Self-Aggregation, Antimicrobial Activity and Cytotoxicity of Ester-Bonded Gemini Quaternary Ammonium Salts: The Role of the Spacer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Surface Tension
2.2. Conductivity Measurements
2.3. Antimicrobial Activity
2.4. Cytotoxicity of Gemini Surfactants
3. Materials and Methods
3.1. Materials
3.2. Synthesis and Characterization
3.3. Surface Tension Measurement
3.4. Conductivity Measurements
3.5. Antimicrobial Activity
3.6. Cytotoxicity Assay (CCK-8 Test)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Fu, S.Q.; Guo, J.W.; Zhong, X.; Yang, Z.; Lai, X.F. Synthesis, physiochemical property and antibacterial activity of gemini quaternary ammonium salts with a rigid spacer. RSC Adv. 2016, 6, 16507–16515. [Google Scholar] [CrossRef]
- Pérez, L.; Pinazo, A.; Ramon, P.; Infante, M. Gemini surfactants from natural amino acids. Adv. Colloid Interface Sci. 2014, 205, 134–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Q.X.; Yang, D.; Hu, T.Y.; Zhang, L.L.; Jiang, C.Y. Synthesis and properties evaluation of novel Gemini surfactant with temperature tolerance and salt resistance for heavy oil. J. Mol. Liq. 2023, 385, 121851. [Google Scholar] [CrossRef]
- Alexandra, D.V.; Syumbelya, K.G.; Anastasiia, S.S.; Natalia, V.K.; Alla, B.M.; Alla, A.K.; Tatiana, M.P.; Vasilii, A.M.; Lucia, Y.Z.; Oleg, G.S. The structure-activity correlation in the family of dicationic imidazolium surfactants: Antimicrobial properties and cytotoxic effect. BBA-Gen. Subj. 2020, 1864, 129728. [Google Scholar]
- Risa, K.; Shiho, Y.; Tomokazu, Y. Layer structure of quaternary-ammonium-salt-type amphiphilic gemini and trimeric ionic liquids. J. Mol. Liq. 2021, 336, 116459. [Google Scholar]
- Liang, Y.; Li, H.; Li, M.; Mao, X.; Li, Y.; Wang, Z.; Xue, L.; Chen, X.; Hao, X. Synthesis and physicochemical properties of ester-bonded gemini pyrrolidinium surfactants and a comparison with single-tailed amphiphiles. J. Mol. Liq. 2019, 280, 319–326. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, L.; Zhao, Z.; Yang, R.; Wang, J.; Guo, X. Synthesis, physiochemical properties, and antimicrobial activities of a novel gemini surfactants with biphenyl and multiple amide groups. Colloids Surf. A 2020, 593, 124628. [Google Scholar] [CrossRef]
- Samy, M.S.; Asma, M.E.; Ahmed, H.E.; Eluskkary, M.M.; Aiad, I.; Soliman, E.A. Some new phospho-zwitterionic Gemini surfactants as corrosion inhibitors for carbon steel in 1.0 M HCl solution. Environ. Technol. Innov. 2021, 24, 102051. [Google Scholar]
- Azin, R.A.; Pakshid, H.; Atefeh, S.; Somaye, A.; Adrianna, M.S.; Bogumil, E.B. Cationic gemini surfactant properties, its potential as a promising bioapplication candidate, and strategies for improving its biocompatibility: A review. Adv. Colloid Interface Sci. 2022, 299, 102581. [Google Scholar]
- Catarina, C.; Isabel, S.O.; João, P.N.S.; Sandra, G.S.; Cláudia, B.; Vale, M.L.C.; Maria, E.C.D.; Andreia, C.G. Effective cytocompatible nanovectors based on serine-derived gemini surfactants and monoolein for small interfering RNA delivery. J. Colloid Interface Sci. 2021, 584, 34–44. [Google Scholar]
- Olga, K.; Bogumił, B.; Isabel, R.; Francesc, C.; Teresa, M.G. Cationic gemini surfactants containing an O-substituted spacer and hydroxyethyl moiety in the polar heads: Self-assembly, biodegradability and aquatic toxicity. J. Ind. Eng. Chem. 2018, 59, 141–148. [Google Scholar]
- Garcia, M.T.; Ribosa, I.; Kowalczyk, I.; Pakiet, M.; Brycki, B. Biodegradability and aquatic toxicity of new cleavable betainate cationic oligomeric surfactants. J. Hazard. Mater. 2019, 371, 108–114. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, M.; Tang, Y.; Han, Y.; Huang, X.; Hou, Y.; Wang, Y. Aggregation of biodegradable cationic gemini surfactants with amide or ester groups. Acta Phys.-Chim. Sin. 2020, 36, 1909046. [Google Scholar]
- Zhuang, L.; Yu, K.; Wang, G.; Yao, C. Synthesis and properties of novel ester-containing gemini imidazolium surfactants. J. Colloid Interface Sci. 2013, 408, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Ge, X.; Gao, W.; Wei, W.; Qiao, Y.; Chang, H. Synthesis and aggregation properties of ethylene glycol ester-based cationic gemini surfactants. Colloid Interface Sci. 2020, 37, 100274. [Google Scholar] [CrossRef]
- Xu, D.; Ni, X.; Zhang, C.; Mao, J.; Song, C. Synthesis and properties of biodegradable cationic gemini surfactants with diester and flexible spacers. J. Mol. Liq. 2017, 240, 542–548. [Google Scholar] [CrossRef]
- Gab-Allah, M.G.A.; El-Ged, A.H.; Badr, E.A.; Bedair, M.A.; Soliman, S.A.; Bakr, M.F. Three novel gemini amide amphiphilics synthesis, characterization, thermodynamics, surface properties and biological activity. Egypt J. Petrol. 2023, 32, 27–33. [Google Scholar] [CrossRef]
- Mohd, A.; Hira, L.; Mohammad, O.; Farah, A.; Sana, A.; Kabir-ud-Din; Ajaz, A.; Samreen, N.A.; Hadi, M.M.; Abdullah, M.A. An insight view on synthetic protocol, surface activity, and biological aspects of novel biocompatible quaternary ammonium cationic gemini surfactants. J. Surfactants Deterg. 2021, 24, 35–49. [Google Scholar]
- Yang, W.; Cao, Y.; Ju, H.; Wang, Y.; Jiang, Y.; Geng, T. Amide gemini surfactants linked by rigid spacer group 1,4-dibromo-2-butene: Surface properties, aggregate and application properties. J. Mol. Liq. 2021, 326, 115339. [Google Scholar] [CrossRef]
- Nazish, F.; Manorama, P.; Kabir-ud-Din; Muheeb, B. Ester-bonded cationic gemini surfactants: Assessment of their cytotoxicity and antimicrobial activity. J. Mol. Liq. 2016, 222, 390–394. [Google Scholar]
- Tehrani-Bagha, A.R.; Holmberg, K.; Ginkel, C.G.; Kean, M. Cationic gemini surfactants with cleavable spacer: Chemical hydrolysis, biodegradation, and toxicity. J. Colloid Interface Sci. 2015, 449, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ding, S.; Yu, J.; Chen, X.; Lei, Q.; Fang, W. Antibacterial Activity, cytotoxicity, and cell cycle arrest of gemini quaternary ammonium surfactants. Langmuir 2015, 31, 12161–12169. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Tong, Q. Synthesis, surface adsorption, micellization behavior and antibacterial activity of novel gemini surfactants with morpholinium headgroup and benzene-based spacer. J. Mol. Liq. 2021, 331, 115781. [Google Scholar] [CrossRef]
- Hou, S.; Wang, Y.; Li, J.; Wang, Z.; Jiang, Y.; Geng, T. Effects of the number of cationic sites on the surface/interfacial activity and application properties of quaternary ammonium surfactants. Colloids Surf. A 2023, 656, 130523. [Google Scholar] [CrossRef]
- Martin, V.; Rodriguez, A.; Graciani, M.; Robina, I.; Moya, M. Study of the micellization and micellar growth in pure alkanediyl-a,ω-bis(dodecyldimethyl ammonium) bromide and MEGA10 surfactant solutions and their mixtures. Influence of the spacer on the enthalpy change accompanying sphere-to-rod transitions. J. Phys. Chem. B 2008, 114, 7817–7829. [Google Scholar] [CrossRef]
- Zana, R. Dimeric (Gemini) surfactants: Effect of the spacer group on the association behavior in aqueous solution. J. Colloid Interface Sci. 2002, 248, 203–220. [Google Scholar] [CrossRef]
- Zhu, D.; Cheng, F.; Chen, Y.; Jiang, S. Preparation, characterization and properties of anionic gemini surfactants with long rigid or semi-rigid spacers. Colloids Surfaces A 2012, 397, 1–7. [Google Scholar] [CrossRef]
- Liu, Z.; Ge, Y.; Zhang, Q.; Gao, X.; Cai, X.; Chen, M.; Yu, X. Adsorption and micellization of gemini surfactants with diethylammonium headgroups: Effect of the spacer rigidity. J. Surfactants Deterg. 2017, 20, 765–775. [Google Scholar] [CrossRef]
- Fu, H.; Li, Y.; Song, Y.; Li, J.; Wang, Z.; Zhao, L. Synthesis and aggregation behaviors of a new bis-quaternary ammonium surfactant. J. Mol. Liq. 2017, 230, 329–336. [Google Scholar] [CrossRef]
