Synthesis, Structure, and Characterization of Thiacalix[4]-2,8-thianthrene
Abstract
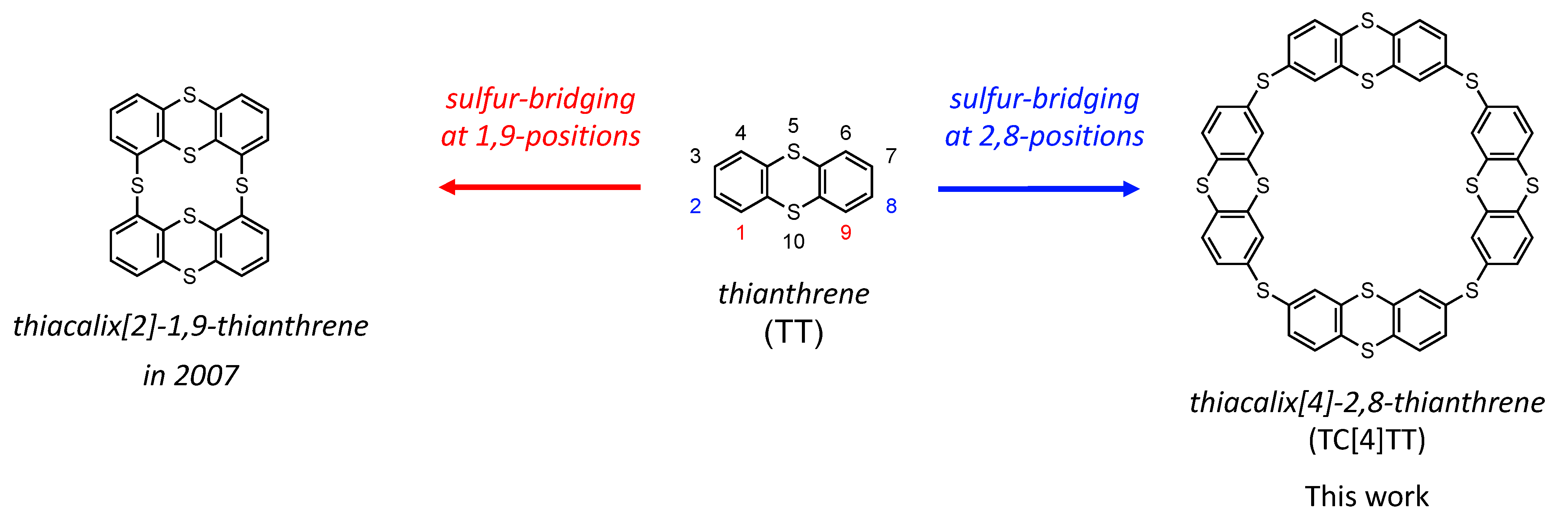
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Crystal Structure
2.3. Photophysical Properties
3. Conclusions
4. Materials and Methods
General Information
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lhoták, P. Chemistry of Thiacalixarenes. Eur. J. Org. Chem. 2004, 2004, 1675–1692. [Google Scholar] [CrossRef]
- Morohashi, N.; Narumi, F.; Iki, N.; Hattori, T.; Miyano, S. Thiacalixarenes. Chem. Rev. 2006, 106, 5291–5316. [Google Scholar] [CrossRef] [PubMed]
- Lang, K.; Prošková, P.; Kroupa, J.; Morávek, J.; Stibor, I.; Pojarová, M.; Lhoták, P. The synthesis and complexation of novel azosubstituted calix[4]arenes and thiacalix[4]arenes. Dye. Pigment. 2008, 77, 646–652. [Google Scholar] [CrossRef]
- Patel, M.H.; Patel, V.B.; Shrivastav, P.S. Design, synthesis, characterization, and preliminary complexation studies of chromogenic vanadophiles: 1,3-alternate thiacalix[4]arene tetrahydroxyamic acids. Tetrahedron 2008, 64, 2057–2062. [Google Scholar] [CrossRef]
- Zaghbani, A.; Fontàs, C.; Hidalgo, M.; Tayeb, R.; Dhahbi, M.; Vocanson, F.; Lamartine, R.; Seta, P. Thiacalix[4]arene derivatives as extractants for metal ions in aqueous solutions: Application to the selective facilitated transport of Ag(I). Mater. Sci. Eng. C 2008, 28, 985–989. [Google Scholar] [CrossRef]
- Stoikov, I.I.; Yushkova, E.A.; Zhukov, A.Y.; Zharov, I.; Antipin, I.S.; Konovalov, A.I. The synthesis of p-tert-butyl thiacalix[4]arene functionalized with secondary amide groups at the lower rim and their extraction properties and self-assembly into nanoscale aggregates. Tetrahedron 2008, 64, 7112–7121. [Google Scholar] [CrossRef]
- Stoikov, I.I.; Yushkova, E.A.; Zhukov, A.T.; Zharov, I.; Antipin, I.S.; Konovalov, A.I. Solvent extraction and self-assembly of nanosized aggregates of p-tert-butyl thiacalix[4]arenes tetrasubstituted at the lower rim by tertiary amide groups and monocharged metal cations in the organic phase. Tetrahedron 2008, 64, 7489–7497. [Google Scholar] [CrossRef]
- Stoikov, I.I.; Yushkova, E.A.; Bukharaev, A.A.; Biziaev, D.A.; Ziganshina, S.A.; Zharov, I. Self-Assembly of Stereoisomers of p-tert-Butyl Thiacalix[4]arenes Tetrasubstituted at the Lower Rim by a Tertiary Amide Group with Cations of p- and d-Elements in the Organic Phase. J. Phys. Chem. 2009, 113, 15838–15844. [Google Scholar] [CrossRef]
- Torgov, V.G.; Us, T.V.; Korda, T.M.; Kostin, G.A.; Kal’checnko, V.I. Extraction of Palladium with Thiacalix[4]arenes from Nitric Acid Nitrate–Nitrite Solution. Russ. J. Inorg. Chem. 2012, 57, 1621–1629. [Google Scholar] [CrossRef]
- Gandhi, M.R.; Yamada, M.; Kondo, Y.; Sato, R.; Hamada, F. Synthesis and Characterization of Dimethylthiocarbomoyl-Modified Thiacalix[n]arenes for Selective Pd(II)-Ion Extraction. Ind. Eng. Chem. Res. 2014, 53, 2559–2565. [Google Scholar] [CrossRef]
