindole skeleton of ketimines allowed these substrates to react effectively with 2a regardless of their different positions, resulting in the corresponding products 3ea–la in yields ranging from 62–91% with acceptable dr values. Moreover, the substrate with trifluoromethyl at the C7-position of the N-2,2,2-trifluoroethylisatin ketimine provided cycloaddition product 3ma in 69% yield with 83:17 dr. In addition, a variety of isatin-derived ketimines with electron-donating groups (-OMe and -Me) worked well, affording the corresponding products 3na–pa with good results (91–96% yields and 80:20–85:15 dr). Disubstituted substrate, such as 5,7-dimethyl-substituted trifluoroethylisatin ketimine, was successfully employed in this transformation under the standard conditions, generating product 3qa in 80% yield with 83:17 dr.
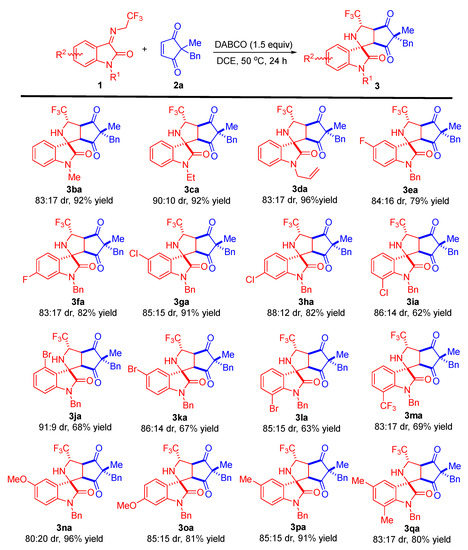
Scheme 2.
Substrate scope investigation of the metal-free diastereoselective formal 1,3-dipolar cycloaddition of different N-2,2,2-trifluoroethylisatin ketimines 1 and cyclopentene-1,3-dione 2a. Reaction conditions: the reactions were carried out with 1 (0.15 mmol), 2a (0.1 mmol), and 1.5 equivalents of DABCO in 1.0 mL of DCE at 50 °C for 24 h. The dr values were determined by 1H NMR analysis of the crude product. The yields refer to isolated yields as a mixture of diastereoisomers.
Next, a range of differently substituted cyclopentenediones 2 were examined by reaction with N-2,2,2-trifluoroethylisatin ketimine 1a to further explore the substrate scope of the 1,3-dipolar cycloaddition reaction (Table 2). With electron-donating groups such as methyl and methoxy on the phenyl moiety of 2-benzyl-2-methylcyclopentenediones 2, the reactions afforded cycloaddition products 3ab–ad in almost quantitative yields (94–96%), with good diastereoselectivities (up to 85:15 dr) in the reaction with N-2,2,2-trifluoroethylisatin ketimine 1a (Table 2, entries 1–3). Similarly, the reaction system showed good compatibility with cyclopentenediones with different electron-withdrawing groups (such as F-, Cl- and Br-) on the phenyl moiety, as demonstrated by the synthesis of cycloaddition products 3ae–ai in good to excellent yields and high diastereoselectivities (Table 2, entries 4–8). Meanwhile, when the aromatic ring of the benzyl group of 2 was substituted with bulky naphthyl groups, the reactions furnished the corresponding tetracyclic spirooxindoles 3aj and 3ak in 98% yield with 89:11 dr and 99% yield with 86:14 dr, respectively (Table 2, entries 9 and 10). In addition, the change of the R2 group of cyclopentenediones from methyl to ethyl was tolerated by the reaction system, providing efficient access to products 3al–an with acceptable results (up to 88% yield and 88:12 dr) (Table 2, entries 11–13).

