Biological Activity and Structural Diversity of Steroids Containing Aromatic Rings, Phosphate Groups, or Halogen Atoms
Abstract
:1. Introduction
2. Steroids Bearing Aromatic Ring(s)
2.1. Steroids Bearing Aromatic Ring A in Plants
Steroids Bearing A, B, C, or D Aromatic Ring
2.2. Steroids Bearing Two or Three Aromatic Rings Derived from Natural Sources
3. Steroids Bearing Phosphate Esters
Steroid Phosphate Esters in Marine Invertebrates



4. Steroids Bearing a Halogen Atom (Cl, Br, or I)
4.1. Chlorinated Plant Steroids
4.2. Halogenated Steroids Derived from Marine Sources
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fahy, E.; Cotter, D.; Sud, M.; Subramaniam, S. Lipid classification, structures, and tools. Biochim. Biophys. Acta 2011, 1811, 637–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reszczyńska, E.; Hanaka, A. Lipids composition in plant membranes. Cell Biochem. Biophys. 2020, 78, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Bishop, G.J.; Koncz, C. Brassinosteroids and plant steroid hormone signaling. Plant Cell 2002, 14, S97–S110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, D.; Loi, B.; Porte, C. Biosynthesis and metabolism of steroids in molluscs. J. Steroid Biochem. Mol. Biol. 2011, 127, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, N.; Auchus, R.J. Steroid biosynthesis and prostate cancer. Steroids 2012, 77, 719–726. [Google Scholar] [CrossRef]
- Li, J.; Papadopoulos, V.; Vihma, V. Steroid biosynthesis in adipose tissue. Steroids 2015, 103, 89–104. [Google Scholar] [CrossRef]
- Moss, G.P. Nomenclature of steroids. Pure Appl. Chem. 1989, 61, 1783–1822. [Google Scholar] [CrossRef]
- Russel, C.A. Organic chemistry: Natural products, steroids. In Chemical History: Reviews of the Recent Literature; Russell, C.A., Roberts, G.K., Eds.; RSC Publ.: Cambridge, UK, 2005. [Google Scholar]
- Kirk, D.N.; Marples, B.A. The structure and nomenclature of steroids. In Steroid Analysis; Makin, H.L.J., Gower, D.B., Kirk, D.N., Eds.; Springer: Dordrecht, The Netherlands, 1995. [Google Scholar]
- Li, H.M.; Chen, X.J.; Luo, D.; Fan, M.; Zhang, Z.J.; Peng, L.Y.; Wu, X.D.; Li, R.T.; Ji, X.; Zhao, Q.S. Protostane-type triterpenoids from Alisma orientale. Chem. Biodivers. 2017, 14, e1700452. [Google Scholar] [CrossRef]
- Gribble, G.W. Biological activity of recently discovered halogenated marine natural products. Mar. Drugs 2015, 13, 4044–4136. [Google Scholar] [CrossRef] [Green Version]
- Cabrita, M.T.; Vale, C.; Rauter, A.P. Halogenated compounds from marine algae. Mar. Drugs 2010, 8, 2301–2317. [Google Scholar] [CrossRef] [Green Version]
- Ermolenko, E.V.; Imbs, A.B.; Gloriozova, T.A.; Poroikov, V.V.; Sikorskaya, T.V.; Dembitsky, V.M. Chemical diversity of soft coral steroids and their pharmacological activities. Mar. Drugs 2020, 18, 613. [Google Scholar] [CrossRef] [PubMed]
- Mello, F.V.; Kasper, D.; Alonso, M.B.; Torres, J.P.M. Halogenated natural products in birds associated with the marine environment: A review. Sci. Total Environ. 2020, 717, 137000. [Google Scholar] [CrossRef] [PubMed]
- Morais, T.; Cotas, J.; Pacheco, D.; Pereira, L. Seaweeds compounds: An ecosustainable source of cosmetic ingredients? Cosmetics 2021, 8, 8. [Google Scholar] [CrossRef]
- Sanjeewa, K.A.; Jeon, Y.-J. Edible brown seaweeds: A review. J. Food Bioact. 2018, 2, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Sohn, S.-I.; Rathinapriya, P.; Balaji, S.; Jaya Balan, D.; Swetha, T.K.; Durgadevi, R.; Alagulakshmi, S.; Singaraj, P.; Pandian, S. Phytosterols in seaweeds: An overview on biosynthesis to biomedical applications. Int. J. Mol. Sci. 2021, 22, 12691. [Google Scholar] [CrossRef]
- Gnanavel, V.; Roopan, S.M.; Rajeshkumar, S. Aquaculture: An overview of chemical ecology of seaweeds (food species) in natural products. Aquaculture 2019, 507, 1–6. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, W.; Huang, Y. Global seaweed farming and processing in the past 20 years. Food Prod. Process. Nutr. 2022, 4, 23. [Google Scholar] [CrossRef]
- Sakthivel, R.; Devi, K.D. Antioxidant, anti-inflammatory and anticancer potential of natural bioactive compounds from seaweeds. Stud. Nat. Prod. Chem. 2019, 63, 113–160. [Google Scholar]
- Dembitsky, V.M.; Savidov, N.; Poroikov, V.V. Naturally occurring aromatic steroids and their biological activities. Appl. Microbiol. Biotechnol. 2018, 102, 4663–4674. [Google Scholar] [CrossRef]
- Kadis, B.M. Synthesis of Steroid Precursors. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 1957. [Google Scholar]
- Taub, D. Naturally occurring aromatic steroids. In Total Synthesis of Natural Products; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1973; Volume 2. [Google Scholar]
- Rutherford, F.J. Ceric Oxidations of Aromatic Steroids and Related Compounds. Ph.D. Thesis, University of Edinburgh, Edinburgh, UK, 1972. [Google Scholar]
- Niven, S.J. The Origins and Occurrence of Estrogenic A-Ring Aromatic Steroids in U.K. Sewage Treatment Works Effluents. Ph.D. Thesis, University of Plymouth, Plymouth, UK, 1999. [Google Scholar]
- Gupta, R.R.; Jain, M. Aliphatic and Aromatic Hydrocarbons, Steroids, Carbohydrates; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Huang, H.; Yin, M.; Han, D. Novel parameters derived from alkylchrysenes to differentiate severe biodegradation influence on molecular compositions in crude oils. Fuel 2020, 268, 117366. [Google Scholar] [CrossRef]
- Matyasik, I.; Bieleń, W. Aromatic steroids as a tool in geochemical interpretation. Nafta-Gaz 2015, 71, 376–383. [Google Scholar]
- Yang, C.; Wang, Z.; Liu, Y.; Yang, Z.; Li, Y.; Shah, K. Aromatic steroids in crude oils and petroleum products and their applications in forensic oil spill identification. Environ. Forensics 2013, 14, 278–293. [Google Scholar] [CrossRef]
- Barbanti, S.M.; Moldowan, J.M.; Watt, D.S.; Kolaczkowska, E. New aromatic steroids distinguish Paleozoic from Mesozoic oil. Org. Geochem. 2011, 42, 409–424. [Google Scholar] [CrossRef]
- Li, L.; Jiang, L.; George, S.C.; Liu, Z. Aromatic compounds in lacustrine sediments from the Lower Cretaceous Jiufotang formation, Chaoyang basin (NE China). Mar. Pet. Geol. 2021, 129, 105111. [Google Scholar] [CrossRef]
- Lednicer, D. Steroid Chemistry at a Glance; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; p. 152. [Google Scholar]
- Pantoja, S.; Wakeham, S. Marine organic geochemistry: A general overview. In Chemical Processes in Marine Environments; Gianguzza, A., Pelizetti, E., Sammartano, S., Eds.; Environmental Science; Springer: Berlin/Heidelberg, Germany, 2000; pp. 43–74. [Google Scholar]
- Killops, S.; Killops, V. FrontMatter. In Front Matter, in Introduction to Organic Geochemistry; Blackwell Publishing Ltd.: Malden, MA, USA, 2004. [Google Scholar]
- Fluhmann, C.F. Estrogenic hormones: Their clinical usage. Calif. West. Med. 1938, 49, 362–366. [Google Scholar]
- Edgar, A.; Doisy, E.A. An ovarian hormone: Preliminary report on its localization, extraction and partial purification, and action in test animals. J. Am. Med. Assoc. 1923, 81, 819–821. [Google Scholar]
- Doisy, E.A.; Clement, D.V.; Sidney, T. Folliculin from urine of pregnant women. Am. J. Phys. 1929, 90, 329–330. [Google Scholar]
- Butenandt, A. Über “Progynon” ein krystallisiertes weibliches Sexualhormon. Naturwissenschaften 1929, 17, 879. [Google Scholar] [CrossRef]
- Butenandt, A. Über physikalische und chemische Eigenschaften des krystallisierten Follikelhormons. Untersuchungen über das weibliche Sexualhormon. Hoppe-Seyler’s Zeit. Physiol. Chem. 1930, 191, 140–156. [Google Scholar] [CrossRef]
- Dohrn, M.; Faure, W.; Poll, H.; Blotevogel, W. Tokokinine, Stoff mit sexualhormonartiger Wirkung aus Pflanzenzellen. Med. Klin. 1926, 22, 1417–1419. [Google Scholar]
