Kinetics and Optimization of Metal Leaching from Heat-Resistant Nickel Alloy Solid Wastes
Abstract
1. Introduction
2. Results and Discussion
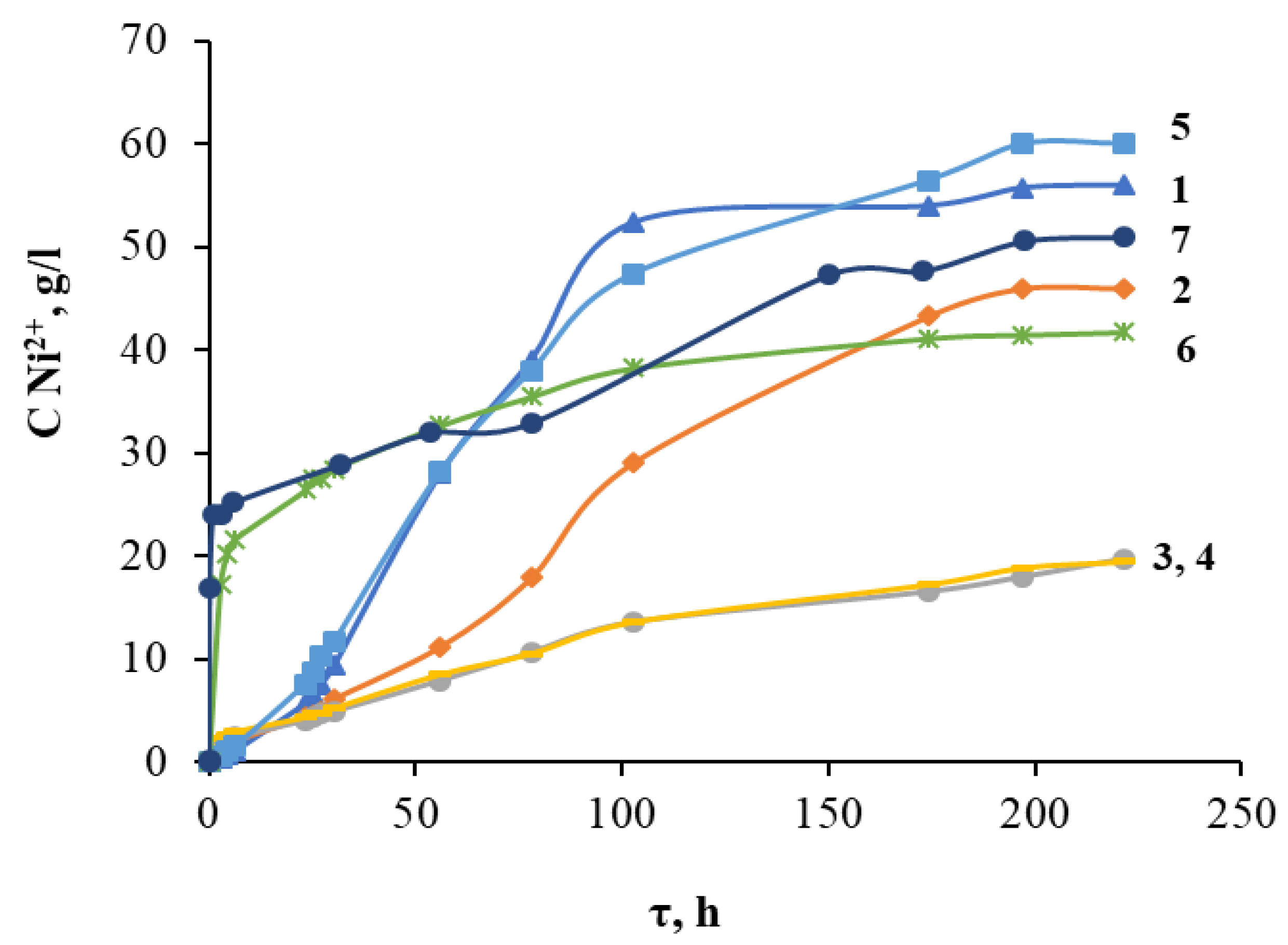
2.1. Optimization of the Acid Decomposition Process of Grinding Waste
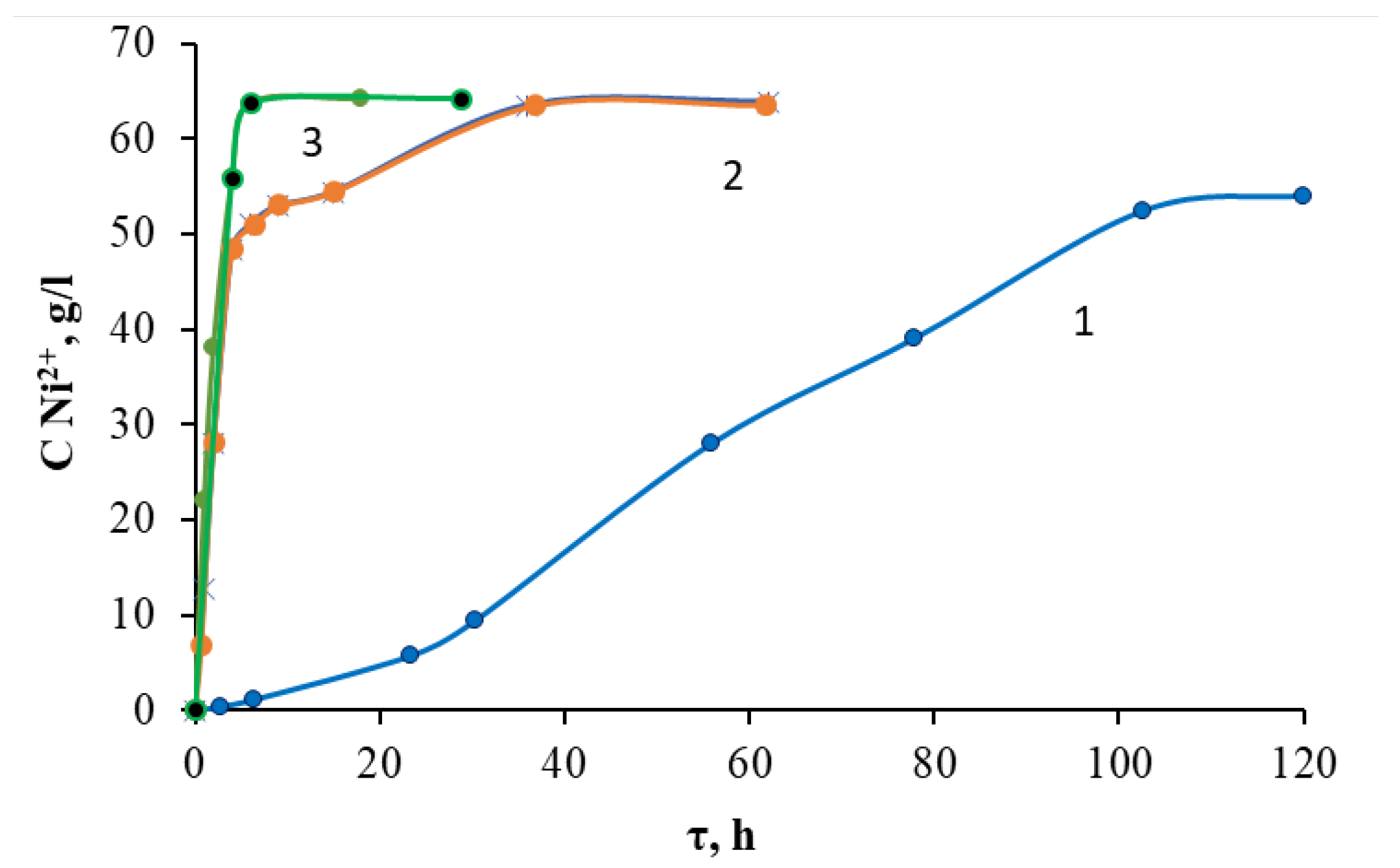
2.2. Kinetics of Acid Decomposition of Grinding Waste
3. Experimental
3.1. Chemicals Used
3.2. Instruments Used
3.3. Procedure
3.3.1. Sample Preparation
3.3.2. Characterization of Solid Waste Samples
3.3.3. Samples Leaching
3.3.4. Method of Metal Ion Determination
3.3.5. Description of Kinetic Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ali, I.; Aboul-Enein, H.Y. Speciation of metal ions by capillary electrophoresis. Critic. Rev. Anal. Chem. 2002, 32, 337–350. [Google Scholar] [CrossRef]
- Ali, I.; Aboul-Enein, H.Y. Determination of metal ions in water, soil and sediment by capillary electrophoresis. Anal. Lett. 2002, 35, 2053–2076. [Google Scholar] [CrossRef]
- Ali, I.; Hasan, M.A.; Alharbi, O.M.L. Toxic metal ions contamination in the groundwater, Kingdom of Saudi Arabia. J. Taibah Univ. Sci. 2020, 14, 1571–1579. [Google Scholar] [CrossRef]
- Bartzas, G.; Tsakiridis, P.E.; Komnitsas, K. Nickel industry: Heavy metal(loid)s contamination—Sources, environmental impacts and recent advances on waste valorization. Curr. Opin. Environ. Sci. Health 2021, 21, 100253. [Google Scholar] [CrossRef]