- Mollard, A.; Ibragimova, D.; Antipin, I.S.; Konovalov, A.I.; Stoikov, I.; Zharov, I. Molecular transport in thiacalix[4]arene-modified nanoporous colloidal films. Macroporous Mesoporous Mater. 2010, 131, 378–384. [Google Scholar] [CrossRef]
- Hoppe, E.; Limberg, C. Oxovanadium(V) tetrathiacalix[4]arene Complexes and Their Activity as Oxidation Catalyst. Chem. Eur. J. 2007, 13, 7006–7016. [Google Scholar] [CrossRef]
- Kozlova, M.N.; Ferlay, S.; Solovieva, S.E.; Antipin, I.S.; Konovalov, A.I.; Kyritsakas, N.; Hosseini, M.W. Molecular tectonics: On the formation of 1-D silver coordination networks by thiacalixarenes bearing nitrile groups. Dalton Trans. 2007, 44, 5126–5131. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.-S.; Liu, Y. A Novel Supramolecular Assembly Constructed by Cu/imidazole Complex with 1,2-Alternate p-Sulfonatothiacalix[4]arene. Cryst. Growth Des. 2007, 7, 1038–1041. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, K.; Guo, D.-S.; Li, O.; Song, H.-B. Comparable Inclusion and Aggregation Structures of p-Sulfonatothiacalix[4]arene and p-Sulfonatocalix[4]arene upon Complexation with Quinoline Guests. Cryst. Growth Des. 2007, 7, 2601–2608. [Google Scholar] [CrossRef]
- Kozlova, M.N.; Ferlay, S.; Kyritsakas, N.; Hosseini, M.W.; Solovieva, S.E.; Antipin, I.S.; Konovalov, A.I. Molecular tectonics: 3-D organisation of decanuclear silver nanoclusters. Chem. Commun. 2009, 18, 2514–2516. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Liao, W.; Wang, X.; Deng, R.; Zhang, H. Self-Assembly from Two-Dimensional Layered Networks to Tetranuclear Structures: Syntheses, Structures, and Properties of Four Copper–Thiacalix[[4]arene Compounds. Eur. J. Inorg. Chem. 2009, 2009, 4989–4994. [Google Scholar] [CrossRef]
- Yamada, M.; Shimakawa, Y.; Kondo, Y.; Hamada, F. Thiacalix[4]arene–rubidium assembly: Supramolecular architecture based on alkali metal coordination and cation-π interactions. CrystEngComm 2010, 12, 1311–1315. [Google Scholar] [CrossRef]
- Chen, T.; Chen, Q.; Zhang, X.; Wang, D.; Wan, L.-J. Chiral Kagome Network from Thiacalix[4]arene Tetrasulfonate at the Interface of Aqueous Solution/Au(111) Surface: An in Situ Electrochemical Scanning Tunneling Microscopy Study. J. Am. Chem. Soc. 2010, 132, 5598–5599. [Google Scholar] [CrossRef]
- Hamada, F.; Yamada, M.; Kondo, Y.; Ito, S.-I.; Akiba, U. Channel structure for guest inclusion based on hexametric assembly of thiacalix[4]arene analogue. CrystEngComm 2011, 13, 6920–6922. [Google Scholar] [CrossRef]
- Yang, Y.L.W.; Chen, Y.; Gong, S. Pendant orientation and its influence on the formation of hydrogen-bonded thiacalixarene nanotubes. CrystEngComm 2011, 13, 259–268. [Google Scholar]
- Yamada, M.; Hamada, F. Thiacalix[4]arene potassium assemblies: A supramolecular architecture based on coordination polymers and a dimeric structure based on triangular pyramidal arrangements. CrystEngComm 2011, 13, 2494–2499. [Google Scholar] [CrossRef]
