Potential Application of the Plant-Derived Essential Oils for Atherosclerosis Treatment: Molecular Mechanisms and Therapeutic Potential
Abstract
:1. Introduction
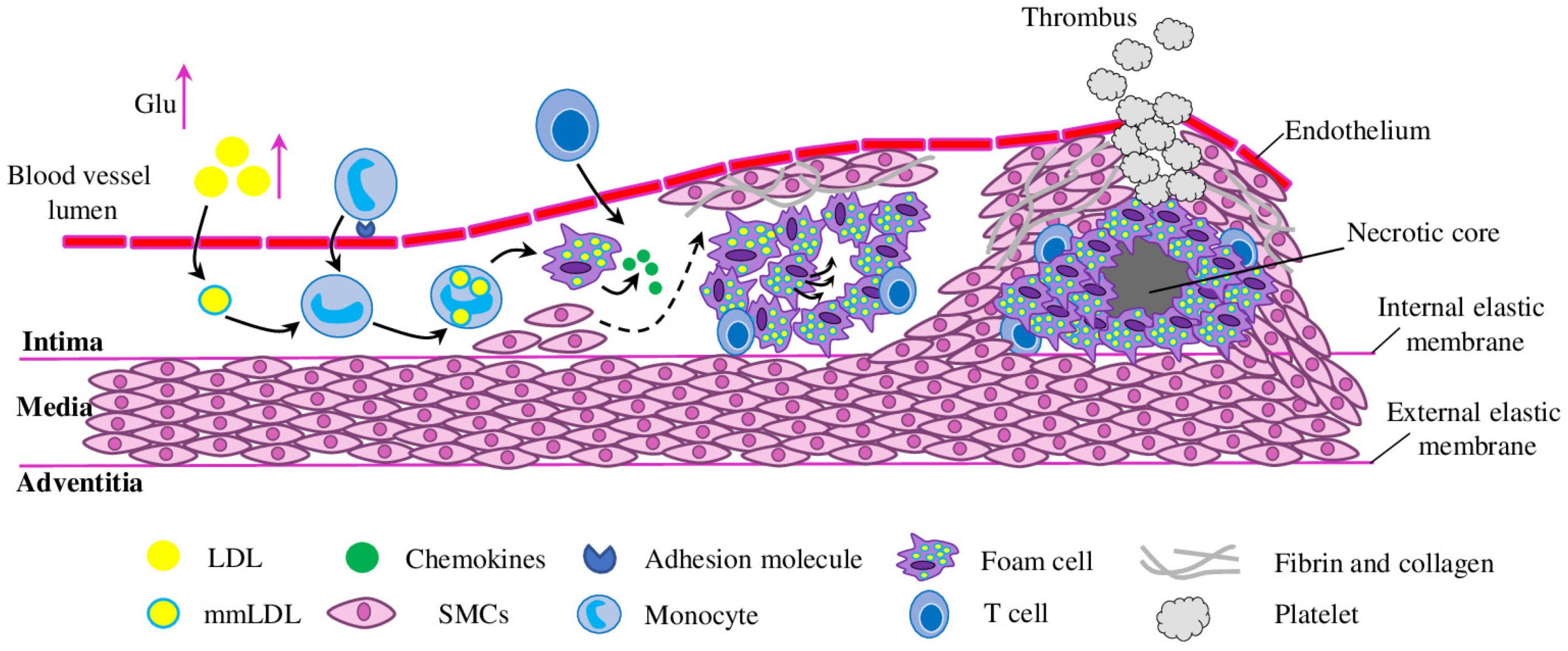
Initiation of Atherosclerosis
2. Anti-Inflammatory Effect of EOs
2.1. In Vitro Raw 264.7 Macrophage Test System
| Species | Major Component | Effect | Experiment Setup | References |
|---|---|---|---|---|
| Artemisia judaica | piperitone, camphor, ethyl cinnamate | inhibits NO production | LPS treated Raw 264.7 macrophage | [54] |
| Hibiscus sabdariffa | n-Hexadecanoic acid | inhibits NO, IL-1, IL-6, TNF-α and COX-2 production; JNK and ERK1/2 pathways | [56] | |
| Waldheimia glabra | α-bisabolol, valeranone and chamazulene | inhibits NO production | [55] | |
| Citrus aurantium | linalool, α-terpineol and (R)-limonene | inhibits NO production; reduces IL-1β, IL-6, TNF-α and COX-2 protein levels and gene expression; inhibits NF-κB/65 and IκB activation | [57] | |
| Siegesbeckia pubescens | β-caryophyllene oxide, trans-longipinocarveol and dehydrosaussurea lactone | inhibits NO production | [59] | |
| Siegesbeckia orientalis | β-caryophyllene, spathulenol and β-caryophyllene oxide | inhibits IL-6 release | ||
| Origanum vulgare | carvacrol, thymol, γ-terpinene and ρ-cymene | inhibits IL-1β, IL-6 and TNF-α expression and secretion | LPS treated Raw 264.7 macrophage | [63] [64,65] |
| Thymus camphoratus | 1,8-cineole and borneol | inhibits NO production; reduces expression of iNOS and COX-2 genes | [60] | |
| Thymus carnosus | borneol and camphene | |||
| Santolina rosmarinifolia | β-pinene, borneol, myrcene and limonene | decreases NO and pro-IL-1β release and expression of iNOS | [61] | |
| Rosa rugosa | citronellol and geraniol | reduces production of IL-1β, IL-6 and TNF-α; increases CAT and SOD activities; inhibits MDA section; normalises iNOS, NO and ROS levels | [66] | |
| Cirsium japonicum | - | inhibit NO production | [62] | |
| decreases lipid accumulation | ox-LDL-induced Raw 264.7 macrophages | |||
| Lavandula pedunculata | 1,8-cineole and fenchone | inhibits NO production | LPS treated Raw 264.7 macrophage | [58] |
| Lavandula luisieri | 1,8-cineole and fenchone; trans-α-necrodol and trans-α-necrodyl acetate | reduces iNOS and increases pro-IL-1β expression; impairs nuclear translocation of NF-κB/p65 | ||
| 5-Methylene-2,3,4,4- tetramethylcyclopent-2- enone; 1,8-cineole | reduces TNF-α and inhibits CCL2 release | LPS-stimulated THP-1 cells | [67] | |
| Thymbra capitata | carvacrol | reduces TNF-α release | ||
| Cymbopogon commutatus | geraniol | inhibits IL-1β, IL-6 and TNF-α; down-regulates ICAM-1 mRNA level; suppresses IkBα phosphorylation; NF-κB/p65 activation and nuclear translocation; increases HO-1 expression | ox-LDL-induced HUVECs | [68] |
| Lavandula angustifolia | linalool, linalyl acetate and terpinen-4-ol | decreases IL-1β, IL-6, IL-8 and TNF-α mRNA and protein levels | LPS-treated THP-1 macrophages | [69] |
| linalool, linalyl acetate | suppresses TNF-α-induced expression of E-selectin, P-selectin, VCAM-1, ICAM-1 and phosphorylated NF-κB/p65 in the nucleus translocation; inhibits TNF-α-induced increase in E-selectin mRNA levels in HUVECs | TNF-α-stimulated bEnd.3 and HUVEC | [70] | |
| Rosmarinus officinalis | 1,8-cineole, camphor, limonene and α-pinene | reduces total cholesterol, LDL, triglycerides, abdominal fat gain | triton and coconut fat-induced rat model of dyslipidaemia | [71] |
| α-pinene, camphor, and 1,8cineole | increases IL-10 level | LPS treated THP-1 macrophage | [72] [73] | |
| camphor, borneol, α-pinene, 1,8-cineole | reduces glucose, triglyceride, total cholesterol; increases HDL; improves serum enzymes (AST, ALT and ALP); ameliorates lipid deposition, macrophage infiltration and stenosis of hepatic vein | rats on HFD | [74] | |
| Zingiber officinale | 1,8-cineole, terpineol, linalool, borneol | |||
| Monarda didyma | carvacrol, p-cymene and thymol | decreases IL-6 and increases miR-146a expression | LPS treated U937 lymphoma cell line | [75] |
| Platycladus orientalis | - | increases IL-10 and reduces IL-1β and TNF-α content in the serum; reduces the p65 and IκB phosphorylation level | mice and rats’ models | [76] |
