Efficient Fe3C-CF Cathode Catalyst Based on the Formation/Decomposition of Li2−xO2 for Li-O2 Batteries
Abstract
:1. Introduction
2. Results and Discussion
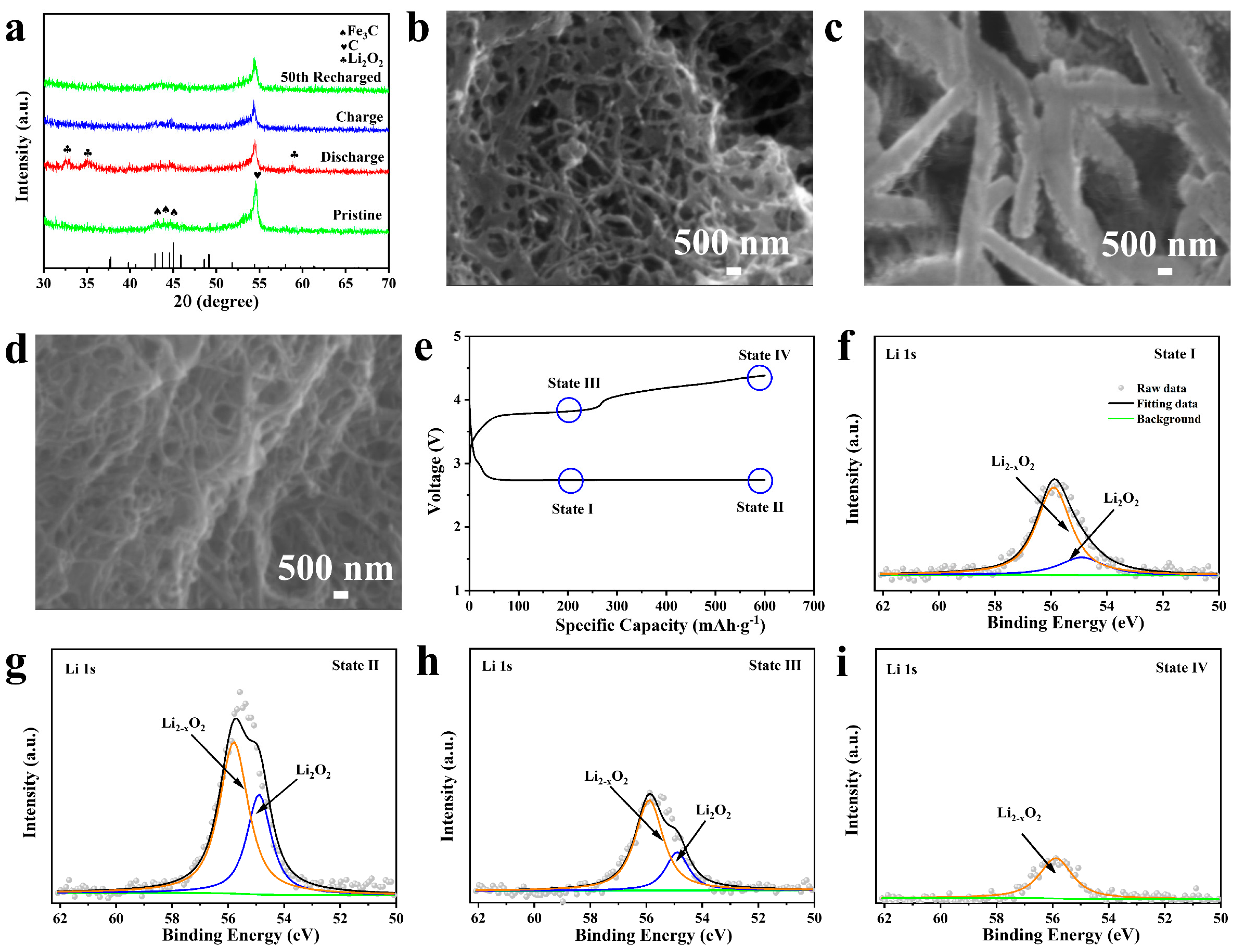
2.1. Synthesis and Characterization
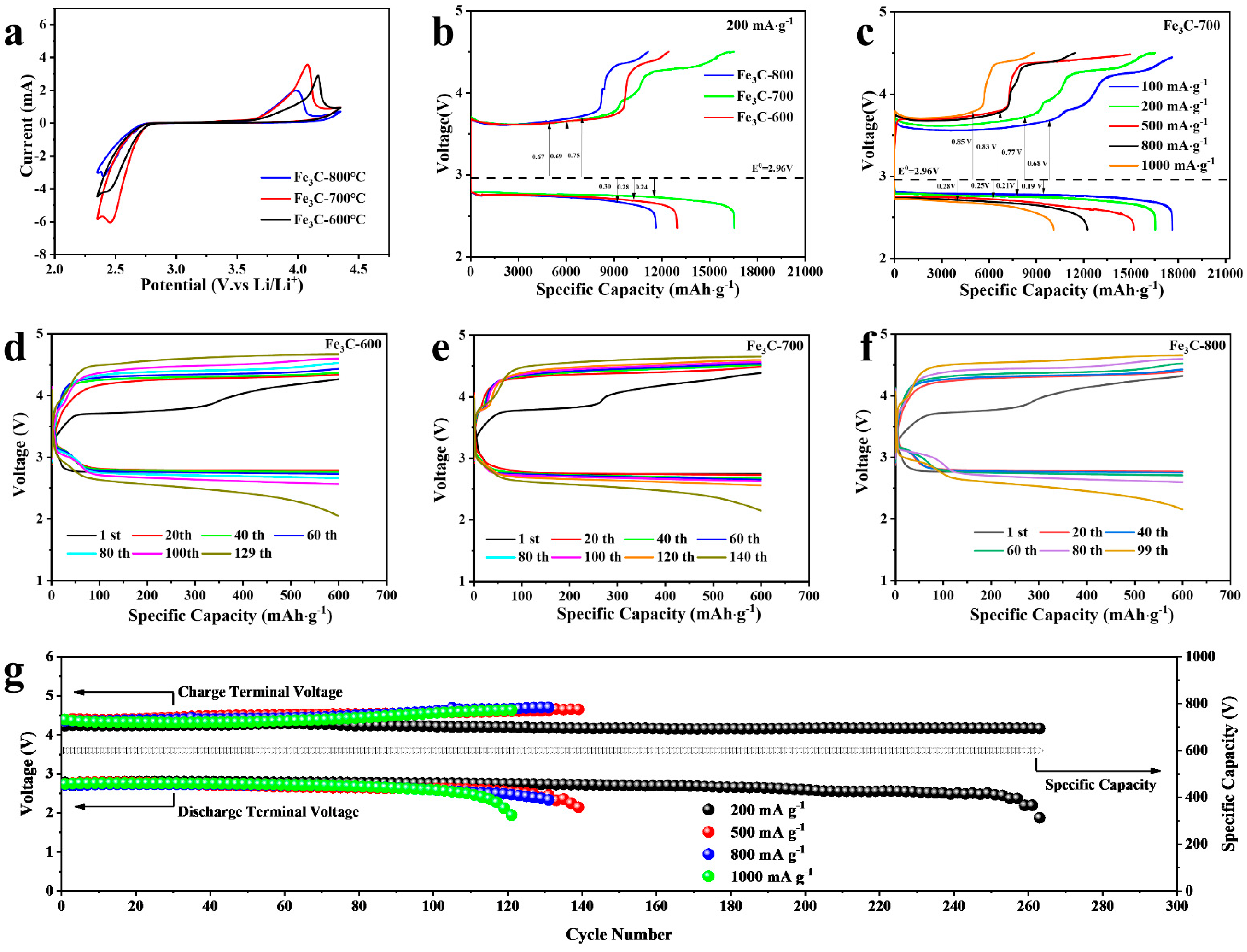
2.2. Electrochemical Performance of the Fe3C Cathode
2.3. Discharge/Charge Characteristics of Fe3C Cathode
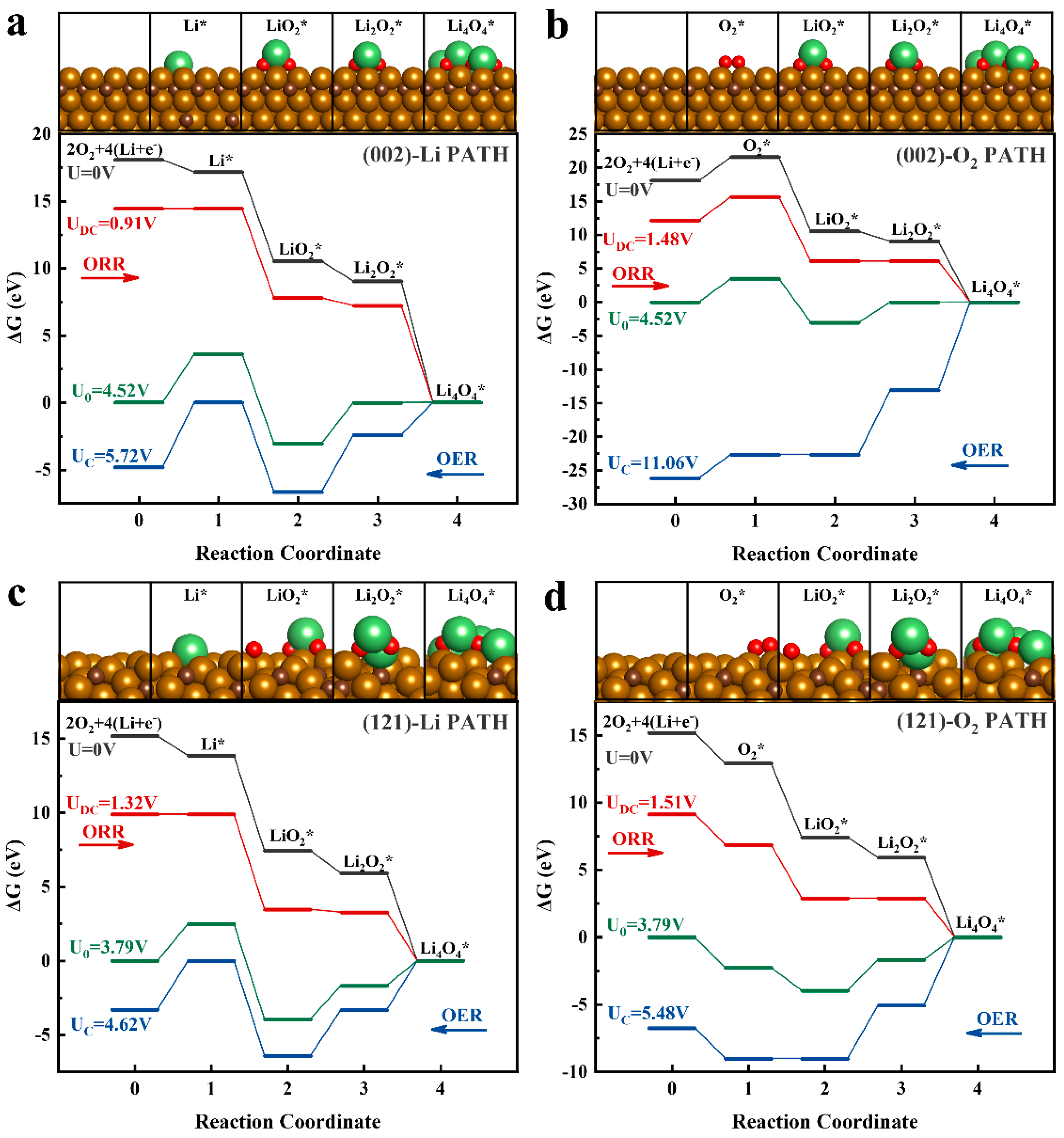
2.4. Theoretical Calculations
- (i)
- (Li+ + e−) → Li*
- (ii)
- Li* + O2 → LiO2*
- (iii)
- LiO2* + (Li+ + e−) → Li2O2*
- (iv)
- Li2O2* + 2(Li+ + e−) + O2 → Li4O4*
- (i)
- O2→ O2*
- (ii)
- O2* + (Li+ + e−) → LiO2*
- (iii)
- LiO2* + (Li+ + e−) → Li2O2*
- (iv)
- Li2O2* + 2(Li+ + e−) + O2 → Li4O4*
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.M. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 2011, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.; Wang, Y.; He, B.; Zhang, W.; Dang, F.; Wang, H.; Du, Y. Novel MoSi2 catalysts featuring surface activation as highly efficient cathode materials for long-life Li-O2 batteries. J. Mater. Chem. A 2020, 8, 259–267. [Google Scholar] [CrossRef]
- Girishkumar, G.; McCloskey, B.; Luntz, A.C.; Swanson, S.; Wilcke, W. Lithium–Air Battery: Promise and Challenges. J. Phys. Chem. Lett. 2010, 1, 2193–2203. [Google Scholar] [CrossRef]
- Wan, J.; Xie, J.; Kong, X.; Liu, Z.; Liu, K.; Shi, F.; Pei, A.; Chen, H.; Chen, W.; Chen, J.; et al. Ultrathin, flexible, solid polymer composite electrolyte enabled with aligned nanoporous host for lithium batteries. Nat. Nanotechnol. 2019, 14, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.R.; Jung, J.-W.; Kim, I.-D. Recent Progress in 1D Air Electrode Nanomaterials for Enhancing the Performance of Nonaqueous Lithium-Oxygen Batteries. ChemNanoMat 2016, 2, 616–634. [Google Scholar] [CrossRef]
- Aurbach, D.; McCloskey, B.D.; Nazar, L.F.; Bruce, P.G. Advances in understanding mechanisms underpinning lithium–air batteries. Nat. Energy 2016, 1, 2058–7546. [Google Scholar] [CrossRef]
- Asadi, M.; Kumar, B.; Liu, C.; Phillips, P.; Yasaei, P.; Behranginia, A.; Zapol, P.; Klie, R.F.; Curtiss, L.A.; Salehi-Khojin, A. Cathode Based on Molybdenum Disulfide Nanoflakes for Lithium-Oxygen Batteries. ACS Nano 2016, 10, 2167–2175. [Google Scholar] [CrossRef]
- Assary, R.S.; Lu, J.; Du, P.; Luo, X.; Zhang, X.; Ren, Y.; Curtiss, L.A.; Amine, K. The effect of oxygen crossover on the anode of a Li-O2 battery using an ether-based solvent: Insights from experimental and computational studies. ChemSusChem 2013, 6, 51–55. [Google Scholar] [CrossRef]
- Fan, W.; Wang, B.; Guo, X.; Kong, X.; Liu, J. Nanosize stabilized Li-deficient Li2−xO2 through cathode architecture for high performance Li-O2 batteries. Nano Energy 2016, 27, 577–586. [Google Scholar] [CrossRef]
- Shao, Y.; Park, S.; Xiao, J.; Zhang, J.-G.; Wang, Y.; Liu, J. Electrocatalysts for Nonaqueous Lithium–Air Batteries: Status, Challenges, and Perspective. ACS Catal. 2012, 2, 844–857. [Google Scholar] [CrossRef]
- He, B.; Li, G.; Li, J.; Wang, J.; Tong, H.; Fan, Y.; Wang, W.; Sun, S.; Dang, F. MoSe2@CNT Core–Shell Nanostructures as Grain Promoters Featuring a Direct Li2O2 Formation/Decomposition Catalytic Capability in Lithium-Oxygen Batteries. Adv. Energy Mater. 2021, 11, 2003263. [Google Scholar] [CrossRef]
- He, B.; Wang, J.; Fan, Y.; Jiang, Y.; Zhai, Y.; Wang, Y.; Huang, Q.; Dang, F.; Zhang, Z.; Wang, N. Mesoporous CoO/Co–N–C nanofibers as efficient cathode catalysts for Li-O2 batteries. J. Mater. Chem. A 2018, 6, 19075–19084. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, J.; Liu, J.; Hou, C.; Xiu, Z.; Fan, Y.; Zhao, L.; Zhai, Y.; Li, H.; Zeng, J.; et al. Interfacial Super-Assembled Porous CeO2 /C Frameworks Featuring Efficient and Sensitive Decomposing Li2O2 for Smart Li-O2 Batteries. Adv. Energy Mater. 2019, 9, 1901751. [Google Scholar] [CrossRef]
- Jian, Z.; Liu, P.; Li, F.; He, P.; Guo, X.; Chen, M.; Zhou, H. Core-shell-structured CNT@RuO2 composite as a high-performance cathode catalyst for rechargeable Li-O2 batteries. Angew. Chem. Int. Ed. 2014, 53, 442–446. [Google Scholar] [CrossRef]
- Lim, H.D.; Park, K.Y.; Song, H.; Jang, E.Y.; Gwon, H.; Kim, J.; Kim, Y.H.; Lima, M.D.; Ovalle Robles, R.; Lepro, X.; et al. Enhanced power and rechargeability of a Li-O2 battery based on a hierarchical-fibril CNT electrode. Adv. Mater. 2013, 25, 1348–1352. [Google Scholar] [CrossRef]
- Gu, Y.; Li, C.; Bai, J.; Wang, J.; Ma, T. One-step solvothermal synthesis of Au-TiO2 loaded electrospun carbon fibers to enhance photocatalytic activity. Vacuum 2016, 130, 1–6. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.; Zou, M.; Wu, C.; Huang, Z.; Guan, L. An effective integrated design for enhanced cathodes of Ni foam-supported Pt/carbon nanotubes for Li-O2 batteries. ACS Appl. Mater. Interfaces 2014, 6, 12479–12485. [Google Scholar] [CrossRef]
- Martinez Crespiera, S.; Amantia, D.; Knipping, E.; Aucher, C.; Aubouy, L.; Amici, J.; Zeng, J.; Francia, C.; Bodoardo, S. Electrospun Pd-doped mesoporous carbon nanofibres as catalysts for rechargeable Li-O2 batteries. RSC Adv. 2016, 6, 57335–57345. [Google Scholar] [CrossRef]
- Cao, Y.; Wei, Z.; He, J.; Zang, J.; Zhang, Q.; Zheng, M.; Dong, Q. α-MnO2 nanorods grown in situ on graphene as catalysts for Li-O2 batteries with excellent electrochemical performance. Energy Environ. Sci. 2012, 5, 9765–9768. [Google Scholar] [CrossRef]
- Liu, J.; Li, D.; Wang, Y.; Zhang, S.; Kang, Z.; Xie, H.; Sun, L. MoO2 nanoparticles/carbon textiles cathode for high performance flexible Li-O2 battery. J. Energy Chem. 2020, 47, 66–71. [Google Scholar] [CrossRef]
- Shang, C.; Dong, S.; Hu, P.; Guan, J.; Xiao, D.; Chen, X.; Zhang, L.; Gu, L.; Cui, G.; Chen, L. Compatible interface design of CoO-based Li-O2 battery cathodes with long-cycling stability. Sci. Rep. 2015, 5, 8335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Wu, X.; Shen, C.; Wen, Z. Facile synthesis of Fe@Fe2O3 core-shell nanowires as O2 electrode for high-energy Li-O2 batteries. J. Solid State Electrochem. 2016, 20, 1831–1836. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Zhao, X.; Liu, Z.; Chen, W. Chen Porous perovskite LaNiO3 nanocubes as cathode catalysts for Li-O2 batteries with low charge potential. Sci. Rep. 2014, 4, 6005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Gao, R.; Li, Z.; Hu, Z.; Liu, H.; Liu, X. Enhancing the Performance of CoO as Cathode Catalyst for Li-O2 Batteries through Confinement into Bimodal Mesoporous Carbon. Electrochim. Acta 2016, 201, 134–141. [Google Scholar] [CrossRef]
- Jeong, M.G.; Kwak, W.J.; Shin, H.J.; Sun, Y.K.; Jung, H.G. Perpendicularly aligned TiC-coated carbon cloth cathode for high-performance Li-O2 batteries. Chem. Eng. J. 2020, 399, 125699. [Google Scholar] [CrossRef]
- Li, G.; Li, N.; Peng, S.; He, B.; Wang, J.; Du, Y.; Zhang, W.; Han, K.; Dang, F. Highly Efficient Nb2C MXene Cathode Catalyst with Uniform O-Terminated Surface for Lithium–Oxygen Batteries. Adv. Energy Mater. 2021, 11, 2002721. [Google Scholar] [CrossRef]
- Qiu, F.; He, P.; Jiang, J.; Zhang, X.; Tong, S.; Zhou, H. Ordered mesoporous TiC-C composites as cathode materials for Li-O2 batteries. Chem. Commun. (Camb.) 2016, 52, 2713–2716. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-S.; Sun, Z.; Cui, Z.; Jianga, F.-L.; Denga, J.-W.; Zhang, T. Inward growth of superthin TiC skin on carbon nanotube framework as stable cathode support for Li-O2 batteries. Energy Stor. Mater. 2020, 30, 59–66. [Google Scholar] [CrossRef]
- Yang, Y.; Xue, X.; Qin, Y.; Wang, X.; Yao, M.; Qin, Z.; Huang, H. Oxygen Evolution Reaction on Pristine and Oxidized TiC (100) Surface in Li-O2 Battery. J. Phy. Chem. C 2018, 122, 12665–12672. [Google Scholar] [CrossRef]
- Yang, Z.-D.; Chang, Z.-W.; Zhang, Q.; Huang, K.; Zhang, X.-B. Decorating carbon nanofibers with Mo2C nanoparticles towards hierarchically porous and highly catalytic cathode for high-performance Li-O2 batteries. Sci. Bull. 2018, 63, 433–440. [Google Scholar] [CrossRef]
- Li, J.; Zou, M.; Chen, L.; Huang, Z.; Guan, L. An efficient bifunctional catalyst of Fe/Fe3C carbon nanofibers for rechargeable Li-O2 batteries. J. Mater. Chem. A 2014, 2, 10634–10638. [Google Scholar] [CrossRef]
- Liang, H.; Gai, Z.; Chen, F.; Jing, S.; Kan, W.; Zhao, B.; Yin, S.; Tsiakaras, P. Fe3C decorated wood-derived integral N-doped C cathode for rechargeable Li-O2 batteries. Appl. Catal. B 2023, 324, 122203. [Google Scholar] [CrossRef]
- Sun, T.; Wang, C.; Jiao, D.; Zhu, M.; Lv, S.; Xiang, J.; Qin, C. Facile preparation of porous N-doped carbon via a one-step carbonization/activation treatment of polyvinylpyrrolidone/melamine formaldehyde resin with ammonium carbonate and its enhanced electrochemical performances for supercapacitors. J. Mater. Sci. Mater. Electron. 2017, 28, 8993–9002. [Google Scholar] [CrossRef]
- Hao, X.; Bi, J.; Wang, W.; Chen, Y.; Gao, X.; Sun, X.; Zhang, J. Bimetallic carbide Fe2MoC as electrode material for high-performance capacitive energy storage. Ceram. Int. 2018, 44, 21874–21881. [Google Scholar] [CrossRef]
- Hao, X.; Bi, J.; Wang, W.; Yan, W.; Gao, X.; Sun, X.; Liu, R. Electrospun Fe2MoC/C nanofibers as an efficient electrode material for high-performance supercapacitors. J. Power Sources 2020, 451, 227802. [Google Scholar] [CrossRef]
- Hu, Y.; Jensen, J.O.; Zhang, W.; Martin, S.; Chenitz, R.; Pan, C.; Xing, W.; Bjerrum, N.J.; Li, Q. Fe3C-based oxygen reduction catalysts: Synthesis, hollow spherical structures and applications in fuel cells. J. Mater. Chem. A 2015, 3, 1752–1760. [Google Scholar] [CrossRef]
- Shebanova, O.N.; Lazor, P. Raman spectroscopic study of magnetite (FeFe2O4): A new assignment for the vibrational spectrum. J. Solid State Chem. 2003, 174, 424–430. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, D.-W.; Li, F.; Zhang, L.; Li, N.; Wu, Z.-S.; Wen, L.; Lu, G.Q.; Cheng, H.-M. Graphene-Wrapped Fe3O4 Anode Material with Improved Reversible Capacity and Cyclic Stability for Lithium Ion Batteries. Chem. Mater. 2010, 22, 5306–5313. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Chen, L.; Zhang, P.; Fu, W.; Zhao, H.; Ma, Y.; Pan, X.; Zhang, Z.; Han, W.; et al. Highly Flexible Freestanding Porous Carbon Nanofibers for Electrodes Materials of High-Performance All-Carbon Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 23515–23520. [Google Scholar] [CrossRef]
- Yang, L.; Yang, P.; Yang, R.; Wang, J.; Hao, Y.; Zhao, X. Low-temperature fabrication of carbon nanofibers with improved graphitization via incorporating carbonaceous inclusions. Polyhedron 2019, 164, 13–16. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, Y.; Li, Y.; Ling, L.; Wu, F.; Wu, C. Mille-feuille shaped hard carbons derived from polyvinylpyrrolidone via environmentally friendly electrostatic spinning for sodium ion battery anodes. RSC Adv. 2017, 7, 5519–5527. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Wang, J.; Liu, J.; Li, Y.; Huang, Q.; Hou, Y.; Li, G.; Li, J.; Zhang, R.; Zhou, J.; et al. Superassembly of Porous Fetet(NiFe)octO Frameworks with Stable Octahedron and Multistage Structure for Superior Lithium–Oxygen Batteries. Adv. Energy Mater. 2020, 10, 1904262. [Google Scholar] [CrossRef]
- Li, J.; Han, K.; Huang, J.; Li, G.; Peng, S.; Li, N.; Wang, J.; Zhang, W.; Du, Y.; Fan, Y.; et al. Polarized nucleation and efficient decomposition of Li2O2 for Ti2C MXene cathode catalyst under a mixed surface condition in lithium-oxygen batteries. Energy Stor. Mater. 2021, 35, 669–678. [Google Scholar] [CrossRef]
- Guo, L.; Tan, L.; Xu, A.; Li, G.; Zhang, G.; Liu, R.; Wang, J.; Du, Y.; Dang, F. Highly efficient two-dimensional Ag2Te cathode catalyst featuring a layer structure derived catalytic anisotropy in lithium-oxygen batteries. Energy Stor. Mater. 2022, 50, 96–104. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, G.; Li, G.; Jiang, S.; Zhang, G.; Guo, L.; Zhang, X.; Zhao, Z.; Zou, Z.; Ma, H.; Fu, X.; et al. Efficient Fe3C-CF Cathode Catalyst Based on the Formation/Decomposition of Li2−xO2 for Li-O2 Batteries. Molecules 2023, 28, 5597. https://doi.org/10.3390/molecules28145597
Yi G, Li G, Jiang S, Zhang G, Guo L, Zhang X, Zhao Z, Zou Z, Ma H, Fu X, et al. Efficient Fe3C-CF Cathode Catalyst Based on the Formation/Decomposition of Li2−xO2 for Li-O2 Batteries. Molecules. 2023; 28(14):5597. https://doi.org/10.3390/molecules28145597
Chicago/Turabian StyleYi, Guanyu, Gaoyang Li, Shuhuai Jiang, Guoliang Zhang, Liang Guo, Xiuqi Zhang, Zhongkui Zhao, Zhongping Zou, Hailong Ma, Xiaojiao Fu, and et al. 2023. "Efficient Fe3C-CF Cathode Catalyst Based on the Formation/Decomposition of Li2−xO2 for Li-O2 Batteries" Molecules 28, no. 14: 5597. https://doi.org/10.3390/molecules28145597
APA StyleYi, G., Li, G., Jiang, S., Zhang, G., Guo, L., Zhang, X., Zhao, Z., Zou, Z., Ma, H., Fu, X., Liu, Y., & Dang, F. (2023). Efficient Fe3C-CF Cathode Catalyst Based on the Formation/Decomposition of Li2−xO2 for Li-O2 Batteries. Molecules, 28(14), 5597. https://doi.org/10.3390/molecules28145597






