Computational Studies on Microreactors for the Decomposition of Formic Acid for Hydrogen Production Using Heterogeneous Catalysts
Abstract
:1. Introduction
2. Results and Discussion
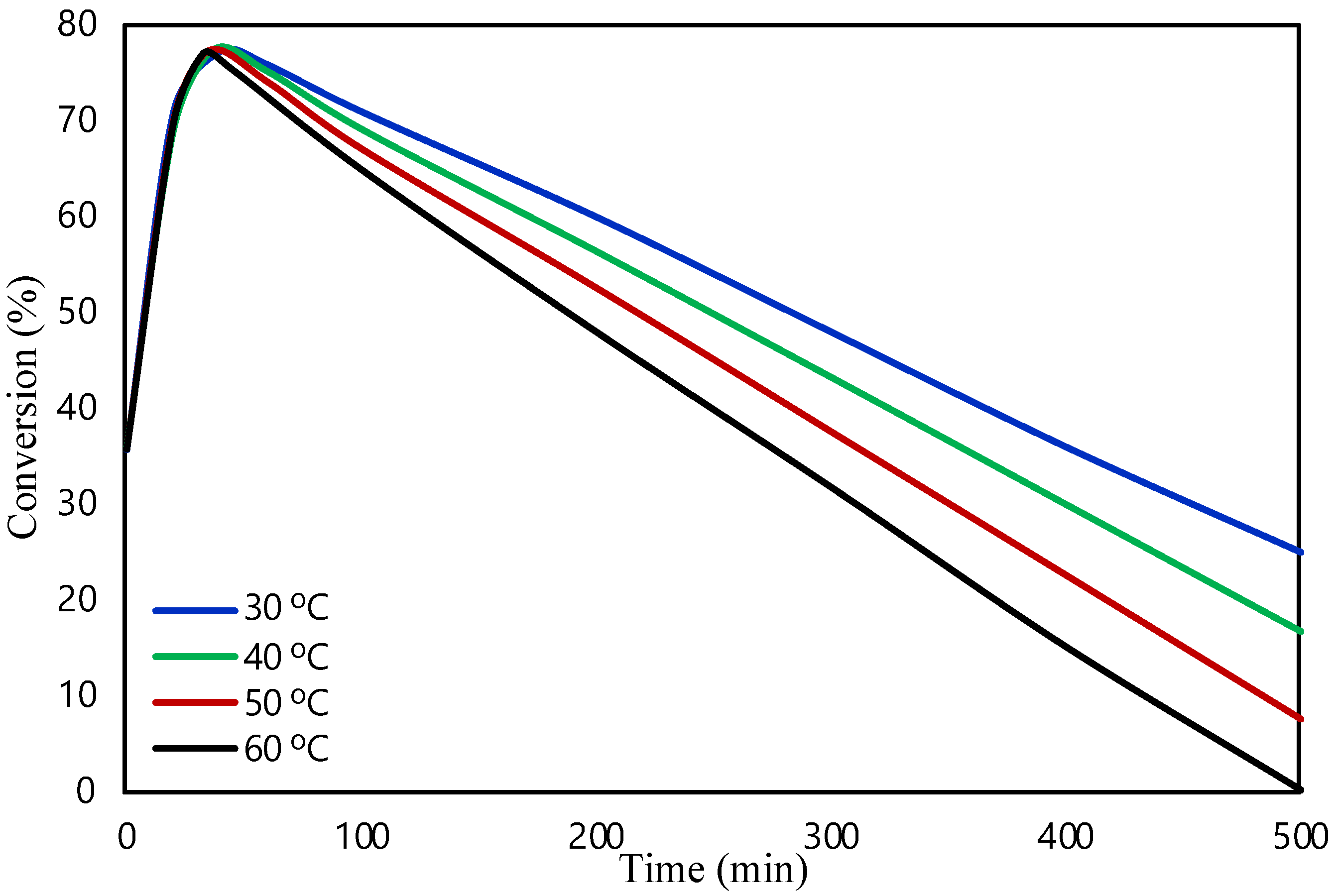
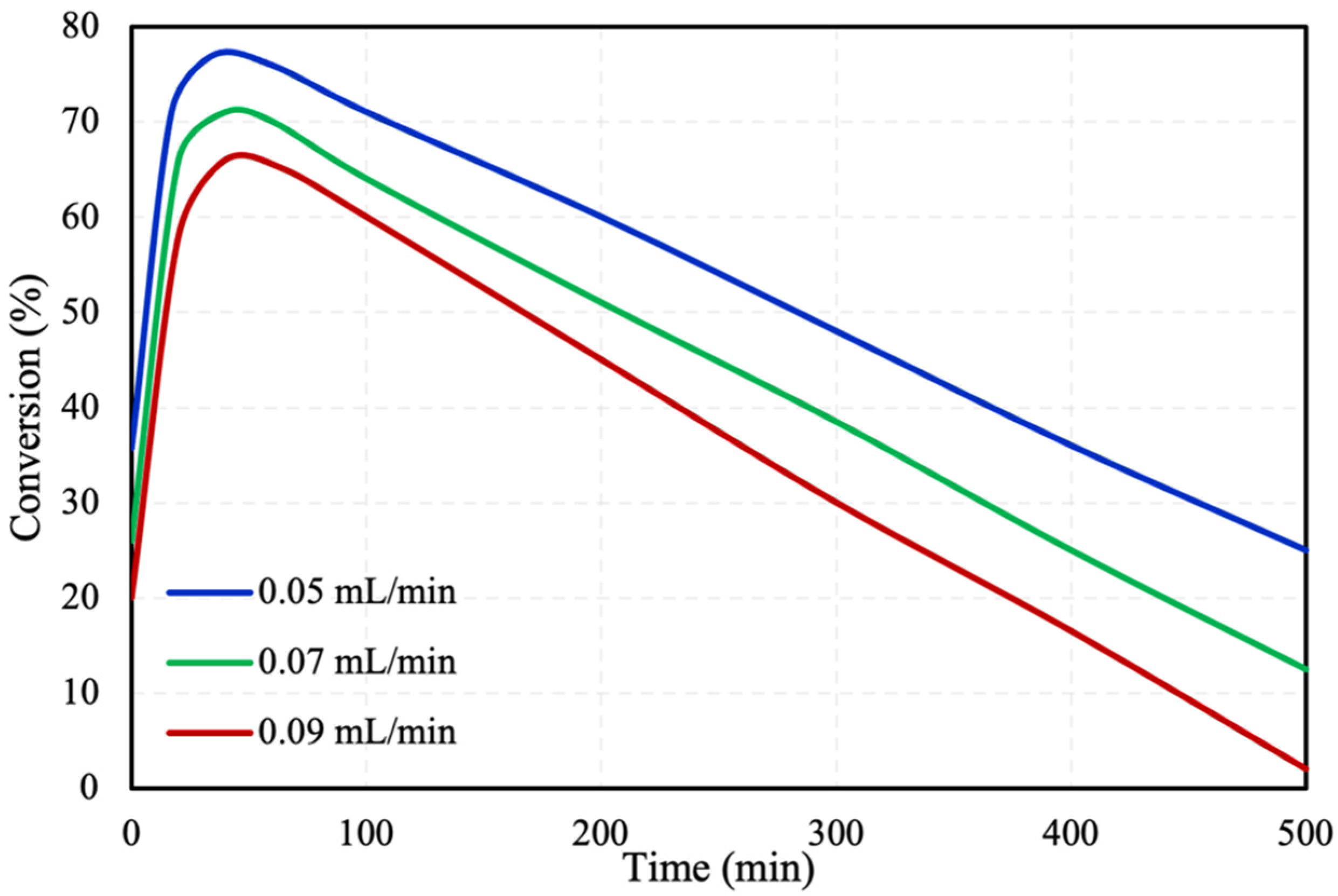
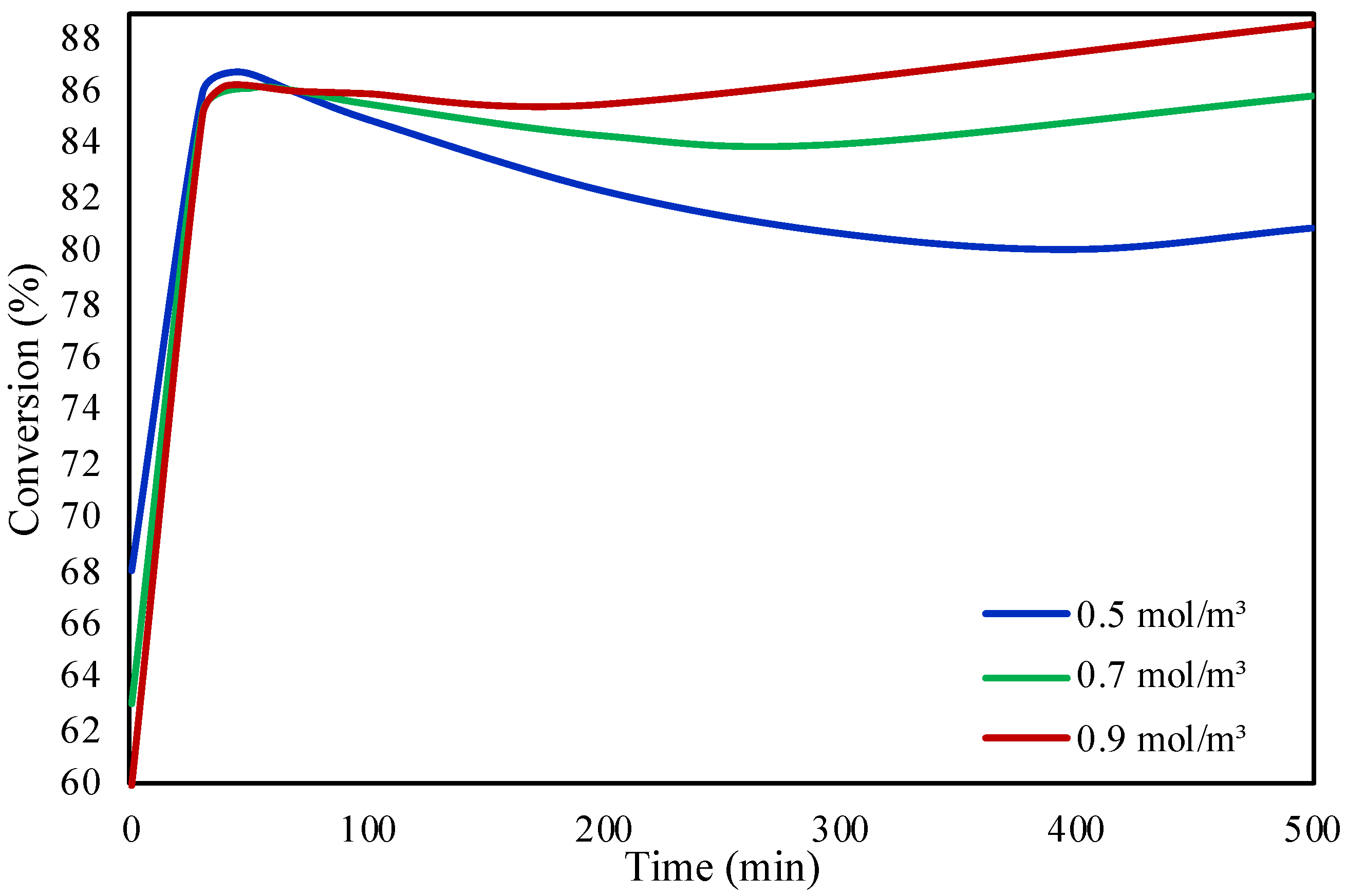
2.1. Packed Bed Microreactor
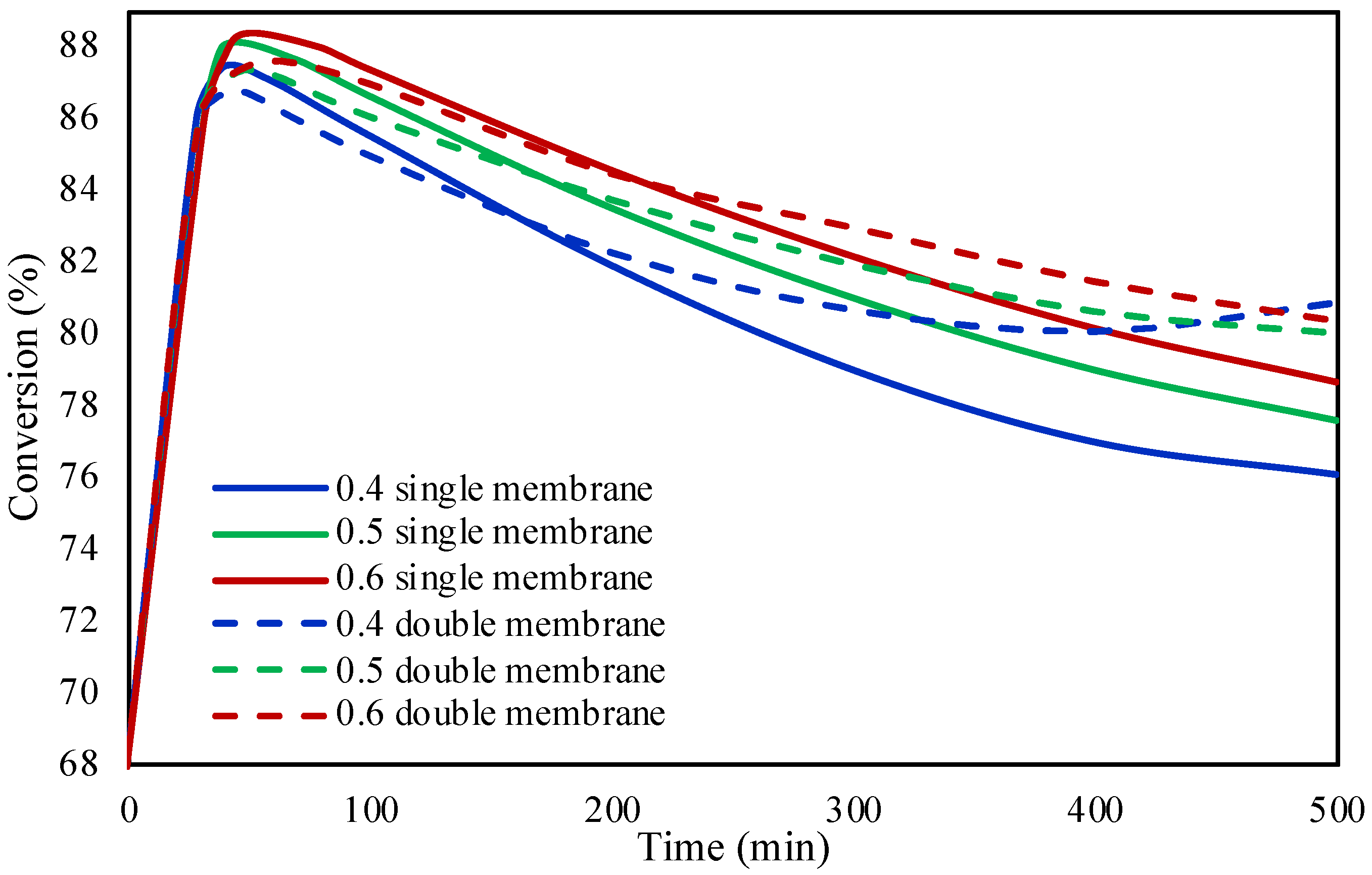
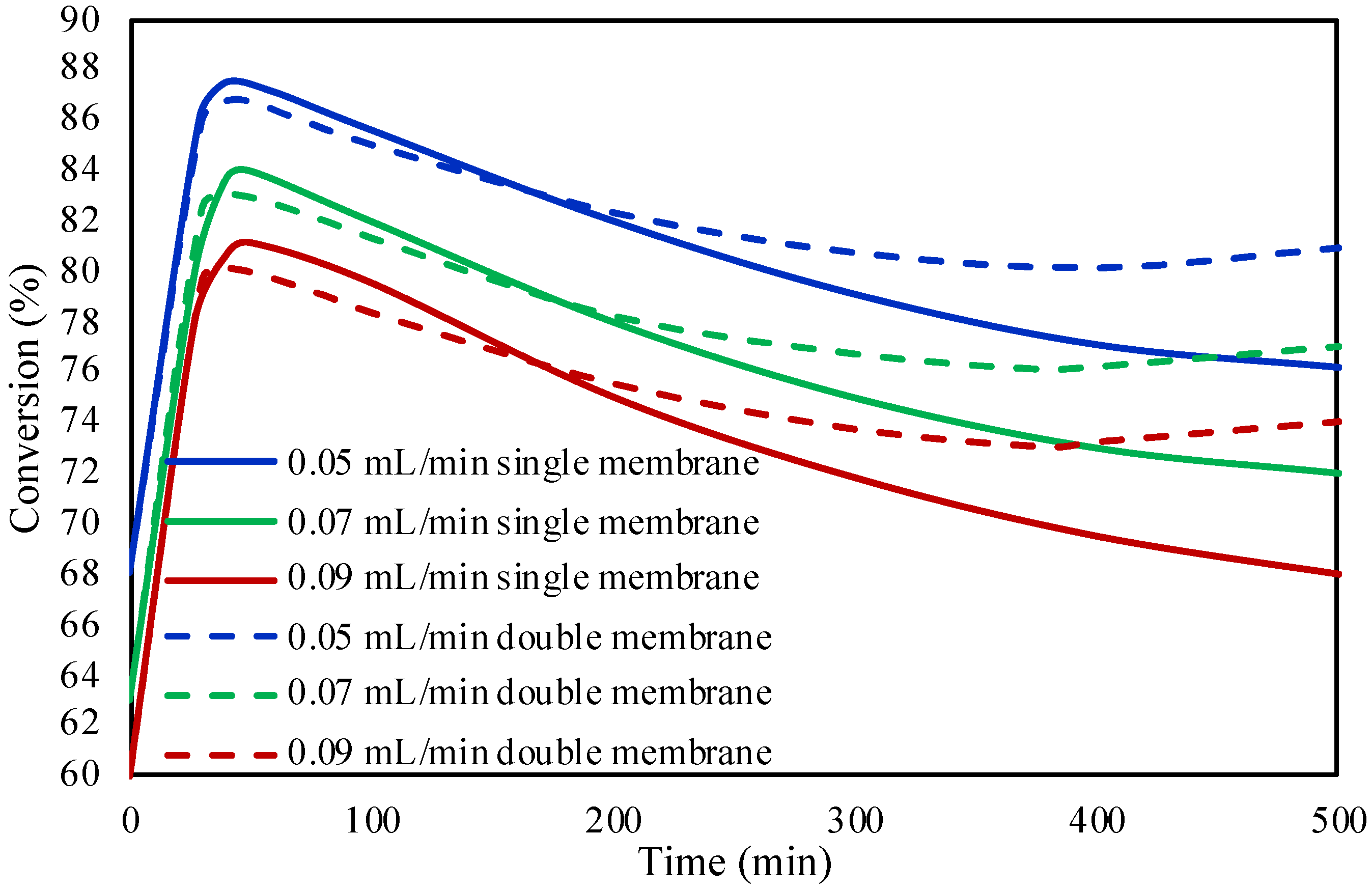
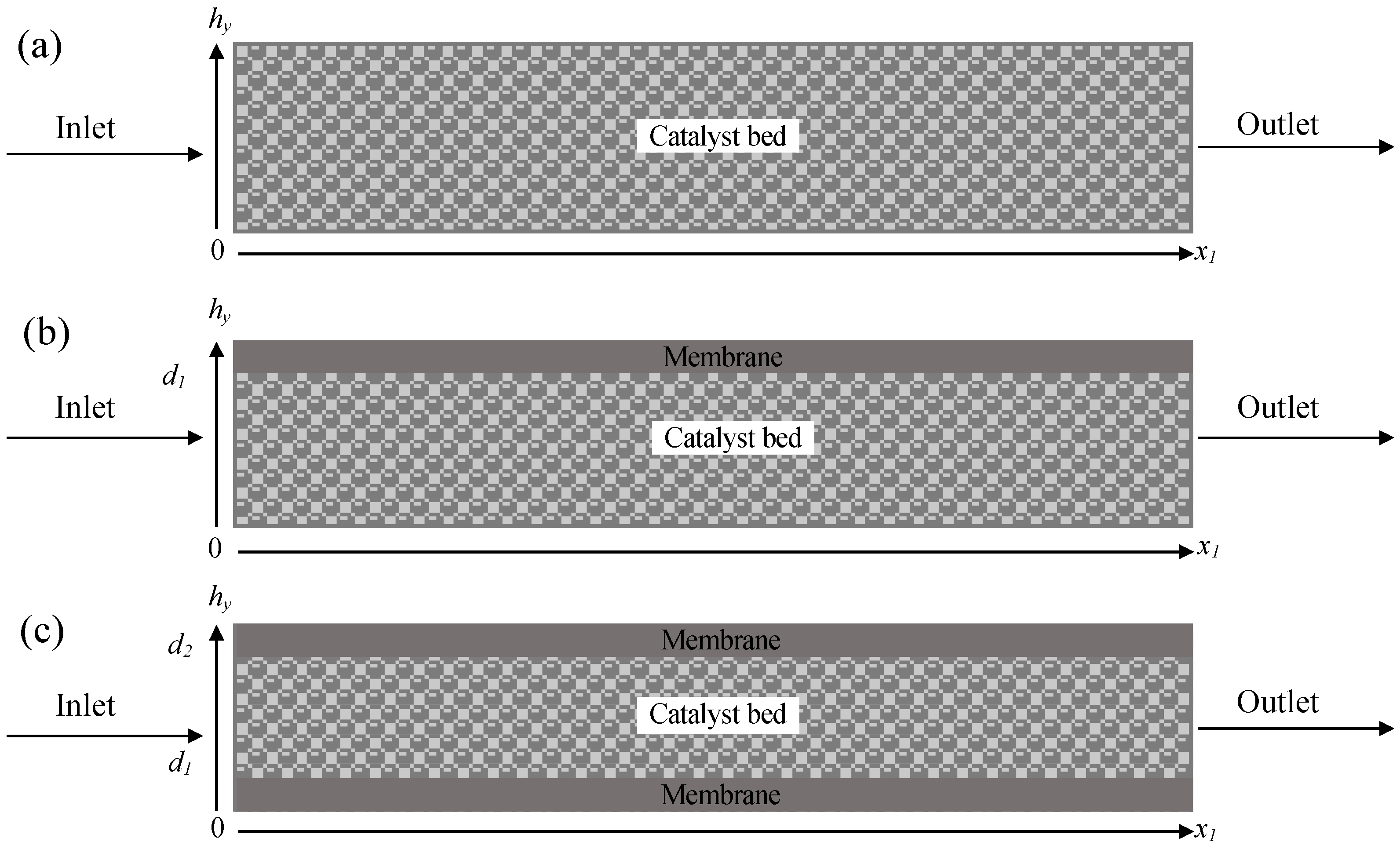
2.2. Single and Double Membrane Packed Bed Microreactor
3. Materials and Methods
3.1. Modelling Methodology
3.2. Reaction Kinetics
3.3. Conservation Equations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Martins, F.; Felgueiras, C.; Smitkova, M.; Caetano, N. Analysis of fossil fuel energy consumption and environmental impacts in European countries. Energies 2019, 12, 964. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.; Shah, H.H.; Fareed, A.G.; Khan, W.U.; Chung, E.; Zia, A.; Farooqi, Z.U.R.; Lee, C. Hydrogen production through renewable and non-renewable energy processes and their impact on climate change. Int. J. Hydrogen Energy 2022, 47, 33112–33134. [Google Scholar] [CrossRef]
- Stančin, H.; Mikulčić, H.; Wang, X.; Duić, N. A review on alternative fuels in future energy system. Renew. Sustain. Energy Rev. 2020, 128, 109927. [Google Scholar] [CrossRef]
- Bulushev, D.A. Progress in catalytic hydrogen production from formic acid over supported metal complexes. Energies 2021, 14, 1334. [Google Scholar] [CrossRef]