| Salvia officinalis | 1,8-Cineole, α-caryophyllene | reduces body weight gain, liver and kidney weight; prevents lipid accumulation and focal necrosis in the liver; attenuates haemorrhage foci, reduces Bowman’s space and necrotic epithelial cells lining the tubules in the kidney; normalises antioxidant enzymes (SOD, CAT and Gpx) activity, the lipid profile (total cholesterol, triglyceride, LDL, VLDL and total lipids) and blood biochemical parameters (GGT, pancreatic lipase, AST, ALT, LDH, ALP and CPK; reduces the levels of TBARS and protein carbonyls; increases cholesterol and triglyceride faecal excretion | HFD mice model | [77] |
| Psidium guineense | spathulenol | in vitro antioxidant activities (MDA, ABTS and DPPH); reduces inflammation in carrageenan-induced paw oedema and pleurisy models | in vitro test systems and mice | [78] |
| Alpinia zerumbet | β-pinene, α-cadinol and camphor | decreases ICAM-1 and VCAM-1 expression, NF-κB/p65 phosphorylation and nuclear translocation, IKKα/β phosphorylation and increases IκBα protein levels; reduces lactate dehydrogenase release and caspase-3 activation | LPS-induced HAEC and mice | [79] [80] |
| inhibits p65 subunit nuclear translocation, secretion of IL-8, TNF-α, ICAM-1 and VCAM-1 | high-glucose treated HUVEC | [81] [80] | ||
| Eucalyptus globulus | 1,8-cineole | reduces levels of TNF-α, NO, IL-1α and IL-1β; reduces phosphorylation of NF-κB/p65 and p38, and increases of ERK1/2 and JNK1/2; down-regulates TREM-1, NLRP3 and MKP-1 | LPS-treated alveolar macrophage cell line MH-S | [82] |
| - | 1,8-cineole (purified) | reduces levels of IL-1 and IL-6; reduces phosphorylation of JNK1/2, and increases of NF-κB/p65; down-regulates MKP-1 | ||
| - | 1,8-cineole (purified) | reduces IL-6 and IL-8 secretion; normalises NO levels; improves iNOS expression and eNOS protein levels; decreases p65 phosphorylation and nuclear translocation | LPS-induced HUVEC | [83] |
| - | 1,8-cineole and α-pinene (purified) | decreases TNF-α, IL-1β, IL-6 and eNOS mRNA levels | [84] | |
| - | 1,8-cineole (purified) | inhibits IL-1β, IL-6 and IL-8, and promotes IL-10 release; reduces NF-κB/p65 and VCAM-1, and increases PPAR-γ expression | LPS-treated mice | [85] |
| - | bornyl acetate | reduces E-selectin, ICAM-1 and VCAM-1, IL-1β and TNF-α expression; ameliorates reduction in cell viability | ox-LDL-induced HUVECs | [86] |
| - | β-Elemene | reduces aortic root lesion sizes and necrotic core areas; increases the plaque stability score; increases expression of eNOS, CAT, Gpx and GSH; reduces levels of p22phox, IL-1β, TNF-α, INF-γ, MCP-1 and ICAM-1 | ApoE−/− mice | [87] |
| - | citronellal | reduces atherosclerotic plaque size; alleviates arterial stenosis; reduces production of IL-1, IL-6, IACM-1 and VACM-1; normalises levels of NO, MDA and SOD activity | rats on HFD | [88] |
| - | β-caryophyllene | inhibits VCAM-1; reduces total cholesterol and triglycerides in serum | TNF-α-stimulated HUVECs and mice | [89] |
| - | geraniol | increases Gpx, GST, mtSOD, GSH and NQO1; reduces TBARS; enhances Nrf2 and HO-1 expression; decreases IL-1β, IL-18 and TNF-α levels, NF-κB/65 activation and nuclear translocation | doxorubicin-treated rats | [90] |
2.2. Other In Vitro Test Systems
2.3. EO Effect on Adhesion Molecules and Leukocytes Recruitment In Vitro and In Vivo
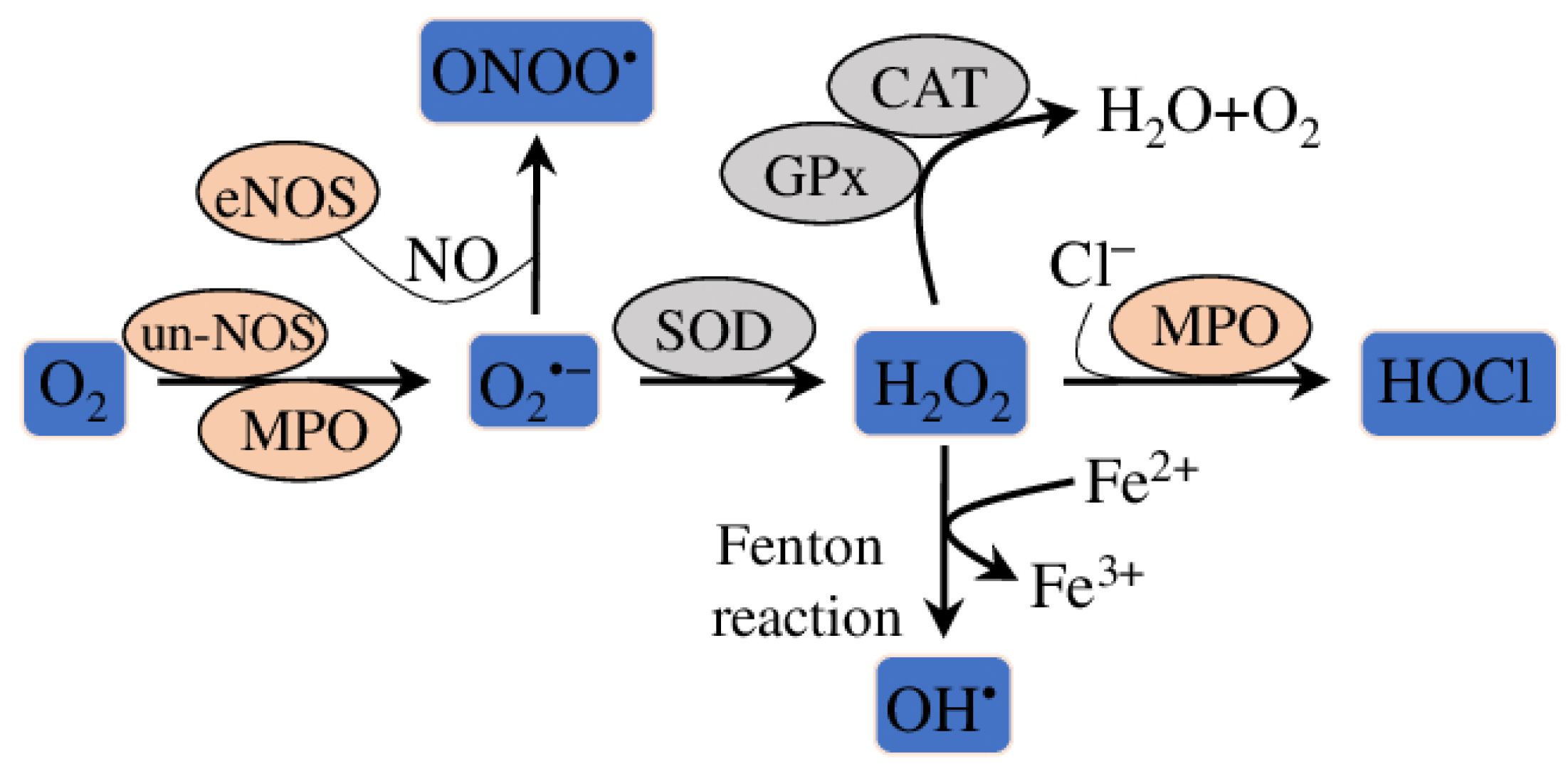
3. Anti-Oxidative Effect of EOs
4. Lipid-Lowering Properties of EOs
5. Clinical Trials Confirming Anti-Inflammatory and Anti-Oxidative Properties of EOs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- WHO CVDs Fact Sheets, R Cardiovascular Diseases (CVDs). Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 11 June 2023).
- Fan, J.; Watanabe, T. Atherosclerosis: Known and Unknown. Pathol. Int. 2022, 72, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Arnesen, E.K.; Retterstøl, K. Secular Trends in Serum Lipid Profiles in Young Adults in Norway, 2001–2019. Atheroscler. Plus 2022, 48, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Georgoulis, M.; Chrysohoou, C.; Georgousopoulou, E.; Damigou, E.; Skoumas, I.; Pitsavos, C.; Panagiotakos, D. Long-Term Prognostic Value of LDL-C, HDL-C, Lp(a) and TG Levels on Cardiovascular Disease Incidence, by Body Weight Status, Dietary Habits and Lipid-Lowering Treatment: The ATTICA Epidemiological Cohort Study (2002–2012). Lipids Health Dis. 2022, 21, 141. [Google Scholar] [CrossRef] [PubMed]