- Krasikov, S.A.; Zhilina, E.M.; Russkih, A.S.; Osinkina, T.V. Selection of elements at the dissolution of heat resistant nickel alloys in mineral acid solutions: IV Congress “Fundamental research and applied developing of recycling and utilization processes of technogenic formations”. KnE Mater. Sci. 2020, 307–311. [Google Scholar] [CrossRef]
- Targanov, I.E.; Troshkina, I.D. Kinetics of sulfuric-Acid nickel leaching from the grinding wastes of rhenium-containing superalloys. Russ. J. Non-Ferr. Met. 2021, 62, 508–513. [Google Scholar] [CrossRef]
- Jäger, S.; Weber, S.; Röttger, A. Potential of the recycling of grinding sludge by various powder metallurgical processes. Procedia CIRP 2021, 104, 893–899. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mate. 2008, 158, 228–256. [Google Scholar] [CrossRef]
- Agapova, L.Y.; Kilibayeva, S.K.; Abisheva, Z.S.; Sharipova, A.S. Complex electrochemical processing of technogenic wastes of rhenium-containing heat-resistant nickel alloys. Non-Ferr. Met. 2020, 1, 24–30. [Google Scholar] [CrossRef]
- Chen, W.-S.; Chen, Y.-C.; Lee, C.-H. Hydrometallurgical recovery of iron, nickel, and chromium from stainless steel sludge with emphasis on solvent extraction and chemical precipitation. Processes 2022, 10, 748. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, J.C.; Park, H.S.; Jun, M.J.; Kim, B.S. A multistep leaching of nickel-based superalloy scrap for selective dissolution of its constituent metals in hydrochloric acid solutions. Hydrometallurgy 2018, 176, 235–242. [Google Scholar] [CrossRef]
