Influence of the Emulsifier Sodium Caseinate–Xanthan Gum Complex on Emulsions: Stability and Digestive Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. pH Stability
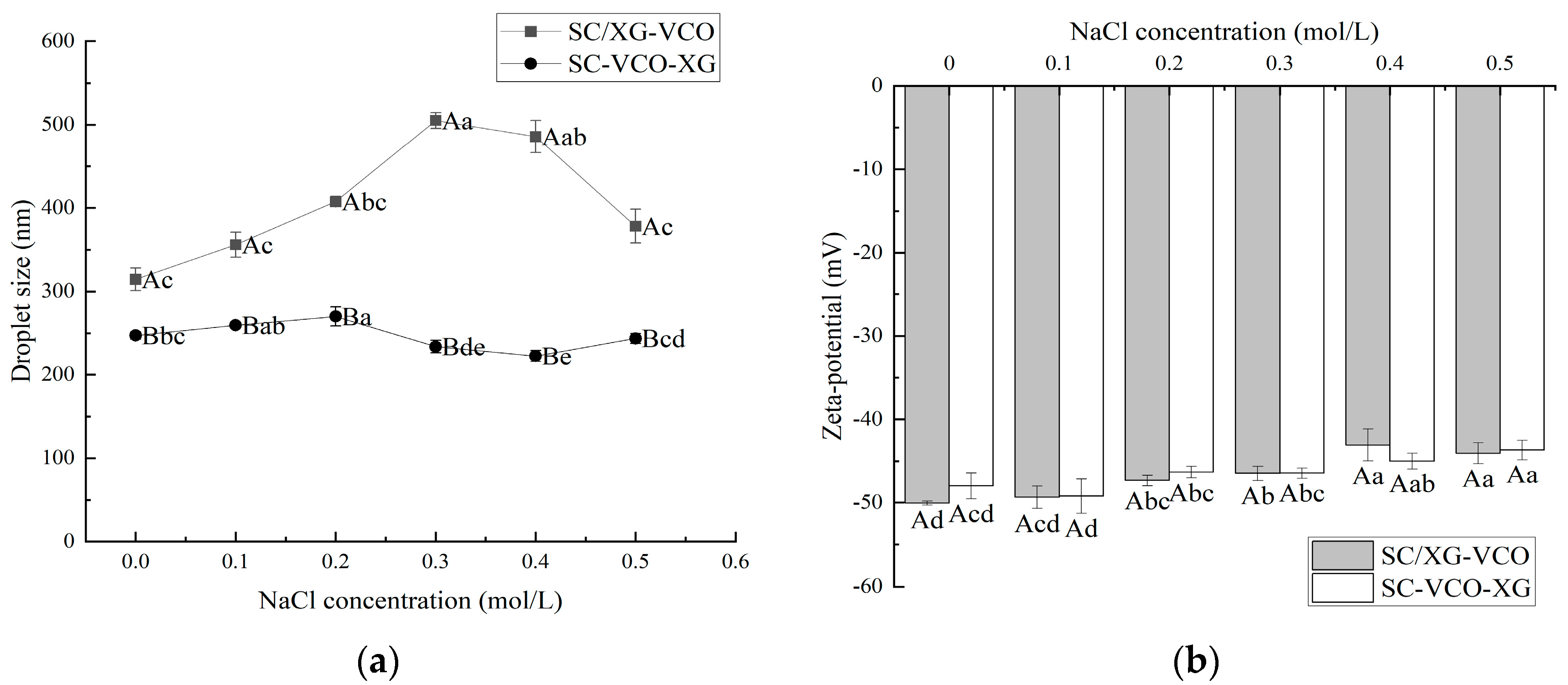
2.2. Ionic Strength Stability
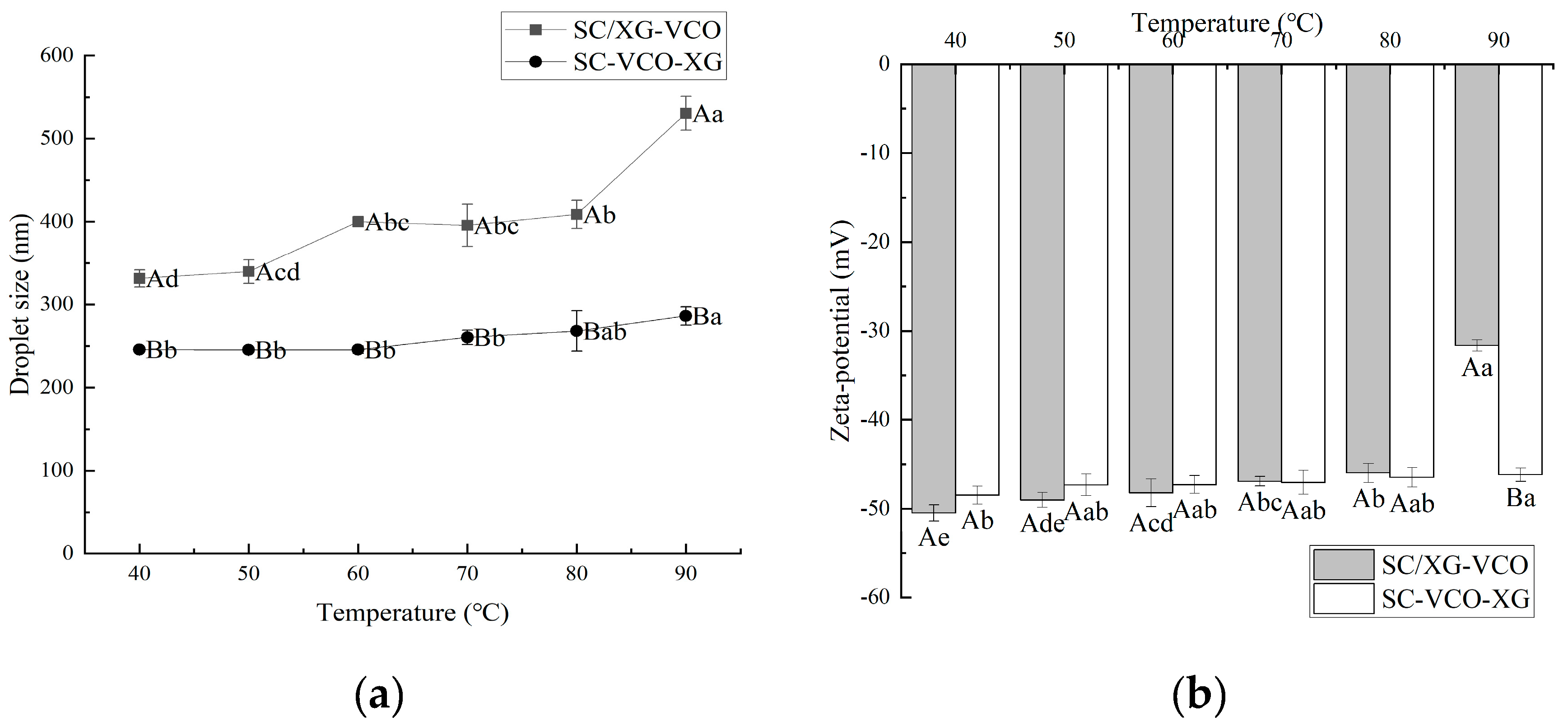
2.3. Thermal Stability
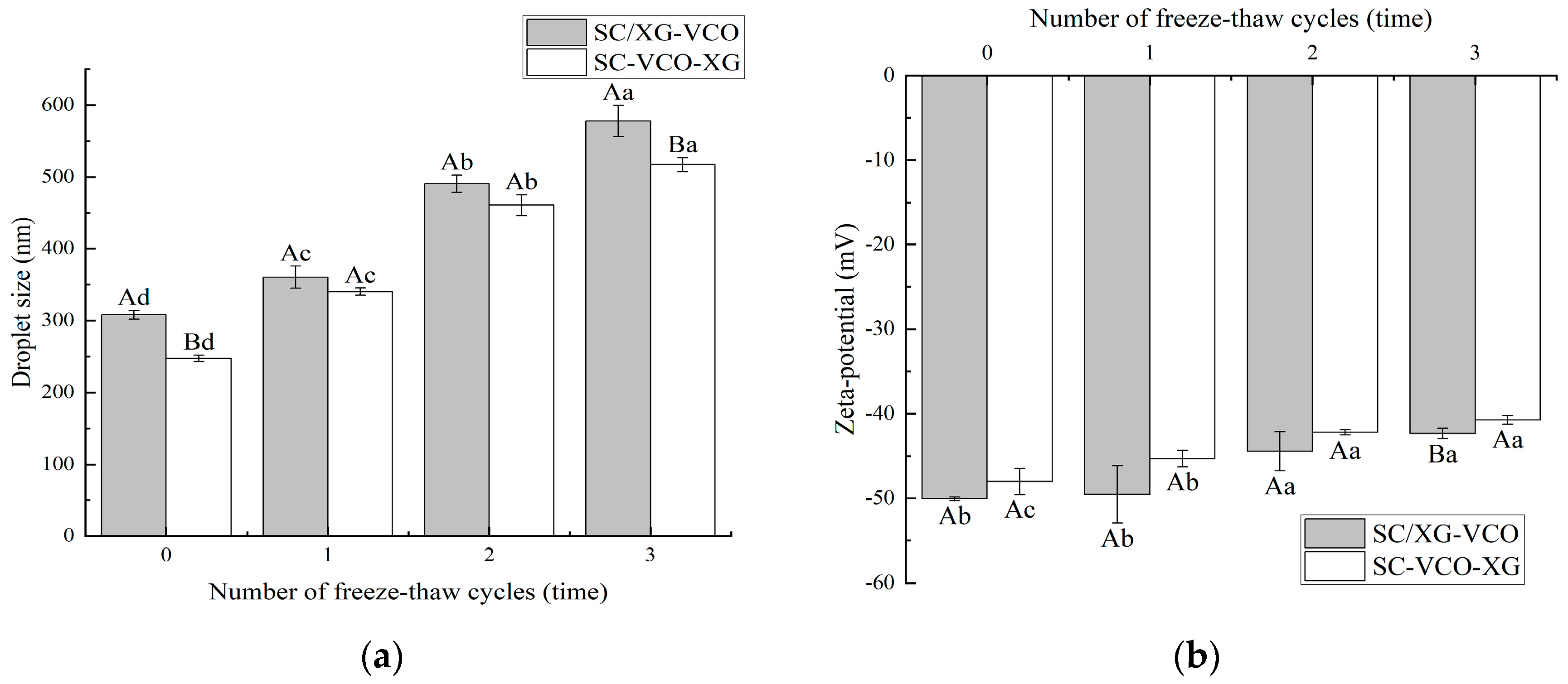
2.4. Freeze-Thaw Stability
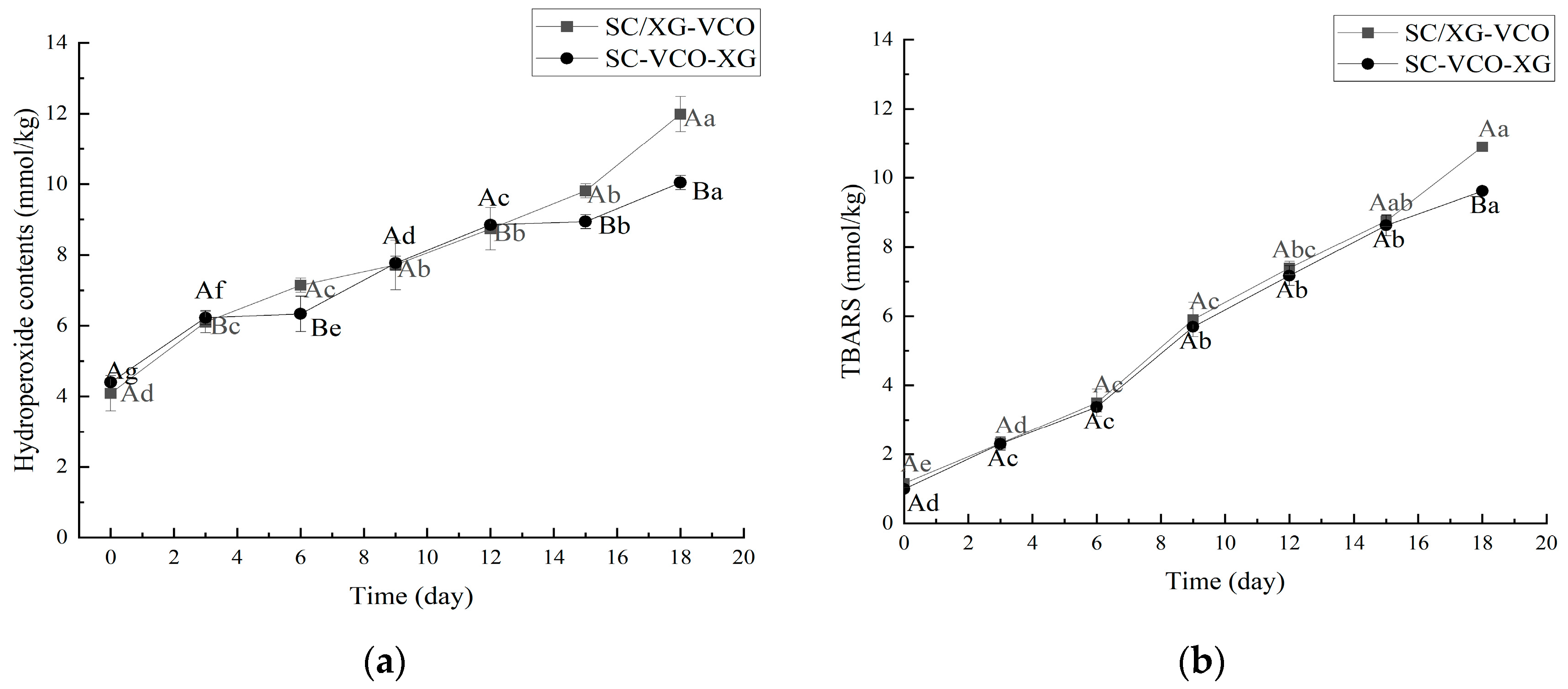
2.5. Oxidative Stability
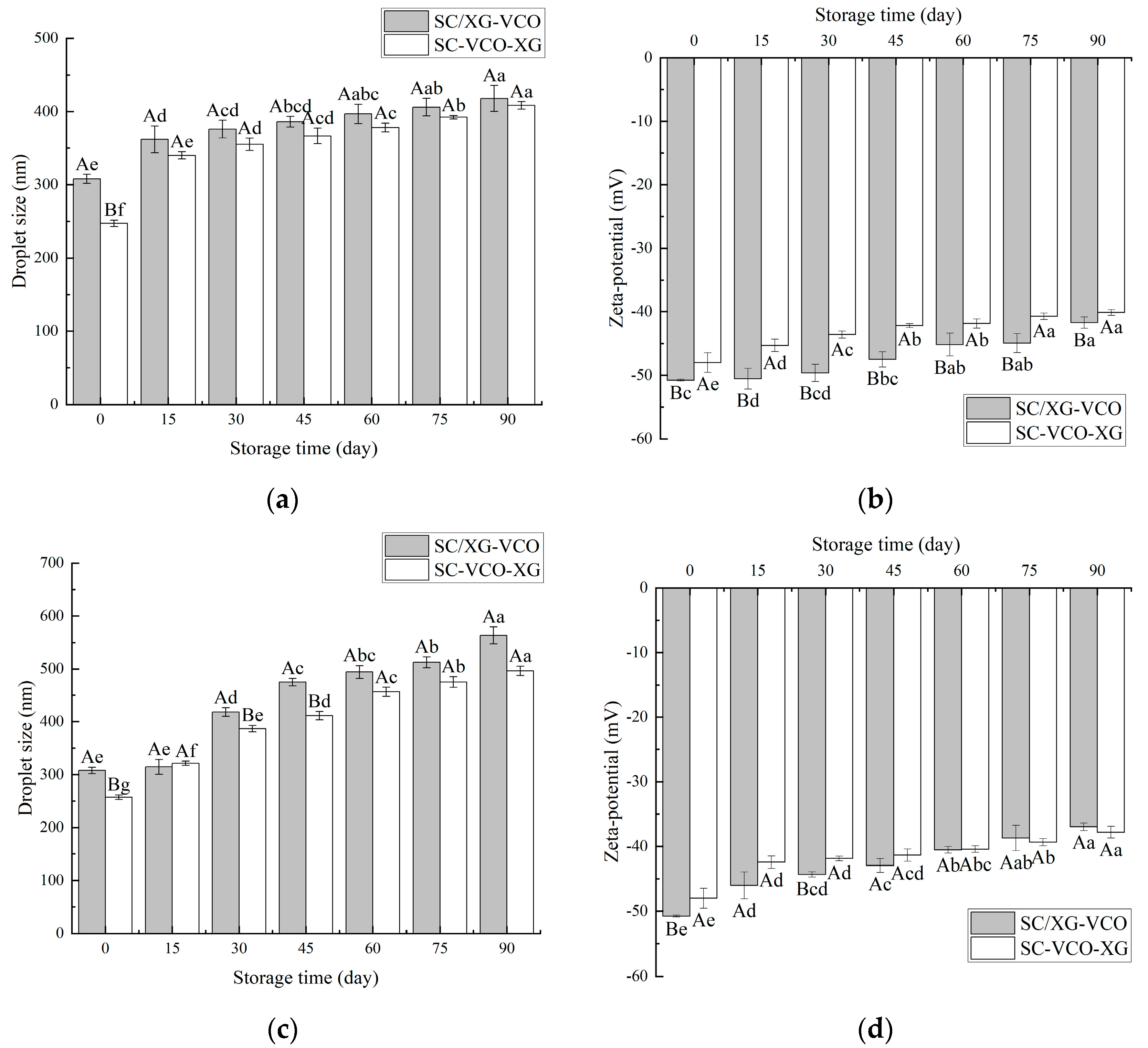
2.6. Storage Stability
2.7. Digestive Properties
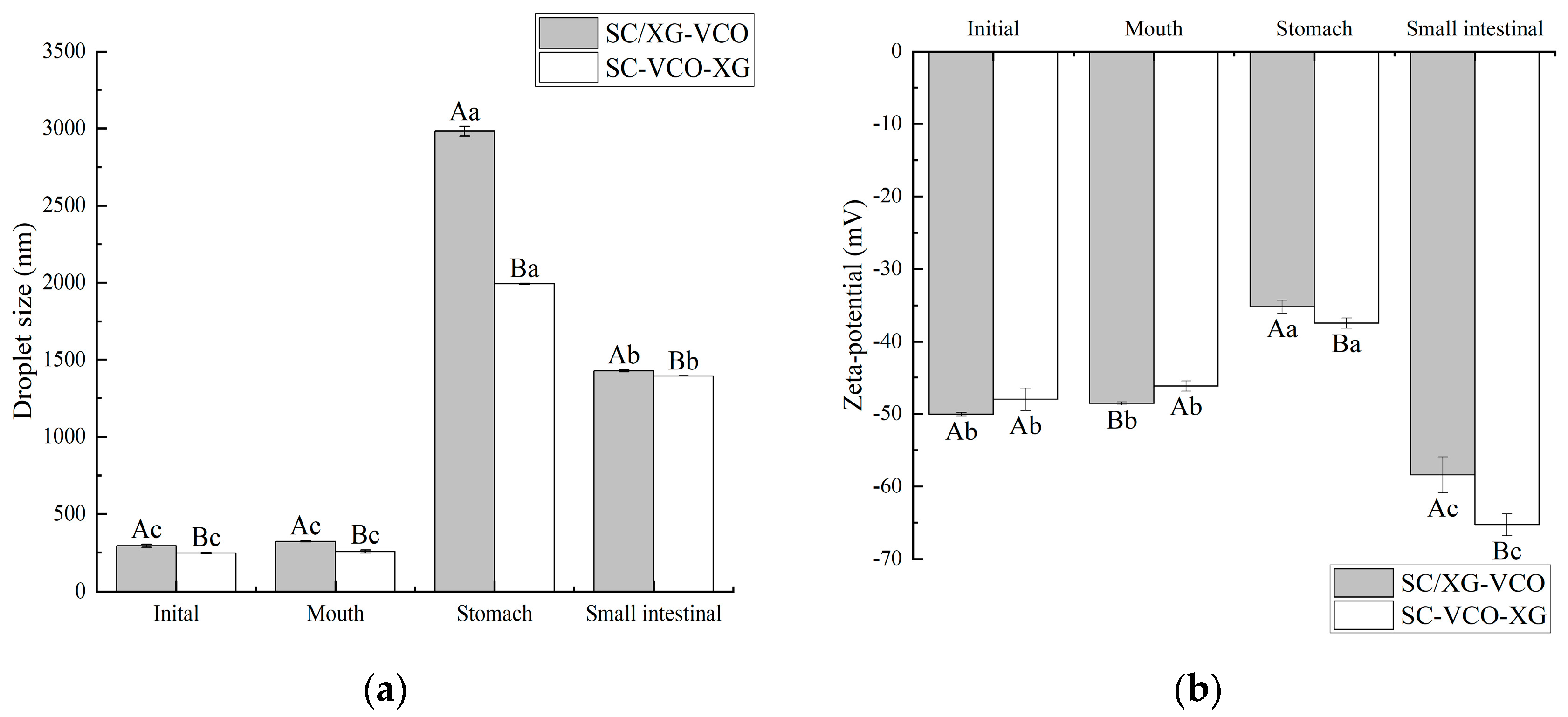
2.7.1. Changes in Droplet Size and Zeta Potential during In Vitro Digestion
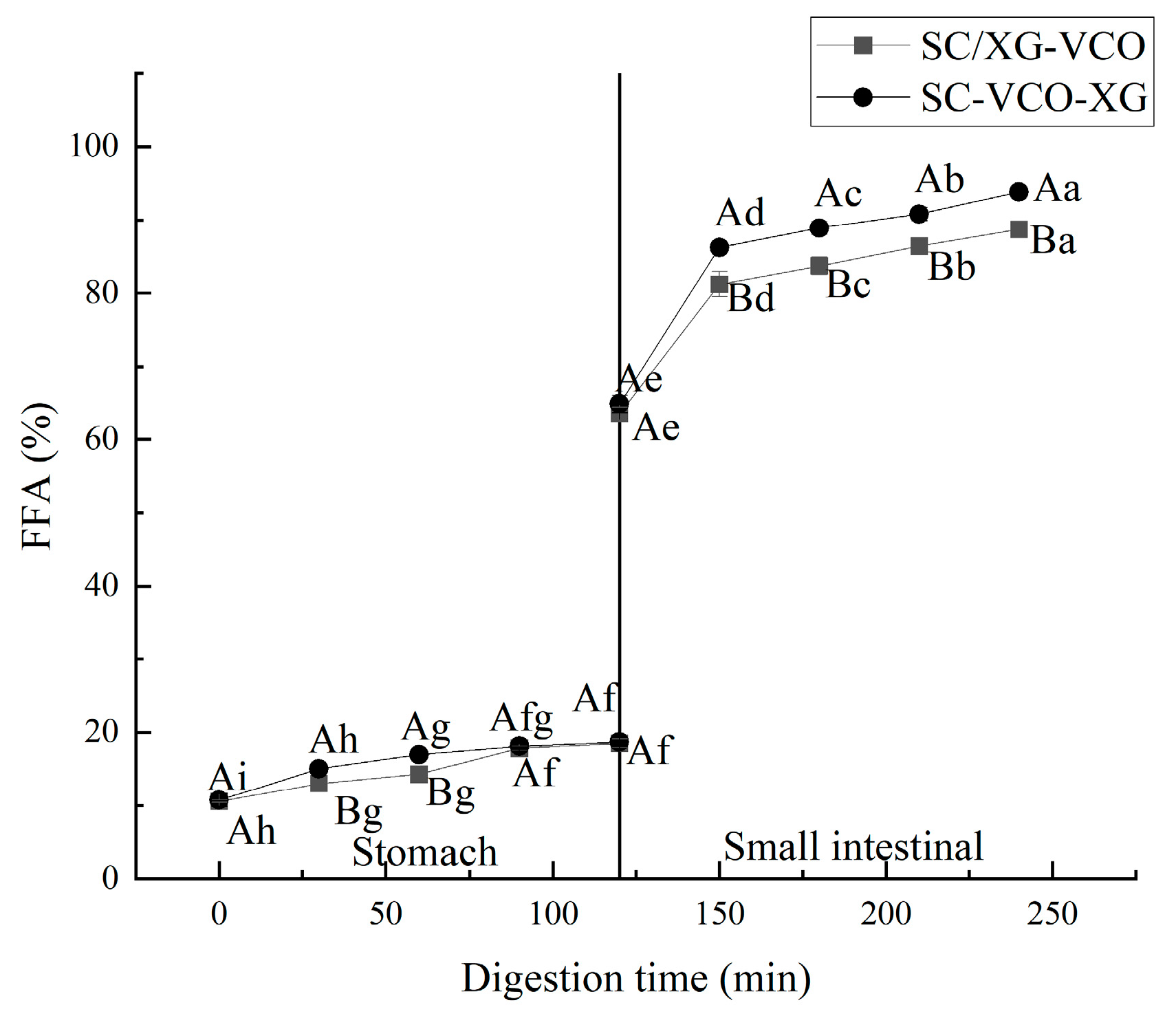
2.7.2. Digestibility
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Coconut Oil Nanoemulsion
3.3. Stability Experiments
3.3.1. pH Stability
3.3.2. Ionic Strength Stability
3.3.3. Thermal Stability
3.3.4. Freeze–Thaw Stability
3.3.5. Oxidation Stability
3.3.6. Storage Stability
3.3.7. Determination of Droplet Size and Zeta Potential
3.4. In Vitro Digestion Analysis
3.4.1. Construction of In Vitro Digestion Model
3.4.2. Changes in Droplet Size and Potential during In Vitro Digestion
3.4.3. Determination of Free Fatty Acids
3.5. Data Processing and Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Solans, C.; Solé, I. Nano-emulsions: Formation by low-energy methods. Curr. Opin. Colloid Interface Sci. 2012, 17, 246–254. [Google Scholar]