- Navlani-García, M.; Salinas-Torres, D.; Cazorla-Amorós, D. Hydrogen production from formic acid attained by bimetallic heterogeneous PdAg catalytic systems. Energies 2019, 12, 4027. [Google Scholar] [CrossRef] [Green Version]
- Joó, F. Breakthroughs in Hydrogen Storage—Formic Acid as a Sustainable Storage Material for Hydrogen. ChemSusChem 2008, 1, 805–808. [Google Scholar] [CrossRef]
- Loges, B.; Boddien, A.; Gärtner, F.; Junge, H.; Beller, M. Catalytic Generation of Hydrogen from Formic acid and its Derivatives: Useful Hydrogen Storage Materials. Top. Catal. 2010, 53, 902–914. [Google Scholar] [CrossRef]
- Valentini, F.; Kozell, V.; Petrucci, C.; Marrocchi, A.; Gu, Y.; Gelman, D.; Vaccaro, L. Formic acid, a biomass-derived source of energy and hydrogen for biomass upgrading. Energy Environ. Sci. 2019, 12, 2646–2664. [Google Scholar] [CrossRef]
- Gan, W.; Dyson, P.J.; Laurenczy, G. Hydrogen storage and delivery: Immobilization of a highly active homogeneous catalyst for the decomposition of formic acid to hydrogen and carbon dioxide. React. Kinet. Catal. Lett. 2009, 98, 205–213. [Google Scholar] [CrossRef]
- Bhandari, S.; Rangarajan, S.; Maravelias, C.T.; Dumesic, J.A.; Mavrikakis, M. Reaction mechanism of vapor-phase formic acid decomposition over platinum catalysts: DFT, reaction kinetics experiments, and microkinetic modeling. Acs Catal. 2020, 10, 4112–4126. [Google Scholar] [CrossRef]
- Wang, X.; Meng, Q.; Gao, L.; Jin, Z.; Ge, J.; Liu, C.; Xing, W. Recent progress in hydrogen production from formic acid decomposition. Int. J. Hydrogen Energy 2018, 43, 7055–7071. [Google Scholar] [CrossRef]