- Mamo, S.K.; Elie, M.; Baron, M.G.; Simons, A.M.; Gonzalez-Rodriguez, J. Leaching kinetics, separation, and recovery of rhenium and component metals from CMSX-4 superalloys using hydrometallurgical processes. Sep. Purif. Technol. 2019, 212, 150–160. [Google Scholar] [CrossRef]
- Ottink, T.; Vieceli, N.; Foreman, M.R.J.; Petranikova, M. Novel approach to recycling of steel swarf using hydrometallurgy resources. Conserv. Recycl. 2022, 185, 106450. [Google Scholar] [CrossRef]
- Zante, G.; Boltoeva, M. Review on hydrometallurgical recovery of metals with deep eutectic solvents. Sustain. Chem. 2020, 1, 238–255. [Google Scholar] [CrossRef]
- Krishnan, S.; Zulkapli, N.S.; Kamyab, H.; Taib, S.M.; Din, M.F.B.M.; Majid, Z.A.; Chaiprapat, S.; Kenzo, I.; Ichikawa, Y.; Nasrullah, M.; et al. Current technologies for recovery of metals from industrial wastes: An overview. Environ. Technol. Innov. 2021, 22, 101525. [Google Scholar] [CrossRef]
- Santoso, P.D.; Prasetyo, A.B.; Maksum, A.; Ulum, R.M. The effect of leaching time and concentration of sulfuric acid on increasing nickel and cobalt content from ferronickel slag waste after alkaline fusion using sodium carbonate. AIP Conf. Proc. 2020, 2255, 040031. [Google Scholar]