- Butenandt, A.; Jacobi, H. Über die Darstellung eines krystallisierten pflanzlichen Tokokinins (Thelykinins) und seine Identifizierung mit dem α-Follikelhormon. Untersuchungen über das weibliche Sexualhormon. Hoppe Seyler’s Z. Physiol. Chem. 1933, 218, 104–112. [Google Scholar] [CrossRef]
- Skarzynski, B. An oestrogenic substance from plant material. Nature 1933, 131, 766. [Google Scholar]
- Janeczko, A.; Skoczowski, A. Mammalian sex hormones in plants. Folia Histochem. Cytobiol. 2005, 43, 71–79. [Google Scholar] [PubMed]
- Zhang, J.S.; Yang, Z.H.; Tsao, T.H. The occurrence of estrogens in relation to reproductive processes in flowering plants. Sex. Plant Reprod. 1991, 4, 193–196. [Google Scholar] [CrossRef]
- Zhong-han, Y.; Yin, T.; Zong-xun, C.; Tsao, T.H. The changes of steroidal sex hormone—Testosterone contents in reproductive organs of Lilium davidii Duch. Acta Bot. Sin. 1994, 36, 215–220. [Google Scholar]
- Janot, M.M.; Devissaguet, P.; Khuong-Huu, Q.; Goutarel, R. Steroid alkaloids. LXVI. New alkaloids from the husks of Holarrhena floribunda (G. Don) Dur. and Schinz: Holarrheline, holadienine, holaromine and holaline. Ann. Pharm. Fr. 1967, 25, 733–748. [Google Scholar]
- Cain, J.C. Miroestrol—An estrogen fromthe plant Pueraria mirifica. Nature 1960, 188, 774–777. [Google Scholar] [CrossRef]
- Misico, R.I.; Veleiro, A.S.; Burton, G.; Oberti, J.C. Withanolides from Jaborosa leucotricha. Phytochemistry 1997, 45, 1045–1048. [Google Scholar] [CrossRef]
- Cirigliano, A.M.; Veleiro, A.S.; Misico, R.I.; Tettamanzi, M.C.; Oberti, J.C.; Burton, G. Withanolides from Jaborosa laciniata. J. Nat. Prod. 2007, 70, 1644–1646. [Google Scholar] [CrossRef]
- Valente, L.M.; Gunatilaka, A.A.; Glass, T.E.; Kingston, D.G.; Pinto, A.C. New norcucurbitacin and heptanorcucurbitacin glucosides from Fevillea trilobata. J. Nat. Prod. 1993, 56, 1772–1778. [Google Scholar] [CrossRef]
- Igarashi, K. Studies on the steroidal components of domestic plants. XXXV. Structure of meteogenin. Chem. Pharm. Bull. 1961, 9, 722–729. [Google Scholar] [CrossRef] [Green Version]
- Minato, H.; Shimaoka, A. Studies on the steroidal components of domestic plants. XLII. Narthogenin, isonarthogenin and neonogiragenin, three new sapogenins of metanarthecium luteoviride MAXIM. Chem. Pharm. Bull. 1961, 9, 729–734. [Google Scholar]
- Pkheidze, T.A.; Gvazava, L.N.; Kemertelidze, É.P. Luvigenin and hecogenin from the leaves of Yucca gloriosa. Chem. Nat. Compd. 1991, 27, 376. [Google Scholar] [CrossRef]
- Sobolewska, D.; Michalska, K.; Podolak, I.; Grabowska, K. Steroidal saponins from the genus Allium. Phytochem. Rev. 2016, 15, 1–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Himeno, E.; Nagao, T.; Honda, J.; Okabe, H.; Irino, N.; Nakasumi, T. Structures of cayaponosides A, B, C and D, glucosides of new norcucurbitacins in the roots of Cayaponia tayuya. Chem. Pharm. Bull. 1992, 40, 2885–2887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Himeno, E.; Nagao, T.; Nonda, J.; Okabe, H.; Irino, N.; Nakasumi, T. Studies on the constituents of the root of Cayaponia tayuya (Vell) Cogn. I. Structures of cayaponosides, new 29-Nor-1,2,3,4,5,10-hexadehydro-cucurbitacin glucosides. Chem. Pharm. Bull. 1994, 42, 2295–2300. [Google Scholar] [CrossRef] [Green Version]
- Konoshima, T.; Takasaki, M.; Kozuka, M.; Nagao, T.; Okabe, H. Inhibitory effects of cucurbitane triterpenoids on Epstein-Barr virus activation and two stage carcinogenesis of skin tumor. II. Biol. Pharm. Bull. 1995, 18, 284–287. [Google Scholar] [CrossRef]
- Shirota, O.; Sekita, S.; Satake, M.; Morita, H.; Takeya, K.; Itokawa, H. Two cangorosin A type triterpene dimers from Maytenus chuchuhuasca. Chem. Pharm. Bull. 2004, 52, 1148–1150. [Google Scholar] [CrossRef] [Green Version]
- Araújo Júnior, R.F.; Oliveira, A.L.; Pessoa, J.B.; Garcia, V.B. Maytenus ilicifolia dry extract protects normal cells, induces apoptosis, and regulates Bcl-2 in human cancer cells. Exp. Biol. Med. 2013, 238, 1251–1258. [Google Scholar] [CrossRef]
- Vendruscolo, G.S.; Simoes, C.M.O.; Mentz, L.A. Etnobotanica no Rio Grande do Sul: Analise comparative entre o conhecimento original eatual sobre as plantas medicinais nativas. Pesqui. Bot. 2005, 56, 285–320. [Google Scholar]
- Goncalves, M.I.A.; Martins, D.T.O. Plantas medicinais usadas pela populacao do municipio de Santo Antonio de Leverger, Mato Grosso, Brasil. Rev. Bras. Farm. 1998, 79, 56–61. [Google Scholar]
- Si, Y.; Yao, X.H.; Zhang, C.K.; Tu, Z.B. C-32 triterpenes from Taxodium ascendens. Biochem. Syst. Ecol. 2005, 33, 211–214. [Google Scholar]
- Otto, A.; White, J.D.; Simoneit, B.R.T. Natural product terpenoids in Eocene and Miocene conifer fossils. Science 2012, 297, 1543–1545. [Google Scholar] [CrossRef]
- Guo, J.; Xue, J.; Hua, J.; Yin, Y.; Creech, D.L.; Han, J. Research status and trends of Taxodium distichum. HortScience 2023, 58, 317–326. [Google Scholar] [CrossRef]
- Lu, Z.; Van Wagoner, R.M.; Harper, M.K.; Hooper, J.N.A.; Ireland, C.M. Two ring-A aromatized bile acids from the marine sponge Sollasella moretonensis. Nat. Prod. Commun. 2010, 5, 1571–1574. [Google Scholar] [PubMed] [Green Version]
- Goddard, P.; Hill, M.J. The dehydrogenation of the steroid nucleus by human-gut bacteria. Biochem. Soc. Trans. 1973, 1, 1113–1115. [Google Scholar] [CrossRef]
- Yeung, B.K.S.; Hamann, M.T.; Scheuer, P.J.; Kelly-Borges, M. Hapaioside: A 19-norpregnane glycoside from the sponge Cribrochalina olemda. Tetrahedron 1994, 50, 12593–12598. [Google Scholar] [CrossRef]
- Di Girolamo, J.A.; Li, X.-C.; Jacob, M.R.; Clark, A.M.; Ferreira, D. Reversal of fluconazole resistance by sulfated sterols from the marine sponge Topsentia sp. J. Nat. Prod. 2009, 72, 1524–1528. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.-H.; Liu, H.-L.; Huang, H.; Li, X.-B.; Guo, Y.-W. Steroids with aromatic A rings from the Hainan soft coral Dendronephthya studeri Ridley. J. Nat. Prod. 2011, 74, 175–180. [Google Scholar] [CrossRef]
- Poza, J.J.; Fernández, R.; Reyes, F.; Rodríguez, J.; Jiménez, C. Isolation, biological significance, synthesis, and cytotoxic evaluation of new natural parathiosteroids A-C and analogues from the soft coral Paragorgia sp. J. Org. Chem. 2008, 73, 7978–7984. [Google Scholar] [CrossRef]
- Barrero, A.F.; Oltra, J.E.; Poyatos, J.A.; Jiménez, D.; Oliver, E. Phycomysterols and other sterols from the fungus Phycomyces blakesleeanus. J. Nat. Prod. 1998, 61, 1491–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.H.; Tang, X.Z.; Miao, F.P.; Ji, N.Y. A new pyrrolidine derivative and steroids from an algicolous Gibberella zeae strain. Nat. Prod. Commun. 2011, 6, 1243–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, F.R.; Gu, B.B.; Zhu, H.R.; Zhang, Y.; Liu, K.C.; Zhang, W.; Shi-Hai, H.H.; Lin, H.W. Asperfloketals A and B, the first two ergostanes with rearranged A and D rings: From the sponge-associated Aspergillus flocculosus. J. Org. Chem. 2021, 86, 10954–10961. [Google Scholar] [CrossRef]
- Luo, X.; Li, F.; Shinde, P.B.; Hong, J.; Lee, C.-O.; Im, K.S.; Jung, J.H. 26,27-Cyclosterols and other polyoxygenated sterols from a marine sponge Topsentia sp. J. Nat. Prod. 2006, 69, 1760–1768. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.L.; Li, J.L.; Hong, J.; Yoon, W.D.; Kim, H.S.; Liu, Y.; Wei, X.; Jung, J.H. An unusual 1(10→19)abeo steroid from a jellyfish-derived fungus. Tetrahedron Lett. 2016, 57, 2803–2806. [Google Scholar] [CrossRef]
- Li, G.; Kusari, S.; Kusari, P.; Kayser, O.; Spiteller, M. Endophytic Diaporthe sp. LG23 produces a potent antibacterial tetracyclic triterpenoid. J. Nat. Prod. 2015, 78, 2128–2132. [Google Scholar] [CrossRef]
- Rowland, S.J.; West, C.E.; Jones, D.; Scarlett, A.G.; Frank, R.A.; Hewitt, L.M. Steroidal aromatic ‘naphthenic acids’ in oil sands process affected water: Structural comparisons with environmental estrogens. Environ. Sci. Technol. 2011, 45, 9806–9815. [Google Scholar] [CrossRef]