- Blom, D.J.; Almahmeed, W.; Al-Rasadi, K.; Azuri, J.; Daclin, V.; Kayikcioglu, M.; Mercier, F.; Ruiz, A.J.; Santos, R.D. Low-Density Lipoprotein Cholesterol Goal Achievement in Patients with Familial Hypercholesterolemia in Countries Outside Western Europe: The International ChoLesterol Management Practice Study. J. Clin. Lipidol. 2019, 13, 594–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopewell, J.C.; Malik, R.; Valdés-Márquez, E.; Worrall, B.B.; Collins, R. METASTROKE Collaboration of the ISGC Differential Effects of PCSK9 Variants on Risk of Coronary Disease and Ischaemic Stroke. Eur. Heart J. 2018, 39, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Mezentsev, A.; Bezsonov, E.; Kashirskikh, D.; Baig, M.S.; Eid, A.H.; Orekhov, A. Proatherogenic Sialidases and Desialylated Lipoproteins: 35 Years of Research and Current State from Bench to Bedside. Biomedicines 2021, 9, 600. [Google Scholar] [CrossRef]
- Gisterå, A.; Hansson, G.K. The Immunology of Atherosclerosis. Nat. Rev. Nephrol. 2017, 13, 368–380. [Google Scholar] [CrossRef]
- Tardif, J.-C.; McMurray, J.J.; Klug, E.; Small, R.; Schumi, J.; Choi, J.; Cooper, J.; Scott, R.; Lewis, E.F.; L’Allier, P.L.; et al. Effects of Succinobucol (AGI-1067) after an Acute Coronary Syndrome: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2008, 371, 1761–1768. [Google Scholar] [CrossRef]
- Toledo-Ibelles, P.; Mas-Oliva, J. Antioxidants in the Fight Against Atherosclerosis: Is This a Dead End? Curr. Atheroscler. Rep. 2018, 20, 36. [Google Scholar] [CrossRef] [Green Version]
- Poznyak, A.V.; Nikiforov, N.G.; Markin, A.M.; Kashirskikh, D.A.; Myasoedova, V.A.; Gerasimova, E.V.; Orekhov, A.N. Overview of OxLDL and Its Impact on Cardiovascular Health: Focus on Atherosclerosis. Front. Pharmacol. 2021, 11, 613780. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-Density Lipoproteins Cause Atherosclerotic Cardiovascular Disease. 1. Evidence from Genetic, Epidemiologic, and Clinical Studies. A Consensus Statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [Green Version]
- Musunuru, K.; Kathiresan, S. Surprises From Genetic Analyses of Lipid Risk Factors for Atherosclerosis. Circ. Res. 2016, 118, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Ference, B.A.; Staley, J.R.; Freitag, D.F.; Mason, A.M.; Nielsen, S.F.; Willeit, P.; Young, R.; Surendran, P.; Karthikeyan, S.; et al. Association of LPA Variants With Risk of Coronary Disease and the Implications for Lipoprotein(a)-Lowering Therapies: A Mendelian Randomization Analysis. JAMA Cardiol. 2018, 3, 619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordestgaard, B.G. Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease: New Insights From Epidemiology, Genetics, and Biology. Circ. Res. 2016, 118, 547–563. [Google Scholar] [CrossRef]
- Libby, P.; Loscalzo, J.; Ridker, P.M.; Farkouh, M.E.; Hsue, P.Y.; Fuster, V.; Hasan, A.A.; Amar, S. Inflammation, Immunity, and Infection in Atherothrombosis. J. Am. Coll. Cardiol. 2018, 72, 2071–2081. [Google Scholar] [CrossRef]
- Biros, E.; Reznik, J.E.; Moran, C.S. Role of Inflammatory Cytokines in Genesis and Treatment of Atherosclerosis. Trends Cardiovasc. Med. 2022, 32, 138–142. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Bharadwaj, D.; Prasad, G.; Grechko, A.V.; Sazonova, M.A.; Orekhov, A.N. Anti-Inflammatory Therapy for Atherosclerosis: Focusing on Cytokines. IJMS 2021, 22, 7061. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.R.; Silva, S.O.; Soares, S.C. The Use of High Sensitivity C-Reactive Protein in Cardiovascular Disease Detection. J. Pharm. Pharm. Sci. 2018, 21, 496–503. [Google Scholar] [CrossRef] [Green Version]
- Saigusa, R.; Winkels, H.; Ley, K. T Cell Subsets and Functions in Atherosclerosis. Nat. Rev. Cardiol. 2020, 17, 387–401. [Google Scholar] [CrossRef]
- Nus, M.; Mallat, Z. Immune-Mediated Mechanisms of Atherosclerosis and Implications for the Clinic. Expert Rev. Clin. Immunol. 2016, 12, 1217–1237. [Google Scholar] [CrossRef] [Green Version]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef] [PubMed]
- Petrucci, G.; Rizzi, A.; Hatem, D.; Tosti, G.; Rocca, B.; Pitocco, D. Role of Oxidative Stress in the Pathogenesis of Atherothrombotic Diseases. Antioxidants 2022, 11, 1408. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef] [PubMed]
- Glanz, V.; Bezsonov, E.E.; Soldatov, V.; Orekhov, A.N. Thirty-Five-Year History of Desialylated Lipoproteins Discovered by Vladimir Tertov. Biomedicines 2022, 10, 1174. [Google Scholar] [CrossRef]