- Kamberovi, Z.; Ranitovi, M.; Kora, M.; Andji, Z. Hydrometallurgical Process for Selective Metals Recovery from Waste-Printed Circuit Boards. Metals 2018, 8, 441. [Google Scholar] [CrossRef]
- Behnamfard, A.; Mohammad Mehdi Salarirad, M.M.; Veglio, F. Process development for recovery of copper and precious metals from waste printed circuit boards with emphasize on palladium and gold leaching and precipitation. Waste Manag. 2013, 33, 2354–2363. [Google Scholar] [CrossRef]
- Gaydukova, A.; Kon’kova, T.; Kolesnikov, V.; Pokhvalitova, A. Adsorption of Fe3+ ions onto carbon powder followed by adsorbent electroflotation. Environ. Technol. Innov. 2021, 23, 101722. [Google Scholar] [CrossRef]
- Barragan, J.A.; Ponce de León, C.; Alemán Castro, J.R.; Peregrina-Lucano, A.; Gómez-Zamudio, F.; Larios-Durán, E.R. Copper and Antimony Recovery from Electronic Waste by Hydrometallurgical and Electrochemical Techniques. ACS Omega 2020, 5, 12355–12363. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Pan, M.-W.; Lo, S.-L. Hydrometallurgical metal recovery from waste printed circuit boards pretreated by microwave pyrolysis. Resour. Conserv. Recycl. 2020, 163, 105090. [Google Scholar] [CrossRef]
- Shenxu, B.; Yongping, T.; Yimin, Z.; Liang, L. Recovery and Separation of Metal Ions from Aqueous Solutions by Solvent-Impregnated Resins. Chem. Eng. Technol. 2016, 39, 1377–1392. [Google Scholar]