- Pounina, T.A.; Gloriozova, T.A.; Savidov, N.; Dembitsky, V.M. Sulfated and sulfur-containing steroids and their pharmacological profile. Mar. Drugs 2021, 19, 240. [Google Scholar] [CrossRef]
- Wang, W.; Lee, Y.; Lee, T.G.; Mun, B.; Giri, A.G.; Lee, J.; Kim, H. Phorone A and isophorbasone A, sesterterpenoids isolated from the marine sponge Phorbas sp. Org. Lett. 2012, 14, 4486–4489. [Google Scholar] [CrossRef]
- Gao, S.; Wang, Q.; Huang, L.J.S.; Lum, L.; Chen, C. Chemical, and biological studies of nakiterpiosin and nakiterpiosinone. J. Am. Chem. Soc. 2010, 132, 371–383. [Google Scholar] [CrossRef] [Green Version]
- Venugopal, J.R.; Mukku, V.; Edrada, R.A.; Schmitz, F.J.; Shanks, M.K.; Chaudhuri, B.; Fabbro, D. New sesquiterpene quinols from a Micronesian sponge, Aka sp. J. Nat. Prod. 2003, 66, 686–689. [Google Scholar]
- Misico, R.I.; Nicotra, V.E.; Oberti, J.C.; Barboza, G.; Gil, R.R.; Burton, G. Withanolides and related steroids. Prog. Chem. Org. Nat. Prod. 2011, 94, 127–229. [Google Scholar]
- Crews, P.; Harrison, B. New triterpene-ketides (Merotriterpenes), haliclotriol A and B, from an Indo–Pacific Haliclona sponge. Tetrahedron 2000, 56, 9039–9046. [Google Scholar] [CrossRef]
- Williams, D.E.; Steinø, A.; de Voogd, N.J.; Mauk, A.G.; Andersen, R.J. Halicloic acids A and B isolated from the marine sponge Haliclona sp. collected in the Philippines inhibit indoleamine 2,3-dioxygenase. J. Nat. Prod. 2012, 75, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Falk, H.; Wolkenstein, K. Natural product molecular fossils. In Progress in the Chemistry of Organic Natural Products; Kinghorn, A., Falk, H., Gibbons, S., Kobayashi, J., Eds.; Springer: Cham, Switzerland, 2017; Volume 104. [Google Scholar]
- Oliveira, C.R.; Oliveira, C.J.F.; Ferreira, A.A.; Azevedo, D.A.; Neto, F.R.A. Characterization of aromatic steroids and hopanoids in marine and lacustrine crude oils using comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GCxGC-TOFMS). Org. Geochem. 2012, 53, 131–136. [Google Scholar] [CrossRef]
- Jacob, J.; Disnar, J.-R.; Boussafir, M.; Albuquerque, A.L.S.; Sifeddine, A. Contrasted distributions of triterpene derivatives in the sediments of Lake Caçó reflect paleoenvironmental changes during the last 20,000 yrs in NE Brazil. Org. Geochem. 2007, 38, 180–197. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, K. Steroids. In Natural Products Chemistry; Nakanishi, K., Goto, T., Itô, S., Natori, S., Nozoe, S., Eds.; Academic Press: Cambridge, MA, USA, 1974; pp. 421–545. [Google Scholar]
- Zuhrotun, A.; Suganda, A.G.; Nawawi, A. Phytochemical study of ketapang bark (Terminalia catappa L.). In Proceedings of the International Conference on Medicinal Plants, Surabaya, Indonesia, 21–22 July 2010. [Google Scholar]
- Beall, D. Some notes on the isolation of oestrone and equilin from the urine of pregnant mares. Biochem. J. 1936, 30, 577–581. [Google Scholar] [CrossRef] [Green Version]
- Schachter, B.; Marrian, G.F. Pregnant mares’ sulfate from the urine of the isolation of estrone. J. Biol. Chem. 1938, 126, 663–669. [Google Scholar] [CrossRef]
- Bachmann, W.E.; Cole, W.; Wilds, A.L. The total synthesis of the sex hormone equilenin. J. Am. Chem. Soc. 1939, 61, 974–975. [Google Scholar] [CrossRef]
- Fritz, M.A.; Speroff, L. Clinical Gynecologic Endocrinology and Infertility; Lippincott Williams & Wilkin: Philadelphia, PA, USA, 2012; p. 751. [Google Scholar]
- Toghueo, R.M.K.; Zabalgogeazco, I.; Vázquez de Aldana, B.R.; Boyoma, F.F. Enzymatic activity of endophytic fungi from the medicinal plants Terminalia catappa, Terminalia mantaly and Cananga odorata. S. Afr. J. Bot. 2017, 109, 146–153. [Google Scholar] [CrossRef]
- Toghueo, R.M.K.; Ejiya, E.I.; Sahal, D.; Yazdani, S.S.; Boyom, F.F. Production of cellulolytic enzymes by endophytic fungi isolated from Cameroonian medicinal plants. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1264–1271. [Google Scholar] [CrossRef] [Green Version]
- Parrish, S.M.; Yoshida, W.Y.; Williams, P.G. New diterpene isolated from a sponge of genus Strongylophora. Planta Med. 2016, 82, S1–S381. [Google Scholar] [CrossRef]
- Qin, X.D.; Liu, J.K. Natural aromatic steroids as potential molecular fossils from the fruiting bodies of the ascomycete Daldinia concentrica. J. Nat. Prod. 2004, 67, 2133–2135. [Google Scholar] [CrossRef] [PubMed]
- Brassell, S.C.; Eglinton, G.; Maxwell, J.R. The geochemistry of terpenoids and steroids. Biochem. Soc. Trans. 1983, 11, 575–586. [Google Scholar] [CrossRef]
- Breger, I.A. Geochemistry of lipids. J. Am. Oil Chem. Soc. 1966, 43, 197–221. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, S.; Su, J. Palaeozoic oil–source correlation in the Tarim Basin, NW China: A review. Org. Geochem. 2016, 94, 32–46. [Google Scholar] [CrossRef]
- Cheng, B.; Zhao, J.; Yang, C.; Tian, Y.; Liao, Z. Geochemical evolution of occluded hydrocarbons inside geomacromolecules: A review. Energy Fuel 2017, 31, 8823–8832. [Google Scholar] [CrossRef]
- Machida, K.; Abe, T.; Arai, D.; Okamoto, M.; Shimizu, I.; de Voogd, N.J.; Fusetani, N.; Nakao, Y. Cinanthrenol A, an estrogenic steroid containing phenanthrene nucleus, from a marine sponge Cinachyrella sp. Org. Lett. 2014, 16, 1539–1541. [Google Scholar] [CrossRef]
- Ludwig, B.; Gussler, G.; Wehrung, P.; Albrecht, P. C26-C29 triaromatic steroid derivatives in sediments and petroleum. Tetrahedron Lett. 1981, 22, 3313–3316. [Google Scholar] [CrossRef]
- Mackenzie, A.S.; Brassell, S.C.; Eglinton, G.; Maxwell, J.R. Chemical fossils: The geological fate of steroids. Science 1982, 217, 491–504. [Google Scholar] [CrossRef]
- Schnell, G.; Schaeffer, P.; Motscha, E.; Adam, P. Triterpenoids functionalized at C-2 as diagenetic transformation products of 2,3-dioxygenated triterpenoids from higher plants in buried wood. Org. Biomol. Chem. 2012, 10, 8276–8282. [Google Scholar] [CrossRef] [PubMed]
- Le Milbeau, C.; Schaeffer, P.; Connan, J.; Albrecht, P.; Adam, P. Aromatized C-2 oxygenated triterpenoids as indicators for a new transformation pathway in the environment. Org. Lett. 2010, 12, 1504–1507. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Yuan, W.; Wang, P.; Li, S. Ethnobotany, phytochemistry, and biological activities of Taxodium rich. Pharm. Crops 2013, 4, 1–14. [Google Scholar]
- Turekian, K.K.; Wedepohl, K.H. Distribution of the elements in some major units of the Earth’s crust. GSA Bull. 1961, 72, 175–192. [Google Scholar] [CrossRef]
- Worsfold, P.; McKelvie, I.; Monbet, P. Determination of phosphorus in natural waters: A historical review. Anal. Chim. Acta 2016, 918, 8–20. [Google Scholar] [CrossRef] [Green Version]
- Dhuime, B.; Wuestefeld, A.; Hawkesworth, C.J. Emergence of modern continental crust about 3 billion years ago. Nat. Geosci. 2015, 8, 552–555. [Google Scholar] [CrossRef]
- Krafft, F. Phosphorus: From elemental light to chemical element. Angew. Chem. Int. Ed. 1969, 8, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.R. Comparison of phosphorus and silicon: Hypervalency, stereochemistry, and reactivity. Chem. Rev. 1996, 96, 927–950. [Google Scholar] [CrossRef]
- Su, J.; Dong, S.; Zhang, Y.; Li, Y.; Chen, X.; Li, J. Apatite fission track geochronology of the Southern Hunan province across the Shi-Hang Belt: Insights into the Cenozoic dynamic topography of South China. Int. Geol. Rev. 2017, 59, 981–995. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates: Occurrence, properties, biomineralization, pathological calcification and biomimetic applications. Biomatter 2011, 1, 121–164. [Google Scholar] [CrossRef]
- Oelkers, E.H.; Montel, J.-M. Phosphates, and nuclear waste storage. Elements 2008, 4, 113–116. [Google Scholar] [CrossRef]
- Ewing, R.C.; Wang, L. Phosphates as nuclear waste forms. Rev. Mineral. Geochem. 2002, 48, 67399. [Google Scholar] [CrossRef]
- Portnov, A.M.; Gorobets, B.S. Luminescence of apatite from different rock types. Dokl. Akad. Nauk SSSR 1969, 184, 110–113. [Google Scholar]