- Canet-Soulas, E.; Bessueille, L.; Mechtouff, L.; Magne, D. The Elusive Origin of Atherosclerotic Plaque Calcification. Front. Cell Dev. Biol. 2021, 9, 622736. [Google Scholar] [CrossRef]
- Gliozzi, M.; Scicchitano, M.; Bosco, F.; Musolino, V.; Carresi, C.; Scarano, F.; Maiuolo, J.; Nucera, S.; Maretta, A.; Paone, S.; et al. Modulation of Nitric Oxide Synthases by Oxidized LDLs: Role in Vascular Inflammation and Atherosclerosis Development. Int. J. Mol. Sci. 2019, 20, 3294. [Google Scholar] [CrossRef] [Green Version]
- Dabravolski, S.A.; Sukhorukov, V.N.; Kalmykov, V.A.; Grechko, A.V.; Shakhpazyan, N.K.; Orekhov, A.N. The Role of KLF2 in the Regulation of Atherosclerosis Development and Potential Use of KLF2-Targeted Therapy. Biomedicines 2022, 10, 254. [Google Scholar] [CrossRef]
- Loscalzo, J. Nitric Oxide Signaling and Atherothrombosis Redux: Evidence From Experiments of Nature and Implications for Therapy. Circulation 2018, 137, 233–236. [Google Scholar] [CrossRef]
- Alonso-Piñeiro, J.A.; Gonzalez-Rovira, A.; Sánchez-Gomar, I.; Moreno, J.A.; Durán-Ruiz, M.C. Nrf2 and Heme Oxygenase-1 Involvement in Atherosclerosis Related Oxidative Stress. Antioxidants 2021, 10, 1463. [Google Scholar] [CrossRef]
- Cui, W.; Leng, B.; Wang, G. Klotho Protein Inhibits H2O2-Induced Oxidative Injury in Endothelial Cells via Regulation of PI3K/AKT/Nrf2/HO-1 Pathways. Can. J. Physiol. Pharmacol. 2019, 97, 370–376. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Kondo, K.; Momiyama, Y. The Protective Role of Heme Oxygenase-1 in Atherosclerotic Diseases. IJMS 2019, 20, 3628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorelli, S.; Porro, B.; Cosentino, N.; Di Minno, A.; Manega, C.M.; Fabbiocchi, F.; Niccoli, G.; Fracassi, F.; Barbieri, S.; Marenzi, G.; et al. Activation of Nrf2/HO-1 Pathway and Human Atherosclerotic Plaque Vulnerability: An In Vitro and In Vivo Study. Cells 2019, 8, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Cai, W.; Fan, Z.; Yang, C.; Wang, W.; Xiong, M.; Ma, C.; Yang, J. MicroRNA-24 Inhibits the Oxidative Stress Induced by Vascular Injury by Activating the Nrf2/Ho-1 Signaling Pathway. Atherosclerosis 2019, 290, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Basatemur, G.L.; Jørgensen, H.F.; Clarke, M.C.H.; Bennett, M.R.; Mallat, Z. Vascular Smooth Muscle Cells in Atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 727–744. [Google Scholar] [CrossRef]
- Yurdagul, A.; Doran, A.C.; Cai, B.; Fredman, G.; Tabas, I.A. Mechanisms and Consequences of Defective Efferocytosis in Atherosclerosis. Front. Cardiovasc. Med. 2018, 4, 86. [Google Scholar] [CrossRef] [Green Version]
- Leszczynska, A.; O’Doherty, A.; Farrell, E.; Pindjakova, J.; O’Brien, F.J.; O’Brien, T.; Barry, F.; Murphy, M. Differentiation of Vascular Stem Cells Contributes to Ectopic Calcification of Atherosclerotic Plaque. Stem Cells 2016, 34, 913–923. [Google Scholar] [CrossRef] [Green Version]
- Hutcheson, J.D.; Goettsch, C.; Bertazzo, S.; Maldonado, N.; Ruiz, J.L.; Goh, W.; Yabusaki, K.; Faits, T.; Bouten, C.; Franck, G.; et al. Genesis and Growth of Extracellular-Vesicle-Derived Microcalcification in Atherosclerotic Plaques. Nat. Mater. 2016, 15, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Russo, M.; Fracassi, F.; Kurihara, O.; Kim, H.O.; Thondapu, V.; Araki, M.; Shinohara, H.; Sugiyama, T.; Yamamoto, E.; Lee, H.; et al. Healed Plaques in Patients With Stable Angina Pectoris. ATVB 2020, 40, 1587–1597. [Google Scholar] [CrossRef]
- Pasterkamp, G.; den Ruijter, H.M.; Libby, P. Temporal Shifts in Clinical Presentation and Underlying Mechanisms of Atherosclerotic Disease. Nat. Rev. Cardiol. 2017, 14, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Franck, G. Role of Mechanical Stress and Neutrophils in the Pathogenesis of Plaque Erosion. Atherosclerosis 2021, 318, 60–69. [Google Scholar] [CrossRef]
- Libby, P. The Changing Landscape of Atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Sharif, H.; Akash, M.S.H.; Rehman, K.; Irshad, K.; Imran, I. Pathophysiology of Atherosclerosis: Association of Risk Factors and Treatment Strategies Using Plant-Based Bioactive Compounds. J. Food Biochem. 2020, 44, e13449. [Google Scholar] [CrossRef] [PubMed]