- Page, M.J.; Soldenhoff, K.; Ogdenb, M.D. Comparative study of the application of chelating resins for rare earth recovery. Hydrometallurgy 2017, 169, 275–281. [Google Scholar] [CrossRef]
- Li, H.; Dai, J.; Wang, A.; Zhao, S.; Ye, H.; Zhang, J. Recycling and Treatment of Waste Batteries. IOP Conf. Ser. Mater. Sci. Eng. 2019, 61, 0520201. [Google Scholar] [CrossRef]
- Lia, H.; Eksteena, J.; Orabya, E. Hydrometallurgical recovery of metals from waste printed circuit boards, (WPCBs): Current status and perspectives—A review Resources. Conserv. Recycl. 2018, 139, 122–139. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, F.; Su, Z.; Liu, S.; Anderson, G.; Jiang, T. Hydrometallurgical recovery of rare earth elements from NdFeB permanent magnet scrap: A review. Metals 2020, 10, 841. [Google Scholar] [CrossRef]
- Sethurajan, M.; van Hullebusch, E.D.; Fontana, D.; Akcil, A.; Deveci, H.; Batinic, B.; Leal, J.P.; Gasche, T.A.; Kucuker, M.A.; Kuchta, K.; et al. Recent advances on hydrometallurgical recovery of critical and precious elements from end of life electronic wastes—A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 212–275. [Google Scholar] [CrossRef]
- Levchuk, O.M.; Levin, A.M.; Bryukvin, V.A.; Troshkina, I.D. Electrochemical processing of W-Re alloy wastes in alkaline electrolytes under the action of alternating current. Tsvetnye Met. 2016, 6, 80–84. [Google Scholar] [CrossRef]