- Pinazo, A.; Petrizelli, V.; Bustelo, M.; Pons, R.; Vinardell, M.P.; Mitjans, M.; Manresa, A.; Perez, L. New cationic vesicles prepared with double chain surfactants from arginine: Role of the hydrophobic group on the antimicrobial activity and cytotoxicity. Colloid Surfaces B 2016, 141, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Mohd, A.; Sana, A.; Farah, A.; Imtiyaz, A.B.; Kabir-ud-Din. Bio-physicochemical analysis of ethylene oxide-linked diester- functionalized green cationic gemini surfactants. RSC Adv. 2016, 6, 21697–21705. [Google Scholar]
- Zhou, C.C.; Wang, Y.L. Structure-activity relationship of cationic surfactants as antimicrobial agents. Curr. Opin. Colloid Interface Sci. 2020, 45, 28–43. [Google Scholar] [CrossRef]
- Ma, J.L.; Si, T.T.; Yan, C.X.; Li, Y.J.; Qiang, L.; Lu, X.F.; Guo, Y. Near-Infrared fluorescence probe for evaluating acetyl-cholinesterase activity in PC12 cells and in situ tracing AChE distribution in Zebrafish. ACS Sens. 2020, 5, 83–92. [Google Scholar] [CrossRef] [PubMed]

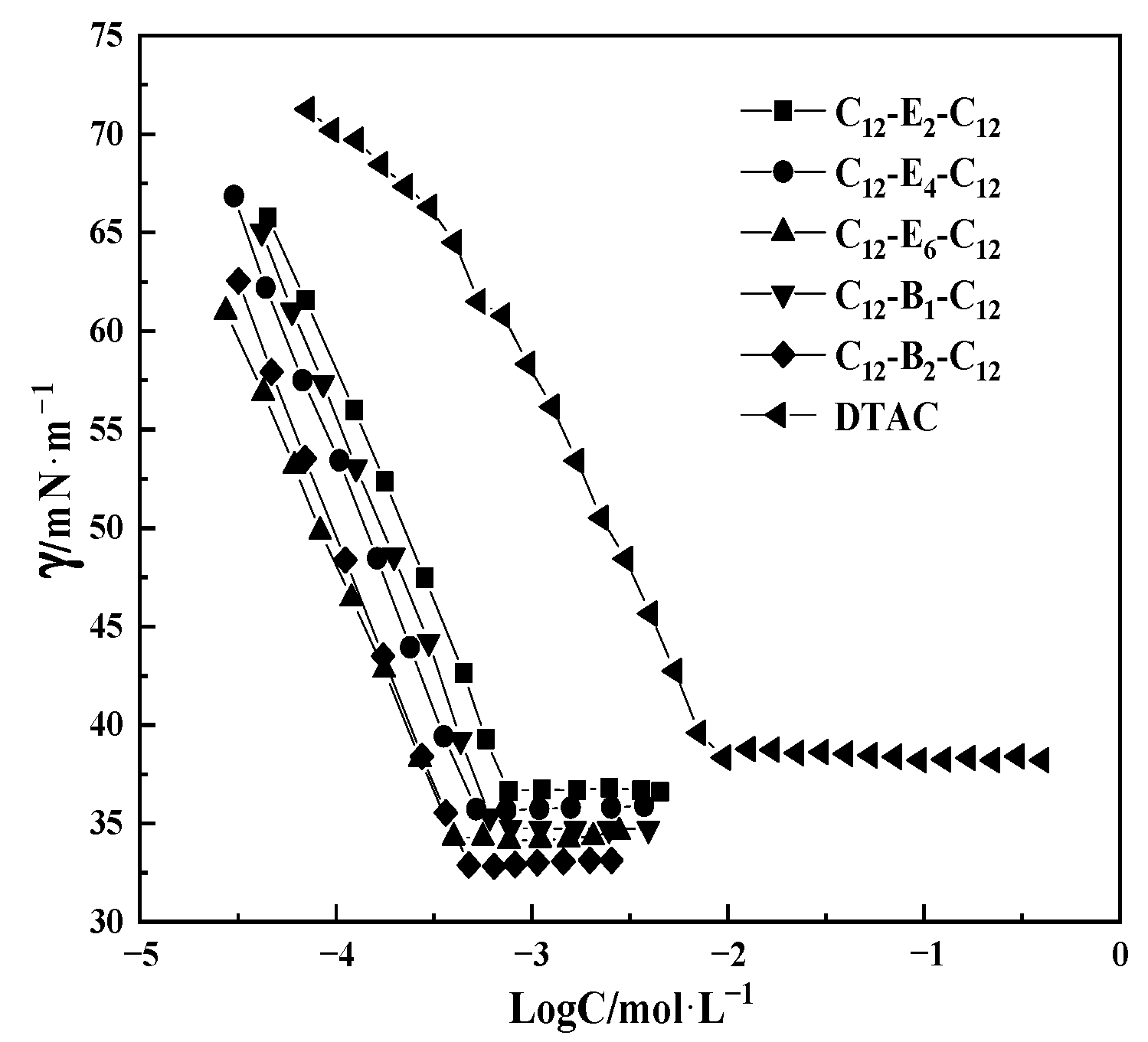


| Surfactant | CMC 1 (mmol·L−1) | CMC 2 (mmol·L−1) | γCMC (mN·m−1) | Γmax × 106 (mol·m−2) | Amin (nm2) | pC20 | (kJ·mol−1) | |
|---|---|---|---|---|---|---|---|---|
| C12-E2-C12 | 0.815 | 0.895 | 0.62 | 36.92 | 0.91 | 1.82 | 3.07 | −25.97 |
| C12-E4-C12 | 0.528 | 0.706 | 0.51 | 35.58 | 1.01 | 1.65 | 3.19 | −28.20 |
| C12-E6-C12 | 0.378 | 0.502 | 0.45 | 34.29 | 1.17 | 1.42 | 3.36 | −32.22 |
| C12-B1-C12 | 0.618 | 0.813 | 0.49 | 34.58 | 0.94 | 1.77 | 3.15 | −27.30 |
| C12-B2-C12 | 0.467 | 0.597 | 0.58 | 32.82 | 1.05 | 1.58 | 3.28 | −30.61 |
| DTAC | 10.25 | 11.88 | 0.66 | 38.57 | 2.32 | 0.72 | 2.95 | −22.78 |
| Strains | C12-E2-C12 | C12-E4-C12 | C12-E6-C12 | C12-B1-C12 | C12-B2-C12 | DTAC |
|---|---|---|---|---|---|---|
| S. aureus | 12 | 10 | 5 | 11 | 8 | 16 |
| E. coli | 22 | 15 | 13 | 19 | 17 | 68 |
| Surfactants | C12-E2-C12 | C12-E4-C12 | C12-E6-C12 | C12-B1-C12 | C12-B2-C12 | DTAC |
|---|---|---|---|---|---|---|
| IC50 (μmol·L−1) | 36.34 ± 2.04 | 29.36 ± 3.11 | 21.01 ± 2.11 | 14.55 ± 1.23 | 12.75 ± 2.45 | 5.04 ± 1.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Li, H.; Ji, J.; Wang, J.; Ji, Y. Self-Aggregation, Antimicrobial Activity and Cytotoxicity of Ester-Bonded Gemini Quaternary Ammonium Salts: The Role of the Spacer. Molecules 2023, 28, 5469. https://doi.org/10.3390/molecules28145469
Liang Y, Li H, Ji J, Wang J, Ji Y. Self-Aggregation, Antimicrobial Activity and Cytotoxicity of Ester-Bonded Gemini Quaternary Ammonium Salts: The Role of the Spacer. Molecules. 2023; 28(14):5469. https://doi.org/10.3390/molecules28145469
Chicago/Turabian StyleLiang, Yaqin, Hui Li, Jiahui Ji, Jiayu Wang, and Yujie Ji. 2023. "Self-Aggregation, Antimicrobial Activity and Cytotoxicity of Ester-Bonded Gemini Quaternary Ammonium Salts: The Role of the Spacer" Molecules 28, no. 14: 5469. https://doi.org/10.3390/molecules28145469