- Dorozhkin, S.V. Calcium orthophosphate cements for biomedical application. J. Mater. Sci. 2008, 43, 3028–3043. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef] [PubMed]
- Yeagle, P.L. The Membranes of Cells; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- De Riccardis, F.; Minale, L.; Riccio, R.; Giovannitti, B.; Iorizzi, M.; Debitus, C. Phosphated and sulfated marine polyhydroxylated steroids from the starfish Tremaster novaecaledoniae. Gazz. Chim. Ital. 1993, 123, 79–86. [Google Scholar]
- Fujita, M.; Nakao, Y.; Matsunaga, S.; Seiki, M.; Itoh, Y.; van Soest, R.W.M.; Heubes, M.; Faulkner, D.J.; Fusetani, N. Isolation and structure elucidation of two phosphorylated sterol sulfates, MT1-MMP inhibitors from a marine sponge Cribrochalina sp.: Revision of the structures of haplosamates A and B. Tetrahedron 2001, 57, 3885–3890. [Google Scholar] [CrossRef]
- Chianese, G.; Fattorusso, E.; Taglialatela-Scafati, O.; Bavestrello, G.; Calcinai, B.; Dien, H.A.; Ligresti, A.; Di Marzo, V. Desulfohaplosamate, a new phosphate-containing steroid from Dasychalina sp., is a selective cannabinoid CB2 receptor ligand. Steroids 2011, 76, 998–1002. [Google Scholar] [CrossRef]
- Van Dullemen, H.M.; Tytgat, G.N.J. Colonoscopy in ileocolitis. In Procedures in Hepatogastroenterology; Tytgat, G.N.J., Mulder, C.J.J., Eds.; Developments in Gastroenterology; Springer: Dordrecht, The Netherlands, 1997; Volume 15. [Google Scholar]
- Delrio, G.; Botte, V. Testosterone 17-phosphate and 19-nortestosterone 17-phosphate as substrate for rabbit prostate phosphatases. Biochim. Biophys. Acta 1970, 218, 327–332. [Google Scholar] [CrossRef]
- Kokado, A.; Tsuji, A.; Maeda, M. Chemiluminescence assay of alkaline phosphatase using cortisol-21-phosphate as substrate and its application to enzyme immunoassays. Anal. Chim. Acta 1997, 337, 335–340. [Google Scholar] [CrossRef]
- Ellam, T.J.; Chico, T.J. Phosphate: The new cholesterol? The role of the phosphate axis in non-uremic vascular disease. Atherosclerosis 2012, 220, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.C.; Szoka, F.C., Jr. Cholesterol phosphate derivatives: Synthesis and incorporation into a phosphatase and calcium-sensitive triggered release liposome. Bioconjug. Chem. 1998, 9, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Sachs-Barrable, K.; Darlington, J.W.; Wasan, K.M. The effect of two novel cholesterol-lowering agents, disodium ascorbyl phytostanol phosphate and nanostructured aluminosilicate on the expression and activity of P-glycoprotein within Caco-2 cells. Lipids Health Dis. 2014, 13, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Kutney, J.P.; Pritchard, H.P.; Lukic, T. Novel Compounds and Compositions Comprising Sterols and/or Stanols and Cholesterol Biosynthesis Inhibitors and Use Thereof in Treating or Preventing a Variety of Diseases and Conditions. Japan Patent EP1644399A2, 7 September 2003. [Google Scholar]
- Somogyi, G.; Nishitani, S.; Nomi, D.; Buchwald, P.; Prokai, L.; Bodor, N. Targeted drug delivery to the brain via phosphonate derivatives I Design, synthesis and evaluation of an anionic chemical delivery system for testosterone. Int. J. Pharm. 1998, 166, 15–26. [Google Scholar] [CrossRef]
- Gunnarsson, P.O.; Norlén, B.J. Clinical pharmacology of polyestradiol phosphate. Prostate 1988, 13, 299–304. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, X.; Lic, H.; Du, N.; Song, S.; Hou, W. Preparation and characterization of betamethasone sodium phosphate intercalated layered double hydroxide liposome nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 824–831. [Google Scholar] [CrossRef]
- Isaac, R.E.; Rees, H.H. Isolation, and identification of ecdysteroid phosphates and acetylecdysteroid phosphates from developing eggs of the locust, Schistocerca gregaria. Biochem. J. 1984, 221, 459–464. [Google Scholar] [CrossRef] [Green Version]
- Chopra, A.; Doiphode, V.V. Ayurvedic medicine: Core concept, therapeutic principles, and current relevance. Med. Clin. 2002, 86, 75–89. [Google Scholar] [CrossRef]
- Patwardhan, B.; Warude, D.; Pushpangadan, P.; Bhatt, N. Ayurveda, and traditional Chinese medicine: A comparative overview. Evid.-Based Complement. Altern. Med. 2005, 2, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A. Traditional methods of food habits and dietary preparations in Ayurveda—The Indian system of medicine. J. Ethn. Foods 2019, 6, 14–24. [Google Scholar]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Alvarez, J.A. Functional properties of honey, propolis, and royal jelly. J. Food Sci. 2008, 73, R117–R124. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. In silico prediction of steroids and triterpenoids as potential regulators of lipid metabolism. Mar. Drugs 2021, 19, 650. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V.; Koola, M.M. QSAR study of some natural and synthetic platelet aggregation inhibitors and their pharmacological profile. J. Appl. Pharm. Sci. 2022, 12, 039–058. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Al-Ghamdi, A. Bioactive compounds and health-promoting properties of royal jelly: A review. J. Funct. Foods 2012, 4, 39–52. [Google Scholar] [CrossRef]
- Gribble, G.W. Naturally occurring organohalogen compounds—A comprehensive survey. Prog. Chem. Org. Nat. Prod. 1996, 68, 1–496. [Google Scholar]
- Gribble, G.W. Naturally occurring organohalogen compounds. Acc. Chem. Res. 1998, 31, 141–152. [Google Scholar] [CrossRef]
- Gribble, G.W. The diversity of naturally occurring organobromine compounds. Chem. Soc. Rev. 1999, 28, 335–346. [Google Scholar] [CrossRef]
- Gribble, G.W. Naturally occurring organohalogen compounds—A comprehensive update. Prog. Chem. Org. Nat. Prod. 2010, 91, 1–613. [Google Scholar]
- Wang, C.; Du, W.; Lu, H.; Lan, J.; Liang, K.; Cao, S. A Review: Halogenated compounds from marine Actinomycetes. Molecules 2021, 26, 2754. [Google Scholar] [CrossRef]
- Wang, C.; Lu, H.; Lan, J.; Ahammad Zaman, K.H.; Cao, S. A Review: Halogenated compounds from marine fungi. Molecules 2021, 26, 458. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Tolstikov, G.A. Chlorine containing sesquiterpenes of higher plants. Chem. Sustain. Dev. 2002, 10, 363–370. [Google Scholar]
- Dembitsky, V.M.; Tolstikov, G.A. Natural Organic Halogenated Compounds; Geo-Science: Novosibirsk, Russia, 2003; p. 367. [Google Scholar]
- Dembitsky, V.M.; Tolstikov, G.A. Natural halogenated alkanes, cycloalkanes, and their derivatives. Chem. Sustain. Dev. 2003, 11, 803–810. [Google Scholar]
- Dembitsky, V.M.; Tolstikov, G.A. Natural halogenated alkaloids. Chem. Sustain. Dev. 2003, 11, 451–466. [Google Scholar]
- Dembitsky, V.M.; Tolstikov, G.A. Natural halogenated furanones, higher terpenes and steroids. Chem. Sustain. Dev. 2003, 11, 697–703. [Google Scholar]
- Dembitsky, V.M.; Tolstikov, G.A. Natural halogenated non-terpenic C15-acetogenins of sea organisms. Chem. Sustain. Dev. 2003, 11, 329–339. [Google Scholar]
- Dembitsky, V.M.; Tolstikov, G.A. Halogenated phenol compounds in lichens and fungi. Chem. Sustain. Dev. 2003, 11, 557–565. [Google Scholar]
- Dembitsky, V.M.; Tolstikov, G.A. Natural halogenated mononuclear phenol compounds and their derivatives. Chem. Sustain. Dev. 2003, 11, 567–575. [Google Scholar]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Chlorinated plant steroids and their biological activities. Int. J. Curr. Res. Biosci. Plant Biol. 2017, 4, 70–85. [Google Scholar] [CrossRef] [Green Version]
- Tschesche, R.; Baumgarth, M.; Welzel, P. Weitere inhaltsstoffe aus Jaborosa integrifolia Lam. III: Zur Struktur der Jaborosalactone C, D, und E. Tetrahedron 1968, 24, 5169–5179. [Google Scholar] [CrossRef]
- Chen, L.X.; He, H.; Qiu, F. Natural withanolides: An overview. Nat. Prod. Rep. 2011, 28, 705–740. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Sahai, M.; Ray, A.B.; Slatkin, D.J. Physalolactone C, a new withanolide from Physalis peruviana. J. Nat. Prod. 1984, 47, 648–651. [Google Scholar] [CrossRef]