Table 2.
Substrate scope investigation of metal-free diastereoselective 1,3-dipolar cycloaddition of N-2,2,2-trifluoroethylisatin ketimines 1a and diverse cyclopentene-1,3-diones 2 [a].
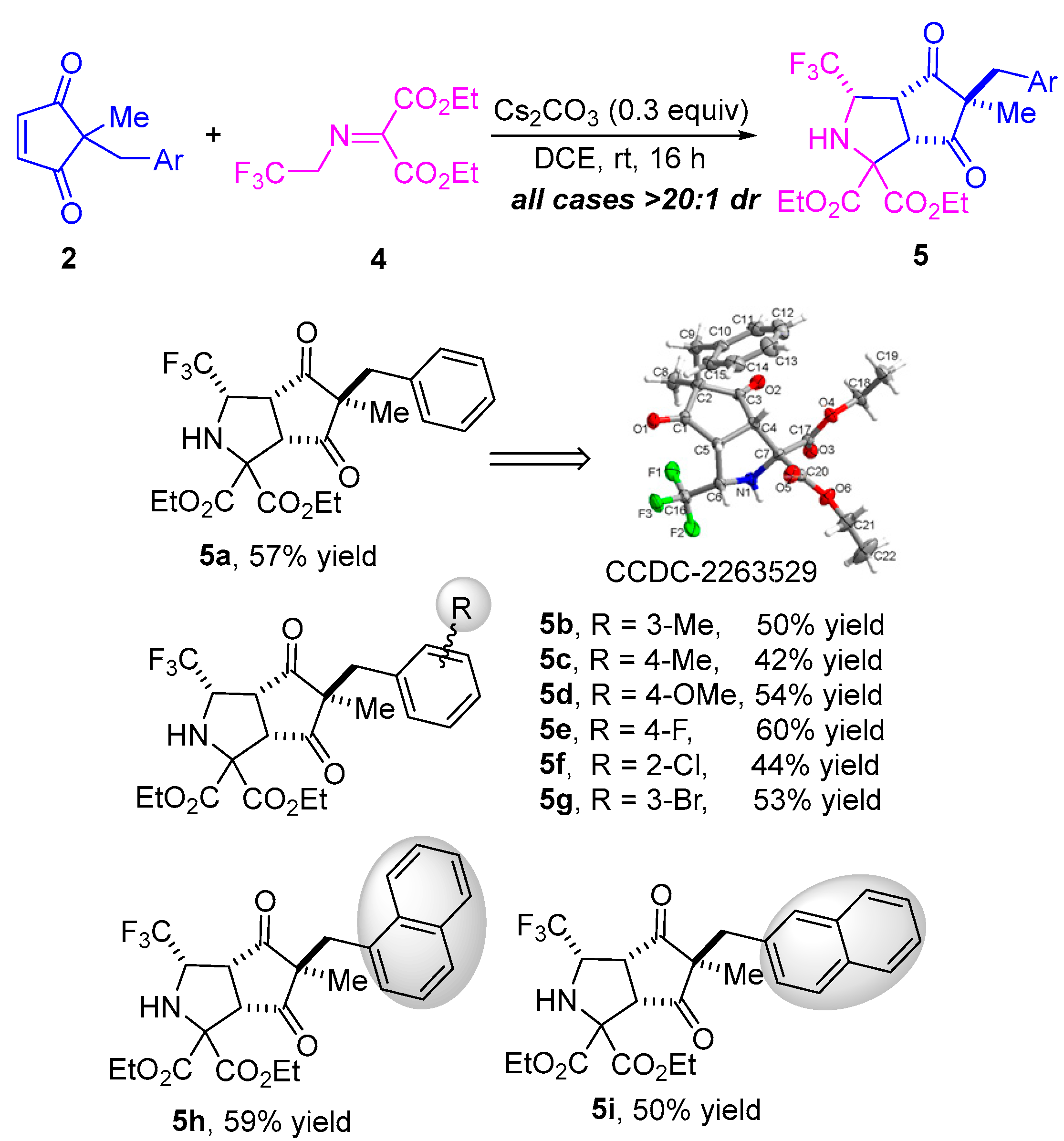
Having established the general scope of the metal-free diastereoselective formal 1,3-dipolar cycloaddition of N-2,2,2-trifluoroethylisatin ketimines 1 and diverse cyclopentene-1,3-diones 2, we next attempted the formal 1,3-dipolar cycloaddition of trifluoromethyl-substituted iminomalonate 4 and cyclopentenediones 2 through a desymmetrization strategy. As illustrated in Scheme 3, using Cs2CO3 as a catalyst in DCE at room temperature for 16 h, the reaction of 2-benzyl-2-methylcyclopentenedione 2a and trifluoromethyl-substituted iminomalonate 4 proceeded well, providing the desired bicyclic heterocyclic product 5a in 57% yield with >20:1 dr. The generality of this transformation of various cyclopentene-1,3-diones 2 and trifluoromethyl-substituted iminomalonate 4 was then investigated, and it was found that all reactions resulted in the corresponding cyclization products with excellent diastereoselectivities (all cases > 20:1 dr) (Scheme 3). As cyclopentenediones bearing different electron-donating substituents such as methyl and methoxyl on the phenyl, these substrates worked well in the reaction with trifluoromethyl-substituted iminomalonate 4 under the standard conditions, providing the desired cycloaddition products 5b–d in moderate yields (42–54% yield). Likewise, cyclopentenediones with various electron-withdrawing substituents (F-, Cl, and Br-) on the phenyl ring, regardless of the position, were compatible with the catalytic system in the reaction with 4, affording the desired cycloaddition products 5e–g in yields ranging from 44–60%. Cyclopentenedione substrates bearing a sterically hindered naphthyl group proceeded successfully as well, yielding the corresponding bicyclic heterocycle products 5h and 5i in 59% and 50% yield, respectively. The structure and the relative configuration of product 5a was unambiguously determined by single-crystal X-ray analysis, and the configurations of all other products in Scheme 3 were assigned by analogy due to their formation through a common reaction pathway [45].
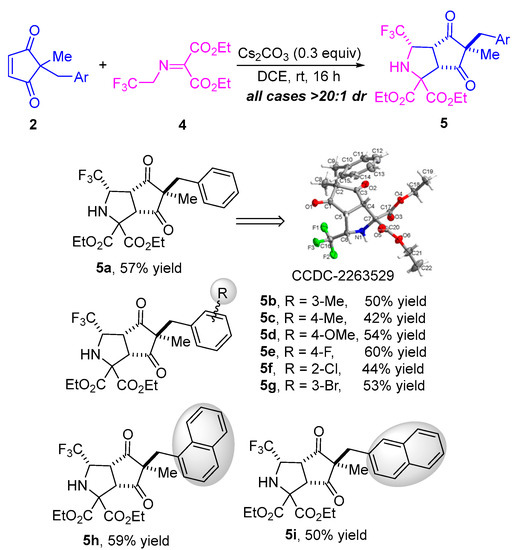
Scheme 3.
Substrate scope investigation of 1,3-dipolar cycloaddition of trifluoromethyl-substituted iminomalonate and different cyclopentenediones. The dr values were determined by 1H NMR analysis of the crude product. The yields refer to isolated yields as a mixture of diastereoisomers.
2.3. Scale-Up Experiment and Versatile Transformations of the Products
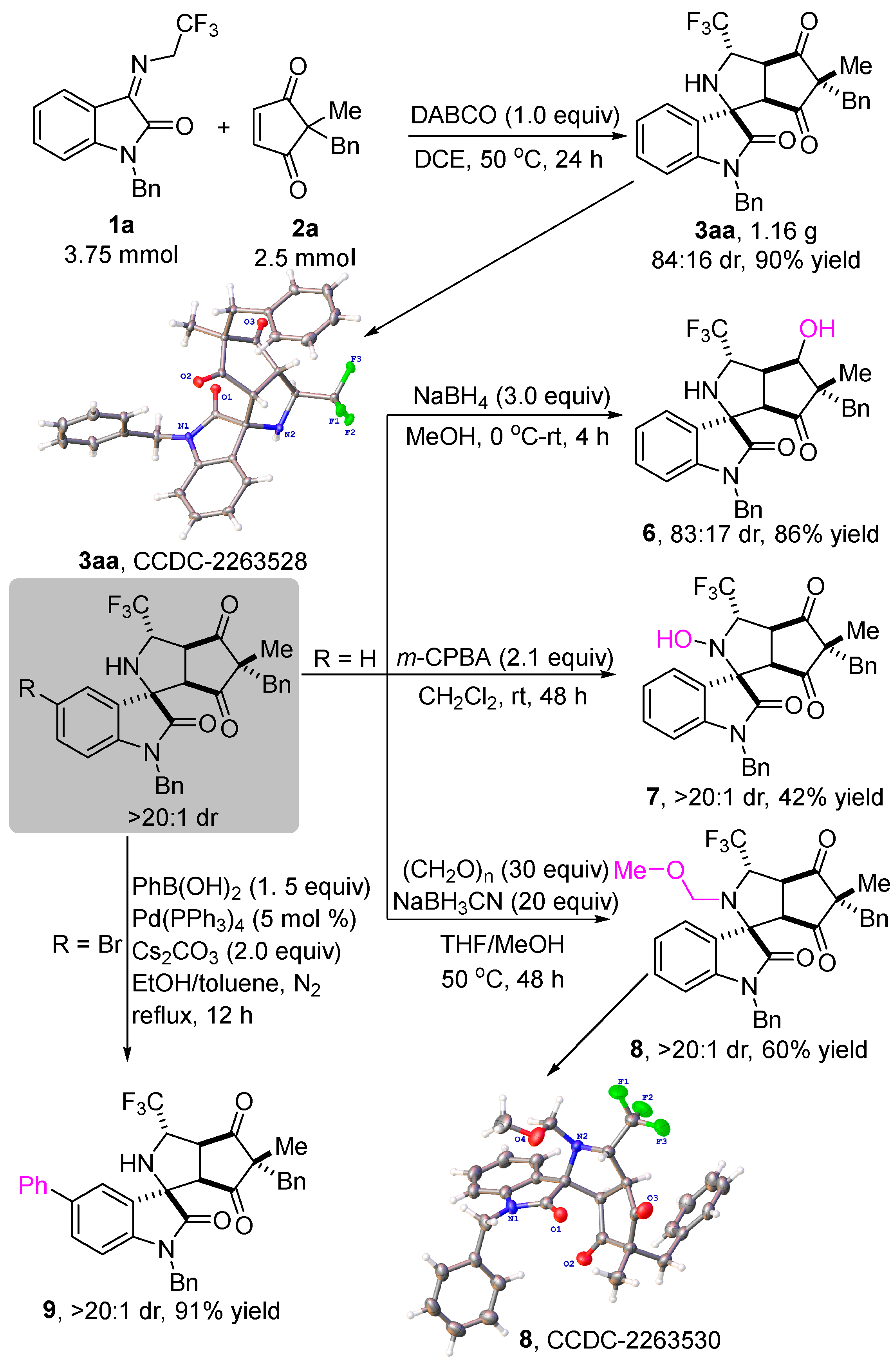
In order to demonstrate the synthetic potential and utility of the metal-free diastereoselective formal 1,3-dipolar cycloaddition of N-2,2,2-trifluoroethylisatin ketimines and cyclopentene-1,3-diones, a gram-scale experiment between 1a and 2a was carried out under standard reaction conditions, which was 25-fold larger than the scale of the model reaction. As shown in Scheme 4, the reaction was able to proceed to completion at 50 °C after 24 h in DCE, smoothly providing product 3aa in 90% yield with 84:16 dr. The structure and the relative configuration of the product 3aa were unambiguously determined by single-crystal X-ray analysis. Assuming a common reaction pathway, the relative configuration of the other products in Scheme 2 and Table 2 was assigned by analogy [45]. Next, various transformations of the products to other heterocyclic compounds were investigated. When 3aa was treated with NaBH4 in MeOH for 4 h, the reduction product 6 was obtained in 86% yield with 83:17 dr. The structure and relative configuration of product 6 were determined with certainty by NMR and NOESY spectra (see Supporting Information). The treatment of 3aa with m-CPBA in CH2Cl2 for 48 h gave rise to the formation of the hydroxylamine product 7 in 42% yield with >20:1 dr. In addition, product 8 was obtained with acceptable results from 3aa with (CH2O)n and NaBH3CN in the mixed solvents of MeOH and THF at 50 °C for 48 h. Notably, the structure of product 8 was unambiguously determined by single-crystal X-ray analysis [45]. Ultimately, the Suzuki coupling reaction between 3ka and phenylboronic acid was conducted with 5 mol % Pd(PPh3)4 as the catalyst to afford the product 9 in 91% yield with >20:1 dr.
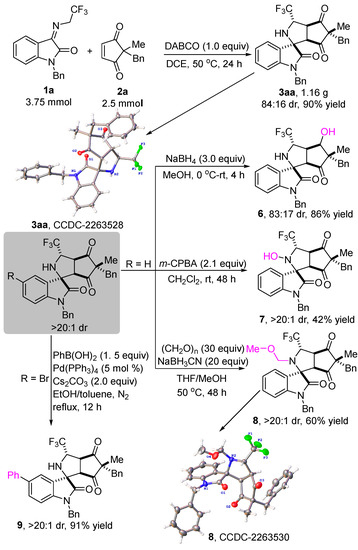
Scheme 4.
Scale-up experiment and different transformations of products.
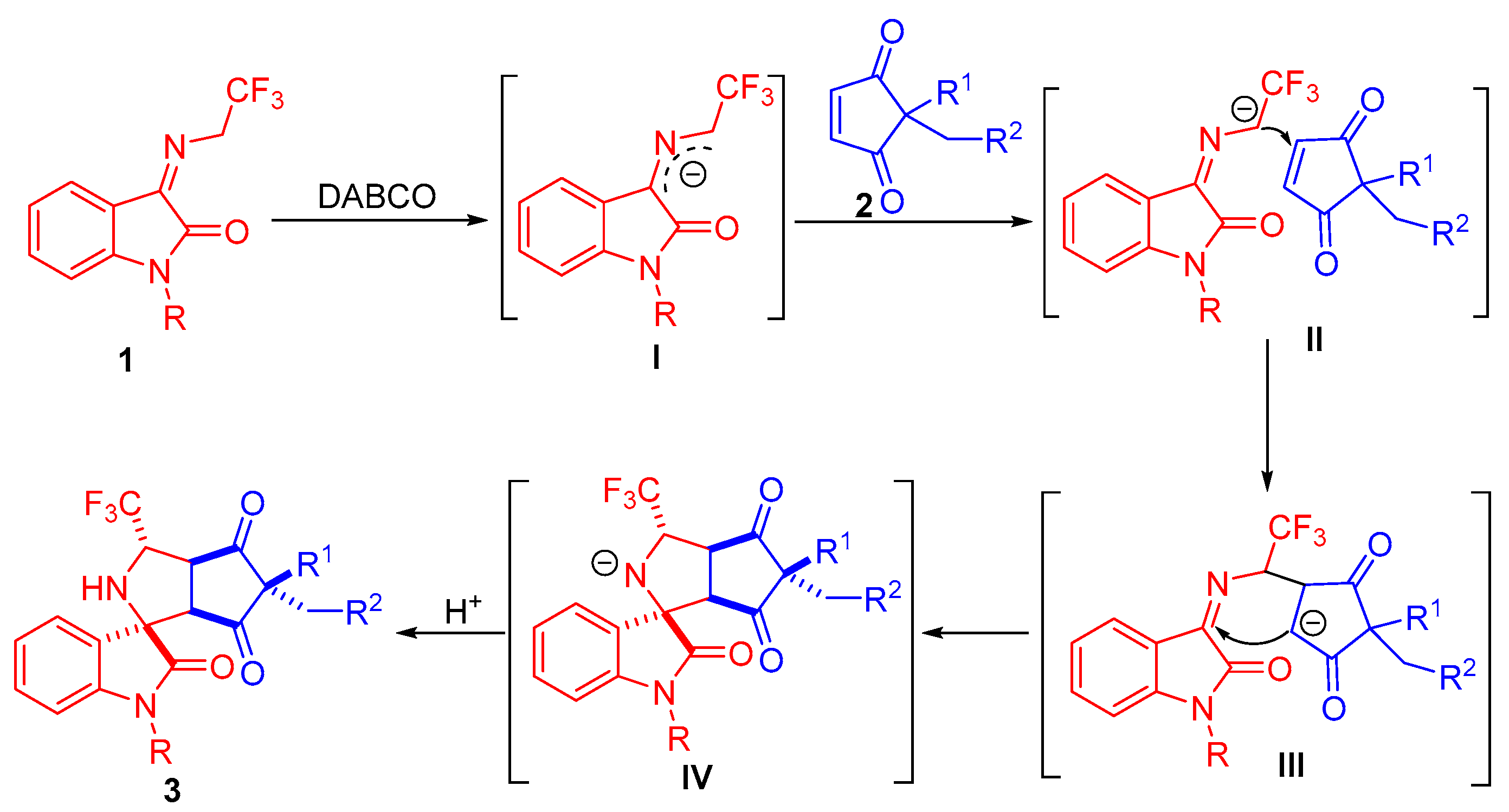
2.4. Proposed Mechanism for the 1,3-Dipolar Cycloaddition Reaction Accompanied by Desymmetrization Process
Based on our experimental results and previous related reports [30,31,32,33], a plausible catalytic mechanism involving a stepwise reaction process was assumed to explain the stereoselectivity of the 1,3-dipolar cycloaddition of N-2,2,2-trifluoroethylisatin ketimines and cyclopentene-1,3-diones. As depicted in Scheme 5, the N-2,2,2-trifluoroethylisatin ketimine 1 is deprotonated under a basic condition to yield the 2-azaallyl anion intermediate I. As illustrated in transition state II, the α-carbon anion of intermediate I approaches the carbon–carbon double bond of cyclopentene-1,3-diones, resulting in addition of the intermediate III. Subsequently, intramolecular cyclization from the carbon anion to the C=N of azomethine-ylide occurs, delivering the intermediate IV, which is protonated to form products 3.
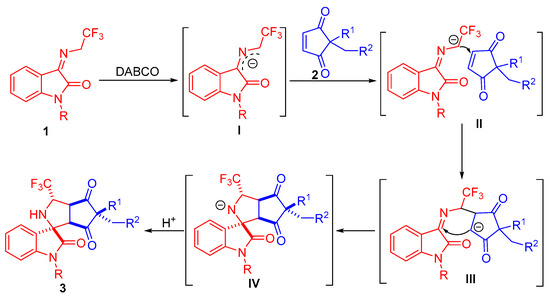
Scheme 5.
Proposed mechanism for 1,3-dipolar cycloaddition of N-2,2,2-trifluoroethylisatin ketimines and cyclopentene-1,3-diones.
3. Materials and Methods
3.1. General Information
Reagents were purchased from commercial sources and were used as received unless mentioned otherwise. Reactions were monitored by thin-layer chromatography (TLC). 1H NMR and 13C NMR spectra were recorded in CDCl3 and DMSO-d6. 1H NMR chemical shifts are reported in ppm relative to tetramethylsilane (TMS), with the solvent resonance employed as the internal standard (CDCl3 at 7.26 ppm, DMSO-d6 at 2.50 ppm). Data are reported as follows: chemical shift, multiplicity (s = singlet, br s = broad singlet, d = doublet, t = triplet, q = quartet, m = multiplet), coupling constants (Hz), and integration. 13C NMR chemical shifts are reported in ppm from tetramethylsilane (TMS), with the solvent resonance as the internal standard (CDCl3 at 77.20 ppm, DMSO-d6 at 39.52 ppm). Melting points of the products were recorded on a Büchi Melting Point B-545. The HRMS were recorded by The HRMS were recorded using an Agilent 6545 LC/Q-TOF mass spectrometer.
3.2. General Experimental Procedure for the 1,3-Dipolar Cycloaddition Reaction of N-2,2,2-Trifluoroethylisatin Ketimines and Cyclopentene-1,3-Diones for the Synthesis of Compounds 3 (Scheme 2 and Table 2)
N-2,2,2-trifluoroethylisatin ketimines 1 (0.3 mmol) and DABCO (33.6 mg, 0.3 mmol, 1.5 equiv.) were added to a solution of cyclopentene-1,3-diones 2 (40.2 mg, 0.2 mmol, 1.0 equiv.) in DCE (2.0 mL), then the mixture was stirred for 24 h at 50 °C. After completion, the reaction mixture was directly purified by flash chromatography on silica gel (petroleum ether/ethyl acetate, 15:1–10:1) to yield the corresponding products 3.
3aa, white solid; 99.5 mg, 96% yield; 85:15 dr, mp 194.4–195.3 °C; 1H NMR (400 MHz, DMSO-d6) δ 7.38 (d, J = 7.3 Hz, 1H), 7.35–7.19 (m, 9H), 7.11 (m, 1H), 7.07–7.01 (m, 2H), 6.74 (d, J = 7.8 Hz, 1H), 4.85 (d, J = 15.9 Hz, 1H), 4.66 (d, J = 5.6 Hz, 1H), 4.59 (d, J = 15.9 Hz, 1H), 4.44 (m, 1H), 4.04 (d, J = 3.1 Hz, 2H), 3.01 (d, J = 13.3 Hz, 1H), 2.85 (d, J = 13.3 Hz, 1H), 0.95 (s, 3H). 13C{1H} NMR (101 MHz, DMSO-d6) δ 212.8, 210.8, 178.0, 143.1, 135.5, 134.5, 130.2, 129.6, 128.6, 128.4, 128.08, 127.4, 127.2, 127.1, 125.6 (q, J = 276.8 Hz, 1C), 124.1, 123.1, 109.7, 70.9, 62.0, 58.7 (q, J = 30.4 Hz, 1C), 57.8, 51.3, 42.7, 41.4, 14.3. HRMS (ESI) m/z: [M+H]+ calcd. for C30H26F3N2O3, 519.1890, found: 519.1895
3.3. General Experimental Procedure for the 1,3-Dipolar Cycloaddition Reaction of Trifluoromethyl-Substituted Iminomalonate and Cyclopentene-1,3-Diones for the Synthesis of Compounds 5 (Scheme 3)
First, 2-((2,2,2-trifluoroethyl)imino) malonate 4 (0.1 mmol) and Cs2CO3 (31.8 mg, 0.09 mmol) were added to a solution of cyclopentene-1,3-diones 2 (60.2mg, 0.3 mmol, 3.0 equiv.) in DCE (3.0 mL). Then, the mixture was stirred at room temperature for 24 h. After completion, the reaction mixture was directly purified by flash chromatography on silica gel (petroleum ether/ethyl acetate = 15:1–10:1) to yield the corresponding products 5.
5a, white solid; 77.8 mg, 57% yield; >20:1 dr, mp 162.1–162.9 °C; 1H NMR (400 MHz, CDCl3) δ 7.25–7.12 (m, 3H), 6.98–6.86 (m, 2H), 4.26 (q, 2H), 4.21–4.06 (m, 2H), 3.63–3.50 (m, 1H), 3.08 (d, J = 10.1 Hz, 1H), 2.99 (d, J = 12.9 Hz, 1H), 2.86 (t, J = 9.7 Hz, 1H), 2.83–2.76 (m, 2H), 1.26 (t, J = 7.2 Hz, 3H), 1.20–1.12 (m, 6H). 13C{1H} NMR (101 MHz, CDCl3) δ 213.6, 212.9, 168.7, 166.2, 135.3, 129.6, 128.9 127.5, 123.6 (q, J = 280.5 Hz, 1C), 74.7, 63.0, 62.4, 61.4 (q, J = 32.6 Hz, 1C), 60.7, 56.2, 49.8, 44.2, 19.5, 13.9, 13.8. HRMS (ESI) m/z: [M+H]+ calcd. for C22H25F3NO6, 456.1628, found: 456.1635.
3.4. Procedure for the Scale-Up Experiment
N-2,2,2-trifluoroethylisatin ketimines 1a (1.2 g, 3.75 mmol, 1.5 equiv.) and DABCO (0.4 g, 3.75 mmol,1.5 equiv.) were added to a solution of cyclopentene-1,3-diones 2a (0.5 g, 2.5 mmol, 1.0 equiv.) in DCE (25.0 mL), then the mixture was stirred for 24 h at 50 °C. After completion, the reaction mixture was directly purified by flash chromatography on silica gel (petroleum ether/ethyl acetate = 15:1) to yield the corresponding product 3aa.
3.5. Procedure for the Synthesis of Compound 6
Compound 3aa (0.1 g, 0.2 mmol) was dissolved in MeOH (2.0 mL), then NaBH4 (22.7 mg, 3.0 equiv.) was added slowly at 0 °C. The reaction mixture was allowed to stir at room temperature for 4 h. The reaction system was monitored by TLC until 3aa disappeared completely. After that, the reaction mixture was quenched by a drop of water and directly purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 15:1) to provide compound 6 as a white solid (89.5 mg, 86% yield).
6, 1H NMR (300 MHz, DMSO-d6) δ 7.44–7.27 (m, 5H), 7.28–7.18 (m, 5H), 7.13 (dd, J = 7.5, 1.1 Hz, 1H), 7.11–7.01 (m, 2H), 6.79–6.70 (m, 1H), 5.58 (s, 1H), 4.92 (d, J = 15.9 Hz, 1H), 4.70 (d, J = 16.0 Hz, 1H), 4.63–4.50 (m, 1H), 3.95 (d, J = 11.6 Hz, 2H), 3.68 (ddd, J = 11.1, 6.3, 3.8 Hz, 2H), 2.86–2.69 (m, 2H), 0.90 (s, 3H). 13C NMR{1H} (75 MHz, CDCl3) δ 218.1, 180.1, 141.7, 136.2, 135.2, 132.5, 130.3, 129.1, 128.5, 127.9, 127.4, 127.3, 126.7 (q, J = 277.5 Hz, 1C),126.6, 123.88, 123.6, 110.0, 75.5, 70.8, 59.4, 59.3, 58.7 (q, J = 30.0 Hz, 1C), 44.2, 43.2, 41.2, 14.8. HRMS (ESI) m/z [M+H]+ calcd. for C30H28F3N2O3, 521.2047, found: 521.2046.
3.6. Procedure for the Synthesis of Compound 7
The solution of compound 3aa (0.1 g, 0.20 mmol) in DCM (2.0 mL) was stirred at room temperature in a sealed tube. Subsequently, m-CPBA (76.1 mg, 0.44 mmol) was added to the above solution. The reaction mixture was then allowed to stir for 48 h. The reaction mixture was quenched by the addition of NaHCO3 aq (15 mL) and diluted with EtOAc (15 mL). The organic layer was separated and the aqueous layer was extracted twice with EtOAc (2 × 15 mL). The combined organic layers were dried over Na2SO4. Subsequently, the organic phase was concentrated under reduced pressure. The crude product was purified by flash column chromatography on silica gel (petroleum ether/ethyl acetate = 15:1) to afford the desired product 7 as a white solid (44.9 mg, 42% yield).
7, 1H NMR (300 MHz, CDCl3) δ 7.37–7.24 (m, 7H), 7.24–7.14 (m, 3H), 7.07 (m, J = 7.6, 1.0 Hz, 1H), 7.04–6.93 (m, 2H), 6.60 (d, J = 7.8 Hz, 1H), 5.07 (s, 1H), 4.76 (s, 2H), 4.60 (m, J = 6.2 Hz, 1H), 3.00 (d, J = 11.9 Hz, 1H), 2.93 (d, J = 12.9 Hz, 1H), 2.84 (d, J = 12.9 Hz, 1H), 2.46 (dd, J = 11.9, 6.0 Hz, 1H), 1.27 (s, 3H). 13C NMR{1H} (75 MHz, CDCl3) δ 214.2, 211.2, 174.7, 144.3, 134.9, 134.6, 130.8, 129.5, 129.1, 128.9, 128.2, 127.8, 127.2, 126.6, 125.8, 124.9 (q, J = 277.2 Hz, 1C), 124.2, 123.6, 110.1, 65.6 (q, J = 30.5 Hz, 1C), 62.3, 54.3, 48.2, 46.4, 43.8, 18.2. HRMS (ESI) m/z [M+H]+ calcd. for C30H26F3N2O4, 535.1839, found: 535.1845.
3.7. Procedure for the Synthesis of Compound 8
Compound 3aa (0.10 g, 0.20 mmol) and polyformaldehyde (0.5 g, 30.0 equiv.) were dissolved in the mixture solvents MeOH and THF (1:1, 4.0 mL). Then, NaBH3CN (0.3 g, 20.0 equiv.) was added to the mixture at room temperature for 30 min. The reaction mixture was allowed to stir for 72 h at 50 °C. After completion, the reaction mixture was quenched by the addition of 1.0 M NaOH (10.0 mL). The aqueous layer was extracted with DCM (2 × 10.0 mL). The combined organic layers were dried over anhydrous Na2SO4. After filtration, the solution was concentrated under reduced pressure and the resulting crude mixture was purified by hexane beating twice to provide compound 8 as a white solid (100.1 mg, 89% yield).
8, 1H NMR (400 MHz, CDCl3) δ 7.31 (m, 5H), 7.27 (d, J = 6.5 Hz, 3H), 7.22 (d, J = 7.8 Hz, 2H), 7.08 (m, 1H), 7.04–6.96 (m, 2H), 6.65 (d, J = 7.8 Hz, 1H), 4.72 (s, 3H), 4.06 (d, J = 11.0 Hz, 1H), 3.73 (d, J = 11.0 Hz, 1H), 3.13 (d, J = 10.2 Hz, 1H), 2.99 (s, 3H), 2.96 (d, J = 12.7 Hz, 1H), 2.84 (d, J = 12.7 Hz, 1H), 2.60 (dd, J = 12.0, 4.0 Hz, 1H), 1.28 (s, 3H). 13C NMR{1H} (101 MHz, CDCl3) δ 214.5, 211.0, 177.5, 144.0, 135.3, 134.9, 130.6, 129.5, 129.0, 128.9, 128.1, 127.8, 127.4, 126.1, 125.7(q, J = 304.0 Hz, 1C),124.4, 123.6, 110.0, 79.2, 73.8, 63.3, 60.6(q, J = 30.0 Hz, 1C), 58.5, 56.4, 52.1, 46.4, 43.9, 17.7. HRMS (ESI) m/z [M+H]+ calcd. for C32H30F3N2O4, 563.2152, found: 563.2163.
3.8. Procedure for the Synthesis of Compound 9
To an oven-dried Schlenk tube, 3ak (119.2 mg, 0.20 mmol), PhB(OH)2 (36.6 mg, 0.30mmol), and Cs2CO3 (130.4 mg, 0.40 mmol) were combined and then the mixture solvents ethanol (0.4 mL) and toluene (2.0 mL) were added to the reaction tube. The reaction mixture was stirred at 120 °C under N2 atmosphere for 12 h. The heat source was an oil bath. When the mixture was cooled to room temperature, the brine (10 mL) was added to quench the reaction. The aqueous layer was extracted with DCM (2 × 10.0 mL) and the combined organic layers were dried over anhydrous Na2SO4. After filtration, the organic phase was concentrated and purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 15:1) to afford product 9 as a white solid (108.1 mg, 91% yield).
9, 1H NMR (400 MHz, DMSO-d6) δ 7.64–7.53 (m, 2H), 7.46 (m, 3H), 7.43–7.39 (m, 1H), 7.39–7.37 (m, 1H), 7.36–7.23 (m, 8H), 7.13–6.95 (m, 3H), 4.93 (d, J = 15.9 Hz, 1H), 4.73 (d, J = 15.9 Hz, 1H), 4.69 (d, J = 5.6 Hz, 1H), 4.52–4.38 (m, 1H), 4.06 (d, J = 3.9 Hz, 2H), 3.02 (d, J = 13.3 Hz, 1H), 2.87 (d, J = 13.3 Hz, 1H), 0.96 (s, 3H). 13C NMR{1H} (101 MHz, DMSO-d6) δ 212.8, 210.8, 178.2, 143.9, 142.0, 139.9, 135.6, 134.5, 130.2, 129.0, 128.4, 128.1, 127.8, 127.7, 127.3, 127.3, 127.2, 126.7, 125.6 (q, J = 276.4 Hz, 1C), 124.6, 121.7, 108.1, 70.8, 62.0, 58.7 (q, J = 31.0 Hz, 1C)57.8, 51.3, 42.7, 41.5, 14.3. HRMS (ESI) m/z [M+H]+ calcd. for C36H30F3N2O3, 595.2203 found: 595.2209
4. Conclusions
In conclusion, we have developed a highly diastereoselective formal 1,3-dipolar cycloaddition reaction of N-2,2,2-trifluoroethylisatin ketimines and cyclopentene-1,3-diones that affords a series of tetracyclic spirooxindoles with good results (up to 96% yield and 91:9 dr). This reaction can lead to the desymmetrization of cyclopentene-1,3-diones, and provides an efficient method for constructing tetracyclic spirooxindoles containing fused pyrrolidine-cyclopentane subunits, which could be useful in the development of new pharmaceuticals. In addition, this 1,3-dipolar cycloaddition reaction could be extended to trifluoromethyl-substituted iminomalonate for the synthesis of bicyclic heterocycles bearing fused pyrrolidine-cyclopentane moieties in moderate yields with >20:1 dr under mild conditions. The potential applications of the protocol have been demonstrated by performing the gram-scale reaction and versatile transformations of the products. We believe that these novel compounds based on fused pyrrolidine–cyclopentane scaffolds can provide novel therapeutic agents and useful biological tools for the research and development of new drugs. Efforts toward the development of a catalytic asymmetric version of this 1,3-dipolar cycloaddition reaction and the application of this strategy to synthesize more promising drug discovery candidates, as well as the biological evaluation of these compounds, are currently underway in our laboratory.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28145372/s1, Characterization data for obtained products; X-ray data for products 3aa, 5a and 8; copies of 1H, 13C NMR and HRMS spectra. References [31,39,46,47] are cited in the supplementary materials.
Author Contributions
Conceptualization, J.-Q.Z. and W.-C.Y.; methodology, L.-Q.L.; investigation, L.-Q.L., Y.-P.Z., Y.Y., Z.-H.W., Z.-Z.G. and M.-Q.Z.; writing—original draft preparation, J.-Q.Z. and W.-C.Y.; writing—review and editing, J.-Q.Z., M.-Q.Z. and W.-C.Y.; supervision, M.-Q.Z. and W.-C.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of China, grant number 22271027, 22171029 and 21901024; the Sichuan Science and Technology Program, grant number 2021YFS0315 and 2023NSFSC1080; and the Talent Program of Chengdu University, grant number 2081919035, 2081921038.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the required data are reported in the manuscript and Supplementary Materials.
Acknowledgments
This work was performed using the equipment of Chengdu University and Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References and Note
- García-Urdiales, E.; Alfonso, I.; Gotor, V. Enantioselective Enzymatic Desymmetrizations in Organic Synthesis. Chem. Rev. 2005, 105, 313–354. [Google Scholar] [CrossRef] [PubMed]
- Borissov, A.; Davies, T.Q.; Ellis, S.R.; Fleming, T.A.; Richardson, M.S.W.; Dixon, D.J. Organocatalytic enantioselective desymmetrisation. Chem. Soc. Rev. 2016, 45, 5474–5540. [Google Scholar] [CrossRef] [PubMed]
- Shu, T.; Cossy, J. Asymmetric desymmetrization of alkene-, alkyne- and allene-tethered cyclohexadienones using transition metal catalysis. Chem. Soc. Rev. 2021, 50, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.Y.; Han, T.; Huang, E.H.; Ye, L.W. Research Progress on Enantioselective Desymmetrization Reactions Involving Metal Carbenes. Chin. J. Org. Chem. 2023, 42, 3295–3301. [Google Scholar] [CrossRef]
- Enríquez-García, Á.; Kündig, E.P. Desymmetrisation of meso-diols mediated by non-enzymatic acyl transfer catalysts. Chem. Soc. Rev. 2012, 41, 7803–7831. [Google Scholar] [CrossRef]
- Zeng, X.-P.; Cao, Z.-Y.; Wang, Y.-H.; Zhou, F.; Zhou, J. Catalytic Enantioselective Desymmetrization Reactions to All-Carbon Quaternary Stereocenters. Chem. Rev. 2016, 116, 7330–7396. [Google Scholar] [CrossRef]
- Nimmagadda, S.K.; Mallojjala, S.C.; Woztas, L.; Wheeler, S.E.; Antilla, J.C. Enantioselective Synthesis of Chiral Oxime Ethers: Desymmetrization and Dynamic Kinetic Resolution of Substituted Cyclohexanones. Angew. Chem. Int. Ed. 2017, 56, 2454–2458. [Google Scholar] [CrossRef]
- Sietmann, J.; Wahl, J.M. Enantioselective Desymmetrization of Cyclobutanones: A Speedway to Molecular Complexity. Angew. Chem. Int. Ed. 2020, 59, 6964–6974. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, X.; Ji, J.; Zhang, Y.; Hu, X.; Lin, L.; Feng, X. Enantioselective Baeyer–Villiger Oxidation: Desymmetrization of Meso Cyclic Ketones and Kinetic Resolution of Racemic 2-Arylcyclohexanones. J. Am. Chem. Soc. 2012, 134, 17023–17026. [Google Scholar] [CrossRef]
- Ren, L.; Lei, T.; Gong, L.-Z. Brønsted acid-catalyzed enantioselective Friedländer condensations: Achiral amine promoter plays crucial role in the stereocontrol. Chem. Commun. 2011, 47, 11683–11685. [Google Scholar] [CrossRef]
- Müller, S.; Webber, M.J.; List, B. The Catalytic Asymmetric Fischer Indolization. J. Am. Chem. Soc. 2011, 133, 18534–18537. [Google Scholar] [CrossRef]
- Gu, Q.; You, S.-L. Desymmetrization of cyclohexadienones viacinchonine derived thiourea-catalyzed enantioselective aza-Michael reaction and total synthesis of (-)-Mesembrine. Chem. Sci. 2011, 2, 1519–1522. [Google Scholar] [CrossRef]
- Jadhav, S.B.; Chegondi, R. Diastereoselective Desymmetrization of p-Quinamines through Regioselective Ring Opening of Epoxides and Aziridines. Org. Lett. 2020, 21, 10115–10119. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Tan, C.; Tang, J.; Zhou, J. Asymmetric Copper(I)-Catalyzed Azide–Alkyne Cycloaddition to Quaternary Oxindoles. J. Am. Chem. Soc. 2013, 135, 10994–10997. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, Y.; Zhang, S.; Cai, Q. Copper-Catalyzed Intramolecular Desymmetric Aryl C-O Coupling for the Enantioselective Construction of Chiral Dihydrobenzofurans and Dihydrobenzopyrans. Angew. Chem. Int. Ed. 2015, 54, 8805–8808. [Google Scholar] [CrossRef]
- Huang, Z.J.; Huang, X.; Li, B.S.; Mou, C.L.; Yang, S.; Song, B.A.; Chi, Y.R. Access to P-Stereogenic Phosphinates via N-Heterocyclic Carbene-Catalyzed Desymmetrization of Bisphenols. J. Am. Chem. Soc. 2016, 138, 7524–7527. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, C.; You, S.-L. Iridium-Catalyzed Asymmetric Allylic Dearomatization by a Desymmetrization Strategy. Angew. Chem. Int. Ed. 2017, 56, 15093–15097. [Google Scholar] [CrossRef]
- Delcourt, M.-L.; Felder, S.; Benedetti, E.; Micouin, L. Highly Enantioselective Desymmetrization of Centrosymmetric pseudo-para-Diformyl [2.2]paracyclophane via Asymmetric Transfer Hydrogenation. ACS Catal. 2018, 8, 6612–6616. [Google Scholar] [CrossRef]
- Yang, B.M.; Dai, J.; Luo, Y.; Lau, K.K.; Lan, Y.; Shao, Z.; Zhao, Y. Desymmetrization of 1,3-Diones by Catalytic Enantioselective Condensation with Hydrazine. J. Am. Chem. Soc. 2021, 143, 4179–4186. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, M.; Yan, Q.; Lin, X.; Li, X.; Fang, X.; Sung, H.H.Y.; Williams, I.D.; Sun, J.W. Asymmetric Synthesis of Pyrrolidines via Oxetane Desymmetrization. Org. Lett. 2022, 24, 2359–2364. [Google Scholar] [CrossRef]
- Gao, J.; Mai, P.-L.; Ge, Y.; Yuan, W.; Li, Y.; He, C. Copper-Catalyzed Desymmetrization of Prochiral Silanediols to Silicon-Stereogenic Silanols. ACS Catal. 2022, 12, 8476–8483. [Google Scholar] [CrossRef]
- Zhi, Y.; Zhao, K.; Wang, A.; Englert, U.; Raabe, G.; Enders, D. Asymmetric Synthesis of Cyclopentane-Substituted Oxindoles via Organocatalytic Desymmetrization of Cyclopent-4-ene-1,3-diones. Adv. Synth. Catal. 2017, 359, 1867–1871. [Google Scholar] [CrossRef]
- Vetica, F.; Bailey, S.; Chauhan, P.; Turberg, M.; Ghaur, A.; Raabe, G.; Enders, D. Desymmetrization of Cyclopentenediones via Organocatalytic Cross-Dehydrogenative Coupling. Adv. Synth. Catal. 2017, 359, 3729–3734. [Google Scholar] [CrossRef]
- Liang, H.; Zhou, X.; Zheng, L.; Wang, J. Enantioselective Organocatalytic Desymmetrization of Cyclopentene-1,3-diones through Formal C(sp(2))-H Amidation. J. Org. Chem. 2019, 84, 11306–11315. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.; Zhu, Z.; Fan, Y.; Chen, X.; Song, X. Chiral Phosphoric Acid Catalyzed Desymmetrization of Cyclopentendiones via Friedel-Crafts Conjugate Addition of Indolizines. Org. Lett. 2022, 23, 9548–9553. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-M.; Zhang, J.-Q.; Sun, B.-B.; Chen, J.-B.; Yu, J.-Q.; Yang, X.-P.; Lv, H.-P.; Wang, Z.; Wang, X.-W. Chiral N-Heterocyclic-Carbene-Catalyzed Cascade Asymmetric Desymmetrization of Cyclopentenediones with Enals: Access to Optically Active 1,3-Indandione Derivatives. Org. Lett. 2019, 21, 8582–8586. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Kim, H.Y.; Oh, K. Silver-Catalyzed Asymmetric Desymmetrization of Cyclopentenediones via [3+2] Cycloaddition with alpha-Substituted Isocyanoacetates. Org. Lett. 2018, 20, 2249–2252. [Google Scholar] [CrossRef]
- Zhou, H.-Q.; Gu, X.-W.; Zhou, X.-H.; Li, L.; Ye, F.; Yin, G.-W.; Xu, Z.; Xu, L.-W. Enantioselective palladium-catalyzed C(sp2)–C(sp2) s bond activation of cyclopropenones by merging desymmetrization and (3 + 2) spiroannulation with cyclic 1,3-diketones. Chem. Sci. 2021, 12, 13737–13743. [Google Scholar] [CrossRef]
- Bhajammanavar, V.; Mallik, S.; Choutipalli, V.S.K.; Subramanian, V.; Baidya, M. Diastereoselective access to [4,4]-carbospirocycles: Governance of thermodynamic enolates with an organocatalyst in vinylogous cascade annulation. Chem. Commun. 2022, 58, 2188–2191. [Google Scholar] [CrossRef]
- Liu, H.-C.; Liu, K.; Xue, Z.-Y.; He, Z.-L.; Wang, C.-J. Silver(I)-Catalyzed Enantioselective Desymmetrization of Cyclopentenediones: Access to Highly Functionalized Bicyclic Pyrrolidines. Org. Lett. 2015, 17, 5440–5443. [Google Scholar] [CrossRef]
- Das, T.; Saha, P.; Singh, V.K. Silver(I)−Ferrophox Catalyzed Enantioselective Desymmetrization of Cyclopentenedione: Synthesis of Highly Substituted Bicyclic Pyrrolidines. Org. Lett. 2015, 17, 5088–5091. [Google Scholar] [CrossRef]
- Sahoo, S.C.; Joshi, M.; Pan, S.C. Diastereoselective Desymmetrization of Prochiral Cyclopentenediones via Cycloaddition Reaction with N-Phenacylbenzothiazolium Bromides. J. Org. Chem. 2017, 82, 12763–12770. [Google Scholar] [CrossRef]
- Liu, H.-C.; Wei, L.; Huang, R.; Tao, H.-Y.; Cong, H.; Wang, C.-J. Ag(I)-Catalyzed Kinetic Resolution of Cyclopentene- 1,3-diones. Org. Lett. 2018, 20, 3482–3486. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.; Kauffman, R.S.; Beaumont, M.; van Heeswijk, R.P.G. Telaprevir: Pharmacokinetics and drug interactions. Antivir. Ther. 2012, 17, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, L.-N.; Leng, Y. Acute and chronic administration of SHR117887, a novel and specific dipeptidyl peptidase-4 inhibitor, improves metabolic control in diabetic rodent models. Acta Pharmacol. Sin. 2012, 33, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, H.; Hiratate, A.; Takahashi, M.; Saito-Hori, M.; Munetomo, E.; Kitano, K.; Saito, H.; Takaoka, Y.; Yamamoto, K. Synthesis and Structure–Activity Relationships of Potent 1-(2-Substituted-aminoacetyl)-4-fluoro-2-cyanopyrrolidine Dipeptidyl Peptidase IV Inhibitors. Chem. Pharm. Bull. 2008, 56, 1110–1117. [Google Scholar] [CrossRef]
- Schröder, F.; Sinnwell, V.; Baumann, H.; Kaib, M.; Francke, W. Myrmicarin 663: A New Decacylic Alkaloid from Ants. Angew. Chem. Int. Ed. 1997, 36, 77–80. [Google Scholar] [CrossRef]
- Pietanza, M.C.; Lynch, T.J., Jr.; Lara, P.N., Jr.; Cho, J.; Yanagihara, R.H.; Vrindavanam, N.; Chowhan, N.M.; Gadgeel, S.M.; Pennell, N.A.; Funke, R.; et al. XL647—A multitargeted tyrosine kinase inhibitor: Results of a phase II study in subjects with non-small cell lung cancer who have progressed after responding to treatment with either gefitinib or erlotinib. Thorac. Oncol. 2012, 7, 219–226. [Google Scholar] [CrossRef]
- Ma, M.-X.; Zhu, Y.-Y.; Sun, Q.-T.; Li, X.-Y.; Su, J.-H.; Zhao, L.; Zhao, Y.-Y.; Qiu, S.; Yan, W.-J.; Wang, K.-R.; et al. The asymmetric synthesis of CF3-containing spiro[pyrrolidin-3,2′-oxindole] through the organocatalytic 1,3-dipolar cycloaddition reaction. Chem. Commun. 2015, 51, 8789–8793. [Google Scholar] [CrossRef]
- Gui, H.-Z.; Wei, Y.; Shi, M. Recent Advances in the Construction of Trifluoromethyl-Containing Spirooxindoles through Cycloaddition Reactions. Chem. Asian J. 2020, 15, 1225–1233. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, C.; Chen, L.; Xie, H.; Liu, B.; Liu, D. Recent Advances in Catalytic Asymmetric Reactions involving Trifluoroethyl Ketimines. Chin. J. Org. Chem. 2020, 40, 1789–1803. [Google Scholar] [CrossRef]
- Zhao, J.-Q.; Zhou, S.; Yang, L.; Du, H.-Y.; You, Y.; Wang, Z.-H.; Zhou, M.-Q.; Yuan, W.-C. Catalytic Asymmetric Dearomative 1,3-Dipolar Cycloaddition of 2-Nitrobenzothiophenes and Isatin-Derived Azomethine Ylides. Org. Lett. 2021, 23, 8600–8605. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Liu, J.-H.; Zhang, Y.-P.; Zhao, J.-Q.; You, Y.; Zhou, M.-Q.; Han, W.-Y.; Yuan, W.-C. Cu-Catalyzed Asymmetric 1,3-Dipolar Cycloaddition of N-2,2,2-Trifluoroethylisatin Ketimines Enables the Desymmetrization of N-Arylmaleimides: Access to Enantioenriched F3C-Containing Octahydropyrrolo[3,4-c]pyrroles. Org. Lett. 2022, 24, 4050–4057. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.-C.; Yang, L.; Zhao, J.-Q.; Du, H.-Y.; Wang, Z.-H.; You, Y.; Zhang, Y.-P.; Liu, J.; Zhang, W.; Zhou, M.-Q. Copper-Catalyzed Umpolung of N-2,2,2-Trifluoroethylisatin Ketimines for the Enantioselective 1,3-Dipolar Cycloaddition with Benzo[b]thiophene Sulfones. Org. Lett. 2022, 24, 4603–4608. [Google Scholar] [CrossRef] [PubMed]
- CCDC-2263528 (3aa), -2263529 (5a), -2263530 (8) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Center.
- Ryu, H.; Seo, J.; Ko H., M. Synthesis of Spiro[oxindole-3,2′-pyrrolidine] Derivatives from Benzynes and Azomethine Ylides through 1,3-Dipolar Cycloaddition Reactions. J. Org. Chem. 2018, 83, 14102. [Google Scholar] [CrossRef]
- Zhi, Y.; Zhao, K.; Liu, Q.; Wang, A.; Enders, D. Asymmetric synthesis of functionalized trifluoromethyl-substituted pyrrolidines: Via an organocatalytic domino Michael/Mannich [3+2] cycloaddition. Chem. Commun. 2016, 52, 14011. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).