- Lunz, K.; Stappen, I. Back to the Roots-An Overview of the Chemical Composition and Bioactivity of Selected Root-Essential Oils. Molecules 2021, 26, 3155. [Google Scholar] [CrossRef]
- Edris, A.E. Pharmaceutical and Therapeutic Potentials of Essential Oils and Their Individual Volatile Constituents: A Review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Ferrentino, G.; Morozova, K.; Horn, C.; Scampicchio, M. Extraction of Essential Oils from Medicinal Plants and Their Utilization as Food Antioxidants. CPD 2020, 26, 519–541. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Heinbockel, T. The Effects of Essential Oils and Terpenes in Relation to Their Routes of Intake and Application. IJMS 2020, 21, 1558. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.A.P.; Santos, D.M.; de Carvalho, F.O.; Menezes, I.A.C.; Barreto, A.S.; Souza, D.S.; Quintans-Júnior, L.J.; Santos, M.R.V. Monoterpenes and Their Derivatives as Agents for Cardiovascular Disease Management: A Systematic Review and Meta-Analysis. Phytomedicine 2021, 88, 153451. [Google Scholar] [CrossRef]
- Saljoughian, S.; Roohinejad, S.; Bekhit, A.E.-D.A.; Greiner, R.; Omidizadeh, A.; Nikmaram, N.; Mousavi Khaneghah, A. The Effects of Food Essential Oils on Cardiovascular Diseases: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1688–1705. [Google Scholar] [CrossRef]
- Roe, K. An Inflammation Classification System Using Cytokine Parameters. Scand. J. Immunol. 2021, 93, e12970. [Google Scholar] [CrossRef]
- García, R.D.; Asensio, J.A.; Perdicaro, D.J.; de los Ángeles Peral, M. The Role of Inflammation as a Preponderant Risk Factor in Cardiovascular Diseases. CVP 2022, 20, 244–259. [Google Scholar] [CrossRef]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.-Y.; Huffel, C.V.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS Signaling in C3H/HeJ and C57BL/10ScCr Mice: Mutations in Tlr4 Gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Darwish, M.S.; Cabral, C.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Zulfiqar, A.; Khan, I.A.; Efferth, T.; Salgueiro, L. Chemical Composition and Biological Activities of Artemisia judaica Essential Oil from Southern Desert of Jordan. J. Ethnopharmacol. 2016, 191, 161–168. [Google Scholar] [CrossRef]
- De, J.; Lu, Y.; Ling, L.; Peng, N.; Zhong, Y. Essential Oil Composition and Bioactivities of Waldheimia glabra (Asteraceae) from Qinghai-Tibet Plateau. Molecules 2017, 22, 460. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.-Y.; Zhang, T.-T.; Zhang, W.-L.; Jiang, J.-G. Anti-Inflammatory Activities of Essential Oil Isolated from the Calyx of Hibiscus sabdariffa L. Food Funct. 2016, 7, 4451–4459. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-Y.; Jiang, J.-G.; Zhu, W.; Ou-Yang, Q. Anti-Inflammatory Effect of Essential Oil from Citrus aurantium L. Var. Amara Engl. J. Agric. Food Chem. 2017, 65, 8586–8594. [Google Scholar] [CrossRef]
- Zuzarte, M.; Sousa, C.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. The Anti-Inflammatory Response of Lavandula luisieri and Lavandula pedunculata Essential Oils. Plants 2022, 11, 370. [Google Scholar] [CrossRef]
- Gao, X.; Wei, J.; Hong, L.; Fan, S.; Hu, G.; Jia, J. Comparative Analysis of Chemical Composition, Anti-Inflammatory Activity and Antitumor Activity in Essential Oils from Siegesbeckia orientalis, S. Glabrescens and S. Pubescens with an ITS Sequence Analysis. Molecules 2018, 23, 2185. [Google Scholar] [CrossRef] [Green Version]
- Zuzarte, M.; Alves-Silva, J.M.; Alves, M.; Cavaleiro, C.; Salgueiro, L.; Cruz, M.T. New Insights on the Anti-Inflammatory Potential and Safety Profile of Thymus carnosus and Thymus camphoratus Essential Oils and Their Main Compounds. J. Ethnopharmacol. 2018, 225, 10–17. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Gonçalves, M.J.; Silva, A.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. Chemical Profile, Anti-Microbial and Anti-Inflammaging Activities of Santolina rosmarinifolia L. Essential Oil from Portugal. Antibiotics 2023, 12, 179. [Google Scholar] [CrossRef]
- Ma, Q.; Jiang, J.-G.; Yuan, X.; Qiu, K.; Zhu, W. Comparative Antitumor and Anti-Inflammatory Effects of Flavonoids, Saponins, Polysaccharides, Essential Oil, Coumarin and Alkaloids from Cirsium japonicum DC. Food Chem. Toxicol. 2019, 125, 422–429. [Google Scholar] [CrossRef]