- Wei, Y.; Tong, Z.; Dai, L.; Ma, P.; Zhang, M.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Novel colloidal particles and natural small molecular surfactants co-stabilized Pickering emulsions with hierarchical interfacial structure: Enhanced stability and controllable lipolysis. J. Colloid Interface Sci. 2020, 563, 291–307. [Google Scholar]
- Albano, K.M.; Cavallieri, Â.L.F.; Nicoletti, V.R. Electrostatic interaction between proteins and polysaccharides: Physicochemical aspects and applications in emulsion stabilization. Food Rev. Int. 2019, 35, 54–89. [Google Scholar] [CrossRef]
- Kapiamba, K.F. Mini-review of the microscale phenomena during emulsification of highly concentrated emulsions. Colloid Interface Sci. Commun. 2022, 47, 100597. [Google Scholar]
- Masalova, I.; Kapiamba, F.; Tshilumbu, N.; Malkin, A.Y. Shear Stability of Highly Concentrated Emulsions. Colloid J. 2018, 80, 54–58. [Google Scholar] [CrossRef]
- Masalova, I.; Fabrice, K.K.; Tshilumbu, N.N.; George, N.; Malkin, A.Y. Emulsification of highly concentrated emulsions—A criterion of shear stability. J. Rheol. 2018, 62, 781–790. [Google Scholar] [CrossRef]
- Liu, J.; Verespej, E.; Alexander, M.; Corredig, M. Comparison on the Effect of High-Methoxyl Pectin or Soybean-Soluble Polysaccharide on the Stability of Sodium Caseinate-Stabilized Oil/Water Emulsions. J. Agric. Food Chem. 2007, 55, 6270–6278. [Google Scholar] [CrossRef]