- Frolow, F.; Ray, B.; Sahai, A.; Glotter, M.; Gottlieb, E.E.; Kirson, H.I. Withaperuvin and 4-deoxy-physalolactone, two new ergostane-type steroids from Physalis peruviana (Solanaceae). J. Chem. Soc. Perkin Trans. 1981, 112, 1029–1032. [Google Scholar] [CrossRef]
- Shingu, K.; Yahara, S.; Okabe, H.; Nohara, T. Three new withanolides, physagulins E, F and G from Physalis angulata L. Chem. Pharm. Bull. 1992, 40, 2448–2451. [Google Scholar] [CrossRef] [Green Version]
- Nittala, S.S.; Vande Velde, V.; Frolow, F.; Lavie, D. Chlorinated withanolides from Withania somnifera and Acnistus breviflorus. Phytochemistry 1981, 20, 2547–2552. [Google Scholar] [CrossRef]
- Bessalle, R.; Lavie, D. Withanolide C, A chlorinated withanolide from Withania somnifera. Phytochemistry 1992, 31, 3648–3651. [Google Scholar] [CrossRef]
- Kirson, I.; Glotter, E.E. Recent Developments in naturally occurring ergostane-type steroids. A Review. J. Nat. Prod. 1981, 44, 633–647. [Google Scholar] [CrossRef]
- Fajardo, V.; Podesta, F.; Shamma, M.; Freyer, A.J. New withanolides from Jaborosa magellanica. J. Nat. Prod. 1991, 54, 554–563. [Google Scholar] [CrossRef]
- Triana, J.; López, M.; Pérez, F.J.; Rico, M.; López, A.; Estévez, F.; Marrero, M.T.; Brouard, I.; León, F. Secondary metabolites from two species of Tolpis and their biological activities. Molecules 2012, 17, 12895–12909. [Google Scholar] [CrossRef] [Green Version]
- Pramanick, S.; Roy, A.; Ghosh, S.; Majumder, H.K.; Mukhopadhyay, S. Withanolide Z, new chlorinated withanolide from Withania somnifera. Planta Med. 2008, 74, 1745–1753. [Google Scholar] [CrossRef]
- Dinan, L.N.; Sarker, S.D.; Sik, V. 28-Hydroxywithanolide E from Physalis peruviana. Phytochemistry 1997, 44, 509–512. [Google Scholar] [CrossRef]
- Lan, Y.-H.; Chang, F.-R.; Pan, M.-J.; Wu, C.-C.; Wu, S.-J.; Chen, S.-L. New cytotoxic withanolides from Physalis peruviana. Food Chem. 2009, 116, 462–471. [Google Scholar] [CrossRef]
- Hsieh, P.-W.; Huang, Z.-Y.; Chen, J.-H.; Chang, F.-R.; Wu, C.-C. Cytotoxic withanolides from Tubocapsicum anomalum. J. Nat. Prod. 2007, 70, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Nagafuji, S.; Okabe, H.; Akahane, H.; Abe, F. Trypanocidal constituents in plants. 4. Withanolides from the aerial parts of Physalis angulata. Biol. Pharm. Bull. 2004, 27, 193–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicotra, V.E.; Gil, R.R.; Vaccarini, C.; Oberti, J.C.; Burton, G. 15,21-Cyclowithanolides from Jaborosa bergii. J. Nat. Prod. 2003, 66, 1471–1475. [Google Scholar] [CrossRef] [PubMed]
- Nicotra, V.E.; Gil, R.R.; Oberti, J.C.; Burton, G. Withanolides with phytotoxic activity from Jaborosa caulescens var. caulescens and J. caulescens var. bipinnatifida. J. Nat. Prod. 2007, 70, 808–813. [Google Scholar] [CrossRef]
- Bonetto, G.M.; Gil, R.R.; Oberti, J.C.; Veleiro, A.S.; Burton, G. Novel withanolides from Jaborosa sativa. J. Nat. Prod. 1995, 58, 705–719. [Google Scholar] [CrossRef]
- Kiyota, N.; Shingu, K.; Yamaguchi, K.; Yoshitake, Y.; Harano, K.; Yoshimitsu, H.; Ikeda, T.; Nohara, T. New C28 steroidal glycosides from Tubocapsicum anomalum. Chem. Pharm. Bull. 2007, 55, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Kiyota, N.; Shingu, K.; Yamaguchi, K.; Yoshitake, Y.; Harano, K.; Yoshimitsu, H. New C28 steroidal glycosides from Tubocapsicum anomalum. Chem. Pharm. Bull. 2008, 56, 1038–1044. [Google Scholar] [CrossRef] [Green Version]
- Glotter, E.; Abraham, A.; Guenzberg, G.; Kirson, I. Naturally occurring steroidal lactones with a 17a-oriented side chain. Structure of withanolide E and related compounds. J. Chem. Soc. Perkin Trans. 1977, 1, 341–344. [Google Scholar] [CrossRef]
- Shingu, K.; Marubayashi, N.; Ueda, I.; Yahara, S.; Nohara, T. Two new ergostane derivatives from Tubocapsicum anomalum (Solanaceae). Chem. Pharm. Bull. 1990, 38, 1107–1111. [Google Scholar] [CrossRef] [Green Version]
- Cirigliano, A.M.; Veleiro, A.S.; Oberti, J.C.; Burton, G. Spiranoid withanolides from Jaborosa odonelliana. J. Nat. Prod. 2002, 65, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Cirigliano, A.M.; Misico, R.I. Spiranoid withanolides from Jaborosa odonelliana and Jaborosa runcinata. Z. Naturforschung B Chem. Sci. 2005, 60, 867–873. [Google Scholar] [CrossRef] [Green Version]
- Garcia, M.E.; Navarro-Vazquez, S.P.A.; Phillips, D.D.; Gayathri, C.; Krakauer, H.; Stephens, P.W.; Nicotra, V.E.; Gil, R.R. Stereochemistry determination by powder X-ray diffraction analysis and NMR spectroscopy residual dipolar couplings. Angew. Chem. Int. Ed. 2009, 48, 5670–5676. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Cao, S.; Chen, L.; Qiu, F. Natural withanolides, an update. Nat. Prod. Rep. 2022, 39, 784–813. [Google Scholar] [CrossRef]
- Moujir, L.M.; Llanos, G.G.; Araujo, L.; Amesty, A.; Bazzocchi, I.L.; Jiménez, I.A. Withanolide-type steroids from Withania aristata as potential anti-leukemic agents. Molecules 2020, 25, 5744. [Google Scholar] [CrossRef]
- Ripperger, H.; Kamperdick, C. First isolation of physalins from the genus Saracha of Solanaceae. Pharmazie 1998, 53, 144–151. [Google Scholar]
- Makino, B.; Kawai, M.; Ogura, T.; Nakanishi, M.; Yamamura, H.; Butsugan, Y. Structural revision of physalin H isolated from Physalis angulata. J. Nat. Prod. 1995, 58, 1668–1673. [Google Scholar] [CrossRef]
- Kawai, M.; Makino, B.; Yamamura, H.; Butsugan, Y. Upon—Physalin L‖ isolated from Physalis minima. Phytochemistry 1996, 43, 661–667. [Google Scholar] [CrossRef]
- Nicotra, V.E.; Ramacciotti, N.S.; Gil, R.R.; Oberti, J.C.; Feresin, G.E.; Guerrero, C.A.; Baggio, R.F.; Garland, M.T.; Burton, G. Phytotoxic withanolides from Jaborosa rotacea. J. Nat. Prod. 2006, 69, 783–801. [Google Scholar] [CrossRef]
- Fattorusso, E.; Taglialatela-Scafati, O.; Petrucci, F.; Bavestrello, G.; Calcinai, B. Polychlorinated androstanes from the burrowing sponge Cliona nigricans. Org. Lett. 2004, 6, 1633–1635. [Google Scholar] [CrossRef] [PubMed]
- Shimura, H.; Iguchi, K.; Yamada, Y.; Nakaike, S.; Yamagishi, T.; Matsumoto, K.; Yokoo, C. Aragusterol C: A novel halogenated marine steroid from an Okinawan sponge, Xestospongia sp., possessing potent antitumor activity. Experientia 1994, 50, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Chen, Y.J.; Higuchi, K.; Aoki, S.; Kitagawa, I. Marine natural products. XXXVII. Aragusteroketals A and C, two novel cytotoxic steroids from a marine sponge of Xestospongia sp. Chem. Pharm. Bull. 1996, 44, 1840–1842. [Google Scholar] [CrossRef] [Green Version]
- Pham, G.N.; Kang, D.Y.; Kim, M.J.; Han, S.J.; Lee, J.H.; Na, M. Isolation of sesquiterpenoids and steroids from the soft coral Sinularia brassica and determination of their absolute configuration. Mar. Drugs 2021, 19, 523. [Google Scholar] [CrossRef] [PubMed]
- Carney, J.R.; Scheuer, P.J.; Kelley-Borges, M. Three unprecedented chloro steroids from the Maui sponge Strongylacidon sp.: Kiheisterones C, D, and E. J. Org. Chem. 1993, 58, 3460–3462. [Google Scholar] [CrossRef]
- Cimino, G.; De Luca, P.; De Stefano, S.; Minale, L. Disidein, a pentacyclic sesterterpene condensed with an hydroxyhydroquinone moiety, from the sponge Disidea pallescens. Tetrahedron 1975, 31, 271–275. [Google Scholar] [CrossRef]
- Teta, R.; Della Sala, G.; Renga, B.; Mangoni, A.; Fiorucci, S.; Costantino, V. Chalinulasterol, a chlorinated steroid disulfate from the caribbean sponge Chalinula molitba. Evaluation of its role as PXR receptor modulator. Mar. Drugs 2012, 10, 1383–1390. [Google Scholar] [CrossRef] [Green Version]
- Teruya, T.; Nakagawa, S.; Koyama, T.; Arimoto, H.; Kita, M.; Uemura, D. Nakiterpiosin and nakiterpiosinone, novel cytotoxic C-nor-D-homosteroids from the Okinawan sponge Terpios hoshinota. Tetrahedron 2004, 60, 6989–6993. [Google Scholar] [CrossRef]
