Incorporation of Poly(TFEMA) in Perovskite Thin Films Using a Supercritical Fluid
Abstract
:1. Introduction
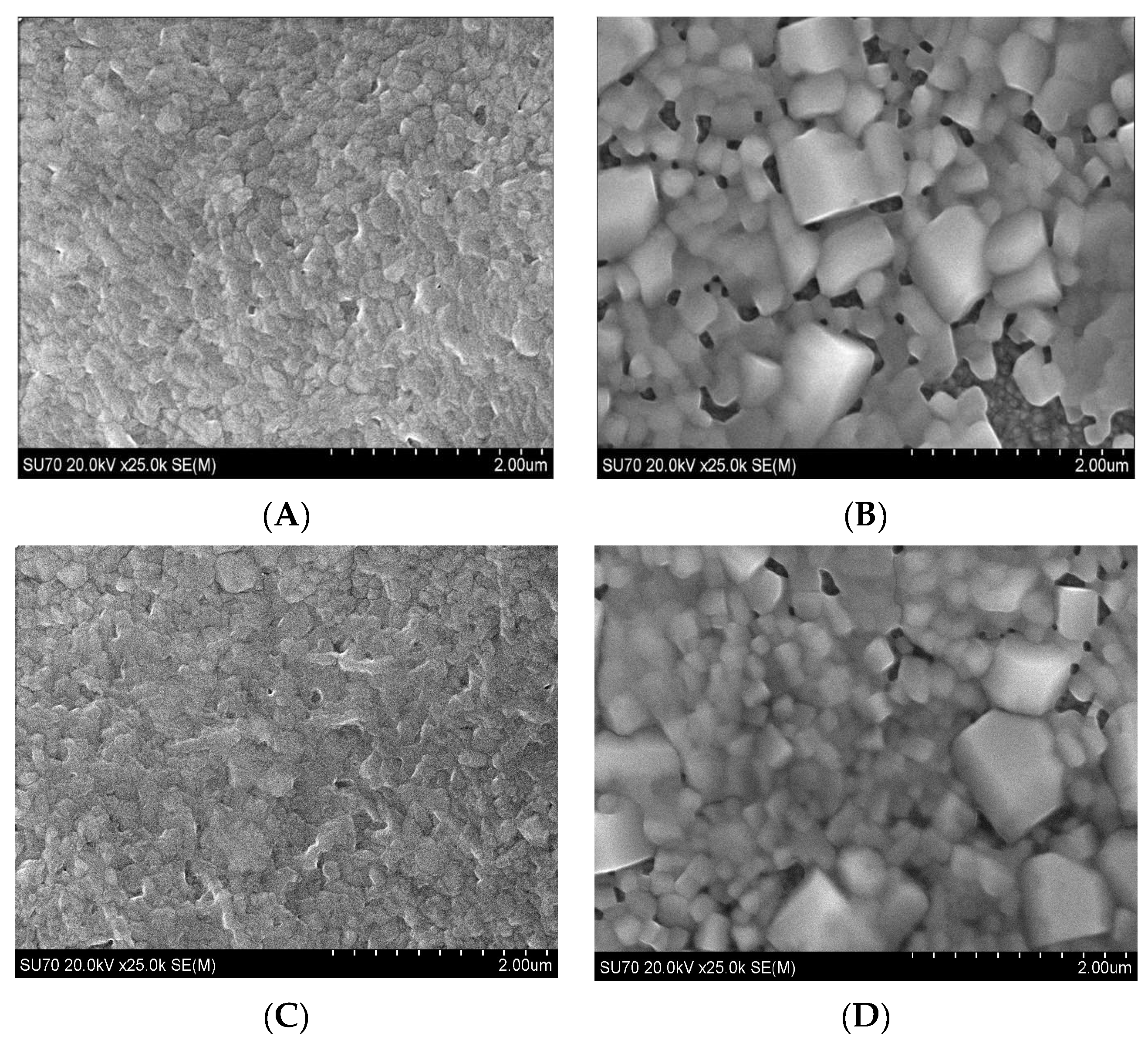
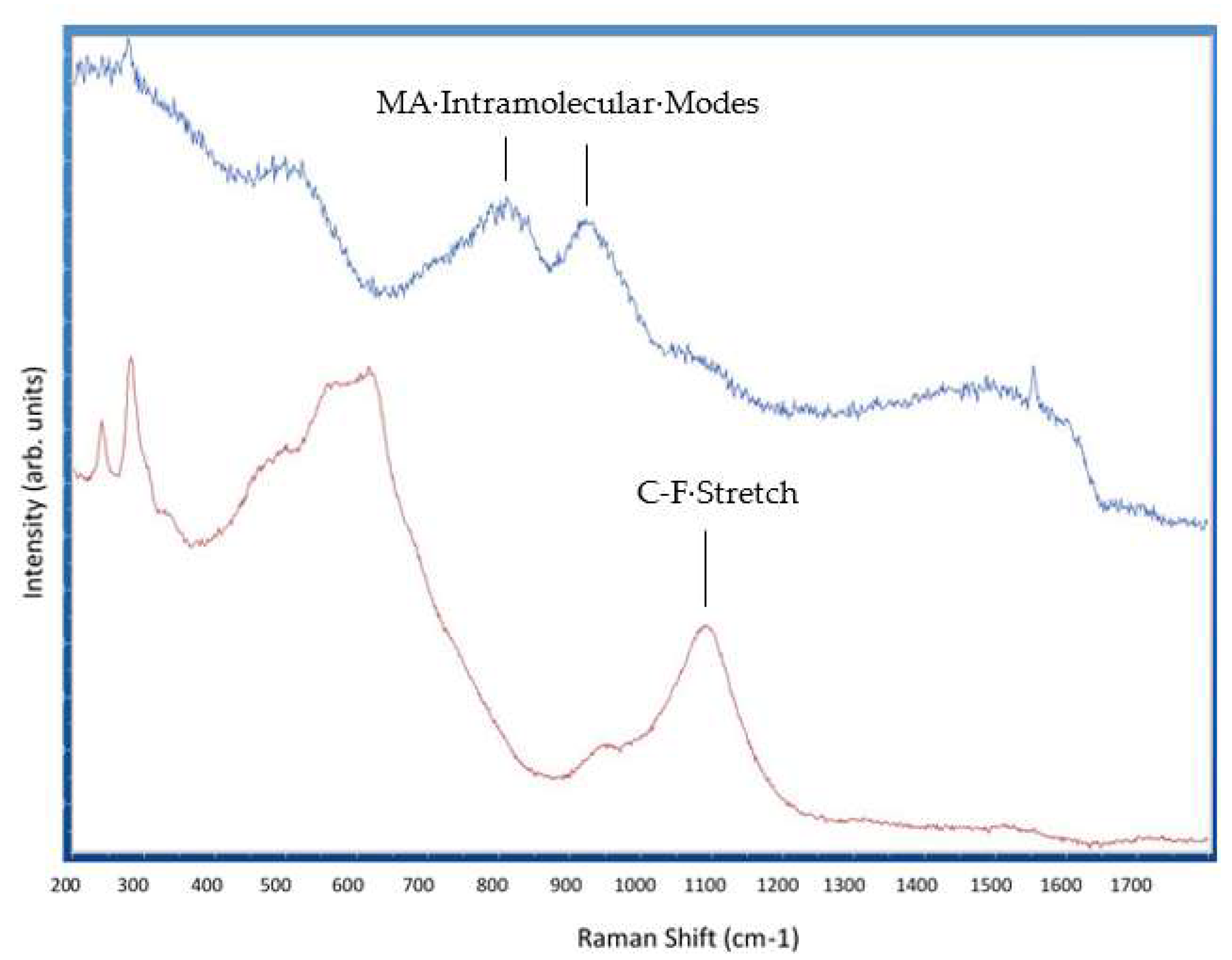
2. Results and Discussion
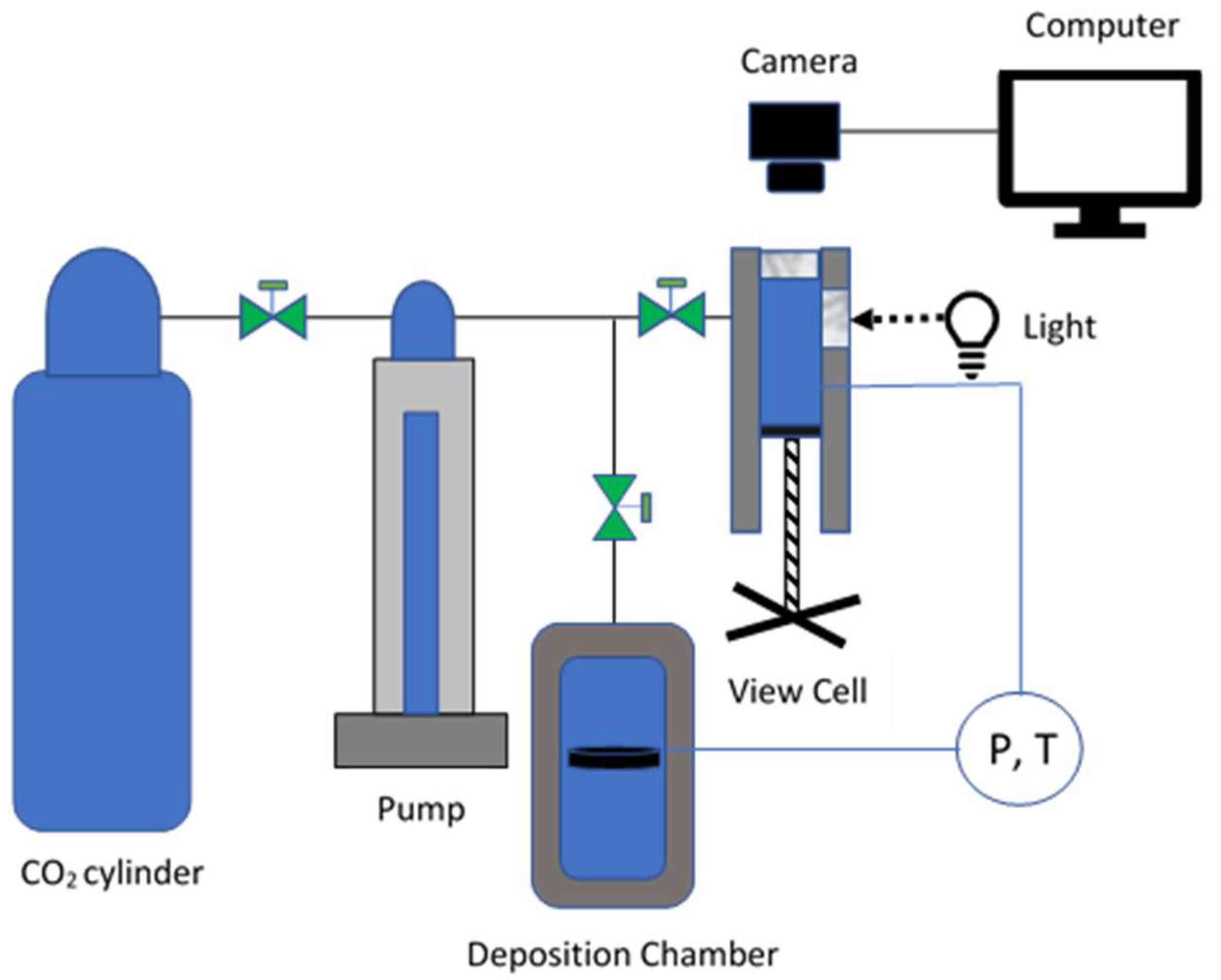
3. Methods
3.1. Substrate Preparation
3.2. Perovskite Layer
3.3. Cloud Point Determination
3.4. Polymer Deposition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Best Research-Cell Efficiency Chart. NREL.gov. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 12 June 2023).
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [Green Version]
- Miyata, A.; Mitioglu, A.; Plochocka, P.; Portugall, O.; Wang, J.T.W.; Stranks, S.D.; Snaith, H.J.; Nicholas, R.J. Direct measurement of the exciton binding energy and effective masses for charge carriers in organic–inorganic tri-halide perovskites. Nat. Phys. 2015, 11, 582–587. [Google Scholar] [CrossRef] [Green Version]
- Eperon, G.E.; Stranks, S.D.; Menelaou, C.; Johnston, M.B.; Herz, L.M.; Snaith, H.J. Formamidinium lead trihalide: A broadly tunable perovskite for efficient planar heterojunction solar cells. Energy Environ. Sci. 2014, 7, 982–988. [Google Scholar] [CrossRef]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steirer, K.X.; Schulz, P.; Teeter, G.; Stevanovic, V.; Yang, M.; Zhu, K.; Berry, J.J. Defect tolerance in methylammonium lead triiodide perovskite. ACS Energy Lett. 2016, 1, 360–366. [Google Scholar] [CrossRef]
- Nie, W.; Tsai, H.; Asadpour, R.; Blancon, J.-C.; Neukirch, A.J.; Gupta, G.; Crochet, J.J.; Chhowalla, M.; Tretiak, S.; Alam, M.A.; et al. High-efficiency solution-processed perovskite solar cells with millimeter-scale grains. Science 2015, 347, 522–525. [Google Scholar] [CrossRef] [Green Version]
- Dhoble, S.J. Chapter 9.2—Perovskite solar cells. In Energy Materials: Fundamentals to Applications; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Gao, F.; Zhao, Y.; Zhang, X.; You, J. Recent Progresses on Defect Passivation toward Efficient Perovskite Solar Cells. Adv. Energy Mater. 2020, 10, 1902650. [Google Scholar] [CrossRef]
- Sun, S.; Salim, T.; Mathews, N.; Duchamp, M.; Boothroyd, C.; Xing, G.; Sum, T.C.; Lam, Y.M. The origin of high efficiency in low-temperature solution-processable bilayer organometal halide hybrid solar cells. Energy Environ. Sci. 2014, 7, 399–407. [Google Scholar] [CrossRef] [Green Version]
- Perovskite Solar Cells. NREL.gov. (n.d.). Available online: https://www.nrel.gov/pv/perovskite-solar-cells.html (accessed on 12 June 2023).
- Zhou, D.; Zhou, T.; Tian, Y.; Zhu, X.; Tu, Y. Perovskite-Based Solar Cells: Materials, Methods, and Future Perspectives. J. Nanomater. 2018, 2018, 8148072. [Google Scholar] [CrossRef]
- Tan, L.; Zhou, J.; Zhao, X.; Wang, S.; Li, M.; Jiang, C.; Li, H.; Zhang, Y.; Ye, Y.; Tress, W.; et al. Combined Vacuum Evaporation and Solution Process for High-Efficiency Large-Area Perovskite Solar Cells with Exceptional Reproducibility. Adv. Mater. 2023, 35, 2205027. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cheng, Y.B.; Ge, Z. Understanding of perovskite crystal growth and film formation in scalable deposition process. R. Soc. Chem. 2020, 49, 1653–1687. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Li, M.; Šiffalovič, P.; Cao, G.; Tian, J. From scalable solution fabrication of perovskite films towards commercialization of solar cells. Energy Environ. Sci. 2019, 12, 518–549. [Google Scholar] [CrossRef]
- Large-Scale Deposition of Organic Solar Cells. Ossila. (n.d.). Available online: https://www.ossila.com/en-us/pages/opv-large-scale-deposition (accessed on 12 June 2023).
- Sanli, D.; Bozbag, S.E.; Erkey, C. Synthesis of nanostructured materials using supercritical CO2: Part I. Physical transformations. J. Mater. Sci. 2012, 47, 2995–3025. [Google Scholar] [CrossRef]
- Khanyile, A.T.; Andrew, J.E.; Paul, V.; Sithole, B.B. A comparative study of supercritical fluid extraction and accelerated solvent extraction of lipophilic compounds from lignocellulosic biomass. Sustain. Chem. Pharm. 2022, 26, 100608. [Google Scholar] [CrossRef]
- Libretexts. 12.4: Phase Diagrams. Chemistry LibreTexts. 2023. Available online: https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_General_Chemistry_%28Petrucci_et_al.%29/12%3A_Intermolecular_Forces%3A_Liquids_And_Solids/12.4%3A_Phase_Diagrams (accessed on 12 June 2023).
- Annohene, G.; Pascucci, J.; Pestov, D.; Tepper, G.C. Supercritical fluid-assisted crystallization of CH3NH3PbI3 perovskite films. J. Supercrit. Fluids 2020, 156, 104684. [Google Scholar] [CrossRef]
- Wu, W.; Ke, J.; Poliakoff, M. Phase boundaries of CO2+ toluene, CO2+ acetone, and CO2+ ethanol at high temperatures and high pressures. J. Chem. Eng. Data 2006, 51, 1398–1403. [Google Scholar] [CrossRef]
- Annohene, G.; Tepper, G.C. Efficient perovskite solar cells processed in supercritical carbon dioxide. J. Supercrit. Fluids 2021, 171, 105203. [Google Scholar] [CrossRef]
- Santo, A.; Siqueira, L.; Almeida, R.; Vargas, R.; Franceschini, G.; Kunde, M.; Cappellari, A.; Morrone, F.; Cassel, E. Decaffeination of yerba mate by supercritical fluid extraction: Improvement, mathematical modelling and infusion analysis. J. Supercrit. Fluids 2021, 168, 105096. [Google Scholar] [CrossRef]
- Kwon, S.; Bae, W.; Lee, K.; Byun, H.S.; Kim, H. High pressure phase behavior of carbon dioxide+ 2, 2, 2-trifluoroethyl methacrylate and+ poly (2, 2, 2-trifluoroethyl methacrylate) systems. J. Chem. Eng. Data 2007, 52, 89–92. [Google Scholar] [CrossRef]
- Ciardelli, F.; Aglietto, M.; Di Mirabello, L.M.; Passaglia, E.; Giancristoforo, S.; Castelvetro, V.; Ruggeri, G. New fluorinated acrylic polymers for improving weatherability of building stone materials. Prog. Org. Coat. 1997, 32, 43–50. [Google Scholar] [CrossRef]
- Kwon, S.; Bae, W.; Kim, H. The effect of CO2 in free-radical polymerization of 2,2,2-trifluoroethyl methacrylate. Korean J. Chem. Eng. 2004, 21, 910–914. [Google Scholar] [CrossRef]
- Ratcharak, O.; Sane, A. Surface coating with poly (trifluoroethyl methacrylate) through rapid expansion of supercritical CO2 solutions. J. Supercrit. Fluids 2014, 89, 106–112. [Google Scholar] [CrossRef]
- Annohene, G.; Tepper, G.C. Low temperature formation of CH3NH3PBI3 perovskite films in supercritical carbon dioxide. J. Supercrit. Fluids 2019, 154, 104604. [Google Scholar] [CrossRef]
- Nakada, K.; Matsumoto, Y.; Shimoi, Y.; Yamada, K.; Furukawa, Y. Temperature-Dependent Evolution of Raman Spectra of Methylammonium Lead Halide Perovskites, CH3NH3PbX3 (X = I,Br). Molecules 2019, 24, 626. [Google Scholar] [CrossRef] [Green Version]
- Berney, C.V.; Cousins, L.R.; Miller, F.A. Vibrational spectra of CF3C-CH3, CF3C-CD and CF3C-CCF3. Spectrochim. Acta 1963, 19, 2019–2032. [Google Scholar] [CrossRef]
- Byun, H.S.; Kim, C.R.; Yoon, S.D. Cloud-point measurement of binary and ternary mixtures for the P(MMA-co-PnFPA) in supercritical fluoric solvents. J. Supercrit. Fluids 2017, 120, 226–239. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Handy, K.; Tepper, G.C. Incorporation of Poly(TFEMA) in Perovskite Thin Films Using a Supercritical Fluid. Molecules 2023, 28, 5385. https://doi.org/10.3390/molecules28145385
Handy K, Tepper GC. Incorporation of Poly(TFEMA) in Perovskite Thin Films Using a Supercritical Fluid. Molecules. 2023; 28(14):5385. https://doi.org/10.3390/molecules28145385
Chicago/Turabian StyleHandy, Kasey, and Gary C. Tepper. 2023. "Incorporation of Poly(TFEMA) in Perovskite Thin Films Using a Supercritical Fluid" Molecules 28, no. 14: 5385. https://doi.org/10.3390/molecules28145385
APA StyleHandy, K., & Tepper, G. C. (2023). Incorporation of Poly(TFEMA) in Perovskite Thin Films Using a Supercritical Fluid. Molecules, 28(14), 5385. https://doi.org/10.3390/molecules28145385