- Yamada, M.; Shimakawa, Y.; Hamada, F. Thiacalix[4]arene–alkali metal assemblies: Crystal structures and guest-binding capabilities of supramolecular architectures supported by metal coordination and cation–π interactions. Tetrahedron 2011, 67, 7392–7399. [Google Scholar] [CrossRef]
- Yushkova, E.A.; Stoikov, I.I.; Puplampu, J.B.; Antipin, I.S.; Konovalov, A.I. Cascade and Commutative Self-Assembles of Nanoscale Three-Component Systems Controlled by the Conformation of Thiacalix[4]arene. Langmuir 2011, 27, 14053–14064. [Google Scholar] [CrossRef]
- Stoikov, I.I.; Yushkova, E.A.; Bukharaev, A.A.; Biziaev, D.A.; Selivanovskaya, S.Y.; Chursina, M.A.; Antipin, I.S.; Konovalov, A.I.; Zharov, I. Slef-assembly of p-tert-butyl thiacalix[4]arenes and metal cations into nanoscale three-dimensional particles. J. Phys. Org. Chem. 2012, 25, 1177–1185. [Google Scholar] [CrossRef]
- Andreyko, E.A.; Padnya, P.L.; Daminova, R.R.; Stoikov, I.I. Supramolecular “containers”: Self-assembly and functionalization of thiacalix[4]arenes for recognition of amino-and dicarboxylic acids. RSC Adv. 2014, 4, 3556–3565. [Google Scholar] [CrossRef]
- Wang, W.; Yang, W.; Guo, R.; Gong, S. ‘Honeycomb’ nanotube assembly based on thiacalix[4]arene derivatives by weak interactions. CrystEngComm 2015, 17, 7663–7675. [Google Scholar] [CrossRef]
- Ju, H.; Park, I.-H.; Lee, E.; Kim, C.; Kim, S.; Kuwahara, S.; Habata, Y.; Lee, S.S. A Thiacalix Basket and Its Anion-Dependent 2-D and 3-D Silver(I) Coordination Polymers via Exo-Coordination. Eur. J. Inorg. Chem. 2020, 2020, 356–360. [Google Scholar] [CrossRef]
- Ju, H.; Lee, J.Y.; Lee, S.S. Influence of anions and mole ratio on the formation of 2-D coordination networks of thiacalix[4]-bis-monothiacrown-5. CrystEngComm 2020, 22, 7617–7622. [Google Scholar] [CrossRef]
- Flood, R.J.; Ramberg, K.O.; Mengel, D.B.; Guagnini, F.; Crowley, P.B. Protein Frameworks with Thiacalixarene and Zinc. Cryst. Growth Des. 2022, 22, 3271–3276. [Google Scholar] [CrossRef]
- Yamada, M.; Kondo, Y.; Iki, N.; Kabuto, C.; Hamada, F. Hydrophobic and metal coordination interacted architecture based on p-tert-butylthiacalix[4]arene–potassium complex and its vapor absorption capability. Tetrahedron Lett. 2008, 49, 3906–3911. [Google Scholar] [CrossRef]
- Morohashi, N.; Noji, S.; Nakayama, H.; Kudo, Y.; Tanaka, S.; Kabuto, C.; Hattori, T. Unique Inclusion Properties of Crystalline Powder p-tert-Butylthiacalix[4]arene toward Alcohols and Carboxylic Acids. Org. Lett. 2011, 13, 3292–3295. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.; Zhang, M.; Hang, X.; Xie, W.; Qin, Y.; Li, Q.; Bi, Y.; Zheng, Z. A 2D metal-thiacalix[4]arene porous coordination polymer with 1D channels: Gas absorption/separation and frequency response. Dalton Trans. 2018, 47, 9008–9013. [Google Scholar] [CrossRef] [PubMed]
- Praveen, L.; Ganga, V.B.; Thirumalai, R.; Sreeja, T.; Reddy, M.L.; Varma, R.L. A New Hg2+-Selective Fluorescent Sensor Based on a 1,3-Alternate Thiacalix[4]arene Anchored with Four 8-Quinolinoloxy Groups. Inorg. Chem. 2007, 46, 6277–6282. [Google Scholar] [CrossRef]
- Bhalla, V.; Kumar, R.; Kumar, M.; Dhir, A. Bifunctional fluorescent thiacalix[4]arene based chemosensor for Cu2+ and F− ions. Tetrahedron 2007, 63, 11153–11159. [Google Scholar] [CrossRef]
- Kumar, R.; Bhalla, V.; Kumar, M. Cu2+ and CN−-selective fluorogenic sensors based on pyrene-appended thiacalix[4]arenes. Tetrahedron 2008, 64, 8095–8101. [Google Scholar] [CrossRef]
- Iki, N.; Ohta, M.; Tanaka, T.; Horiuchi, T.; Hoshino, H. A Supramolecular sensing system for AgI t nanomolar levels by the formation of a luminescent AgI–TbIII–thiacalix[4]arene ternary complex. New. J. Chem. 2009, 33, 23–25. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, R.; Bhalla, V. F−-Induced ‘turn-on’ fluorescent chemosensor based on 1,3-alt thiacalix[4]arene. Tetrahedron 2009, 65, 4340–4344. [Google Scholar] [CrossRef]
- Kumar, M.; Dhir, A.; Bhalla, V. On–Off Switchable Binuclear Chemosensor Based on Thiacalix[4]crown Armed with Pyrene Moieties. Eur. J. Org. Chem. 2009, 2009, 4534–4540. [Google Scholar] [CrossRef]
- Kumar, M.; Dhir, A.; Bhalla, V. Regulation of metal ion recognirion by allosteric effects in thiacalix[4]crown based receptors. Tetrahedron 2009, 65, 7510–7515. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, R.; Bhalla, V. Fluorescent probe for Fe3+ and CN− in aqueous media mimicking a memorized molecular crossword puzzle. RSC Adv. 2011, 1, 1045–1049. [Google Scholar] [CrossRef]
- Ni, X.-L.; Zeng, X.; Redshaw, C.; Yamato, T. Synthesis and evaluation of a novel pyrenyl-appended triazole-based thiacalix[4]arene as a fluorescent sensor for Ag+ ion. Tetrahedron 2011, 67, 3248–3253. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Kumar, R.; Bhalla, V.; Sharma, P.R.; Kaur, T.; Qurishi, Y. Thiacalix[4]arene based fluorescent probe for sensing and imaging of Fe3+ ions. Dalton Trans. 2012, 41, 408–412. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, R.; Bhalla, V. Differential fluorogenic sensing of F− versus CN− based on thiacalix[4]arene derivatives. Tetrahedron Lett. 2013, 54, 1524–1527. [Google Scholar] [CrossRef]
- Tomiyasu, H.; Jin, C.-C.; Ni, X.-L.; Zheng, X.; Redshaw, C.; Yamato, T. A study of allosteric binding behaviour of a 1,3-alternate thiacalix[4]arene-based receptor using fluorescence signal. Org. Biomol. Chem. 2014, 12, 4917–4923. [Google Scholar] [CrossRef]
- Zhao, J.-L.; Tomiyasu, H.; Wu, C.; Cong, H.; Zeng, X.; Rahman, S.; Georghiou, P.E.; Hughes, D.L.; Redshaw, C.; Yamato, T. Synthesis, crystal structure and complexation behaviour study of an efficient Cu2+ ratiometric fluorescent chemosensor based on thiacalix[4]arene. Tetrahedron 2015, 71, 8521–8527. [Google Scholar] [CrossRef]
- Takagiri, Y.; Ikuta, T.; Maehashi, K. Selective Detection of Cu2+ Ions by Immobilizing Thiacalix[4]arene on Graphene Field-Effect Transistors. ACS Omega 2020, 5, 877–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tashiro, S.; Shionoya, M. Cavity-Assembled Porous Solids (CAPSs) for Nanospace-Specific Functions. Bull. Chem. Soc. Jpn. 2014, 87, 643–654. [Google Scholar] [CrossRef]
- Nakayama, J.; Katano, N.; Sugihara, Y.; Ishii, A. Cyclic Oligo(thio-2,5-thioenylenes)(Sulfur-bridged Calixarenes). Chem. Lett. 1997, 26, 897–898. [Google Scholar] [CrossRef]
- Katano, N.; Sugihara, Y.; Ishii, A.; Nakayama, J. Synthesis and Structure of Sulfur-Bridged [1.n](2,5)Thiophenophanes (n = 4–6). Bull. Chem. Soc. Jpn. 1998, 71, 2695–2700. [Google Scholar] [CrossRef]
- Nakabayashi, S.; Fukushima, E.; Baba, R.; Katano, N.; Sugihara, Y.; Nakayama, J. Stereo-electrochemistry by a self assembled monolayer of sulfur-bridged calixthiophene on gold. Electrochem. Commun. 1999, 1, 550–553. [Google Scholar] [CrossRef]
- Inoue, R.; Hasegawa, M.; Nishinaga, T.; Yoza, K. Mazaki. Efficient Synthesis, Structure, and Complexation Studies of Electron-Donating Thiacalix[n]dithienothiophene. Angew. Chem. Int. Ed. 2015, 54, 2734–2738. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Yano, T.; Nishioka, T.; Nakajo, K.; Breedlove, B.K.; Kimura, K.; Kinoshita, I.; Isobe, K. Thia-calix[n]pyridines, synthesis and coordination to Cu (I,II) ions with both N and S donor atoms. Chem. Commun. 2002, 16, 1686–1687. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, I.; Hamazawa, A.; Nishioka, T.; Adachi, H.; Suzuki, H.; Miyazaki, Y.; Tsuboyama, A.; Okada, S.; Hoshino, M. Laser photolysis studies on CuI complexes of thia-calix[3]pyridine. Phosphorescence from the intramolecular charge-transfer excited state. Chem. Phys. Lett. 2003, 371, 451–457. [Google Scholar] [CrossRef]
- Tanaka, R.; Hamazawa, A.; Nishioka, T.; Kinoshita, I.; Takui, T.; Santo, R.; Ichimura, A. Thiacalix[3]pyridine produces a stable mononuclear rhodium(II) complex with mutual Jahn–Teller effect. Dalton Trans. 2006, 11, 1374–1376. [Google Scholar] [CrossRef]
- Tsukahara, Y.; Hirotsu, M.; Hattori, S.-I.; Usuki, Y.; Kinoshita, I. A Thiacalix[3]pyridine Copper(I) Complex as a Highly Active Catalyst for the Olefin Aziridination Reaction. Chem. Lett. 2008, 37, 452–453. [Google Scholar] [CrossRef]
- Maes, W.; Rossom, W.V.; Hecke, K.V.; Meervelt, L.V.; Dehaen, W. Selective Synthesis of Functionalized Thia- and Oxacalix[2]arene[2]pyrimidines. Org. Lett. 2006, 8, 4161–4164. [Google Scholar] [CrossRef]
- Pisagatti, I.; Manganaro, N.; Mirabella, C.F.M.; Pappalardo, A.; Sfrazzetto, G.T.; Nastasi, F.; Notti, A.; Parisi, M.F.; Gattuso, G. How do fluoride ions bind to tetrathiacalix[2]arene[2]triazines? Tetrahedron Lett. 2020, 61, 151911. [Google Scholar] [CrossRef]
- Ueda, M.; Mazaki, Y. Synthesis and crystal structure of a sulphur-bridged molecular hoop consisting of 5,7,12,14-tetrathiapentacene. RSC Adv. 2022, 12, 10870–10874. [Google Scholar] [CrossRef]
- Hinrichs, W.; Berges, P.; Klar, G.; Sánchez-Martínez, E.; Gunsser, W. Structure and electrical conductivity of TCNQ-2,3,7,8-tetramethoxychalcogenanthracene complex. Synth. Met. 1987, 20, 357–364. [Google Scholar] [CrossRef]
- Söderholm, S.; Noreland, J.; Olovsson, G.; Olovsson, I.; Helleberg, J.; Engman, L. 2,3;7,8-Bis(ethylenedioxy)thianthrene-hexafluoroarsenate [(bEDOT)AsF6]: A Mott-Hubbard Insulator Showing Evidence for Appreciable Correlation Effects. Mol. Cryst. Liq. Cryst. 1989, 167, 259–268. [Google Scholar]
- Noreland, J.; Olovsson, G.; Olovsson, I. Structure of the organic semiconducting radical cation salt [2,3;7,8-bis(ethylenedioxy)thianthrene]hexafluoroarsenate. Synth. Met. 1991, 42, 1691–1694. [Google Scholar] [CrossRef]
- Beck, J.; Bredow, T.; Tjahjanto, R.T. Thianthrene Radical Cation Hexafluorophosphate. Z. Naturforsch. B 2009, 64, 145–152. [Google Scholar]
- Tjahjanto, R.T.; Beck, J. Synthesis and Characterization of Semiconductive Dichloridobis(thianthrene)gold(1+) tetrachloridoaurate(1−). Eur. J. Inorg. Chem. 2009, 2009, 2524–2528. [Google Scholar]
- Mao, Y.; Thomas, K. Photoinduced Electron Transfer and Subsequent Chemical Reactions of Absorbed Thianthrene on Clay Surfaces. J. Org. Chem. 1993, 58, 6641–6649. [Google Scholar] [CrossRef]
- Liu, H.; Gao, Y.; Cao, J.; Li, T.; Wen, Y.; Ge, Y.; Zhang, L.; Pan, G.; Zhou, T.; Yang, B. Efficient room-temperature phosphorescence based on a pure organic sulfur-containing heterocycle: Folding-induced spin-orbit coupling enhancement. Mater. Chem. Front. 2018, 2, 1853–1858. [Google Scholar]
- Pander, P.; Swist, A.; Turczyn, R.; Pouget, S.; Djurado, D.; Lazauskas, A.; Pashazadeh, R.; Grazulevicius, J.V.; Motyka, R.; Klimash, A.; et al. Observation of Dual Room Temperature Fluorescence-Phosphorescence in Air, in the Crystal Form of a Thianthrene Derivative. J. Phys. Chem. C 2018, 122, 24958–24966. [Google Scholar] [CrossRef] [Green Version]
- Ong, W.; Bertani, F.; Dalcanale, E.; Swager, T.M. Redox Switchable Thianthrene Cavitands. Synthesis 2017, 49, 358–364. [Google Scholar]
- Burkhart, C.; Haberhauer, G.A. Light- and Electricity-Driven Molecular Pushing Motor. Eur. J. Org. Chem. 2017, 2017, 1308–1317. [Google Scholar]
- Zieba, R.; Desroches, C.; Chaput, F.; Sigala, C.; Jeanneau, E.; Parola, S. The first approach to a new family of macrocycles: Synthesis and characterization of thiacalix[2]thianthrenes. Tetrahedron Lett. 2007, 48, 5401–5405. [Google Scholar] [CrossRef]
- Thabet, W.; Baklouti, L.; Parola, R.Z.S. Cation binding by thiacalixthianthrenes. J. Incl. Phenom. Macrocycl. Chem. 2012, 73, 135–139. [Google Scholar] [CrossRef]
- Gilman, H.; Swayampati, D.R. Bromination in the Thianthrene System. J. Org. Chem. 1958, 23, 313–314. [Google Scholar] [CrossRef]
- Umemoto, T.; Ishihara, S. Power-Variable Electrophilic Trifluoromethylating Agents. J. Am. Chem. Soc. 1993, 115, 2156–2164. [Google Scholar] [CrossRef]
- Leuninger, J.; Trimpin, S.; Räder, H.-J.; Müllen, K. Novel Approach to Ladder-Type Polymers: Polydithiathianthrene via the Intramolecular Acid Induced Cyclization of Methylsulfinyl-Substituted Poly(meta-phenylene sulfide). Macromol. Chem. Phys. 2001, 220, 2832–2842. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, J.; Xie, J.; Lu, Z.; Jiang, L.; Mu, Y.; Huo, Y.; Tsuchido, Y.; Zhu, K. Sulphur-embedded hydrocarbon Belts: Synthesis, Structure and Redox Chemistry of Cyclothianthrenes. Angew. Chem. Int. Ed. 2021, 60, 18443–18447. [Google Scholar] [CrossRef]
- Park, N.; Park, K.; Jang, M.; Lee, S. One-Pot Synthesis of Symmetrical and Unsymmetrical Aryl Sulfides by Pd-Catalyzed Couplings of Aryl Halides and Thioacetates. J. Org. Chem. 2011, 76, 4371–4378. [Google Scholar] [CrossRef]
- Fargher, H.A.; Sherbow, T.J.; Haley, M.M.; Johnson, D.W.; Pluth, M.D. C–H···S hydrogen bonding interactions. Chem. Soc. Rev. 2022, 51, 1454–1469. [Google Scholar]
- CrysAlisPro: Data Collection and Processing Software Package; Rigaku Oxford Diffraction, Rigaku Corporatioin: Yarnton, UK, 2019.
- Dolomanov, O.V.; Nourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Peterson, G.A.; Nakatsuji, H.; et al. (Eds.) Gaussian 16; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Fujiki, K.; Tanifuji, N.; Sasaki, Y.; Yokohama, T. New and Facile Synthesis of Thiosulfonates from Sulfinate/Disulfide/I2 System. Synthesis 2002, 3, 343–348. [Google Scholar] [CrossRef]
- Morita, H.; Oida, Y.; Ariga, T.; Fukumoto, S.; Sheikh, M.C.; Fujii, T.; Yoshimura, T. Synthesis of ‘thianthrene dimer’ by the coupling reaction of stannylthianthrenes in the presence of copper catalysts. Tetrahedron 2011, 67, 4672–4679. [Google Scholar] [CrossRef]
- Chen, T.; Zheng, L.; Yuan, J.; An, Z.; Chen, R.; Tao, Y.; Li, H.; Xie, X.; Huang, W. Understanding the Control of Singlet-Triplet Splitting for Organic Exciton Manipulating: A Combined Theoretical and Experimental Approach. Sci. Rep. 2015, 5, 10923. [Google Scholar]
- Milián-Medina, B.; Gierschner, J. Computational design of low singlet-triplet gap all-organic molecules for OLED application. Org. Electron. 2012, 13, 985–991. [Google Scholar]
- Zhu, H.; Badía-Domínguez, I.; Shi, B.; Li, Q.; Wei, P.; Xing, H.; Delgado, M.C.R.; Huang, F. Cyclization-Promoted Ultralong Low-Temperature Phosphorescence via Boosting Intersystem Crossing. J. Am. Chem. Soc. 2021, 143, 2164–2169. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueda, M.; Isozaki, M.; Mazaki, Y. Synthesis, Structure, and Characterization of Thiacalix[4]-2,8-thianthrene. Molecules 2023, 28, 5462. https://doi.org/10.3390/molecules28145462
Ueda M, Isozaki M, Mazaki Y. Synthesis, Structure, and Characterization of Thiacalix[4]-2,8-thianthrene. Molecules. 2023; 28(14):5462. https://doi.org/10.3390/molecules28145462
Chicago/Turabian StyleUeda, Masafumi, Moe Isozaki, and Yasuhiro Mazaki. 2023. "Synthesis, Structure, and Characterization of Thiacalix[4]-2,8-thianthrene" Molecules 28, no. 14: 5462. https://doi.org/10.3390/molecules28145462