- Younas, M.; Rezakazemi, M.; Arbab, M.S.; Shah, J.; Rehman, W.U. Green hydrogen storage and delivery: Utilizing highly active homogeneous and heterogeneous catalysts for formic acid dehydrogenation. Int. J. Hydrogen Energy 2022, 47, 11694–11724. [Google Scholar] [CrossRef]
- Wang, X.; Meng, Q.; Gao, L.; Liu, J.; Ge, J.; Liu, C.; Xing, W. Metal organic framework derived nitrogen-doped carbon anchored palladium nanoparticles for ambient temperature formic acid decomposition. Int. J. Hydrogen Energy 2019, 44, 28402–28408. [Google Scholar] [CrossRef]
- Ding, T.-Y.; Zhao, Z.-G.; Ran, M.-F.; Yang, Y.-Y. Superior activity of Pd nanoparticles confined in carbon nanotubes for hydrogen production from formic acid decomposition at ambient temperature. J. Colloid Interface Sci. 2019, 538, 474–480. [Google Scholar] [CrossRef]
- Hafeez, S.; Harkou, E.; Spanou, A.; Al-Salem, S.M.; Villa, A.; Dimitratos, N.; Manos, G.; Constantinou, A. Review on recent progress and reactor set-ups for hydrogen production from formic acid decomposition. Mater. Today Chem. 2022, 26, 101120. [Google Scholar] [CrossRef]
- Choi, B.-S.; Song, J.; Song, M.; Goo, B.S.; Lee, Y.W.; Kim, Y.; Yang, H.; Han, S.W. Core–shell engineering of Pd–Ag bimetallic catalysts for efficient hydrogen production from formic acid decomposition. ACS Catal. 2018, 9, 819–826. [Google Scholar] [CrossRef]