- Vil, V.; Gloriozova, T.A.; Zhukova, N.V.; Dembitsky, V.M. Highly oxygenated isoprenoid lipids derived from terrestrial and aquatic sources: Origin, structures and biological activities. Vietnam J. Chem. 2019, 57, 1–15. [Google Scholar] [CrossRef]
- Guzii, A.G.; Makarieva, T.N.; Denisenko, V.A.; Dmitrenok, P.S.; Burtseva, Y.V.; Krasokhin, V.B.; Stonik, V.A. Topsentiasterol sulfates with novel iodinated and chlorinated side chains from the marine sponge Topsentia sp. Tetrahedron Lett. 2008, 49, 7191–7193. [Google Scholar] [CrossRef]
- Areche, C.; Vaca, I.; Labbe, P.; Soto-Delgado, J.; Astudilloc, L.; Silva, M.; Rovirosa, J.; San-Martin, A. Biotransformation of stypotriol triacetate by Aspergillus niger. J. Mol. Struct. 2011, 998, 167–170. [Google Scholar] [CrossRef]
- Minale, L.; Riccio, R.; De Simone, F.; Dini, A.; Pizza, C. Starfish saponins II. 22,23-epoxysteroids, minor genins from the starfish Echinaster sepositus. Tetrahedron Lett. 1979, 20, 645–648. [Google Scholar] [CrossRef]
- Dort, E.; Díaz-Marrero, A.R.; Cueto, M.; D’Croz, L.; Maté, J.L.; San-Martín, A.; Darias, J. Unusual chlorinated pregnanes from the eastern Pacific octocoral Carijoa multiflora. Tetrahedron Lett. 2004, 45, 915–918. [Google Scholar] [CrossRef]
- Iwashima, M.; Nara, K.; Nakamichi, Y.; Iguchi, K. Three new chlorinated marine steroids, yonarasterols G, H and I, isolated from the Okinawan soft coral, Clavularia viridis. Steroids 2001, 66, 25–32. [Google Scholar] [CrossRef] [PubMed]
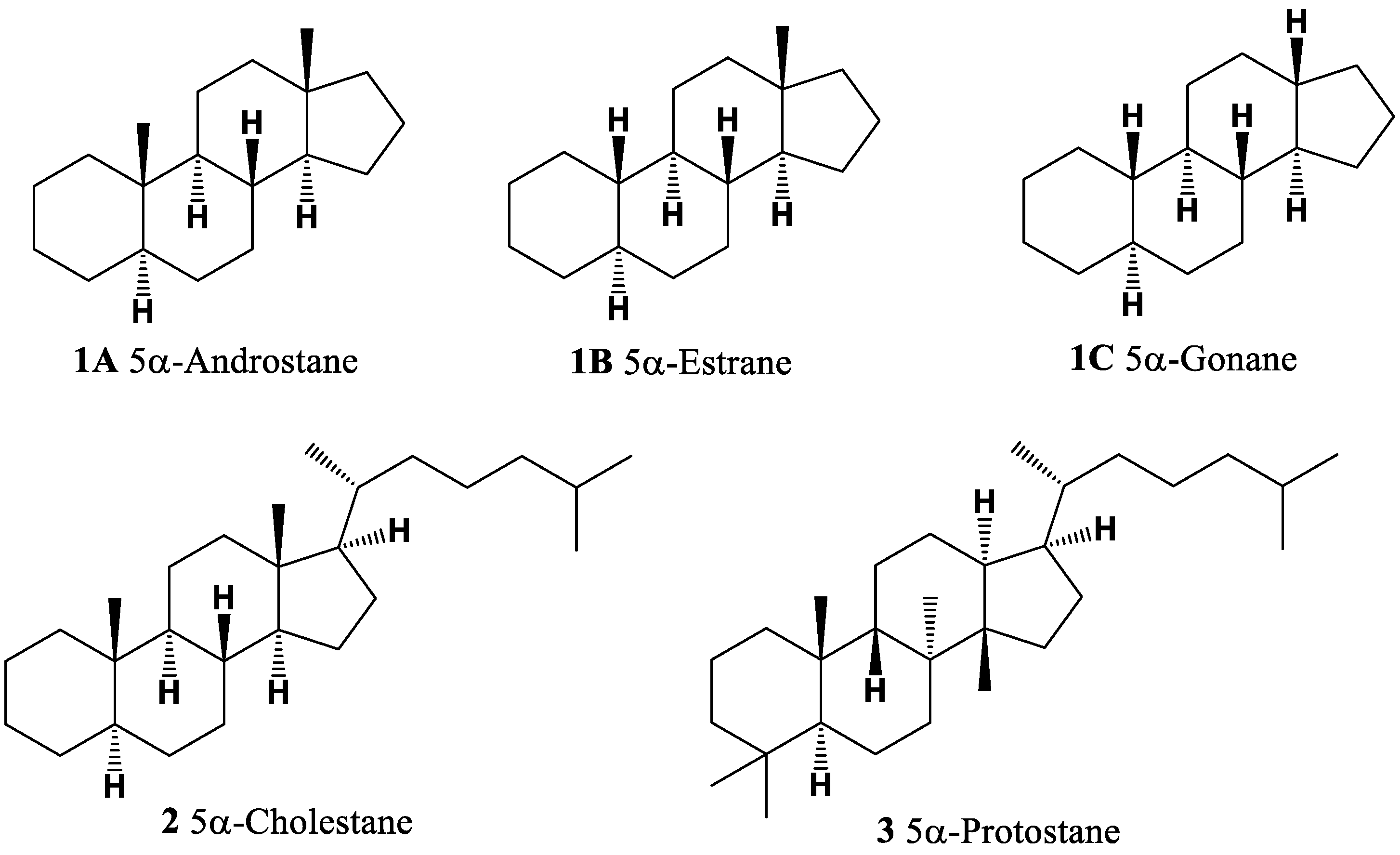













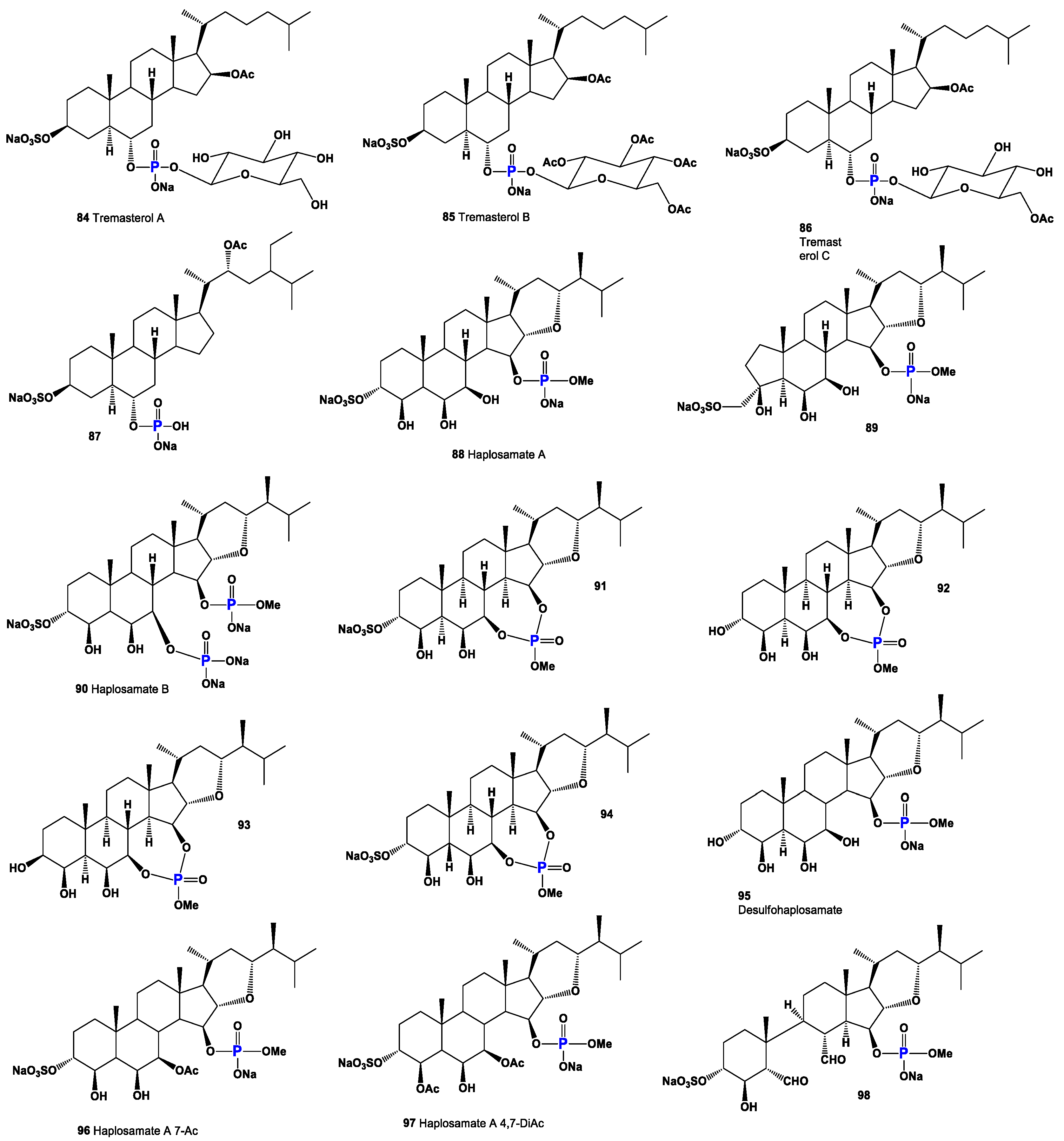
















| No. | Dominated Biological Activity (Pa) * | Additional Predicted Activities (Pa) * |
|---|---|---|
| 4 | Ovulation inhibitor (0.942) Cardiovascular analeptic (0.924) Apoptosis agonist (0.750) | Anti-hypercholesterolemic (0.871) Lipid metabolism regulator (0.788) Prostate disorders treatment (0.737) |
| 5 | Anti-hypercholesterolemic (0.894) Ovulation inhibitor (0.889) Anesthetic general (0.868) | Respiratory analeptic (0.851) Acute neurologic disorders treatment (0.793) Prostate disorders treatment (0.729) |
| 6 | Anesthetic general (0.845) Ovulation inhibitor (0.832) | Acute neurologic disorders treatment (0.822) Neuroprotector (0.815) |
| 7 | Anti-hypercholesterolemic (0.856) Ovulation inhibitor (0.847) Cardiovascular analeptic (0.842) | Lipid metabolism regulator (0.788) Apoptosis agonist (0.750) Prostate disorders treatment (0.725) |
| 8 | Anti-hypercholesterolemic (0.885) Apoptosis agonist (0.801) | Hepatic disorders treatment (0.739) Ovulation inhibitor (0.726) |
| 9 | Acute neurologic disorders treatment (0.871) Respiratory analeptic (0.843) Vasoprotector (0.811) | Neuroprotector (0.785) Anesthetic general (0.753) Ovulation inhibitor (0.740) |
| 10 | Cardiovascular analeptic (0.882) Ovulation inhibitor (0.860) | Respiratory analeptic (0.846) Acute neurologic disorders treatment (0.844) |
| 11 | Respiratory analeptic (0.879) Ovulation inhibitor (0.765) | Neuroprotector (0.762) Cardiovascular analeptic (0.692) |
| 12 | Acute neurologic disorders treatment (0.849) Vasoprotector (0.795) | Anti-inflammatory (0.788) Ovulation inhibitor (0.778) |
| 13 | Psychotropic (0.815) Ovulation inhibitor (0.586) | Attention deficit/hyperactivity disorder treatment (0.744) |
| 14 | Postmenopausal disorders treatment (0.945) | Anti-inflammatory (0.669) |
| 15 | Lipid metabolism regulator (0.913) Cytostatic (0.891) Anti-neoplastic (0.876) | Hepatoprotectant (0.845) Immunosuppressant (0.792) Apoptosis agonist (0.784) |
| 16 | Chemopreventive (0.919) Proliferative diseases treatment (0.914) | Anti-neoplastic (0.837) Vasoprotector (0.824) |
| 17 | Apoptosis agonist (0.893) Anti-neoplastic (0.827) | Anti-inflammatory (0.873) Hypolipemic (0.854) |
| 18 | Apoptosis agonist (0.883) Anti-neoplastic (0.826) | Hypolipemic (0.863) Anti-inflammatory (0.855) |
| 19 | Anti-neoplastic (0.879) Apoptosis agonist (0.775) | Immunosuppressant (0.744) Anti-inflammatory (0.715) |
| 20 | Anti-neoplastic (0.782) | Genital warts treatment (0.736) |
| 21 | Apoptosis agonist (0.896) Anti-neoplastic (0.843) | Hypolipemic (0.850) Anti-inflammatory (0.814) |
| 22 | Chemopreventive (0.887) Anti-neoplastic (0.794) | Anti-inflammatory (0.819) Proliferative diseases treatment (0.784) |
| 23 | Anti-neoplastic (0.909) Apoptosis agonist (0.790) | Anti-inflammatory (0.822) Immunosuppressant (0.727) |
| 24 | Anti-neoplastic (0.888) Apoptosis agonist (0.847) | Anti-inflammatory (0.830) Immunosuppressant (0.739) |
| 25 | Anti-neoplastic (0.802) Apoptosis agonist (0.789) | Anti-inflammatory (0.786) Prostate disorders treatment (0.685) |
| 26 | Acute neurologic disorders treatment (0.867) Anti-neoplastic (0.812) | Diuretic (0.813) Male reproductive dysfunction treatment (0.759) |
| 27 | Anti-hypercholesterolemic (0.959) | Anti-neoplastic (0.832) |
| No. | Dominated Biological Activity (Pa) * | Additional Predicted Activities (Pa) * |
|---|---|---|
| 28 | Anti-hypercholesterolemic (0.961) Proliferative diseases treatment (0.711) | Anti-neoplastic (0.840) Apoptosis agonist (0.787) |
| 29 | Neuroprotector (0.979) Respiratory analeptic (0.970) Anti-neoplastic (0.888) | Anti-hypercholesterolemic (0.953) Anti-infective (0.933) Anti-protozoal (Leishmania) (0.922) |
| 30 | Anti-hypercholesterolemic (0.860) Anti-inflammatory (0.754) | Anti-neoplastic (0.805) Chemopreventive (0.721) |
| 31 | Anti-hypercholesterolemic (0.907) Anti-inflammatory (0.765) | Anti-neoplastic (0.836) Apoptosis agonist (0.788) |
| 32 | Anti-hypercholesterolemic (0.764) | Anti-inflammatory (0.695) |
| 33 | Anti-hypercholesterolemic (0.929) | Respiratory analeptic (0.885) |
| 34 | Anti-hypercholesterolemic (0.935) | Apoptosis agonist (0.850) |
| 35 | Anti-hypercholesterolemic (0.950) Anti-Parkinsonian, rigidity relieving (0.875) | Apoptosis agonist (0.898) Anti-neoplastic (0.880) |
| 36 | Anti-hypercholesterolemic (0.806) | Anti-neoplastic (0.729) |
| 37 | Anti-hypercholesterolemic (0.914) Hypolipemic (0.858) | Apoptosis agonist (0.894) Anti-neoplastic (0.879) |
| 38 | Anti-neoplastic (0.922) | Immunosuppressant (0.774) |
| 39 | Anti-neoplastic (0.899) Apoptosis agonist (0.896) | Anti-inflammatory (0.795) |
| 40 | Neuroprotector (0.829) | Anti-allergic (0.731) |
| 41 | Anti-convulsant (0.877) | |
| 42 | Apoptosis agonist (0.828) Anti-neoplastic (0.798) | Anti-inflammatory (0.813) |
| 43 | Anti-neoplastic (0.782) | Anti-bacterial (0.736) |
| 44 | Acute neurologic disorders treatment (0.867) | Anti-neoplastic (0.797) |
| 45 | Anti-inflammatory (0.825) | Apoptosis agonist (0.793) |
| 46 | Anti-neoplastic (0.884) | Apoptosis agonist (0.848) |
| 47 | Anti-neoplastic (0.799) | Apoptosis agonist (0.716) |
| 48 | Anti-neoplastic (0.858) | Anti-hypercholesterolemic (0.839) |
| 49 | Anti-neoplastic (0.858) | Cell adhesion molecule inhibitor (0.795) |
| 50 | Anti-neoplastic (0.841) | Immunosuppressant (0.722) |
| 51 | Anti-neoplastic (0.844) | Apoptosis agonist (0.792) |
| 52 | Apoptosis agonist (0.706) | Acute neurologic disorders treatment (0.768) |
| No. | Dominated Biological Activities (Pa) * | Additional Predicted Activities (Pa) * |
|---|---|---|
| 53 | Ovulation inhibitor (0.866) | Anti-neoplastic (0.824) |
| 54 | Acute neurologic disorders treatment (0.925) Anti-neoplastic (0.790) | Diuretic (0.824) Male reproductive dysfunction treatment (0.791) |
| 55 | Acute neurologic disorders treatment (0.826) Anti-neoplastic (0.818) | Respiratory analeptic (0.811) Neuroprotector (0.807) |
| 56 | Ovulation inhibitor (0.846); male reproductive dysfunction treatment (0.815) | Anti-neoplastic (0.821) |
| 57 | Neuroprotector (0.837) Anti-neoplastic (0.833) | Acute neurologic disorders treatment (0.828) |
| 58 | Ovulation inhibitor (0.843) Lipid metabolism regulator (0.723) | Anti-neoplastic (0.839) Neuroprotector (0.829) |