- Srivastava, R.R.; Kim, M.; Lee, J. Novel aqueous processing of the reverted turbine-blade superalloy for rhenium recovery. Ind. Eng. Chem. Res. 2016, 55, 8191–8199. [Google Scholar] [CrossRef]
- Liddell, K.C. Shrinking core models in hydrometallurgy: What students are not being told about the pseudo-steady approximation. Hydrometallurgy 2005, 79, 62–68. [Google Scholar] [CrossRef]
- Dickinsona, C.F.; Healb, G.R. Solid-liquid diffusion controlled rate equations. Thermochim. Acta 1999, 340–341, 89–103. [Google Scholar] [CrossRef]
- Krauklis, A.E.; Dreyer, I. A simplistic preliminary assessment of ginstling-brounstein model for solid spherical particles in the context of a diffusion-controlled synthesis. Open Chem. 2018, 16, 64–72. [Google Scholar] [CrossRef]
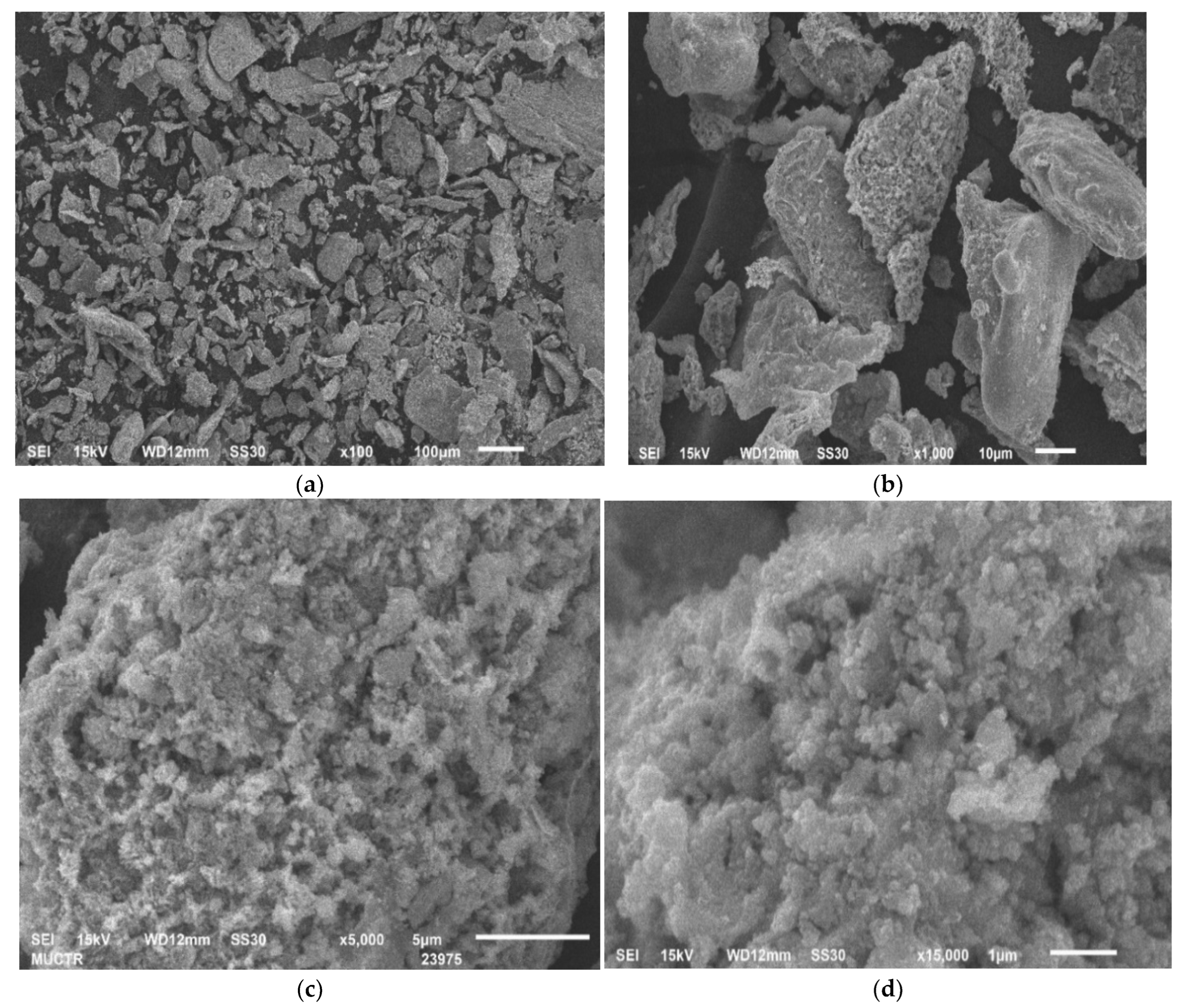
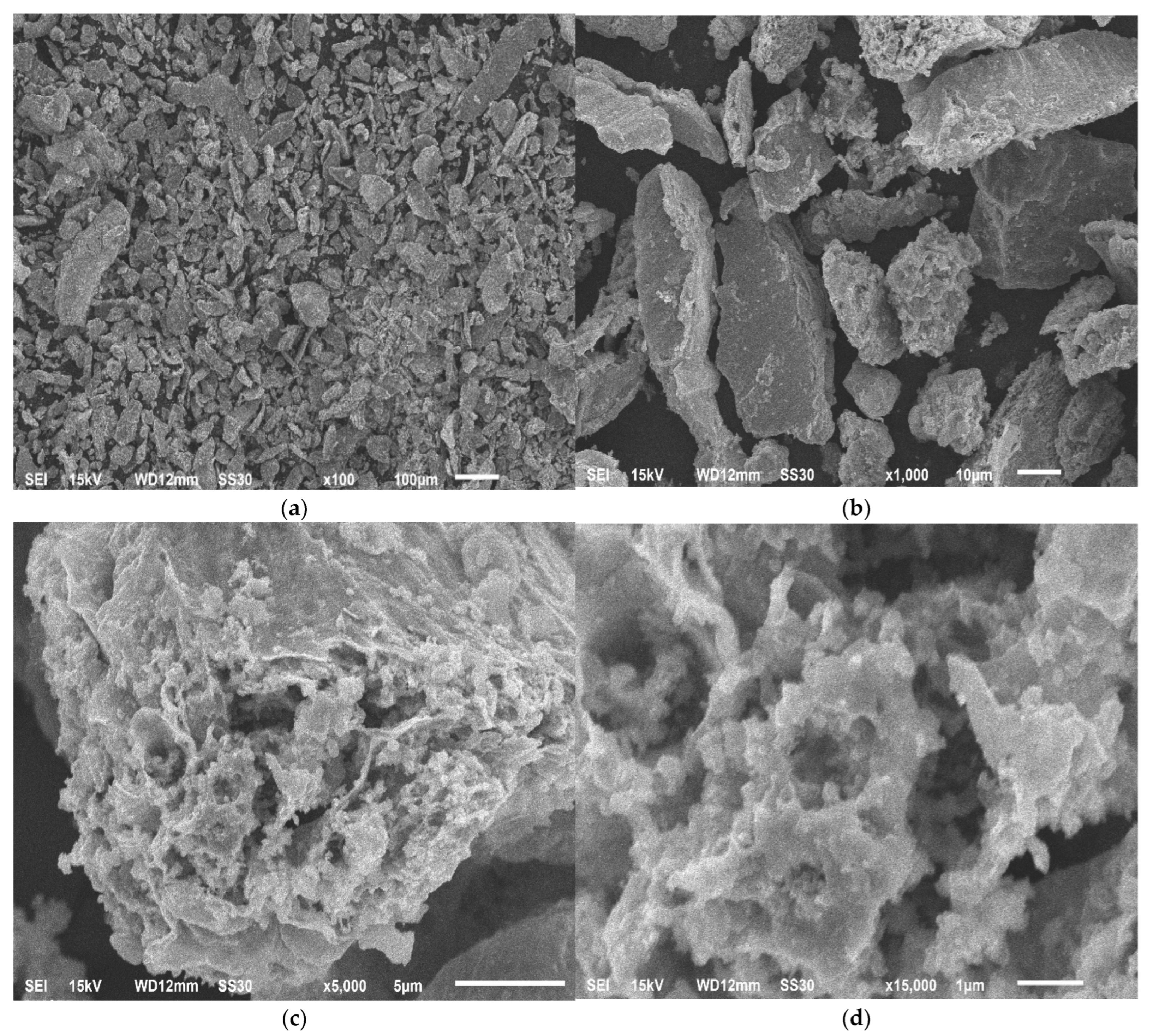
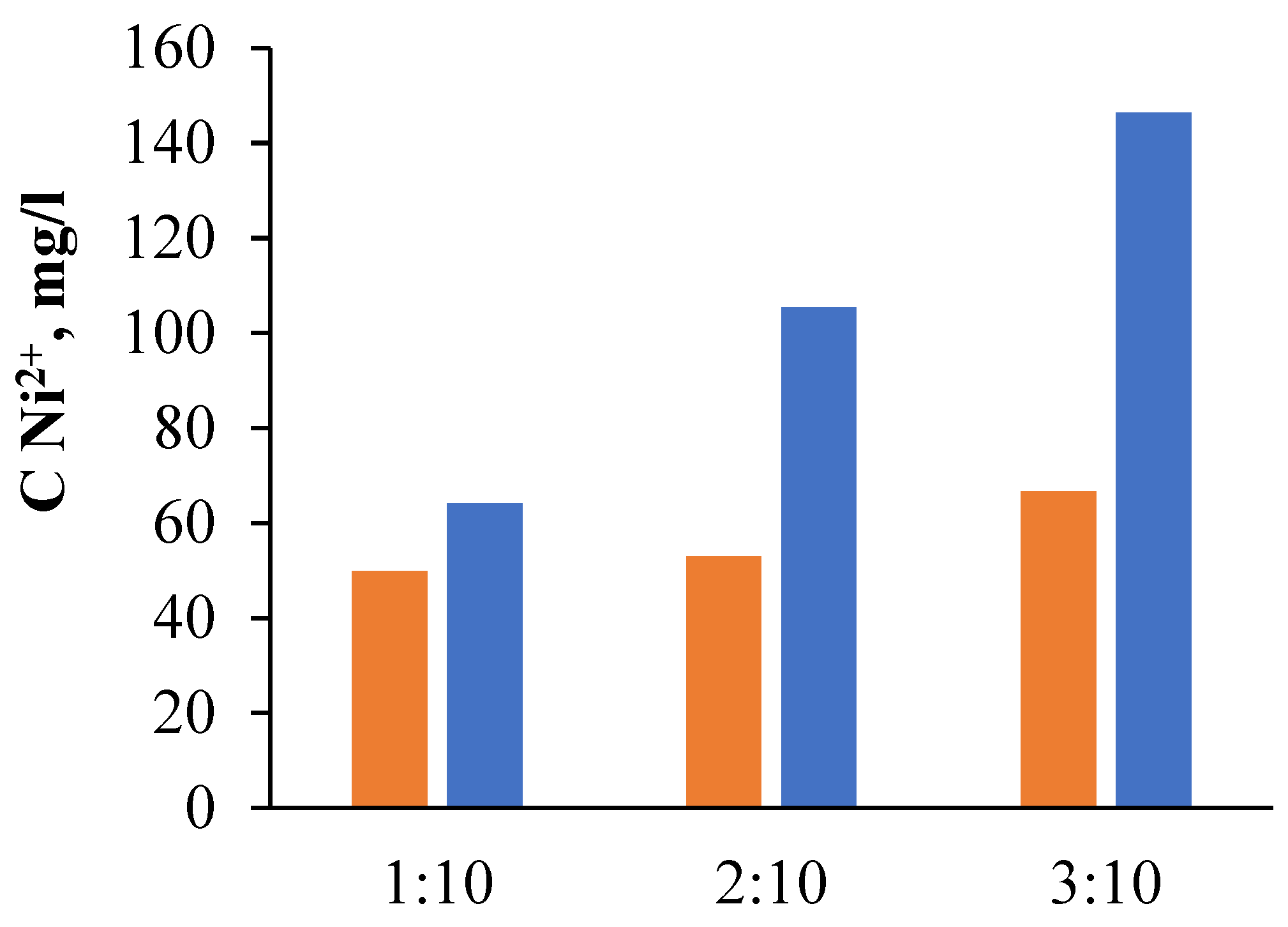

| Size, mm | 0.5–1 | 0.2–0.5 | 0.1–0.2 | 0.063–0.1 | 0.05–0.063 | 0.04–0.05 | <0.04 |
| Original sample | |||||||
| wt.% | 7.2 | 35.1 | 40.2 | 15.7 | 1.9 | 0 | 0 |
| Heat-treated sample | |||||||
| wt.% | 8.27 | 17.98 | 25.83 | 12.65 | 7.90 | 8.28 | 19.08 |
| Elements | C | O | Al | Ti | Cr | Co | Ni | Mo | W |
|---|---|---|---|---|---|---|---|---|---|
| Original samples | |||||||||
| wt.% | 14.92 | 12.50 | 2.33 | 2.88 | 14.57 | 1.15 | 36.74 | 5.13 | 9.78 |
| Heat-treated samples | |||||||||
| Масс.% | 6.72 | 9.19 | 1.36 | 3.07 | 15.9 | 1.12 | 46.94 | 8.08 | 7.62 |