- Cheng, C.; Zou, Y.; Peng, J. Oregano Essential Oil Attenuates RAW264.7 Cells from Lipopolysaccharide-Induced Inflammatory Response through Regulating NADPH Oxidase Activation-Driven Oxidative Stress. Molecules 2018, 23, 1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foti, M.C.; Ingold, K.U. Mechanism of Inhibition of Lipid Peroxidation by γ-Terpinene, an Unusual and Potentially Useful Hydrocarbon Antioxidant. J. Agric. Food Chem. 2003, 51, 2758–2765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Raka, R.N.; Zhiqian, D.; Yue, Y.; Luchang, Q.; Suyeon, P.; Junsong, X.; Hua, W. Pingyin Rose Essential Oil Alleviates LPS-Induced Inflammation in RAW 264.7 Cells via the NF-ΚB Pathway: An Integrated in Vitro and Network Pharmacology Analysis. BMC Complement. Med. Ther. 2022, 22, 272. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G.; da Silva, C.I.; Farah, L.; Castro Braga, F.; Figueiredo, A.C. Effect of Essential Oils on the Release of TNF-α and CCL2 by LPS-Stimulated THP-1 Cells. Plants 2020, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Ammar, R.B.; Mohamed, M.E.; Alfwuaires, M.; Abdulaziz Alamer, S.; Bani Ismail, M.; Veeraraghavan, V.P.; Sekar, A.K.; Ksouri, R.; Rajendran, P. Anti-Inflammatory Activity of Geraniol Isolated from Lemon Grass on Ox-LDL-Stimulated Endothelial Cells by Upregulation of Heme Oxygenase-1 via PI3K/Akt and Nrf-2 Signaling Pathways. Nutrients 2022, 14, 4817. [Google Scholar] [CrossRef] [PubMed]
- Pandur, E.; Balatinácz, A.; Micalizzi, G.; Mondello, L.; Horváth, A.; Sipos, K.; Horváth, G. Anti-Inflammatory Effect of Lavender (Lavandula angustifolia Mill.) Essential Oil Prepared during Different Plant Phenophases on THP-1 Macrophages. BMC Complement. Med. Ther. 2021, 21, 287. [Google Scholar] [CrossRef]
- Aoe, M.; Ueno-Iio, T.; Shibakura, M.; Shinohata, R.; Usui, S.; Arao, Y.; Ikeda, S.; Miyahara, N.; Tanimoto, M.; Kataoka, M. Lavender Essential Oil and Its Main Constituents Inhibit the Expression of TNF-α-Induced Cell Adhesion Molecules in Endothelial Cells. Acta Med. Okayama 2017, 71, 493–503. [Google Scholar]
- Santos Rodrigues, A.P.; Faria e Souza, B.S.; Alves Barros, A.S.; de Oliveira Carvalho, H.; Lobato Duarte, J.; Letícia Elizandra Boettger, M.; Barbosa, R.; Maciel Ferreira, A.; Maciel Ferreira, I.; Fernandes, C.P.; et al. The Effects of Rosmarinus officinalis L. Essential Oil and Its Nanoemulsion on Dyslipidemic Wistar Rats. J. Appl. Biomed. 2020, 18, 126–135. [Google Scholar] [CrossRef]
- Lorenzo-Leal, A.C.; Palou, E.; López-Malo, A.; Bach, H. Antimicrobial, Cytotoxic, and Anti-Inflammatory Activities of Pimenta dioica and Rosmarinus officinalis Essential Oils. BioMed Res. Int. 2019, 2019, 1639726. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo-Leal, A.C.; Palou, E.; López-Malo, A. Evaluation of the Efficiency of Allspice, Thyme and Rosemary Essential Oils on Two Foodborne Pathogens in in-Vitro and on Alfalfa Seeds, and Their Effect on Sensory Characteristics of the Sprouts. Int. J. Food Microbiol. 2019, 295, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Eissa, F.A.; Choudhry, H.; Abdulaal, W.H.; Baothman, O.A.; Zeyadi, M.; Moselhy, S.S.; Zamzami, M.A. Possible Hypocholesterolemic Effect of Ginger and Rosemary Oils in Rats. AJTCAM 2017, 14, 188–200. [Google Scholar] [CrossRef] [Green Version]
- Fraternale, D.; Dufat, H.; Albertini, M.C.; Bouzidi, C.; D’Adderio, R.; Coppari, S.; Di Giacomo, B.; Melandri, D.; Ramakrishna, S.; Colomba, M. Chemical Composition, Antioxidant and Anti-Inflammatory Properties of Monarda didyma L. Essential Oil. PeerJ 2022, 10, e14433. [Google Scholar] [CrossRef] [PubMed]
- Gan, D.; Yao, Y.; Su, H.; Huang, Y.; Shi, J.; Liu, X.; Xiang, M. Volatile Oil of Platycladus orientalis (L.) Franco Leaves Exerts Strong Anti-Inflammatory Effects via Inhibiting the IκB/NF-ΚB Pathway. Curr. Med. Sci. 2021, 41, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Koubaa-Ghorbel, F.; Chaâbane, M.; Turki, M.; Makni-Ayadi, F.; El Feki, A. The Protective Effects of Salvia officinalis Essential Oil Compared to Simvastatin against Hyperlipidemia, Liver, and Kidney Injuries in Mice Submitted to a High-fat Diet. J. Food Biochem. 2020, 44, e13160. [Google Scholar] [CrossRef]
- Do Nascimento, K.F.; Moreira, F.M.F.; Alencar Santos, J.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; Vieira, M.d.C.; Góis Ruiz, A.L.T.; Ann Foglio, M.; de Carvalho, J.E.; et al. Antioxidant, Anti-Inflammatory, Antiproliferative and Antimycobacterial Activities of the Essential Oil of Psidium guineense Sw. and Spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef]