| 59 | Acute neurologic disorders treatment (0.932) Anti-neoplastic (0.810) | Laxative (0.833) Diuretic (0.751) |
| 60 | Apoptosis agonist (0.924) Anti-neoplastic (0.868) | Antioxidant (0.776) Neuroprotector (0.728) |
| 61 | Anti-osteoporotic (0.837) | Anti-neoplastic (0.735) |
| 62 | Anti-hypercholesterolemic (0.860) | Respiratory analeptic (0.847) |
| 63 | Anti-osteoporotic (0.776) | Anti-neoplastic (0.732) |
| 64 | Apoptosis agonist (0.758) Anti-neoplastic (0.733) | Anti-inflammatory (0.744) |
| 65 | Apoptosis agonist (0.758) Anti-neoplastic (0.733) | Anti-inflammatory (0.744) |
| 66 | Anti-inflammatory (0.807) | Apoptosis agonist (0.746); anti-neoplastic (0.726) |
| 67 | Anti-infertility, female (0.796) | Anti-inflammatory (0.794) |
| 68 | Anti-neoplastic (0.697) | Ovulation inhibitor (0.683) |
| 69 | Prostate disorders treatment (0.699) | Anti-inflammatory (0.661) |
| 70 | Anti-neoplastic (0.825) Alzheimer’s disease treatment (0.824) | Neurodegenerative diseases treatment (0.809) Psychotropic (0.700) |
| 71 | Anti-eczematic (0.767) | Anti-dyskinetic (0.670) |
| 72 | Anti-eczematic (0.695) | Autoimmune disorders treatment (0.652) |
| 73 | Anti-eczematic (0.767) | Anti-dyskinetic (0.670) |
| 74 | Anti-eczematic (0.782) Anti-psoriatic (0.619) | Anti-neurotic (0.709) |
| 75 | Neuroprotector (0.685) | Acute neurologic disorders treatment (0.647) |
| 76 | Hypolipemic (0.724) | Anti-convulsant (0.649) |
| 77 | Anti-eczematic (0.885) Anti-psoriatic (0.757) | Anti-inflammatory (0.735) |
| 78 | Anti-eczematic (0.709) Anti-psoriatic (0.632) | Anti-convulsant (0.661) |
| 79 | Anti-eczematic (0.691) Anti-psoriatic (0.622) | Psychotropic (0.611) Anti-convulsant (0.570) |
| 80 | Apoptosis agonist (0.758) Anti-neoplastic (0.733) | Anti-inflammatory (0.744) |
| 81 | Acute neurologic disorders treatment (0.778) | Neuroprotector (0.733) |
| 82 | Anti-inflammatory (0.650) | Menopausal disorders treatment (0.628) |
| 83 | Anti-inflammatory (0.782) | Anti-eczematic (0.771) |
| No. | Dominated Biological Activity (Pa) * | Additional Predicted Activities (Pa) * |
|---|---|---|
| 84 | Wound-healing agent (0.975) Hepatoprotectant (0.961) Analeptic (0.952) Laxative (0.933) | Anti-hypercholesterolemic (0.926) Anti-carcinogenic (0.912) Hemostatic (0.853) Anti-neoplastic (0.841) |
| 85 | Hepatoprotectant (0.874) Analeptic (0.874) | Anti-carcinogenic (0.861) Anti-neoplastic (0.848) |
| 86 | Wound-healing agent (0.947) Analeptic (0.941) Hepatoprotectant (0.932) | Anti-carcinogenic (0.915) Anti-hypercholesterolemic (0.912) Anti-neoplastic (0.843) |
| 87 | Anti-hypercholesterolemic (0.894) Hepatoprotectant (0.853) Wound-healing agent (0.844) | Anti-neoplastic (0.816) Anti-inflammatory (0.782) Cholesterol synthesis inhibitor (0.778) |
| 88 | Anti-hypercholesterolemic (0.894) Hepatoprotectant (0.853) Wound-healing agent (0.844) | Anti-neoplastic (0.816) Anti-inflammatory (0.782) Cholesterol synthesis inhibitor (0.778) |
| 89 | Anti-neoplastic (0.845) Anti-fungal (0.814) | Anti-inflammatory (0.693) Anti-bacterial (0.651) |
| 90 | Anti-fungal (0.837) | Anti-neoplastic (0.824) |
| 91 | Anti-neoplastic (0.827) | Anti-fungal (0.663) |
| 92 | Anti-neoplastic (0.852) Anti-neoplastic (liver cancer) (0.790) | Anti-eczematic (0.730) Anti-allergic (0.650) |
| 93 | Anti-neoplastic (0.852) Anti-neoplastic (liver cancer) (0.790) | Anti-eczematic (0.730) Anti-allergic (0.650) |
| 94 | Anti-neoplastic (0.827) Anti-neoplastic (liver cancer) (0.607) | Anti-fungal (0.663) Anti-bacterial (0.636) |
| 95 | Anti-neoplastic (0.841) | Anti-fungal (0.799) |
| 96 | Anti-fungal (0.850) Anti-bacterial (0.717) | Anti-neoplastic (0.832) Anti-carcinogenic (0.707) |
| 97 | Anti-fungal (0.850) Anti-bacterial (0.717) | Anti-neoplastic (0.832) Anti-carcinogenic (0.707) |
| 98 | Anti-fungal (0.858) Anti-bacterial (0.739) | Anti-neoplastic (0.842) Anti-carcinogenic (0.733) |
| No. | Dominated Biological Activity (Pa) * | Additional Predicted Activities (Pa) * |
|---|---|---|
| 99 | Anti-inflammatory (0.910) Anesthetic general (0.908) | Respiratory analeptic (0.904) Anti-osteoporotic (0.878) |
| 100 | Neuroprotector (0.987) Anesthetic general (0.959) | Respiratory analeptic (0.944) Anti-hypercholesterolemic (0.909) |
| 101 | Anesthetic general (0.991) Neuroprotector (0.976) Anti-inflammatory (0.906) | Respiratory analeptic (0.990) Anti-hypercholesterolemic (0.894) |
| 102 | Respiratory analeptic (0.979) Anesthetic general (0.973) Neuroprotector (0.972) | Anti-hypercholesterolemic (0.971) Wound-healing agent (0.913) Anti-neoplastic (0.826) |
| 103 | Respiratory analeptic (0.995) Anesthetic general (0.948) Wound-healing agent (0.897) | Anti-hypercholesterolemic (0.945) Neuroprotector (0.932) Hemostatic (0.910) |
| 104 | Respiratory analeptic (0.995) Anti-hypercholesterolemic (0.967) Anesthetic general (0.954) | Hemostatic (0.928) Wound-healing agent (0.921) Neuroprotector (0.909) |
| 105 | Anti-hypercholesterolemic (0.996) Cholesterol absorption inhibitor (0.976) Cholesterol synthesis inhibitor (0.952) Lipid metabolism regulator (0.952) | Acute neurologic disorders treatment (0.948) Anti-hyperlipoproteinemic (0.920) Hypolipemic (0.919) Respiratory analeptic (0.908) |
| 106 | Anti-hypercholesterolemic (0.999) Anti-hyperlipoproteinemic (0.986) Hypolipemic (0.974) | Cholesterol absorption inhibitor (0.957) Lipid metabolism regulator (0.954) Cholesterol synthesis inhibitor (0.916) |
| 107 | Anti-neoplastic (0.822) | Anti-inflammatory (0.645) |
| 108 | Neuroprotector (0.982) Anesthetic general (0.931) | Anti-hypercholesterolemic (0.909) |
| 109 | Anesthetic general (0.970) Neuroprotector (0.965) | Respiratory analeptic (0.961) Acute neurologic disorders treatment (0.916) |
| 110 | Anti-inflammatory (0.979) Anti-allergic (0.959) | Anti-asthmatic (0.951) Anti-arthritic (0.944) |
| 111 | Respiratory analeptic (0.929) Anti-ischemic, cerebral (0.907) | Anesthetic general (0.897) Anti-neoplastic (0.847) |
| 112 | Anti-ischemic, cerebral (0.979) Respiratory analeptic (0.919) | Anti-osteoporotic (0.843) Anesthetic general (0.830) |
| 113 | Respiratory analeptic (0.937) Anti-ischemic, cerebral (0.922) | Anesthetic general (0.897) |
| 114 | Anti-ischemic, cerebral (0.978) Respiratory analeptic (0.911) | Anti-osteoporotic (0.852) |
| No. | Dominated Biological Activity (Pa) * | Additional Predicted Activities (Pa) * |
|---|---|---|
| 115 | Hepatic disorders treatment (0.940) Anti-eczematic (0.924) | Macular degeneration treatment (0.921) Cytostatic (0.904) |
| 116 | Hepatic disorders treatment (0.933) Anti-eczematic (0.932) | Macular degeneration treatment (0.926) Cytostatic (0.875) |
| 117 | Anti-eczematic (0.919) Hepatic disorders treatment (0.908) | Cytostatic (0.921) Macular degeneration treatment (0.912) |
| 118 | Anti-diabetic (0.938) Myocardial infarction treatment (0.823) | Anti-eczematic (0.902) Alzheimer’s disease treatment (0.664) |
| 119 | Anti-diabetic (0.981) Lipoprotein disorders treatment (0.938) | Anti-eczematic (0.902) Alzheimer’s disease treatment (0.666) |
| 120 | Anti-diabetic (0.980) Lipoprotein disorders treatment (0.939) | Anti-eczematic (0.897) Alzheimer’s disease treatment (0.696) |
| 121 | Apoptosis agonist (0.888) Anti-neoplastic (0.860) | Anti-eczematic (0.910) Cytostatic (0.643) |
| 122 | Neurodegenerative diseases treatment (0.913) Alzheimer’s disease treatment (0.889) | Anti-eczematic (0.926) Anti-Parkinsonian (0.856) |
| 123 | Lipoprotein disorders treatment (0.968) Anti-diabetic (0.953) | Anti-eczematic (0.912) Alzheimer’s disease treatment (0.670) |
| 124 | Anti-eczematic (0.930) Myocardial infarction treatment (0.872) | Anti-neoplastic (0.866) Cytostatic (0.819) |
| 125 | Anti-eczematic (0.823) Allergic conjunctivitis treatment (0.629) | Anti-neoplastic (0.785) Anti-inflammatory (0.731) |
