An Overview of the Nano-Enhanced Phase Change Materials for Energy Harvesting and Conversion
Abstract
:1. Introduction
2. Preparation Methods of Nano-Enhanced Phase Change Materials
2.1. Microencapsulation Methods
2.2. Dispersion and Sonication
2.3. Autoclave Method
2.4. Gravity Die Casting
2.5. Vacuum Impregnation
3. Phase Change Material Nanofluid Thermophysical Properties
3.1. Thermal Conductivity
3.2. Specific Heat
3.3. Latent Heat
3.4. Viscosity
3.5. Density
3.6. Sub-Cooling and Phase Change Temperature and Time
3.7. Stability of the Nano-Enhanced Phase Change Materials in Acids and Salts
4. Phase Change Influencing Factors
5. Characterization of the Nano-Enhanced Phase Change Materials
6. Thermophysical Properties Modeling
7. Metal–Organic Frameworks and Covalent Organic Frameworks
8. Main Applications of Nano-Enhanced Phase Change Materials
8.1. Historical Background of Nano-Enhanced Phase Change Materials in Energy Applications
8.2. Solar Thermal Energy Storage Systems
8.3. Photovoltaic/Thermal Systems
8.4. Cooling of Electronics
8.5. Water Desalination
8.6. Engine Exhaust Gas Heat Recovery
8.7. Thermal Management of Residential Buildings
- Solar domestic water heating systems;
- Tankless solar water heaters;
- Photovoltaic/thermal;
- Radiant floors;
- Smart double-glazed windows;
- Thermal batteries;
- Radiators.
9. Limitations and Prospects for Further Research
- Further experimental works should be conducted and more accurate preparation routes should be developed for thermal management systems using nano-enhanced phase change materials. In the available published studies, some relevant details are still missing for the sake of the repeatability of results and representative sampling, including the types of base fluids, ideal concentration, optimized synthesis methodologies, safety procedures, and environmental risk assessment.
- Only a few researchers investigating nano-enhanced phase change materials have provided the specific heat capacity determination and discussion. Additionally, the scarce experimental results on the specific heat are not consistent with each other, normally presenting considerable fluctuations. In some of the published studies, the specific heat of the nano-enhanced phase change materials was reported to increase with increasing concentrations of added nanoparticles, and in some others, the opposite trend was verified. Considering this scenario, further investigation studies on specific heat and its influencing factors are recommended.
- Further experimental and numerical works should be conducted to seek further progress in the less-studied areas of application of nano-enhanced phase change materials, such as the cooling of electronics and thermal management of batteries and solar distillers.
- The major concerns about the synthesis, characterization, and employment of the nano-enhanced phase change materials are economic analysis issues. However, the economic viability and investment costs have not been sufficiently assessed and require further in-depth studies.
- It is highly recommended to conduct further analysis on the environmental impact of the nanomaterials incorporated in the nano-enhanced phase change materials to achieve improved knowledge on the subject. Many scientific papers lack the environmental impact in all conceivable stages of the production, use, and final disposal of phase change materials. Also, the available literature does not present extensive guidelines for the safety procedures inherent to the handling, use, and experimental evaluation of these types of materials. Hence, it is strongly suggested to publish the environmental impact evaluation consequences and the description of safety procedures to ensure a safe working environment for the researchers and potential users of the nano-enhanced phase change materials.
- The impact of the added nanoparticles to the base phase change materials on human health is still not yet completely understood. Given this, future experimental works should more intensively explore its potential adverse impacts to establish proper safety guidelines.
- More research studies aiming to develop cost-effective biodegradable nano-enhanced phase change materials should be carried out to produce renewable eco-friendly materials with the ability to be microencapsulated. One potential research path is the analysis of alternative waste-based materials for preparing phase change materials and added nanoparticles.
- Certain issues should be further explored, like the need to lower the cost of the synthesis methods for the nano-enhanced phase change materials and active equipment, and the thermal and long-term stability of these materials to be used in photovoltaic/thermal systems.
- Many of the base phase change materials explored in solar energy storage technology are single-type materials like paraffin wax; consequently, further experimental works involving mixtures of different base phase change materials should be conducted. These works will provide useful insights into the synergistic benefits coming from the improved thermal energy storage capability and stability of such mixtures.
- Most of the studies confirmed that the enhancement route of nano-enhanced phase change materials, incorporating superior thermally conductive nanomaterials, is strongly influenced by their particle shape, size, and constitutive material. Nonetheless, there are considerable discrepancies in the results, which need a better understanding to identify and explain the underlying mechanisms between the nanoparticles and the base phase change materials to infer their impact on the final stability and thermophysical characteristics.