- Wang, S.; Yang, J.; Shao, G.; Liu, J.; Wang, J.; Yang, L.; Li, J.; Liu, H.; Zhu, D.; Li, Y.; et al. pH-induced conformational changes and interfacial dilatational rheology of soy protein isolated/soy hull polysaccharide complex and its effects on emulsion stabilization. Food Hydrocoll. 2020, 109, 106075. [Google Scholar]
- Xu, X.; Luo, L.; Liu, C.; McClements, D.J. Utilization of anionic polysaccharides to improve the stability of rice glutelin emulsions: Impact of polysaccharide type, pH, salt, and temperature. Food Hydrocoll. 2017, 64, 112–122. [Google Scholar] [CrossRef]
- Cabezas, D.M.; Pascual, G.N.; Wagner, J.R.; Palazolo, G.G. Nanoparticles assembled from mixtures of whey protein isolate and soluble soybean polysaccharides. Structure, interfacial behavior and application on emulsions subjected to freeze-thawing. Food Hydrocoll. 2019, 95, 445–453. [Google Scholar] [CrossRef]
- Ho, K.K.; Schroën, K.; San Martín-González, M.F.; Berton-Carabin, C.C. Physicochemical stability of lycopene-loaded emulsions stabilized by plant or dairy proteins. Food Struct. 2017, 12, 34–42. [Google Scholar] [CrossRef]
- Sun, C.; Liang, B.; Sheng, H.; Wang, R.; Zhao, J.; Zhang, Z.; Zhang, M. Influence of initial protein structures and xanthan gum on the oxidative stability of O/W emulsions stabilized by whey protein. Int. J. Biol. Macromol. 2018, 120, 34–44. [Google Scholar]
- Fan, L.; Liu, Y.; Huang, S.; Li, J. Effects of proteins on emulsion stability: The role of proteins at the oil–water interface. Food Chem. 2022, 397, 133726. [Google Scholar]
- Guzey, D.; McClements, D.J. Impact of electrostatic interactions on formation and stability of emulsions containing oil droplets coated by ß-lactoglobulin−pectin complexes. J. Agric. Food Chem. 2007, 55, 475–485. [Google Scholar] [CrossRef]
- Perrechil, F.A.; Cunha, R.L. Stabilization of multilayered emulsions by sodium caseinate and κ-carrageenan. Food Hydrocoll. 2013, 30, 606–613. [Google Scholar] [CrossRef]
- Ray, M.; Rousseau, D. Stabilization of oil-in-water emulsions using mixtures of denatured soy whey proteins and soluble soybean polysaccharides. Food Res. Int. 2013, 52, 298–307. [Google Scholar] [CrossRef]
- Yan, S.; Xu, J.; Liu, G.; Du, X.; Hu, M.; Zhang, S.; Jiang, L.; Zhu, H.; Qi, B.; Li, Y. Emulsions co-stabilized by soy protein nanoparticles and tea saponin: Physical stability, rheological properties, oxidative stability, and lipid digestion. Food Chem. 2022, 387, 132891. [Google Scholar] [CrossRef]
- Wei, Z.H.; Gao, Y.X. Physicochemical properties of β-carotene bilayer emulsions coated by milk proteins and chitosan–EGCG conjugates. Food Hydrocoll. 2016, 52, 590–599. [Google Scholar] [CrossRef]
- Zhao, J.; Xiang, J.; Wei, T.; Yuan, F.; Gao, Y. Influence of environmental stresses on the physicochemical stability of orange oil bilayer emulsions coated by lactoferrin–soybean soluble polysaccharides and lactoferrin–beet pectin. Food Res. Int. 2014, 66, 216–227. [Google Scholar] [CrossRef]
- Azarikia, F.; Abbasi, S.; Scanlon, M.; McClements, D.J. Emulsion stability enhancement against environmental stresses using whey protein–tragacanthin complex: Comparison of layer-by-layer and mixing methods. Int. J. Food Prop. 2017, 20, 2084–2095. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, L.W.; Lei, F.F.; Zheng, J.C.; He, D.P. Preparation of pumpkin seed oil O/W emulsion by whey protein isolate-xanthan gum compound emulsifier and its stability. China Oils Fats 2022, 47, 76–83. [Google Scholar]
- Zang, X.; Wang, J.; Yu, G.; Cheng, J. Addition of anionic polysaccharides to improve the stability of rice bran protein hydrolysate-stabilized emulsions. LWT 2019, 111, 573–581. [Google Scholar] [CrossRef]
- Donsì, F.; Wang, Y.; Huang, Q. Freeze–thaw stability of lecithin and modified starch-based nanoemulsions. Food Hydrocoll. 2011, 25, 1327–1336. [Google Scholar] [CrossRef]
- Hou, P.P. Stability and Bioavailability of Whey Protein-Based Ginsenoside Rg3 Nanoemulsion; Jilin University: Changchun, China, 2020. [Google Scholar]
- Liao, Y.; Sun, Y.; Peng, X.; Wang, Q.; Wu, L.; Yan, S.; Liu, G.; Zhu, H.; Qi, B.; Li, Y. Preparation in vitro digestibility of perilla oil multilayer emulsion. Food Sci. 2022, 43, 58–65. [Google Scholar]
- Sun, Y.X. Study on Construction, Stability and In Vitro Digestion of Camellia Oil Nanoemulsion; Hainan University: Haikou, China, 2020. [Google Scholar]
- Sandra, B.; Stephan, D. Saponins—Self-assembly and behavior at aqueous interfaces. Adv. Colloid Interface Sci. 2017, 243, 105–113. [Google Scholar]
- Zhang, Z.P. Study on the Preparation of Egg White Protein-Chitosan Emulsion and the Stability of Loaded β-Carotene; Wuhan Polytechnic University: Wuhan, China, 2021. [Google Scholar]
- Yi, J.; Li, Y.; Zhong, F.; Yokoyama, W. The physicochemical stability and in vitro bioaccessibility of beta-carotene in oil-in-water sodium caseinate emulsions. Food Hydrocoll. 2014, 35, 19–27. [Google Scholar] [CrossRef]
- Chang, Y.; McClements, D.J. Influence of emulsifier type on the in vitro digestion of fish oil-in-water emulsions in the presence of an anionic marine polysaccharide (fucoidan): Caseinate, whey protein, lecithin, or Tween 80. Food Hydrocoll. 2016, 61, 92–101. [Google Scholar]
- Ozturk, B.; Argin, S.; Ozilgen, M.; McClements, D.J. Nanoemulsion delivery systems for oil-soluble vitamins: Influence of carrier oil type on lipid digestion and vitamin D3 bioaccessibility. Food Chem. 2015, 187, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Luo, X.; Wang, J.; Li, Y.; Feng, F.; Zhao, M. Digestive behavior of unemulsified triglycerides with different chain lengths: In vitro dynamic and static simulated digestion models. LWT 2021, 149, 112006. [Google Scholar] [CrossRef]
- Golding, M.; Wooster, T.J. The influence of emulsion structure and stability on lipid digestion. Curr. Opin. Colloid Interface Sci. 2010, 15, 90–101. [Google Scholar]
- Zhang, J.; Bing, L.; Reineccius, G.A. Formation, optical property and stability of orange oil nanoemulsions stabilized by Quallija saponins. LWT—Food Sci. Technol. 2015, 64, 1063–1070. [Google Scholar] [CrossRef]
- Jiang, L.Z.; Qi, Y.; Ma, C.; Liu, B.; Wang, Z.; Li, Y. Formation and stability of fish oil enriched biocompatible nano-emulsion. Trans. Chin. Soc. Agric. Mach. 2018, 49, 387–395. [Google Scholar]
- Pengon, S.; Chinatangkul, N.; Limmatvapirat, C.; Limmatvapirat, S. The effect of surfactant on the physical properties of coconut oil nanoemulsions. Asian J. Pharm. Sci. 2018, 13, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yuan, F.; Wang, X.; Li, X.; Hou, Z.; Gao, Y. The effect of whey protein isolate-dextran conjugates on the freeze-thaw stability of oil-in-water emulsions. J. Dispers. Sci. Technol. 2010, 32, 77–83. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, Q.; Li, D.; Chen, X.; Liang, L. Impact of gum arabic on the partition and stability of resveratrol in sunflower oil emulsions stabilized by whey protein isolate. Colloids Surf. B Biointerfaces 2019, 181, 749–755. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Verkempinck, S.; Rijal, S.K.; Van Loey, A.; Grauwet, T.; Hendrickx, M. Lipid nanoparticles with fats or oils containing β-carotene: Storage stability and in vitro digestibility kinetics. Food Chem. 2019, 278, 396–405. [Google Scholar] [CrossRef]
- Xu, X.F.; Sun, Q.J.; McClements, D.J. Enhancing the formation and stability of emulsions using mixed natural emulsifiers: Hydrolyzed rice glutelin and quillaja saponin. Food Hydrocoll. 2019, 89, 396–405. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Tian, Y.; Bai, X.; Cao, Y.; Fu, Z. Influence of the Emulsifier Sodium Caseinate–Xanthan Gum Complex on Emulsions: Stability and Digestive Properties. Molecules 2023, 28, 5460. https://doi.org/10.3390/molecules28145460
Huang H, Tian Y, Bai X, Cao Y, Fu Z. Influence of the Emulsifier Sodium Caseinate–Xanthan Gum Complex on Emulsions: Stability and Digestive Properties. Molecules. 2023; 28(14):5460. https://doi.org/10.3390/molecules28145460
Chicago/Turabian StyleHuang, Huan, Yan Tian, Xinpeng Bai, Yumiao Cao, and Zihuan Fu. 2023. "Influence of the Emulsifier Sodium Caseinate–Xanthan Gum Complex on Emulsions: Stability and Digestive Properties" Molecules 28, no. 14: 5460. https://doi.org/10.3390/molecules28145460
APA StyleHuang, H., Tian, Y., Bai, X., Cao, Y., & Fu, Z. (2023). Influence of the Emulsifier Sodium Caseinate–Xanthan Gum Complex on Emulsions: Stability and Digestive Properties. Molecules, 28(14), 5460. https://doi.org/10.3390/molecules28145460