| 126 | Myocardial infarction treatment (0.825) Anti-neoplastic (0.707) | Anti-eczematic (0.815) Allergic conjunctivitis treatment (0.618) |
| 127 | Anti-neoplastic (0.918) Apoptosis agonist (0.793) Anti-neoplastic (myeloid leukemia) (0.520) | Respiratory analeptic (0.757) Anti-secretoric (0.755) Lipid metabolism regulator (0.677) |
| 128 | Anti-neoplastic (0.892) Apoptosis agonist (0.796) Anti-metastatic (0.551) | Hepatoprotectant (0.739) Hepatic disorders treatment (0.701) Dermatologic (0.614) |
| 129 | Cytostatic (0.863) Anti-neoplastic (0.826) Apoptosis agonist (0.797) | Anti-eczematic (0.929) Macular degeneration treatment (0.856) Alzheimer’s disease treatment (0.729) |
| 130 | Lipoprotein disorders treatment (0.952) Anti-diabetic (0.943) Anti-asthmatic (0.593) | Anti-eczematic (0.904) Anti-neoplastic (0.765) Anti-leukemic (0.651) |
| 131 | Insulin promoter (0.986) Myocardial infarction treatment (0.868) Anti-neoplastic (0.833) Apoptosis agonist (0.768) | Anti-eczematic (0.910) Anti-fungal (0.670) Anti-psoriatic (0.582) Anti-bacterial (0.535) |
| 132 | Anti-eczematic (0.914) Anti-fungal (0.795) Anti-parasitic (0.756) | Anti-neoplastic (0.854) Apoptosis agonist (0.786) Cytostatic (0.722) |
| 133 | Anti-neoplastic (0.914) Apoptosis agonist (0.823) | Anti-asthmatic (0.834) Anti-allergic (0.828) |
| No. | Dominated Biological Activity (Pa) * | Additional Predicted Activities (Pa) * |
|---|---|---|
| 134 | Apoptosis agonist (0.806) Anti-neoplastic (0.803) | Genital warts treatment (0.724) Anti-eczematic (0.718) |
| 135 | Insulin promoter (0.981) Myocardial infarction treatment (0.819) | Anti-neoplastic (0.797) Apoptosis agonist (0.695) |
| 136 | Insulin promoter (0.986) Myocardial infarction treatment (0.899) | Anti-neoplastic (0.866) Apoptosis agonist (0.772) |
| 137 | Insulin promoter (0.986) Myocardial infarction treatment (0.899) | Anti-neoplastic (0.839) Apoptosis agonist (0.696) |
| 138 | Anti-neoplastic (0.875) Apoptosis agonist (0.795) | Anti-asthmatic (0.816) Anti-allergic (0.533) |
| 139 | Anti-neoplastic (0.885) Apoptosis agonist (0.824) | Anti-psoriatic (0.595) Anti-allergic (0.539) |
| 140 | Anti-neoplastic (0.806) Apoptosis agonist (0.634) | Myocardial infarction treatment (0.781) Hypolipemic (0.599) |
| 141 | Hepatic disorders treatment (0.934) Immunosuppressant (0.691) | Anti-allergic (0.618) Allergic conjunctivitis treatment (0.543) |
| 142 | Hepatic disorders treatment (0.942) Anti-neoplastic (0.782) | Anti-allergic (0.758) Anti-asthmatic (0.728) |
| 143 | Hepatic disorders treatment (0.930) Anti-neoplastic (0.753) | Anti-allergic (0.711) Allergic conjunctivitis treatment (0.597) |
| 144 | Anti-neoplastic (0.888) Apoptosis agonist (0.761) | Anti-inflammatory (0.815) Anti-fungal (0.629) |
| 145 | Anti-neoplastic (0.907) Apoptosis agonist (0.673) | Anti-inflammatory (0.824) Anti-fungal (0.597) |
| 146 | Anti-eczematic (0.850) Anti-neoplastic (0.765) | Allergic conjunctivitis treatment (0.649) Anti-allergic (0.641) |
| 147 | Anti-eczematic (0.850) Anti-pruritic (0.787) | Allergic conjunctivitis treatment (0.649) Anti-allergic (0.641) |
| 148 | Anti-protozoal (0.956) Genital warts treatment (0.824) | Anti-neoplastic (0.761) Anti-metastatic (0.530) |
| 149 | Anti-protozoal (0.954) Genital warts treatment (0.805) | Anti-neoplastic (0.759) Apoptosis agonist (0.540) |
| 150 | Anti-protozoal (0.958) Anti-protozoal (Plasmodium) (0.953) | Genital warts treatment (0.798) Anti-neoplastic (0.766) |
| 151 | Insulin promoter (0.984) Cytostatic (0.907) | Anti-eczematic (0.907) Anti-fungal (0.752) |
| 152 | Insulin promoter (0.982) Cytostatic (0.921) | Anti-eczematic (0.919) Macular degeneration treatment (0.912) |
| 153 | Anti-eczematic (0.922) Macular degeneration treatment (0.913) | Anti-neoplastic (0.868) Cytostatic (0.866) |
| No. | Dominated Biological Activity (Pa) * | Additional Predicted Activities (Pa) * |
|---|---|---|
| 154 | Anti-neoplastic (0.860) Prostate disorders treatment (0.781) | Bone diseases treatment (0.722) Anti-inflammatory (0.639) |
| 155 | Anti-neoplastic (0.894) Prostate disorders treatment (0.799) | Bone diseases treatment (0.787) Anti-inflammatory (0.731) |
| 156 | Anti-neoplastic (0.934) Prostate cancer treatment (0.885) Anti-neoplastic (sarcoma) (0.875) Anti-neoplastic (renal cancer) (0.820) | Choleretic (0.879) Anti-hypercholesterolemic (0.828) Anti-fungal (0.781) Dermatologic (0.778) |
| 157 | Anti-neoplastic (0.922) Anti-neoplastic (sarcoma) (0.836) | Anti-osteoporotic (0.803) Bone diseases treatment (0.781) |
| 158 | Anti-hypercholesterolemic (0.937) Atherosclerosis treatment (0.831) | Respiratory analeptic (0.878) Anti-infertility, female (0.833) |
| 159 | Anti-neoplastic (0.881) Growth stimulant (0.751) | Dermatologic (0.771) Anti-fungal (0.696) |
| 160 | Anti-hypercholesterolemic (0.885) | Anesthetic general (0.823) |
| 161 | Anti-neoplastic (0.810) Apoptosis agonist (0.776) | Prostate disorders treatment (0.688) Acute neurologic disorders treatment (0.680) |
| 162 | Anti-neoplastic (0.805) Apoptosis agonist (0.744) Cytoprotectant (0.690) Prostate disorders treatment (0.681) | Dermatologic (0.750) Anti-viral (influenza) (0.738) Anti-bacterial (0.736) Anti-fungal (0.728) |
| 163 | Anti-neoplastic (0.805) Apoptosis agonist (0.744) Cytoprotectant (0.690) Prostate disorders treatment (0.681) | Dermatologic (0.750) Anti-viral (influenza) (0.738) Anti-bacterial (0.736) Anti-fungal (0.728) |
| 164 | Anti-neoplastic (0.851) Anti-carcinogenic (0.754) | Biliary tract disorders treatment (0.841) Bone diseases treatment (0.725) |
| 165 | Anti-neoplastic (0.882) Cytostatic (0.793) | Anti-bacterial (0.736) Anti-fungal (0.695) |
| 166 | Anti-neoplastic (0.822) Cytostatic (0.782) | Anti-parasitic (0.718) Anti-protozoal (0.714) |
| 167 | Glucan endo-1,3-b-D-glucosidase inhibitor (0.890) | Biliary tract disorders treatment (0.845) |
| 168 | Anti-neoplastic (0.884) | Anti-inflammatory (0.829) |
| 169 | Anti-inflammatory (0.829) Anti-viral (0.826) | Anti-neoplastic (0.784) Apoptosis agonist (0.763) |
| 170 | Anti-hypercholesterolemic (0.941) Atherosclerosis treatment (0.831) | Anti-infertility, female (0.833) Prostate disorders treatment (0.773) |
| 171 | Anti-neoplastic (0.912) Cytoprotectant (0.764) Prostate disorders treatment (0.767) | Respiratory analeptic (0.894) Erythropoiesis stimulant (0.776) |
| 172 | Anti-neoplastic (0.912) Cytoprotectant (0.764) Prostate disorders treatment (0.767) | Respiratory analeptic (0.894) Erythropoiesis stimulant (0.776) Apoptosis agonist (0.677) |
| 173 | Anti-hypercholesterolemic (0.911) Myocardial infarction treatment (0.900) Atherosclerosis treatment (0.811) | Apoptosis agonist (0.862) Anti-neoplastic (0.846) Prostate disorders treatment (0.823) |
| 174 | Respiratory analeptic (0.911) Myocardial infarction treatment (0.906) | Anti-hypercholesterolemic (0.845) Anti-diabetic (type 2) (0.669) |
| 175 | Myocardial infarction treatment (0.864) Immunosuppressant (0.734) | Dermatologic (0.785) Anti-psoriatic (0.728) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dembitsky, V.M. Biological Activity and Structural Diversity of Steroids Containing Aromatic Rings, Phosphate Groups, or Halogen Atoms. Molecules 2023, 28, 5549. https://doi.org/10.3390/molecules28145549
Dembitsky VM. Biological Activity and Structural Diversity of Steroids Containing Aromatic Rings, Phosphate Groups, or Halogen Atoms. Molecules. 2023; 28(14):5549. https://doi.org/10.3390/molecules28145549
Chicago/Turabian StyleDembitsky, Valery M. 2023. "Biological Activity and Structural Diversity of Steroids Containing Aromatic Rings, Phosphate Groups, or Halogen Atoms" Molecules 28, no. 14: 5549. https://doi.org/10.3390/molecules28145549
APA StyleDembitsky, V. M. (2023). Biological Activity and Structural Diversity of Steroids Containing Aromatic Rings, Phosphate Groups, or Halogen Atoms. Molecules, 28(14), 5549. https://doi.org/10.3390/molecules28145549