- Diverse research groups should conduct more property measurements to attain the repeatability of the results. Each research team reports their measurements individually, and even though discrepancies are frequently found between the thermophysical properties of the nano-enhanced phase change materials, as they were also often found in the thermophysical properties of the base phase change materials, no vigorous attempts have been made to reproduce those findings. Such an acting mode reduces the accuracy and reliability of the reported results.
- There are limited published data on the thermophysical properties of eutectic mixtures of base phase change materials incorporating nanoparticles. In view of this fact, more research studies are needed on the production and evaluation of nano-enhanced composites containing eutectic base phase change materials.
- Many available techniques to evaluate the stability of nano-enhanced phase change materials are suitable only for small samples, the associated measurement uncertainties are not completely explicit, and there are inconsistent findings in the published data. With these facts in mind, it is suggested to develop, implement, and validate a standardized method to carry out characterization and thermal cycling stability tests on these types of materials.
- Only a small number of available studies have analyzed the heat transfer capability of nano-enhanced phase change materials in real thermal management systems. Though it is vital to determine the thermophysical characteristics of these materials, it is also very important to appraise their thermal behavior within a working real thermal storage system. Also, it is convenient to compare the efficiency of the same real system when using the nano-enhanced phase change materials and when exploring only the base phase change material. Such a procedure will enable a more accurate evaluation of the contribution of the incorporation of the nanoparticles to the thermal performance of the system. Hence, further work on this specific matter is most welcome.
10. Conclusions
- The organic phase change materials are very suitable to be applied in solar energy recovery systems because of their intrinsic beneficial features like improved thermal stability and supercooling absence. Hence, it is foreseen that the improved thermal energy storage equipment and systems using phase change materials will have a major role in the research and technological areas of thermal solar energy harvesting and conversion processes.
- The exploration of the nano-enhanced phase change materials greatly enhances the average daily energy storage capability and considerably extends the operating time of solar thermal energy storage systems.
- It was found that most of the nano-enhanced phase change materials’ applications were in the improvement of thermal energy storage systems. The published experimental and numerical studies dealt with the thermal management of energy storage systems, solar collectors, photovoltaic/thermal systems, and engine exhaust gas heat recovery using nano-enhanced phase change materials.
- The average thermal conductivity values when using the nano-enhanced phase change materials were enhanced by up to 100% as compared with those achieved with the traditional thermal fluids themselves.
- The thermal conductivity of the nano-enhanced phase change materials can be adjusted through the different distribution and orientation of the included nanoparticles. Moreover, the addition of porous nanoparticles may aid in the thermal conductivity of the base phase change materials. The porosity of the nanoparticles will decrease the supercooling degree by the great number of active nucleation sites.
- The graphite and graphene-added nanoparticles were extremely good thermal conductivity enhancers for the diverse base phase change materials. Also, the metallic and metal oxide nanoparticles dispersed in the phase change material are good thermal conductivity enhancers, with the induced improvements dependent on their shape, size, and concentration.
- The different types of incorporated nanoparticles cause diverse enhancements in the solidification of the base phase change materials. It is usually observed that the solidification and melting processes of the nano-enhanced phase change materials are influenced by the concentration of the added nanoparticles. However, when a limiting concentration value is surpassed, it can entail some negative results in other thermophysical properties of the phase change material, namely the viscosity. The excessive viscosity of the nano-enhanced phase change materials deteriorates the heat transfer behavior by decreasing the thermal conductivity. Nonetheless, there is the possibility to add solvents to mitigate the viscous effect.
- The latent heat of the phase change materials normally decreases with increasing concentrations of the included nanoparticles, with a few exceptions. The thermophysical properties of the nanoparticles affect latent heat deterioration. Nowadays, there is still no ideal nanoparticle concentration that causes maximum thermal conductivity enhancement and, simultaneously, minor latent heat deterioration.
- The synergistic employment of distinct heat transfer enhancement procedures like the incorporation of nanoparticles, metallic foams, and finned heating surfaces provides an improved heat transport capability of the nano-enhanced phase change materials as compared to that achieved with only one of the heat transfer procedures.
- The combined usage of the nano-enhanced phase change materials and nanofluids is more effective for the thermal management of photovoltaic/thermal cooling than the separate exploration of the nano-enhanced phase change materials. Such a synergetic route normally provides extra heat dissipation to the panels because the heat is extracted in sequence by the phase change material and nanofluid, which are two highly heat-absorbing media. The combined use of the nano-enhanced phase change material and the nanofluid lowers the surface average temperature and improves the temperature uniformity of the photovoltaic panels. Such effects mainly derive from the uniform contact of the nano-enhanced phase change material with the panels.