- Zhang, Y.; Lyu, Y.; Wang, Y.; Li, C.; Jiang, M.; Ding, Y. Highly active and stable porous polymer heterogenous catalysts for decomposition of formic acid to produce H2. Chin. J. Catal. 2019, 40, 147–151. [Google Scholar] [CrossRef]
- Barlocco, I.; Capelli, S.; Lu, X.; Bellomi, S.; Huang, X.; Wang, D.; Prati, L.; Dimitratos, N.; Roldan, A.; Villa, A. Disclosing the role of gold on palladium–gold alloyed supported catalysts in formic acid decomposition. ChemCatChem 2021, 13, 4210–4222. [Google Scholar] [CrossRef]
- Winkler, T.; Baccot, F.; Eränen, K.; Wärnå, J.; Hilpmann, G.; Lange, R.; Peurla, M.; Simakova, I.; Grénman, H.; Murzin, D.Y. Catalytic decomposition of formic acid in a fixed bed reactor—An experimental and modelling study. Catal. Today 2022, 387, 128–139. [Google Scholar] [CrossRef]
- Hafeez, S.; Sanchez, F.; Al-Salem, S.M.; Villa, A.; Manos, G.; Dimitratos, N.; Constantinou, A. Decomposition of additive-free formic acid using a pd/c catalyst in flow: Experimental and cfd modelling studies. Catalysts 2021, 11, 341. [Google Scholar] [CrossRef]
- Hafeez, S.; Barlocco, I.; Al-Salem, S.M.; Villa, A.; Chen, X.; Delgado, J.J.; Manos, G.; Dimitratos, N.; Constantinou, A. Experimental and process modelling investigation of the hydrogen generation from formic acid decomposition using a pd/zn catalyst. Appl. Sci. 2021, 11, 8462. [Google Scholar] [CrossRef]
- Caiti, M.; Padovan, D.; Hammond, C. Continuous Production of Hydrogen from Formic Acid Decomposition over Heterogeneous Nanoparticle Catalysts: From Batch to Continuous Flow. ACS Catal. 2019, 9, 9188–9198. [Google Scholar] [CrossRef]