| Elements | Ni | Cr | Co | Mo | Ti | Al | Fe | Re | Ir | V | Ru | Ca | W |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g/kg | 502.0 | 70.4 | 68.7 | 172.3 | 15.4 | 71.2 | 6.8 | 5.9 | 1.9 | 1.4 | 0.84 | 0.68 | 0.42 |
| T (°C) | Parameter | Ni | Cr | Co | Mo | Ti | Al | Fe | Re | Nb | W | Ta | Na |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 | C, g/L | 45.12 | 7.52 | 5.69 | 1.72 | 1.62 | 4.02 | 0.76 | 0.01 | 0.08 | 1.21 | 0.05 | 0.10 |
| α, % | 70 | 63 | 75 | 10 | 88 | 56 | 82 | 2 | - | - | - | - | |
| 70 | C, g/L | 61.63 | 11.67 | 7.34 | 2.41 | 1.85 | 5.21 | 0.93 | 0.05 | 0.10 | 1.59 | 0.07 | 0.81 |
| α, % | 96 | 98 | 99 | 14 | 99.9 | 73 | 99.9 | 8 | - | - | - | - | |
| 100 | C, g/L | 64.15 | 11.88 | 7.57 | 2.51 | 1.85 | 5.33 | 0.93 | 0.05 | 0.01 | 1.18 | 0.03 | 0.53 |
| α, % | 99.9 | 99.9 | 99.9 | 15 | 99.9 | 75 | 99.9 | 8 | - | - | - | - |
| P:S | Contents of Metal Ions in the Solution (g/L) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni | Cr | Co | Mo | Ti | Al | Fe | Re | Nb | W | Ta | Na | |
| 1:10 | 64.15 | 11.88 | 7.57 | 2.51 | 1.84 | 5.33 | 0.93 | 0.05 | 0.01 | 1.18 | 0.03 | 0.53 |
| 2:10 | 105.46 | 16.03 | 13.46 | 4.14 | 2.34 | 9.18 | 1.45 | 0.02 | 0.60 | 5.22 | 0.25 | 0.84 |
| 3:10 | 146.36 | 23.46 | 18.97 | 6.13 | 4.27 | 12.88 | 2.23 | 0.04 | 0.90 | 8.35 | 0.41 | 1.17 |
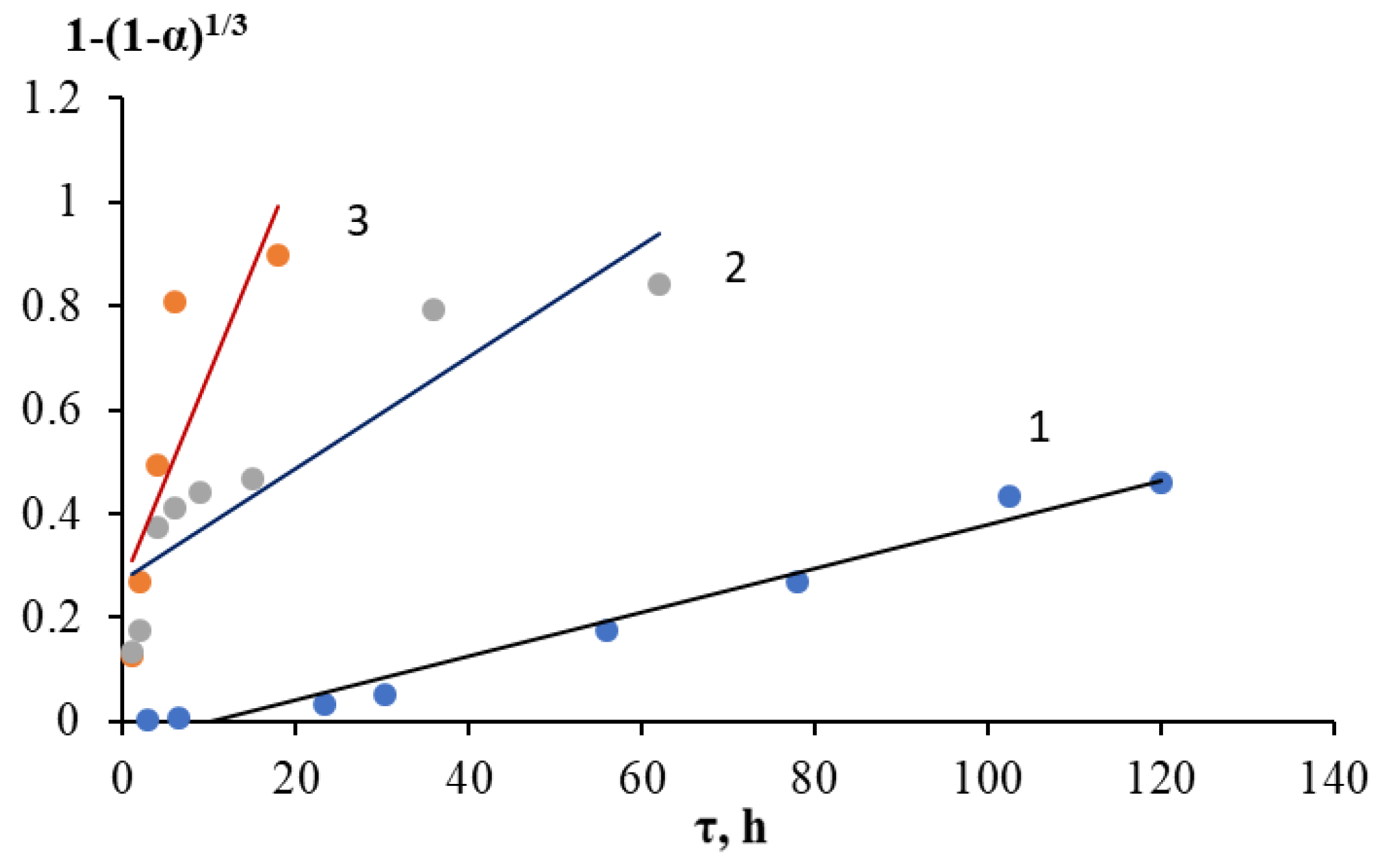
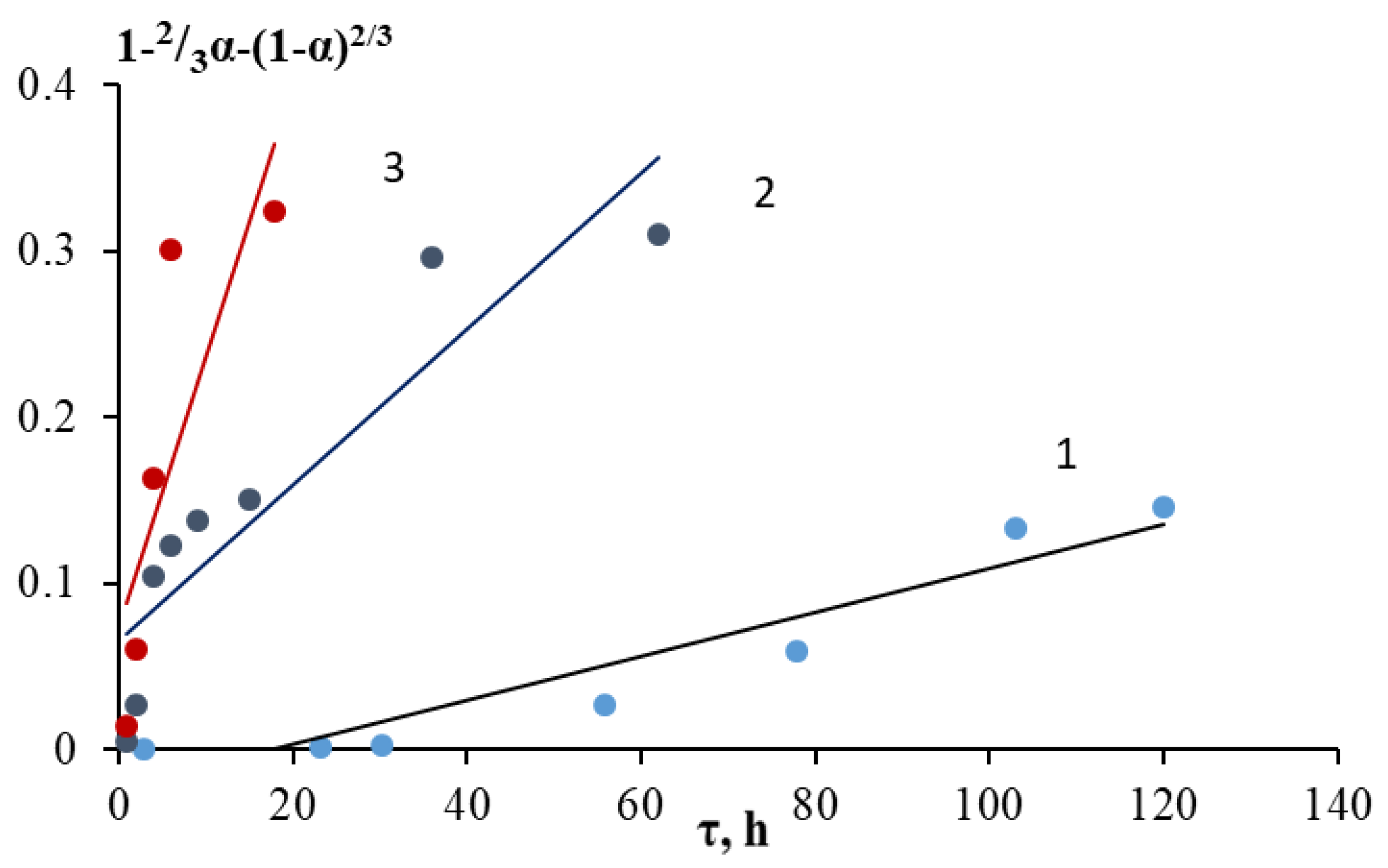