- The unconverted incident thermal solar energy in photovoltaic/thermal systems can be stored by the nano-enhanced phase change materials under the form of latent heat, which may reduce the average temperature of the panels by more than 30 °C. Additionally, the adoption of a particular nano-enhanced phase change material should be based on many factors including the environment's typical temperature values and latitude, solar irradiation intensity, and wind velocity, among others, given that its effectiveness is more intense during summer than in winter because it absorbs more heat in summer, leading to increased efficiency.
- It was already demonstrated that photovoltaic/thermal systems cooled with two distinct phase change materials at a time are more efficient compared to that provided by only one phase change material because of the improved heat regulation and surface temperature uniformity.
- It was found in the available literature that the efficiency of photovoltaic/thermal systems operating with water can be improved by more than 30% when the nano-enhanced phase change materials are included. Moreover, the general use of nano-enhanced phase change materials may decrease the consumption of non-renewable fossil fuels for electricity production purposes and may considerably reduce the carbon footprint and greenhouse gases.
- The combined use of metallic foams and fins provokes a heat transfer performance enhancement in the thermal management processes of the systems using nano-enhanced phase change materials. The thermal transport network constructed by the foams’ diverse constitutive materials and the effect of the finned surfaces that increase the heat exchange rate between the included nano-enhanced phase change materials and the solar collection system ameliorate the energy harvesting and conversion processes.
- Only very few published studies analyzed the long-term stability of the nano-enhanced phase change materials in terms of thermal conductivity. These studies reported a substantial thermal conductivity decrement after the completion of only a few thermal cycles.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Nourafkan, E.; Asachi, M.; Jin, H.; Wen, D.; Ahmed, W. Stability and photo-thermal conversion performance of binary nanofluids for solar absorption refrigeration systems. Renew. Energy 2019, 140, 264–273. [Google Scholar] [CrossRef]
- Siddique, A.R.M.; Kratz, F.; Mahmud, S.; Van Heyst, B. Energy Conversion by Nanomaterial-Based Trapezoidal-Shaped Leg of Thermoelectric Generator Considering Convection Heat Transfer Effect. J. Energy Resour. Technol. 2019, 141, 082001. [Google Scholar] [CrossRef]
- Natividade, P.S.G.; Moura, G.D.M.; Avallone, E.; Filho, E.B.; Gelamo, R.V.; Gonçalves, J.C.D.S.I. Experimental analysis applied to an evacuated tube solar collector equipped with parabolic concentrator using multilayer graphene-based nanofluids. Renew. Energy 2019, 138, 152–160. [Google Scholar] [CrossRef]
- Salem, M.; Elsayed, M.; Abd-Elaziz, A.; Elshazly, K. Performance enhancement of the photovoltaic cells using Al2O3/PCM mixture and/or water cooling-techniques. Renew. Energy 2019, 138, 876–890. [Google Scholar] [CrossRef]
- Ding, Y.; Zheng, S.; Meng, X.; Yang, D. Low Salinity Hot Water Injection with Addition of Nanoparticles for Enhancing Heavy Oil Recovery under Reservoir Conditions. Trans. ASME J. Energy Resour. Technol. 2019, 141, 072904. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, J.; Ma, H.; Chen, L.; Li, R.; Jin, P. VO2/Nickel-bromine-ionic liquid composite film for thermochromic application. Sol. Energy Mater. Sol. Cells 2019, 196, 124–130. [Google Scholar] [CrossRef]
- Adelekan, D.S.; Ohunakin, O.S.; Gill, J.; Atayero, A.A.; Diarra, C.D.; Asuzu, E.A. Experimental performance of a safe charge of LPG refrigerant enhanced with varying concentrations of TiO2 nano-lubricant in a domestic refrigerator. J. Therm. Anal. Calorim. 2019, 136, 2439–2448. [Google Scholar] [CrossRef]
- Martín, M.; Villalba, A.; Fernandez, A.I.; Barreneche, C. Development of new nano-enhanced phase change materials (NEPCM) to improve energy efficiency in buildings: Lab-scale characterization. Energy Build. 2019, 192, 75–83. [Google Scholar] [CrossRef]
- Ren, Q.; Xu, H.; Luo, Z. PCM charging process accelerated with combination of optimized triangle fins and nanoparticles. Int. J. Therm. Sci. 2019, 140, 466–479. [Google Scholar] [CrossRef]
- Balakin, B.V.; Zhdaneev, O.; Kosinska, A.; Kutsenko, K.V. Direct absorption solar collector with magnetic nanofluid: CFD model and parametric analysis. Renew. Energy 2019, 136, 23–32. [Google Scholar] [CrossRef]
- Bonab, H.B.; Javani, N. Investigation and optimization of solar volumetric absorption systems using nanoparticles. Sol. Energy Mater. Sol. Cells 2019, 194, 229–234. [Google Scholar] [CrossRef]
- Kumar, G.; Mathimani, T.; Rene, E.R.; Pugazhendhi, A. Application of nanotechnology in dark fermentation for enhanced biohydrogen production using inorganic nanoparticles. Int. J. Hydrogen Energy 2019, 44, 13106–13113. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, Z.; Maharjan, A. Numerical investigation of coupled optical-electrical-thermal processes for plasmonic solar cells at various angles of incident irradiance. Energy 2019, 174, 110–121. [Google Scholar] [CrossRef]
- Zayed, M.; Zhao, J.; Du, Y.; Kabeel, A.; Shalaby, S. Factors affecting the thermal performance of the flat plate solar collector using nanofluids: A review. Sol. Energy 2019, 182, 382–396. [Google Scholar] [CrossRef]
- Abdelrazik, A.S.; Al-Sulaiman, F.; Saidur, R.; Ben-Mansour, R. Evaluation of the effects of optical filtration and nanoPCM on the performance of a hybrid photovoltaic-thermal solar collector. Energy Convers. Manag. 2019, 195, 139–156. [Google Scholar] [CrossRef]
- Agresti, F.; Fedele, L.; Rossi, S.; Cabaleiro, D.; Bobbo, S.; Ischia, G.; Barison, S. Nano-encapsulated PCM emulsions prepared by a solvent-assisted method for solar applications. Sol. Energy Mater. Sol. Cells 2019, 194, 268–275. [Google Scholar] [CrossRef]
- Rehman, T.U.; Ali, H.M.; Janjua, M.M.; Sajjad, U.; Yan, W.-M. A critical review on heat transfer augmentation of phase change materials embedded with porous materials/foams. Int. J. Heat Mass Transf. 2019, 135, 649–673. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Mahian, O. Enhancement of PCM solidification using inorganic nanoparticles and an external magnetic field with application in energy storage systems. J. Clean. Prod. 2019, 215, 963–977. [Google Scholar] [CrossRef]
- Punniakodi, B.M.S.; Senthil, R. Recent developments in nano-enhanced phase change materials for solar thermal storage. Sol. Energy Mater. Sol. Cells 2022, 238, 111629. [Google Scholar] [CrossRef]
- Kibria, M.; Anisur, M.; Mahfuz, M.; Saidur, R.; Metselaar, I. A review on thermophysical properties of nanoparticle dispersed phase change materials. Energy Convers. Manag. 2015, 95, 69–89. [Google Scholar] [CrossRef] [Green Version]
- Leong, K.Y.; Abdul Rahman, M.R.; Gurunathan, B.A. Nano-enhanced phase change materials: A review of thermo-physical properties, applications and challenges. J. Energy Storage 2019, 21, 18–31. [Google Scholar] [CrossRef]
- Teggar, M.; Arıcı, M.; Mert, M.S.; Ajarostaghi, S.S.M.; Niyas, H.; Tunçbilek, E.; Ismail, K.A.R.; Younsi, Z.; Benhouia, A.T.; Mezaache, E.H. A comprehensive review of micro/nano enhanced phase change materials. J. Therm. Anal. Calorim. 2021, 147, 3989–4016. [Google Scholar] [CrossRef]
- De Matteis, V.; Cannavale, A.; Martellotta, F.; Rinaldi, R.; Calcagnile, P.; Ferrari, F.; Ayr, U.; Fiorito, F. Nano-encapsulation of phase change materials: From design to thermal performance, simulations and toxicological assessment. Energy Build. 2019, 188–189, 1–11. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.M.; Farid, M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y. Investigation of specific heat and latent heat enhancement in hydrate salt based TiO2 nanofluid phase change material. Appl. Therm. Eng. 2017, 124, 533–538. [Google Scholar] [CrossRef]
- Munyalo, J.M.; Zhang, X.; Li, Y.; Chen, Y.; Xu, X. Latent heat of fusion prediction for nanofluid based phase change material. Appl. Therm. Eng. 2018, 130, 1590–1597. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, W.; Li, D.; Qi, H.; Wang, F.; Arici, M. Experimental investigation of thermal radiative properties of Al2O3-paraffin nanofluid. Sol. Energy 2019, 177, 420–426. [Google Scholar] [CrossRef]
- Warzoha, R.J.; Rao, A.; Weigand, R.; Fleischer, A.S. Experimental characterization of the thermal diffusivity of paraffin phase change material embedded with herring-bone style graphite nanofibers. Heat Transf. Summer Conf. Am. Soc. Mech. Eng. 2012, 44786, 307–315. [Google Scholar]
- Nourani, M.; Hamdami, N.; Keramat, J.; Moheb, A.; Shahedi, M. Thermal behavior of paraffin-nano-Al2O3 stabilized by sodium stearoyl lactylate as a stable phase change material with high thermal conductivity. Renew. Energy 2016, 88, 474–482. [Google Scholar] [CrossRef]
- Motahar, S.; Alemrajabi, A.A.; Khodabandeh, R. Experimental study on solidification process of a phase change material containing TiO2 nanoparticles for thermal energy storage. Energy Convers. Manag. 2017, 138, 162–170. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; Fang, X.; Zhang, Z. Graphite nanoparticles-dispersed paraffin/water emulsion with enhanced thermal-physical property and photo-thermal performance. Sol. Energy Mater. Sol. Cells 2016, 147, 101–107. [Google Scholar] [CrossRef]
- Chieruzzi, M.; Cerritelli, G.F.; Miliozzi, A.; Kenny, J.M.; Torre, L. Heat capacity of nanofluids for solar energy storage produced by dispersing oxide nanoparticles in nitrate salt mixture directly at high temperature. Sol. Energy Mater. Sol. Cells 2017, 167, 60–69. [Google Scholar] [CrossRef]
- Ebadi, S.; Tasnim, S.H.; Aliabadi, A.A.; Mahmud, S. Geometry and nanoparticle loading effects on the bio-based nano-PCM filled cylindrical thermal energy storage system. Appl. Therm. Eng. 2018, 141, 724–740. [Google Scholar] [CrossRef]
- Salyan, S.; Suresh, S. Study of thermo-physical properties and cycling stability of d -Mannitol-copper oxide nanocomposites as phase change materials. J. Energy Storage 2018, 15, 245–255. [Google Scholar] [CrossRef]
- Praveen, B.; Suresh, S. Experimental study on heat transfer performance of neopentyl glycol/CuO composite solid-solid PCM in TES based heat sink. Eng. Sci. Technol. Int. J. 2018, 21, 1086–1094. [Google Scholar] [CrossRef]
- Zeng, J.-L.; Gan, J.; Zhu, F.-R.; Yu, S.-B.; Xiao, Z.-L.; Yan, W.-P.; Zhu, L.; Liu, Z.-Q.; Sun, L.-X.; Cao, Z. Tetradecanol/expanded graphite composite form-stable phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2014, 127, 122–128. [Google Scholar] [CrossRef]
- Mandal, S.K.; Kumar, S.; Singh, P.K.; Mishra, S.K.; Singh, D. Performance investigation of nanocomposite based solar water heater. Energy 2020, 198, 117295. [Google Scholar] [CrossRef]
- Kim, S.; Chang, S.J.; Chung, O.; Jeong, S.-G.; Kim, S. Thermal characteristics of mortar containing hexadecane/xGnP SSPCM and energy storage behaviors of envelopes integrated with enhanced heat storage composites for energy efficient buildings. Energy Build. 2014, 70, 472–479. [Google Scholar] [CrossRef]
- Colla, L.; Fedele, L.; Mancin, S.; Danza, L.; Manca, O. Nano-PCMs for enhanced energy storage and passive cooling applications. Appl. Therm. Eng. 2017, 110, 584–589. [Google Scholar] [CrossRef]
- Harish, S.; Orejon, D.; Takata, Y.; Kohno, M. Thermal conductivity enhancement of lauric acid phase change nanocomposite with graphene nanoplatelets. Appl. Therm. Eng. 2015, 80, 205–211. [Google Scholar] [CrossRef]
- Sarı, A.; Biçer, A.; Hekimoğlu, G. Effects of carbon nanotubes additive on thermal conductivity and thermal energy storage properties of a novel composite phase change material. J. Compos. Mater. 2018, 53, 2967–2980. [Google Scholar] [CrossRef]
- Yadav, A.; Barman, B.; Kardam, A.; Narayanan, S.S.; Verma, A.; Jain, V. Thermal properties of nano-graphite-embedded magnesium chloride hexahydrate phase change composites. Energy Environ. 2017, 28, 651–660. [Google Scholar] [CrossRef]
- Sharma, R.; Ganesan, P.; Tyagi, V.; Metselaar, H.; Sandaran, S. Thermal properties and heat storage analysis of palmitic acid-TiO2 composite as nano-enhanced organic phase change material (NEOPCM). Appl. Therm. Eng. 2016, 99, 1254–1262. [Google Scholar] [CrossRef]
- Lin, S.C.; Al-Kayiem, H.H. Evaluation of copper nanoparticles—Paraffin wax compositions for solar thermal energy storage. Sol. Energy 2016, 132, 267–278. [Google Scholar] [CrossRef]
- Sami, S.; Etesami, N. Improving thermal characteristics and stability of phase change material containing TiO2 nanoparticles after thermal cycles for energy storage. Appl. Therm. Eng. 2017, 124, 346–352. [Google Scholar] [CrossRef]
- Barreneche, C.; Mondragon, R.; Ventura-Espinosa, D.; Mata, J.; Cabeza, L.F.; Fernández, A.I.; Julia, J.E. Influence of nanoparticle morphology and its dispersion ability regarding thermal properties of water used as phase change material. Appl. Therm. Eng. 2018, 128, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Harikrishnan, S.; Hussain, S.I.; Devaraju, A.; Sivasamy, P.; Kalaiselvam, S. Improved performance of a newly prepared nano-enhanced phase change material for solar energy storage. J. Mech. Sci. Technol. 2017, 31, 4903–4910. [Google Scholar] [CrossRef]
- Mayilvelnathan, V.; Arasu, A.V. Characterization and thermophysical properties of graphene nanoparticles dispersed erythritol PCM for medium temperature thermal energy storage applications. Thermochim. Acta 2019, 676, 94–103. [Google Scholar] [CrossRef]
- Putra, N.; Amin, M.; Kosasih, E.A.; Luanto, R.A.; Abdullah, N.A. Characterization of the thermal stability of RT 22 HC/graphene using a thermal cycle method based on thermoelectric methods. Appl. Therm. Eng. 2017, 124, 62–70. [Google Scholar] [CrossRef]
- Wang, J.; Xie, H.; Guo, Z.; Guan, L.; Li, Y. Improved thermal properties of paraffin wax by the addition of TiO2 nanoparticles. Appl. Therm. Eng. 2014, 73, 1541–1547. [Google Scholar] [CrossRef]
- Guo, S.; Liu, Q.; Zhao, J.; Jin, G.; Wang, X.; Lang, Z.; He, W.; Gong, Z. Evaluation and comparison of erythritol-based composites with addition of expanded graphite and carbon nanotubes. Appl. Energy 2017, 205, 703–709. [Google Scholar] [CrossRef]
- Wang, J.; Xie, H.; Xin, Z. Thermal properties of paraffin based composites containing multi-walled carbon nanotubes. Thermochim. Acta 2009, 488, 39–42. [Google Scholar] [CrossRef]
- Shaikh, S.; Lafdi, K.; Hallinan, K. Carbon nanoadditives to enhance latent energy storage of phase change materials. J. Appl. Phys. 2008, 103, 094302. [Google Scholar] [CrossRef]
- Mohamed, N.H.; Soliman, F.S.; El Maghraby, H.; Moustfa, Y. Thermal conductivity enhancement of treated petroleum waxes, as phase change material, by α nano alumina: Energy storage. Renew. Sustain. Energy Rev. 2017, 70, 1052–1058. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Li, C.; Zhang, B.; Xie, B.; Zhao, X.; Chen, J.; Chen, Z.; Long, Y. Stearic acid/expanded graphite as a composite phase change thermal energy storage material for tankless solar water heater. Sustain. Cities Soc. 2018, 44, 458–464. [Google Scholar] [CrossRef]
- Zeng, J.L.; Cao, Z.; Yang, D.W.; Xu, F.; Sun, L.X.; Zhang, X.F.; Zhang, L. Effects of MWNTs on phase change enthalpy and thermal conductivity of a solid-liquid organic PCM. J. Therm. Anal. Calorim. 2009, 95, 507–512. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, H.; Sun, L.; Xu, F.; Jiao, Q.; Zhao, Z.; Zhang, J.; Zhou, H.; Sawada, Y.; Liu, Y. Preparation and thermal properties of fatty acids/CNTs composite as shape-stabilized phase change materials. J. Therm. Anal. Calorim. 2013, 111, 377–384. [Google Scholar] [CrossRef]
- Wu, S.; Zhu, D.; Zhang, X.; Huang, J. Preparation and Melting/Freezing Characteristics of Cu/Paraffin Nanofluid as Phase-Change Material (PCM). Energy Fuels 2010, 24, 1894–1898. [Google Scholar] [CrossRef]
- Parameshwaran, R.; Jayavel, R.; Kalaiselvam, S. Study on thermal properties of organic ester phase-change material embedded with silver nanoparticles. J. Therm. Anal. Calorim. 2013, 114, 845–858. [Google Scholar] [CrossRef]
- Ho, C.; Gao, J. Preparation and thermophysical properties of nanoparticle-in-paraffin emulsion as phase change material. Int. Commun. Heat Mass Transf. 2009, 36, 467–470. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, J.; Song, G.; Liu, Y.; Tang, G. The experimental exploration of nano-Si3N4/paraffin on thermal behavior of phase change materials. Thermochim. Acta 2014, 597, 101–106. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Q.; Medina, M.A.; Lee, K.O. On the Natural Convection Enhancement of Heat Transfer during Phase Transition Processes of Solid-liquid Phase Change Materials (PCMs). Energy Procedia 2014, 61, 2062–2065. [Google Scholar] [CrossRef] [Green Version]
- Brinkman, H.C. The Viscosity of Concentrated Suspensions and Solutions. J. Chem. Phys. 1952, 20, 571. [Google Scholar] [CrossRef]
- He, Q.; Wang, S.; Tong, M.; Liu, Y. Experimental study on thermophysical properties of nanofluids as phase-change material (PCM) in low temperature cool storage. Energy Convers. Manag. 2012, 64, 199–205. [Google Scholar] [CrossRef]
- Jesumathy, S.; Udayakumar, M.; Suresh, S. Experimental study of enhanced heat transfer by addition of CuO nanoparticle. Heat Mass Transf. 2011, 48, 965–978. [Google Scholar] [CrossRef]
- Bahiraei, F.; Fartaj, A.; Nazri, G.-A. Experimental and numerical investigation on the performance of carbon-based nanoenhanced phase change materials for thermal management applications. Energy Convers. Manag. 2017, 153, 115–128. [Google Scholar] [CrossRef]
- Daneshazarian, R.; Antoun, S.; Dworkin, S.B. Performance Assessment of Nano-enhanced Phase Change Material for Thermal Storage. Int. J. Heat Mass Transf. 2021, 173, 121256. [Google Scholar] [CrossRef]
- Xu, H.; Sze, J.Y.; Romagnoli, A.; Py, X. Selection of Phase Change Material for Thermal Energy Storage in Solar Air Conditioning Systems. Energy Procedia 2017, 105, 4281–4288. [Google Scholar] [CrossRef]
- Mjallal, I.; Farhat, H.; Hammoud, M.; Ali, S.; Assi, I. Improving the Cooling Efficiency of Heat Sinks through the Use of Different Types of Phase Change Materials. Technologies 2018, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Owolabi, A.L.; Al-Kayiem, H.H.; Baheta, A.T. Nanoadditives induced enhancement of the thermal properties of paraffin-based nanocomposites for thermal energy storage. Sol. Energy 2016, 135, 644–653. [Google Scholar] [CrossRef]
- Abdelrazik, A.; Al-Sulaiman, F.; Saidur, R. Numerical investigation of the effects of the nano-enhanced phase change materials on the thermal and electrical performance of hybrid PV/thermal systems. Energy Convers. Manag. 2020, 205, 112449. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, Y.; Alelyani, S.; Cao, X.; Phelan, P.E. Thermophysical properties enhancement of ternary carbonates with carbon materials for high-temperature thermal energy storage. Sol. Energy 2017, 155, 661–669. [Google Scholar] [CrossRef]
- Wu, X.; Wu, H.; Cheng, P. Pressure drop and heat transfer of Al2O3-H2O nanofluids through silicon microchannels. J. Micromech. Microeng. 2009, 19, 105020. [Google Scholar] [CrossRef]
- Safari, A.; Saidur, R.; Sulaiman, F.; Xu, Y.; Dong, J. A review on supercooling of Phase Change Materials in thermal energy storage systems. Renew. Sustain. Energy Rev. 2017, 70, 905–919. [Google Scholar] [CrossRef]
- Kumar, K.R.S.; Kalaiselvam, S. Experimental investigations on the thermophysical properties of CuO-palmitic acid phase change material for heating applications. J. Therm. Anal. Calorim. 2017, 129, 1647–1657. [Google Scholar] [CrossRef]
- Venkitaraj, K.P.; Suresh, S.; Praveen, B.; Venugopal, A.; Nair, S.C. Pentaerythritol with alumina nano additives for thermal energy storage applications. J. Energy Storage 2017, 13, 359–377. [Google Scholar] [CrossRef]
- Saeed, R.M.; Schlegel, J.; Castano, C.; Sawafta, R. Preparation and enhanced thermal performance of novel (solid to gel) form-stable eutectic PCM modified by nano-graphene platelets. J. Energy Storage 2018, 15, 91–102. [Google Scholar] [CrossRef]
- Warzoha, R.J.; Fleischer, A.S. Improved heat recovery from paraffin-based phase change materials due to the presence of percolating graphene networks. Int. J. Heat Mass Transf. 2014, 79, 314–323. [Google Scholar] [CrossRef]
- Rufuss, D.D.W.; Suganthi, L.; Iniyan, S.; Davies, P. Effects of nanoparticle-enhanced phase change material (NPCM) on solar still productivity. J. Clean. Prod. 2018, 192, 9–29. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-D.; Zhou, Y.-G.; Tong, M.-W.; Zhou, X.-S. Experimental study of thermal conductivity and phase change performance of nanofluids PCMs. Microfluid. Nanofluidics 2009, 7, 579–584. [Google Scholar] [CrossRef]
- Hu, P.; Lu, D.-J.; Fan, X.-Y.; Zhou, X.; Chen, Z.-S. Phase change performance of sodium acetate trihydrate with AlN nanoparticles and CMC. Sol. Energy Mater. Sol. Cells 2011, 95, 2645–2649. [Google Scholar] [CrossRef]
- Cui, W.; Yuan, Y.; Sun, L.; Cao, X.; Yang, X. Experimental studies on the supercooling and melting/freezing characteristics of nano-copper/sodium acetate trihydrate composite phase change materials. Renew. Energy 2016, 99, 1029–1037. [Google Scholar] [CrossRef]
- Soni, V.; Kumar, A.; Jain, V. Performance evaluation of nano-enhanced phase change materials during discharge stage in waste heat recovery. Renew. Energy 2018, 127, 587–601. [Google Scholar] [CrossRef]
- Babapoor, A.; Karimi, G. Thermal properties measurement and heat storage analysis of paraffin nanoparticles composites phase change material: Comparison and optimization. Appl. Therm. Eng. 2015, 90, 945–951. [Google Scholar] [CrossRef]
- Srinivasan, S.; Diallo, M.S.; Saha, S.K.; Abass, O.A.; Sharma, A.; Balasubramanian, G. Effect of temperature and graphite particle fillers on thermal conductivity and viscosity of phase change material n-eicosane. Int. J. Heat Mass Transf. 2017, 114, 318–323. [Google Scholar] [CrossRef]
- Pahamli, Y.; Hosseini, M.; Ranjbar, A.; Bahrampoury, R. Effect of nanoparticle dispersion and inclination angle on melting of PCM in a shell and tube heat exchanger. J. Taiwan Inst. Chem. Eng. 2017, 81, 316–334. [Google Scholar] [CrossRef]
- Weigand, R.; Hess, K.; Fleischer, A.S. Experimental Analysis of the Impact of Nanoinclusions and Surfactants on the Viscosity of Paraffin-Based Energy Storage Materials. J. Heat Transf. 2018, 140, 114502. [Google Scholar] [CrossRef]
- Kumar, M.S.; Krishna, V.M. Experimental investigation on performance of hybrid PCM’s on addition of nano particles in thermal energy storage. Mater Today Proc. 2019, 17, 271–276. [Google Scholar] [CrossRef]
- Paul, J.; Samykano, M.; Pandey, A.K.; Kadirgama, K.; Tyagi, V.V. Nano Engineered Paraffin-Based Phase Change Material for Building Thermal Management. Buildings 2023, 13, 900. [Google Scholar] [CrossRef]
- Marcos, M.A.; Cabaleiro, D.; Guimarey, M.J.G.; Comuñas, M.J.P.; Fedele, L.; Fernández, J.; Lugo, L. PEG 400-Based Phase Change Materials Nano-Enhanced with Functionalized Graphene Nanoplatelets. Nanomaterials 2017, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- George, M.; Pandey, A.; Rahim, N.A.; Tyagi, V.; Shahabuddin, S.; Saidur, R. A novel polyaniline (PANI)/ paraffin wax nano composite phase change material: Superior transition heat storage capacity, thermal conductivity and thermal reliability. Sol. Energy 2020, 204, 448–458. [Google Scholar] [CrossRef]
- Zhou, D.; Yuan, J.; Zhou, Y.; Liu, Y. Preparation and characterization of myristic acid/expanded graphite composite phase change materials for thermal energy storage. Sci. Rep. 2020, 10, 10889. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Q.; Wen, X.; Yin, H.; Liu, J. A novel CNT encapsulated phase change material with enhanced thermal conductivity and photo-thermal conversion performance. Sol. Energy Mater. Sol. Cells 2018, 184, 82–90. [Google Scholar] [CrossRef]
- Al-Gebory, L.; Mengüç, M.P. The effect of pH on particle agglomeration and optical properties of nanoparticle suspensions. J. Quant. Spectrosc. Radiat. Transf. 2018, 219, 46–60. [Google Scholar] [CrossRef]
- Mondragón, R.; Juliá, J.E.; Cabedo, L.; Navarrete, N. On the relationship between the specific heat enhancement of salt-based nanofluids and the ionic exchange capacity of nanoparticles. Sci. Rep. 2018, 8, 7532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Li, G.; Li, T.; Zeng, M.; Sundén, B. Effect of various surfactants on stability and thermophysical properties of nanofluids. J. Therm. Anal. Calorim. 2021, 143, 4057–4070. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, X.; Hu, P.; Hu, G. