DBDMH-Promoted Methylthiolation in DMSO: A Metal-Free Protocol to Methyl Sulfur Compounds with Multifunctional Groups
Abstract
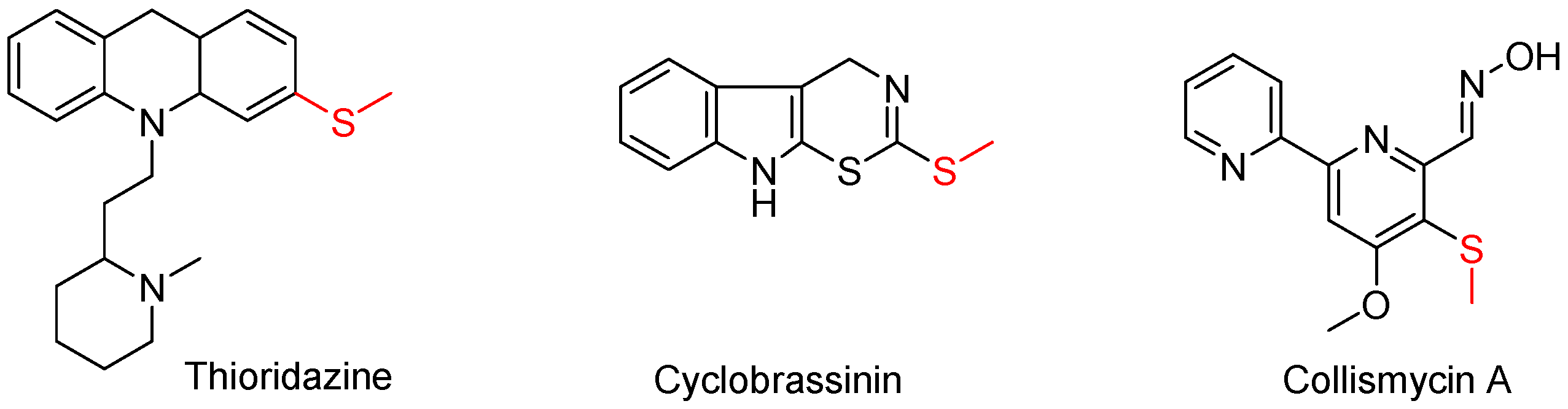
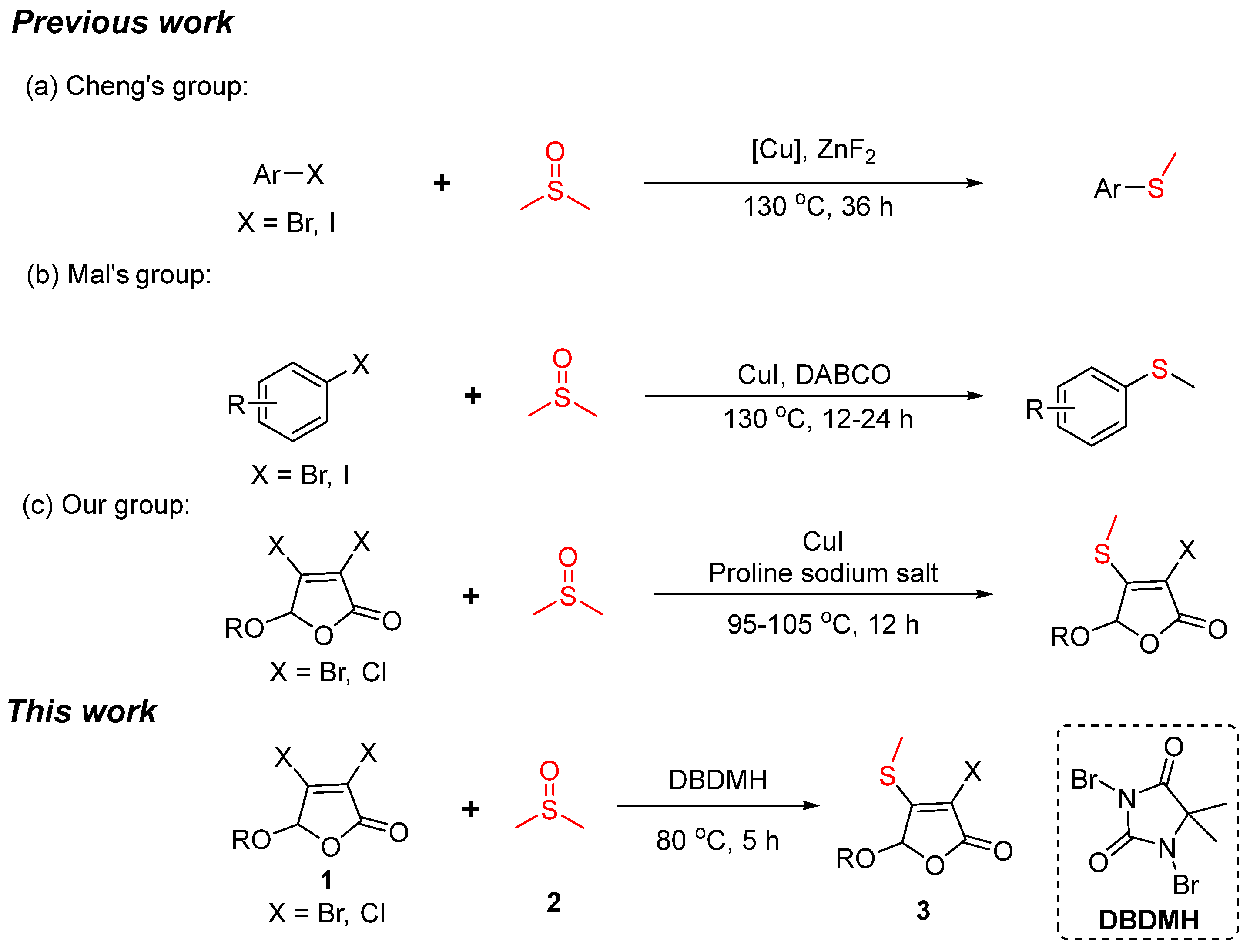
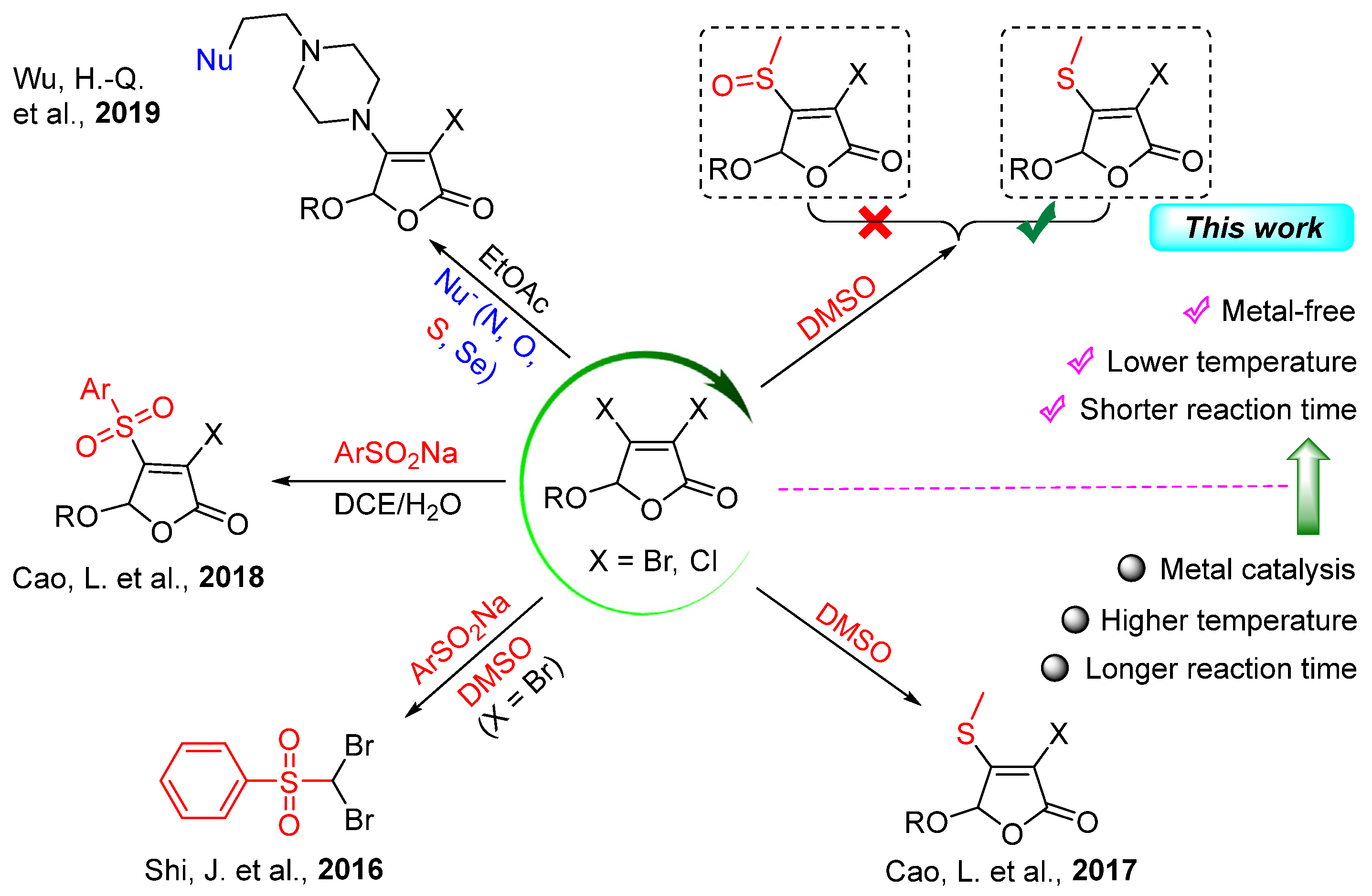
1. Introduction
2. Results and Discussion
2.1. Optimization of Reaction Conditions
2.2. Investigation into the Range of Reaction Substrates
2.3. Structural Characterization Analysis
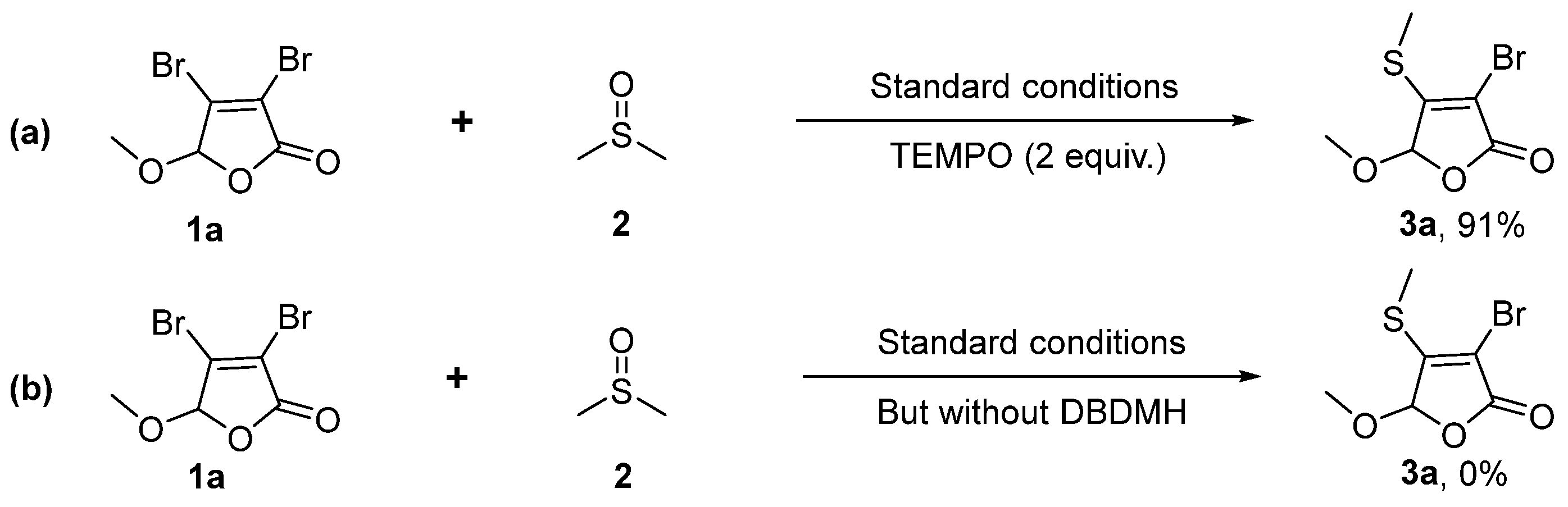
2.4. Mechanism Investigation and Gram-Scale Experiment
3. Materials and Methods
3.1. General Information
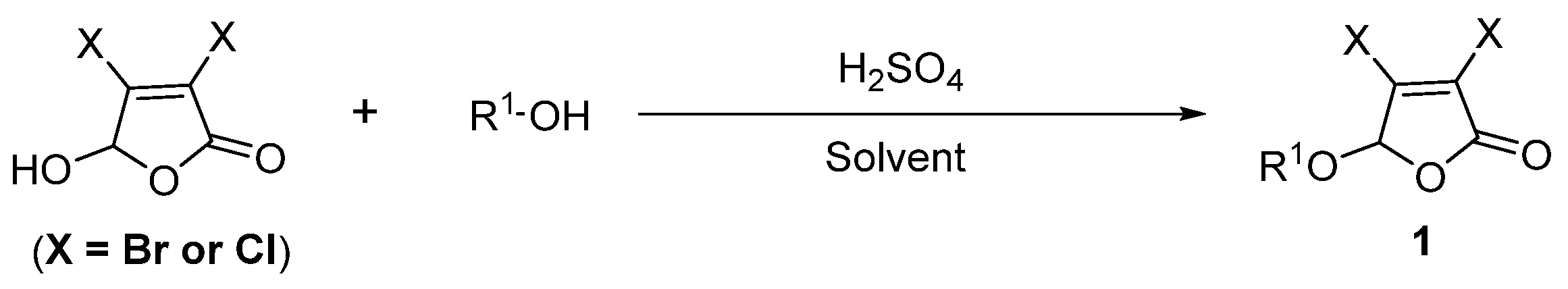
3.2. Experimental Procedure for Intermediate Compounds 1
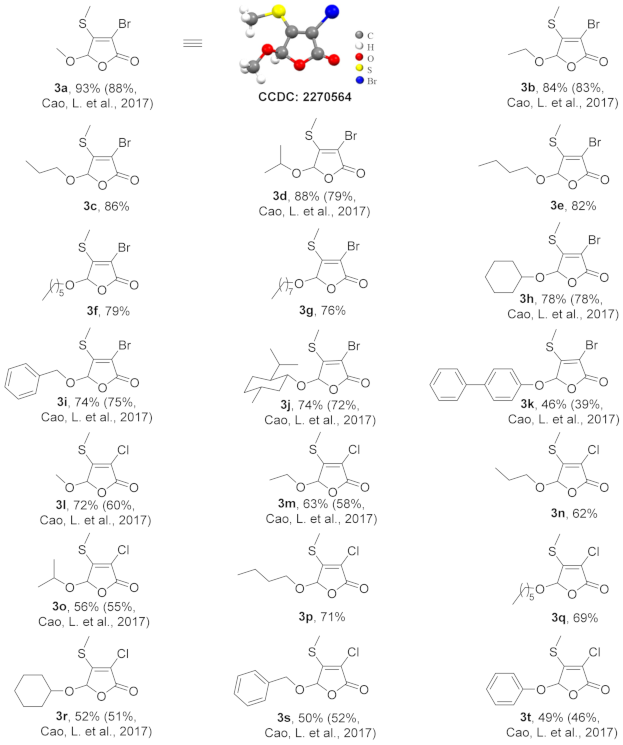
3.3. Experimental Procedure for Compounds 3a–3t
3.4. Structural Characterization Data of Compounds 3a–3t
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dong, B.L.; Lu, Y.R.; Zhang, N.; Song, W.H.; Lin, W.Y. Ratiometric imaging of cysteine level changes in endoplasmic reticulum during H2O2-induced redox imbalance. Anal. Chem. 2019, 91, 5513–5516. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, S.-F.; Zhu, X.-B.; Yuan, Y.F.; Li, Z.; Ye, K.-Y. Electrochemical sulfoxidation of thiols and alkyl halides. J. Org. Chem. 2022, 87, 6942–6950. [Google Scholar] [CrossRef]
- Feng, M.H.; Tang, B.Q.; Liang, S.H.; Jiang, X.F. Sulfur containing scaffolds in drugs: Synthesis and application in medicinal chemistry. Curr. Top. Med. Chem. 2016, 16, 1200–1216. [Google Scholar] [CrossRef] [PubMed]
- Bie, F.S.; Liu, X.J.; Cao, H.; Shi, Y.J.; Zhou, T.L.; Szostak, M.; Liu, C.W. Pd-catalyzed double-decarbonylative aryl sulfide synthesis through aryl exchange between amides and thioesters. Org. Lett. 2021, 23, 8098–8103. [Google Scholar] [CrossRef] [PubMed]
- Stepien, M.; Gonka, E.; Zyla, M.; Sprutta, N. Heterocyclic nanographenes and other polycyclic heteroaromatic compounds: Synthetic routes, properties, and applications. Chem. Rev. 2017, 117, 3479–3716. [Google Scholar] [CrossRef]
- Chen, S.-H.; Luo, S.-H.; Xing, L.-J.; Jiang, K.; Huo, Y.-P.; Chen, Q.; Wang, Z.-Y. Rational design and facile synthesis of dual-state emission fluorophores: Expanding functionality for the sensitive detection of nitroaromatic compounds. Chem. Eur. J. 2022, 28, e202103478. [Google Scholar] [CrossRef]
- Mondal, S.; Di Tommaso, E.M.; Olofsson, B. Transition-metal-free difunctionalization of sulfur nucleophiles. Angew. Chem. Int. Ed. 2023, 62, e202216296. [Google Scholar] [CrossRef] [PubMed]
- Denes, F.; Schiesser, C.H.; Renaud, P. Thiols, thioethers, and related compountandds as sources of C-centred radicals. Chem. Soc. Rev. 2013, 42, 7900–7942. [Google Scholar] [CrossRef] [PubMed]
- Merad, J.; Matyasovsky, J.; Stopka, T.; Brutiu, B.R.; Pinto, A.; Drescher, M.; Maulide, N. Stable and easily available sulfide surrogates allow a stereoselective activation of alcohols. Chem. Sci. 2021, 12, 7770–7774. [Google Scholar] [CrossRef]
- Ahmad, B.S.; Yaqoob, B.M.; Ahmad, R.S.; Salman, J.; Ahmad, B.K.; Naveed, A.Q. I2-DMSO promoted deaminative coupling reactions of glycine esters: Access to 5-(methylthio)pyridazin-3(2H)-ones. Org. Lett. 2023, 25, 2382–2387. [Google Scholar]
- Dunbar, K.L.; Scharf, D.H.; Litomska, A.; Hertweck, C. Enzymatic carbon-sulfur bond formation in natural product biosynthesis. Chem. Rev. 2017, 117, 5521–5577. [Google Scholar] [CrossRef]
- Park, J.K.; Lee, S. Sulfoxide and sulfone synthesis via electrochemical oxidation of sulfides. J. Org. Chem. 2021, 86, 13790–13799. [Google Scholar] [CrossRef]
- Forchetta, M.; Sabuzi, F.; Stella, L.; Conte, V.; Galloni, P. KuQuinone as a highly stable and reusable organic photocatalyst in selective oxidation of thioethers to sulfoxides. J. Org. Chem. 2022, 87, 14016–14025. [Google Scholar] [CrossRef]
- Cao, Y.; Huang, Y.; Blakemore, P.R. Synthesis of thioalkynes by desilylative sonogashira cross-coupling of aryl iodides and 1-methylthio-2-(trimethylsilyl)ethyne. Eur. J. Org. Chem. 2022, 2022, e202200498. [Google Scholar] [CrossRef]
- Cao, W.; Chen, P.; Wang, L.; Wen, H.; Liu, Y.; Wang, W.S.; Tang, Y. A highly regio- and stereoselective syntheses of α-halo enamides, vinyl thioethers, and vinyl ethers with aqueous hydrogen halide in two-phase systems. Org. Lett. 2018, 20, 4507–4511. [Google Scholar] [CrossRef]
- Clarke, A.K.; Parkin, A.; Taylor, R.J.K.; Unsworth, W.P.; Rossi-Ashton, J.A. Photocatalytic deoxygenation of sulfoxides using visible light: Mechanistic investigations and synthetic applications. ACS Catal. 2020, 10, 5814–5820. [Google Scholar] [CrossRef]
- Sakai, N.; Shimada, R.; Ogiwara, Y. Indium-catalyzed deoxygenation of sulfoxides with hydrosilanes. Asian J. Org. Chem. 2021, 10, 845–850. [Google Scholar] [CrossRef]
- Antoniak, D.; Paluba, B.; Basak, T.; Blaziak, K.; Barbasiewicz, M. Alkylation of nitroarenes via vicarious nucleophilic substitution-experimental and DFT mechanistic studies. Chem. Eur. J. 2022, 28, e202201153. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, Z.W.; Chen, X.-Y.; Wu, Y.C.; Wang, D.M.; Peng, Q.; Wang, P. Para-selective borylation of monosubstituted benzenes using a transient mediator. Sci. China Chem. 2020, 63, 336–340. [Google Scholar] [CrossRef]
- Gensch, T.; Klauck, F.J.R.; Glorius, F. Cobalt-catalyzed C-H thiolation through dehydrogenative cross-coupling. Angew. Chem. Int. Ed. 2016, 55, 11287–11291. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Bhat, M.Y.; Hussain, F.; Ahmed, Q.N. BF3-Et2O Promoted heteronucleophilic addition reactions for the synthesis of unsymmetrical gem-diarylmethyl thioethers. Org. Lett. 2023, 25, 5017–5021. [Google Scholar] [CrossRef]
- Li, X.M.; Zhang, B.B.; Zhang, J.R.; Wang, X.; Zhang, D.K.; Du, Y.F.; Zhao, K. Synthesis of 3-methylthioindoles via intramolecular cyclization of 2-alkynylanilines mediated by DMSO/DMSO-d6 and SOCl2. Chin. J. Chem. 2021, 39, 1211–1224. [Google Scholar] [CrossRef]
- Lin, Z.G.; Huang, L.B.; Yuan, G.Q. Electrosynthesis of sulfonamides from DMSO and amines under mild conditions. Chem. Commun. 2021, 57, 3579–3582. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.L.; Li, H.; Nie, Z.W.; Su, M.-D.; Luo, W.-P.; Liu, Q.; Guo, C.-C. [3+1+1+1] Annulation to the Pyridine structure in quinoline molecules based on DMSO as a nonadjacent dual-methine synthon: Simple synthesis of 3-arylquinolines from arylaldehydes, arylamines, and DMSO. J. Org. Chem. 2022, 87, 2797–2808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Wang, W.J.; Wang, B.D.; Tan, H.; Jiao, N.; Song, S. Electrophilic amidomethylation of arenes with DMSO/MeCN reagents. Org. Chem. Front. 2022, 9, 2430–2437. [Google Scholar] [CrossRef]
- Liu, H.; He, G.-C.; Zhao, C.-Y.; Zhang, X.-X.; Ji, D.-W.; Hu, Y.-C.; Chen, Q.-A. Redox-divergent construction of (dihydro)thiophenes with DMSO. Angew. Chem. Int. Ed. 2021, 133, 24486–24493. [Google Scholar] [CrossRef]
- Mukherjee, N.; Chatterjee, T. Iodine-catalyzed methylthiolative annulation of 2-alkynyl biaryls with DMSO: A metal-free approach to 9-sulfenylphen-anthrenes. J. Org. Chem. 2021, 86, 7881–7890. [Google Scholar] [CrossRef]
- Xia, Z.-H.; Gao, Z.-H.; Dai, L.; Ye, S. Visible-light-promoted oxo-difluoroalkylation of alkenes with DMSO as the oxidant. J. Org. Chem. 2019, 84, 7388–7394. [Google Scholar] [CrossRef]
- Kornfeind, J.; Iyer, P.S.; Keller, T.M.; Fleming, F.F. Oxidative DMSO cyclization cascade to bicyclic hydroxyketonitriles. J. Org. Chem. 2022, 87, 6097–6104. [Google Scholar] [CrossRef]
- Luo, F.; Pan, C.D.; Li, L.P.; Chen, F.; Cheng, J. Copper-mediated methylthiolation of aryl halides with DMSO. Chem. Commun. 2011, 47, 5304–5306. [Google Scholar] [CrossRef]
- Ghosh, K.; Ranjit, S.; Mal, D. A convenient method for the synthesis of aryl methyl sulfides via Cu(I)-mediated methylthiolation of haloarenes with DMSO. Tetrahedron Lett. 2015, 56, 5199–5202. [Google Scholar] [CrossRef]
- Cao, L.; Luo, S.-H.; Wu, H.-Q.; Chen, L.-Q.; Jiang, K.; Hao, Z.-F.; Wang, Z.-Y. Copper(I)-catalyzed alkyl- and arylsulfenylation of 3,4-dihalo-2(5H)-furanones (X=Br, Cl) with sulfoxides under mild conditions. Adv. Synth. Catal. 2017, 359, 2961–2971. [Google Scholar] [CrossRef]
- Kaiser, D.; Klose, I.; Oost, R.; Neuhaus, J.; Maulide, N. Bond-forming and-breaking reactions at sulfur (IV): Sulfoxides, sulfonium salts, sulfur ylides, and sulfinate salts. Chem. Rev. 2019, 119, 8701–8780. [Google Scholar] [CrossRef] [PubMed]
- Saito, F. Sulfoxide reagent for one-pot, three-component syntheses of sulfoxides and sulfinamides. Angew. Chem. Int. Ed. 2022, 61, e202213872. [Google Scholar] [CrossRef]
- Wang, N.Z.; Saidhareddy, P.; Jiang, X.F. Construction of sulfur-containing moieties in the total synthesis of natural products. Nat. Prod. Rep. 2020, 37, 246–275. [Google Scholar] [CrossRef]
- Fu, Z.-H.; Tian, H.-D.; Ni, S.-F.; Wright, J.S.; Li, M.; Wen, L.-R.; Zhang, L.-B. Scalable selective electrochemical oxidation of sulfides to sulfoxides. Green Chem. 2022, 24, 4772–4777. [Google Scholar] [CrossRef]
- Wang, B.-W.; Jiang, K.; Li, J.-X.; Luo, S.-H.; Wang, Z.-Y.; Jiang, H.-F. 1,1-diphenyl-vinylsulfide as a functional AIEgen derived from the aggregation-caused-quenching molecule 1,1-diphenylethene through simple thioetherification. Angew. Chem. Int. Ed. 2020, 59, 2338–2343. [Google Scholar] [CrossRef]
- Cao, L.; Luo, S.-H.; Jiang, K.; Hao, Z.-F.; Wang, B.-W.; Pang, C.-M.; Wang, Z.-Y. Disproportionate coupling reaction of sodium sulfinates mediated by BF3·OEt2: An approach to symmetrical/unsymmetrical thiosulfonates. Org. Lett. 2018, 20, 4754–4758. [Google Scholar] [CrossRef]
- Zhou, Y.-J.; Yang, K.; Fang, Y.-G.; Luo, S.-H.; Chen, Q.; Yu, S.-W.; Wang, Z.-Y. A NaHCO3 promoted three-component cyclization: Easy access to benzodisulfide heterocycles. Asian J. Org. Chem. 2022, 11, e202200170. [Google Scholar] [CrossRef]
- Shi, J.; Tang, X.-D.; Wu, Y.-C.; Fang, J.-F.; Cao, L.; Chen, X.-Y.; Wang, Z.-Y. A radical coupling reaction of DMSO with sodium arylsulfinates in air: Mild utilization of DMSO as C1 resource for the synthesis of arylsulfonyl dibromomethane. RSC Adv. 2016, 6, 25651–25655. [Google Scholar] [CrossRef]
- Cao, L.; Li, J.-X.; Wu, H.-Q.; Jiang, K.; Hao, Z.-F.; Luo, S.-H.; Wang, Z.-Y. Metal-free sulfonylation of 3,4-dihalo-2(5H)-furanones (X=Cl, Br) with sodium sulfinates under air atmosphere in aqueous media via a radical pathway. ACS Sustain. Chem. Eng. 2018, 6, 4147–4153. [Google Scholar] [CrossRef]
- Wu, H.-Q.; Yang, K.; Luo, S.-H.; Wu, X.-Y.; Wang, N.; Chen, S.-H.; Wang, Z.-Y. C4-Selective synthesis of vinyl thiocyanates and selenocyanates through 3,4-dihalo-2(5H)-furanones. Eur. J. Org. Chem. 2019, 2019, 4572–4580. [Google Scholar] [CrossRef]
- Wu, H.-Q.; Yang, K.; Chen, X.-Y.; Arulkumar, M.; Wang, N.; Chen, S.-H.; Wang, Z.-Y. A 3,4-dihalo-2(5H)-furanone initiated ring-opening reaction of DABCO in the absence of a metal catalyst and additive and its application in a one-pot two-step reaction. Green Chem. 2019, 21, 3782–3788. [Google Scholar] [CrossRef]
- Yu, S.L.; Hong, C.; Liu, Z.; Zhang, H. Cobalt-catalyzed vinylic C-H addition to formaldehyde: Synthesis of butenolides from acrylic acids and HCHO. Org. Lett. 2021, 23, 8359–8364. [Google Scholar] [CrossRef]
- Irie, T.; Asami, T.; Sawa, A.; Uno, Y.; Hanada, M.; Taniyama, C.; Funakoshi, Y.; Masai, H.; Sawa, M. Discovery of novel furanone derivatives as potent Cdc7 kinase inhibitors. Eur. J. Med. Chem. 2017, 130, 406–418. [Google Scholar] [CrossRef]
- Zeiler, M.J.; Connors, G.M.; Durling, G.M.; Oliver, A.G.; Marquez, L.; Melander, R.J.; Quave, C.L.; Melander, C. Synthesis, stereochemical confirmation, and derivatization of 12(S), 16ϵ-dihydroxycleroda-3,13-dien-15,16-olide, a clerodane diterpene that sensitizes methicillin-resistant staphylococcus aureus to β-lactam antibiotics. Angew. Chem. Int. Ed. 2022, 134, e202117458. [Google Scholar] [CrossRef]
- Byczek-Wyrostek, A.; Kitel, R.; Rumak, K.; Skonieczna, M.; Kasprzycka, A.; Walczak, K. Simple 2(5H)-furanone derivatives with selective cytotoxicity towards non-small cell lung cancer cell line A549–synthesis, structure-activity relationship and biological evaluation. Eur. J. Med. Chem. 2018, 150, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Bailly, F.; Queffelec, C.; Mbemba, G.; Mouscadet, J.-F.; Pommery, N.; Pommery, J.; Henichart, J.-P.; Cotelle, P. Synthesis and biological activities of a series of 4, 5-diaryl-3-hydroxy-2 (5H)-furanones. Eur. J. Med. Chem. 2008, 43, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.-X.; Feng, L.; Li, X.-Q.; Zhou, X.-Z.; Shao, Z.-H. Synthesis of new chiral 2,5-disubstituted 1,3,4-thiadiazoles possessing γ-butenolide moiety and preliminary evaluation of in vitro anticancer activity. Eur. J. Med. Chem. 2009, 44, 3340–3344. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Yang, J.-Q.; Luo, S.-H.; Mei, W.-J.; Lin, J.-Y.; Zhan, J.-Q.; Wang, Z.-Y. Synthesis of N-2(5H)-furanonyl sulfonyl hydrazone derivatives and their biological evaluation in vitro and in vivo activity against MCF-7 breast cancer cells. Bioorg. Chem. 2021, 107, 104518. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-J.; Fang, Y.-G.; Yang, K.; Yu, S.-W.; Chen, Z.-J.; Wang, B.-C.; Zhan, H.-Y.; Wang, Z.-Y. Multicomponent selective thioetherification of KSAc: Easy access to symmetrical/unsymmetrical 4-alkylthio-3-halo-2(5H)-furanones. Asian J. Org. Chem. 2023, 12, e202300038. [Google Scholar] [CrossRef]
- CCDC 2270564 (for 3a) Contains the Supplementary Crystallographic Data for This Paper. These Data Can Be Obtained Free of Charge from The Cambridge Crystallographic Data Centre. Available online: www.ccdc.cam.ac.uk/data_request/cif (accessed on 17 June 2023).
- Murai, K.; Matsushita, T.; Nakamura, A.; Fukushima, S.; Shimura, M.; Fujioka, H. Asymmetric bromolactonization catalyzed by a C3-symmetric chiral trisimidazoline. Angew. Chem. Int. Ed. 2010, 49, 9174–9177. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhai, L.L.; Chen, J.Y.; Gong, Y.L.; Wang, P.; Li, H.L.; She, X.G. Catalyst-free 1,2-dibromination of alkenes using 1,3-dibromo-5,5-dimethylhydantoin (DBDMH) as a bromine source. J. Org. Chem. 2022, 87, 3177–3183. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-Y.; Liao, Q.; Zhang, J.-W.; Lin, G.-Q.; Wang, Z.T.; Gao, D.D.; Li, Q.-H.; Tian, P. Thiourea-promoted cascade dehalogenation-cyclization of cyclohexadienone-containing 1,6-enynes. Adv. Synth. Catal. 2023, 365, 482–489. [Google Scholar] [CrossRef]
- Khazaei, A.; Zolfigol, M.A.; Rostami, A. 1,3-dibromo-5,5-dimethylhydantoin [DBDMH] as an efficient and selective agent for the oxidation of thiols to disulfides in solution or under solvent-free conditions. Synthesis 2004, 2004, 2959–2961. [Google Scholar] [CrossRef]
- Xu, S.H.; Wu, P.; Zhang, W. 1,3-Dibromo-5,5-dimethylhydantoin (DBH) mediated one-pot syntheses of α-bromo/amino ketones from alkenes in water. Org. Biomol. Chem. 2016, 14, 11389–11395. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.W.; Xu, J.Q.; Xu, J.; Zhou, B.C.; Zhang, D.; Yang, Z.; Fang, Z.; Guo, K. Oxidative thioesterification of alkenes mediated by 1,3-dibromo-5,5-dimethylhydantoin and DMSO for the synthesis of α-ketothioesters. Eur. J. Org. Chem. 2019, 2019, 4056–4060. [Google Scholar] [CrossRef]
- Fu, D.; Dong, J.; Du, H.G.; Xu, J.X. Methanesulfinylation of benzyl halides with dimethyl sulfoxide. J. Org. Chem. 2019, 85, 2752–2758. [Google Scholar] [CrossRef]
- Fu, D.; Dong, J.; Wang, J.Y.; Xu, J.X. Direct 1,2-oxosulfenylation of acetylenic sulfones with DMSO. Asian J. Org. Chem. 2021, 10, 1756–1764. [Google Scholar] [CrossRef]


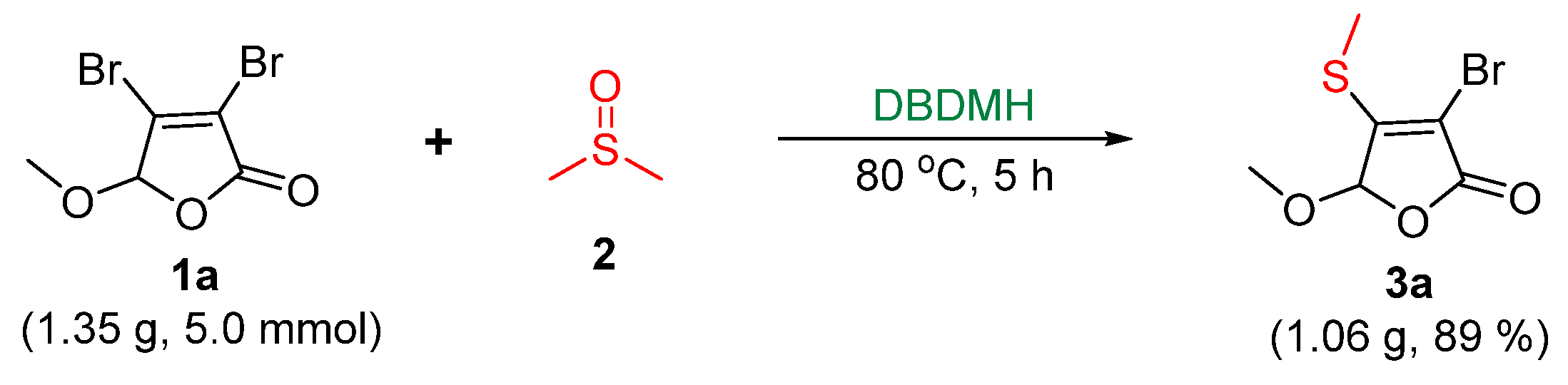

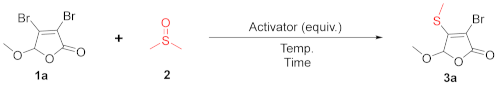 | ||||
|---|---|---|---|---|
| Entry | Activator (Equiv.) | Temp. (°C) | Time (h) | Yield of 3a (%) [b] |
| 1 | NBS (1.0) | 80 | 5 | 65 |
| 2 | NCS (1.0) | 80 | 5 | 43 |
| 3 | DBDMH (1.0) | 80 | 5 | 87 |
| 4 | DBDMH (0.5) | 80 | 5 | 78 |
| 5 | DBDMH (1.5) | 80 | 5 | 93 |
| 6 | DBDMH (2.0) | 80 | 5 | 90 |
| 7 | DBDMH (1.5) | 60 | 5 | 85 |
| 8 | DBDMH (1.5) | 100 | 5 | 87 |
| 9 | DBDMH (1.5) | 80 | 8 | 89 |
| 10 | DBDMH (1.5) | 80 | 3 | 57 |
 |
|---|
 |
| Compound | 3a |
|---|---|
| Empirical formula | C6H7BrO3S |
| Formula weight | 239.09 |
| Temperature (K) | 297 |
| Wavelength (Å) | 0.71073 |
| Crystal system | Monoclinic |
| Space group | P21 |
| Unit cell dimensions (Å, °) | a = 4.1083 (8), b = 7.6804 (19), c = 13.739 (3) α = 90, β = 90.079 (17), γ = 90 |
| Volume (Å3) | 433.22 (16) |
| Z | 2 |
| Density (calculated) (g/cm3) | 1.833 |
| Absorption coefficient (mm−1) | 4.941 |
| F(000) | 236.0 |
| Theta range for data collection | 3.981 to 22.995 |
| Index ranges | −4 ≤ h ≤ 4, −9 ≤ k ≤ 9, 0 ≤ l ≤ 16 |
| Reflections collected | 1464 |
| Independent reflections | 1464 [Rsigma = 0.1075] |
| Completeness to theta = 1.78° | 99.6% |
| Absorption correction | Multiscan |
| Max. and min. transmission | 1.000 and 0.101 |
| Refinement method | Least squares minimization |
| Data/restraints/parameters | 1464/1/103 |
| Goodness-of-fit on F2 | 1.040 |
| Final R indices [I > 2 sigma (I)] | R1 = 0.0545, wR2 = 0.0955 |
| R indices (all data) | R1 = 0.0707, wR2 = 0.1048 |
| Largest diff. peak and hole | 0.58 and −0.84 e.Å−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.-J.; Fang, Y.-G.; Yang, K.; Lin, J.-Y.; Li, H.-Q.; Chen, Z.-J.; Wang, Z.-Y. DBDMH-Promoted Methylthiolation in DMSO: A Metal-Free Protocol to Methyl Sulfur Compounds with Multifunctional Groups. Molecules 2023, 28, 5635. https://doi.org/10.3390/molecules28155635
Zhou Y-J, Fang Y-G, Yang K, Lin J-Y, Li H-Q, Chen Z-J, Wang Z-Y. DBDMH-Promoted Methylthiolation in DMSO: A Metal-Free Protocol to Methyl Sulfur Compounds with Multifunctional Groups. Molecules. 2023; 28(15):5635. https://doi.org/10.3390/molecules28155635
Chicago/Turabian StyleZhou, Yong-Jun, Yong-Gan Fang, Kai Yang, Jian-Yun Lin, Huan-Qing Li, Zu-Jia Chen, and Zhao-Yang Wang. 2023. "DBDMH-Promoted Methylthiolation in DMSO: A Metal-Free Protocol to Methyl Sulfur Compounds with Multifunctional Groups" Molecules 28, no. 15: 5635. https://doi.org/10.3390/molecules28155635
APA StyleZhou, Y.-J., Fang, Y.-G., Yang, K., Lin, J.-Y., Li, H.-Q., Chen, Z.-J., & Wang, Z.-Y. (2023). DBDMH-Promoted Methylthiolation in DMSO: A Metal-Free Protocol to Methyl Sulfur Compounds with Multifunctional Groups. Molecules, 28(15), 5635. https://doi.org/10.3390/molecules28155635