- Xu, R.; Lu, W.; Toan, S.; Zhou, Z.; Russell, C.K.; Sun, Z.; Sun, Z. Thermocatalytic formic acid dehydrogenation: Recent advances and emerging trends. J. Mater. Chem. A 2021, 9, 24241–24260. [Google Scholar] [CrossRef]
- Wang, Z.; Ashok, J.; Pu, Z.; Kawi, S. Low temperature partial oxidation of methane via BaBi0. 05Co0. 8Nb0. 15O3− δ-Ni phyllosilicate catalytic hollow fiber membrane reactor. Chem. Eng. J. 2017, 315, 315–323. [Google Scholar] [CrossRef]
- Shahbaz, M.; Al-Ansari, T.; Aslam, M.; Khan, Z.; Inayat, A.; Athar, M.; Naqvi, S.R.; Ahmed, M.A.; McKay, G. A state of the art review on biomass processing and conversion technologies to produce hydrogen and its recovery via membrane separation. Int. J. Hydrogen Energy 2020, 45, 15166–15195. [Google Scholar] [CrossRef]
- Cechetto, V.; Di Felice, L.; Medrano, J.A.; Makhloufi, C.; Zuniga, J.; Gallucci, F. H2 production via ammonia decomposition in a catalytic membrane reactor. Fuel Process. Technol. 2021, 216, 106772. [Google Scholar] [CrossRef]
- Hafeez, S.; Al-Salem, S.M.; Bansode, A.; Villa, A.; Dimitratos, N.; Manos, G.; Constantinou, A. Computational Investigation of Microreactor Configurations for Hydrogen Production from Formic Acid Decomposition Using a Pd/C Catalyst. Ind. Eng. Chem. Res. 2022, 61, 1655–1665. [Google Scholar] [CrossRef]
- Hafeez, S.; Aristodemou, E.; Manos, G.; Al-Salem, S.M.; Constantinou, A. Computational fluid dynamics (CFD) and reaction modelling study of bio-oil catalytic hydrodeoxygenation in microreactors. React. Chem. Eng. 2020, 5, 1083–1092. [Google Scholar] [CrossRef]
- Hafeez, S.; Aristodemou, E.; Manos, G.; Al-Salem, S.M.; Constantinou, A. Modelling of packed bed and coated wall microreactors for methanol steam reforming for hydrogen production. RSC Adv. 2020, 10, 41680–41692. [Google Scholar] [CrossRef]
- Hafeez, S.; Al-Salem, S.M.; Manos, G.; Constantinou, A. Fuel production using membrane reactors: A review. Environ. Chem. Lett. 2020, 18, 1477–1490. [Google Scholar] [CrossRef]
- Harkou, E.; Hafeez, S.; Manos, G.; Constantinou, A. CFD Study of the Numbering up of Membrane Microreactors for CO2 Capture. Processes 2021, 9, 1515. [Google Scholar] [CrossRef]
- Wu, G.; Cao, E.; Ellis, P.; Constantinou, A.; Kuhn, S.; Gavriilidis, A. Development of a flat membrane microchannel packed-bed reactor for scalable aerobic oxidation of benzyl alcohol in flow. Chem. Eng. J. 2019, 377, 120086. [Google Scholar] [CrossRef]
- Constantinou, A.; Wu, G.; Venezia, B.; Ellis, P.; Kuhn, S.; Gavriilidis, A. Aerobic Oxidation of Benzyl Alcohol in a Continuous Catalytic Membrane Reactor. Top. Catal. 2019, 62, 1126–1131. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, F.; Motta, D.; Roldan, A.; Hammond, C.; Villa, A.; Dimitratos, N. Hydrogen generation from additive-free formic acid decomposition under mild conditions by Pd/C: Experimental and DFT studies. Top. Catal. 2018, 61, 254–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Qi, Y.; Zhang, D.; Liu, C. New Insight into the Decomposition Mechanism of Formic Acid on Pd(111): Competing Formation of CO2 and CO. J. Phys. Chem. C 2014, 118, 2067–2076. [Google Scholar] [CrossRef]
- Kosider, A.; Blaumeiser, D.; Schötz, S.; Preuster, P.; Bösmann, A.; Wasserscheid, P.; Libuda, J.; Bauer, T. Enhancing the feasibility of Pd/C-catalyzed formic acid decomposition for hydrogen generation–catalyst pretreatment, deactivation, and regeneration. Catal. Sci. Technol. 2021, 11, 4259–4271. [Google Scholar] [CrossRef]
- Sandoval, V.H.; Gigola, C.E. Characterization of Pd and Pd Pbα-Al2O3 catalysts. A TPR-TPD study. Appl. Catal. A Gen. 1996, 148, 81–96. [Google Scholar] [CrossRef]
- Richardson, J.; Harker, J.; Backhurst, J. Flow of fluids through granular beds and packed columns. Chem. Eng. 2002, 2, 191–236. [Google Scholar]
- Fogler, H.S. Elements of Chemical Reaction Engineering, 3rd ed.; Prentice Hall PTR: Upper Saddle River, NJ, USA, 1999; Available online: https://search.library.wisc.edu/catalog/999810177702121 (accessed on 10 June 2023).
- Froessling, N. Über die verdunstung fallender tropfen. Gerlands Beiträge Zur Geophys. 1938, 52, 170–216. [Google Scholar]


| Mesh Quality | Time to Compute | Elements | Vertices | Boundary Elements | Degrees of Freedom | Max. Conv. Rate (30 min) | Difference |
|---|---|---|---|---|---|---|---|
| Normal | 5 s | 98 | 68 | 36 | 996 | 77.766% | Reference |
| Fine | 5 s | 154 | 100 | 44 | 1524 | 77.752% | −0.0018% |
| Extra Fine | 10 s | 1010 | 564 | 116 | 9444 | 77.720% | −0.041% |
| Extremely Fine | 23 s | 4030 | 2132 | 232 | 36,972 | 77.703% | −0.022% |
| Symbol | Value | Units | Description |
|---|---|---|---|
| 0.5 | mol m−3 | FA inlet concentration | |
| 0.05 | mL min−1 | Volumetric inlet flow rate | |
| 25 | mm | Microreactor length | |
| 4 | mm | Microreactor height | |
| 1 | mm | Membrane thickness | |
| 303–333 | K | Reaction temperature | |
| 4 | nm | Catalyst pellet size | |
| 1 | kg m−3 | Fluid density | |
| 1300 | kg m−3 | Catalyst density | |
| 0.4 | - | Clean catalyst porosity | |
| 8.314 | J mol−1 K−1 | Ideal gas constant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harkou, E.; Adamou, P.; Georgiou, K.; Hafeez, S.; Al-Salem, S.M.; Villa, A.; Manos, G.; Dimitratos, N.; Constantinou, A. Computational Studies on Microreactors for the Decomposition of Formic Acid for Hydrogen Production Using Heterogeneous Catalysts. Molecules 2023, 28, 5399. https://doi.org/10.3390/molecules28145399
Harkou E, Adamou P, Georgiou K, Hafeez S, Al-Salem SM, Villa A, Manos G, Dimitratos N, Constantinou A. Computational Studies on Microreactors for the Decomposition of Formic Acid for Hydrogen Production Using Heterogeneous Catalysts. Molecules. 2023; 28(14):5399. https://doi.org/10.3390/molecules28145399
Chicago/Turabian StyleHarkou, Eleana, Panayiota Adamou, Kyproula Georgiou, Sanaa Hafeez, Sultan M. Al-Salem, Alberto Villa, George Manos, Nikolaos Dimitratos, and Achilleas Constantinou. 2023. "Computational Studies on Microreactors for the Decomposition of Formic Acid for Hydrogen Production Using Heterogeneous Catalysts" Molecules 28, no. 14: 5399. https://doi.org/10.3390/molecules28145399