| T, °C | Gray–Weddington Equation | Gistling–Brownstein Equation | Kazeev–Erofeev Equation | ||||
|---|---|---|---|---|---|---|---|
| k·103, h–1 | R2 | k·103, h–1 | R2 | n | k·103, h–1 | R2 | |
| 25 | 4.2 | 0.9771 | 1.3 | 0.9105 | 1.55 | 1.1 | 0.9902 |
| 70 | 10.8 | 0.8269 | 4.7 | 0.8278 | 0.61 | 462.0 | 0.9403 |
| 100 | 40.2 | 0.6832 | 16.3 | 0.6483 | 0.91 | 608.6 | 0.8772 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, I.; Gaydukova, A.; Kon’kova, T.; ALOthman, Z.A.; Sillanpää, M. Kinetics and Optimization of Metal Leaching from Heat-Resistant Nickel Alloy Solid Wastes. Molecules 2023, 28, 5545. https://doi.org/10.3390/molecules28145545
Ali I, Gaydukova A, Kon’kova T, ALOthman ZA, Sillanpää M. Kinetics and Optimization of Metal Leaching from Heat-Resistant Nickel Alloy Solid Wastes. Molecules. 2023; 28(14):5545. https://doi.org/10.3390/molecules28145545
Chicago/Turabian StyleAli, Imran, Anastasya Gaydukova, Tatiana Kon’kova, Zeid Abdullah ALOthman, and Mika Sillanpää. 2023. "Kinetics and Optimization of Metal Leaching from Heat-Resistant Nickel Alloy Solid Wastes" Molecules 28, no. 14: 5545. https://doi.org/10.3390/molecules28145545
APA StyleAli, I., Gaydukova, A., Kon’kova, T., ALOthman, Z. A., & Sillanpää, M. (2023). Kinetics and Optimization of Metal Leaching from Heat-Resistant Nickel Alloy Solid Wastes. Molecules, 28(14), 5545. https://doi.org/10.3390/molecules28145545