- Ji, Y.; Shi, T.; Zhang, Y.; Lin, D.; Linghu, K.; Xu, Y.; Tao, L.; Lu, Q.; Shen, X. Essential Oil from Fructus Alpinia zerumbet (Fruit of Alpinia zerumbet (Pers.) Burtt.et Smith) Protected against Aortic Endothelial Cell Injury and Inflammation In Vitro and In Vivo. J. Ethnopharmacol. 2019, 237, 149–158. [Google Scholar] [CrossRef]
- Xiao, R.-Y.; Wu, L.-J.; Hong, X.-X.; Tao, L.; Luo, P.; Shen, X.-C. Screening of Analgesic and Anti-Inflammatory Active Component in Fructus Alpiniae zerumbet Based on Spectrum-Effect Relationship and GC-MS. Biomed. Chromatogr. 2018, 32, e4112. [Google Scholar] [CrossRef]
- Huang, N.; Xu, Y.; Zhou, H.; Lin, D.; Zhang, B.; Zhang, Y.; Pan, D.; Tao, L.; Liu, X.; Shen, X. Essential Oil from Fructus Alpiniae zerumbet Protects Human Umbilical Vein Endothelial Cells In Vitro from Injury Induced by High Glucose Levels by Suppressing Nuclear Transcription Factor-Kappa B Signaling. Med. Sci. Monit. 2017, 23, 4760–4767. [Google Scholar] [CrossRef] [Green Version]
- Yadav, N.; Chandra, H. Suppression of Inflammatory and Infection Responses in Lung Macrophages by Eucalyptus Oil and Its Constituent 1,8-Cineole: Role of Pattern Recognition Receptors TREM-1 and NLRP3, the MAP Kinase Regulator MKP-1, and NFκB. PLoS ONE 2017, 12, e0188232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linghu, K.; Lin, D.; Yang, H.; Xu, Y.; Zhang, Y.; Tao, L.; Chen, Y.; Shen, X. Ameliorating Effects of 1,8-Cineole on LPS-Induced Human Umbilical Vein Endothelial Cell Injury by Suppressing NF-ΚB Signaling in Vitro. Eur. J. Pharmacol. 2016, 789, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Kutlu, Z.; Gulaboglu, M.; Halıcı, Z.; Cınar, İ.; Dıyarbakır, B. Biochemical Research of the Effects of Essential Oil Obtained from the Fruit of Myrtus communis L. on Cell Damage Associated with Lipopolysaccharide-Induced Endotoxemia in a Human Umbilical Cord Vein Endothelial Cells. Biochem. Genet. 2021, 59, 315–334. [Google Scholar] [CrossRef]
- Jiang, F.; Wu, G.; Li, W.; Yang, J.; Yan, J.; Wang, Y.; Yao, W.; Zhou, X.; He, Z.; Wu, L.; et al. Preparation and Protective Effects of 1,8-Cineole-Loaded Self-Microemulsifying Drug Delivery System on Lipopolysaccharide-Induced Endothelial Injury in Mice. Eur. J. Pharm. Sci. 2019, 127, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, J.; Li, Y.; Qi, G. Bornyl Acetate Suppresses Ox-LDL-Induced Attachment of THP-1 Monocytes to Endothelial Cells. Biomed. Pharmacother. 2018, 103, 234–239. [Google Scholar] [CrossRef]
- Liu, M.; Chen, X.; Ma, J.; Hassan, W.; Wu, H.; Ling, J.; Shang, J. β-Elemene Attenuates Atherosclerosis in Apolipoprotein E-Deficient Mice via Restoring NO Levels and Alleviating Oxidative Stress. Biomed. Pharmacother. 2017, 95, 1789–1798. [Google Scholar] [CrossRef]
- Lu, J.; Guo, C.; Ou, W.; Jing, Y.; Niu, H.; Song, P.; Li, Q.; Liu, Z.; Xu, J.; Li, P.; et al. Citronellal Prevents Endothelial Dysfunction and Atherosclerosis in Rats. J. Cell. Biochem. 2019, 120, 3790–3800. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, C.; Dai, X.; Ao, Y.; Li, Y. Inhibitory Effect of Trans-Caryophyllene (TC) on Leukocyte-Endothelial Attachment. Toxicol. Appl. Pharmacol. 2017, 329, 326–333. [Google Scholar] [CrossRef]
- Younis, N.S.; Elsewedy, H.S.; Soliman, W.E.; Shehata, T.M.; Mohamed, M.E. Geraniol Isolated from Lemon Grass to Mitigate Doxorubicin-Induced Cardiotoxicity through Nrf2 and NF-ΚB Signaling. Chem.-Biol. Interact. 2021, 347, 109599. [Google Scholar] [CrossRef]
- Alharbi, K.S.; Fuloria, N.K.; Fuloria, S.; Rahman, S.B.; Al-Malki, W.H.; Javed Shaikh, M.A.; Thangavelu, L.; Singh, S.K.; Rama Raju Allam, V.S.; Jha, N.K.; et al. Nuclear Factor-Kappa B and Its Role in Inflammatory Lung Disease. Chem.-Biol. Interact. 2021, 345, 109568. [Google Scholar] [CrossRef]
- Lin, C.-F.; Chang, Y.-H.; Chien, S.-C.; Lin, Y.-H.; Yeh, H.-Y. Epidemiology of Dyslipidemia in the Asia Pacific Region. Int. J. Gerontol. 2018, 12, 2–6. [Google Scholar] [CrossRef]
- Tan, C.X.; Chong, G.H.; Hamzah, H.; Ghazali, H.M. Effect of Virgin Avocado Oil on Diet-Induced Hypercholesterolemia in Rats via 1H NMR-Based Metabolomics Approach: Hypocholesterolemic Effect of Virgin Avocado Oil. Phytother. Res. 2018, 32, 2264–2274. [Google Scholar] [CrossRef] [PubMed]
- Chalhoub, E.; Emami, E.; Freijé, M.; Kandelman, D.; Campese, M.; St-Georges, A.; Voyer, R.; Rompré, P.; Barbeau, J.; Leduc, A.; et al. Effectiveness of an Alcohol-Free Essential Oil-Containing Mouthwash in Institutionalised Elders Receiving Long-Term Care: A Feasibility Study. Gerodontology 2016, 33, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Jünger, H.; Jaun-Ventrice, A.; Guldener, K.; Ramseier, C.A.; Reissmann, D.R.; Schimmel, M. Anti-Inflammatory Potential of an Essential Oil-Containing Mouthwash in Elderly Subjects Enrolled in Supportive Periodontal Therapy: A 6-Week Randomised Controlled Clinical Trial. Clin. Oral Investig. 2020, 24, 3203–3211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luís, H.; Luís, L.; Bernardo, M.; dos Santos, N. Randomized Controlled Trial on Mouth Rinse and Flossing Efficacy on Interproximal Gingivitis and Dental Plaque. Int. J. Dent. Hyg. 2018, 16, e73–e78. [Google Scholar] [CrossRef] [PubMed]
- Saliasi, I.; Llodra, J.; Bravo, M.; Tramini, P.; Dussart, C.; Viennot, S.; Carrouel, F. Effect of a Toothpaste/Mouthwash Containing Carica Papaya Leaf Extract on Interdental Gingival Bleeding: A Randomized Controlled Trial. IJERPH 2018, 15, 2660. [Google Scholar] [CrossRef] [Green Version]
- Lynch, M.C.; Cortelli, S.C.; McGuire, J.A.; Zhang, J.; Ricci-Nittel, D.; Mordas, C.J.; Aquino, D.R.; Cortelli, J.R. The Effects of Essential Oil Mouthrinses with or without Alcohol on Plaque and Gingivitis: A Randomized Controlled Clinical Study. BMC Oral Health 2018, 18, 6. [Google Scholar] [CrossRef] [Green Version]
- Yaneva, B.K.; Dermendzhieva, Y.B.; Mutafchieva, M.Z.; Stamenov, N.V.; Kavlakova, L.B.; Tanev, M.Z.; Karaslavova, E.; Tomov, G.T. Randomised Controlled Trial Comparing the Clinical Effectiveness of Mouthwashes Based on Essential Oils, Chlorhexidine, Hydrogen Peroxide and Prebiotic in Gingivitis Treatment. FM 2022, 64, 588–595. [Google Scholar] [CrossRef]
- Cortelli, S.C.; Costa, F.O.; Gargioni-Filho, A.; Aquino, D.R.; Cota, L.O.M.; Scherma, A.P.; Miranda, T.B.; Cortelli, J.R. Impact of Gingivitis Treatment for Diabetic Patients on Quality of Life Related to Periodontal Objective Parameters: A Randomized Controlled Clinical Trial. Arch. Oral Biol. 2018, 86, 80–86. [Google Scholar] [CrossRef]
- Aboonabi, A.; Meyer, R.R.; Singh, I. The Association between Metabolic Syndrome Components and the Development of Atherosclerosis. J. Hum. Hypertens. 2019, 33, 844–855. [Google Scholar] [CrossRef]
- Morovati, A.; Pourghassem Gargari, B.; Sarbakhsh, P.; Azari, H.; Lotfi-Dizaji, L. The Effect of Cumin Supplementation on Metabolic Profiles in Patients with Metabolic Syndrome: A Randomized, Triple Blind, Placebo-controlled Clinical Trial. Phytother. Res. 2019, 33, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Morovati, A.; Pourghassem Gargari, B.; Sarbakhsh, P. Effects of Cumin (Cuminum cyminum L.) Essential Oil Supplementation on Metabolic Syndrome Components: A Randomized, Triple-blind, Placebo-controlled Clinical Trial. Phytother. Res. 2019, 33, 3261–3269. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabravolski, S.A.; Sukhorukov, V.N.; Melnichenko, A.A.; Khotina, V.A.; Orekhov, A.N. Potential Application of the Plant-Derived Essential Oils for Atherosclerosis Treatment: Molecular Mechanisms and Therapeutic Potential. Molecules 2023, 28, 5673. https://doi.org/10.3390/molecules28155673
Dabravolski SA, Sukhorukov VN, Melnichenko AA, Khotina VA, Orekhov AN. Potential Application of the Plant-Derived Essential Oils for Atherosclerosis Treatment: Molecular Mechanisms and Therapeutic Potential. Molecules. 2023; 28(15):5673. https://doi.org/10.3390/molecules28155673
Chicago/Turabian StyleDabravolski, Siarhei A., Vasily N. Sukhorukov, Alexandra A. Melnichenko, Victoria A. Khotina, and Alexander N. Orekhov. 2023. "Potential Application of the Plant-Derived Essential Oils for Atherosclerosis Treatment: Molecular Mechanisms and Therapeutic Potential" Molecules 28, no. 15: 5673. https://doi.org/10.3390/molecules28155673
APA StyleDabravolski, S. A., Sukhorukov, V. N., Melnichenko, A. A., Khotina, V. A., & Orekhov, A. N. (2023). Potential Application of the Plant-Derived Essential Oils for Atherosclerosis Treatment: Molecular Mechanisms and Therapeutic Potential. Molecules, 28(15), 5673. https://doi.org/10.3390/molecules28155673










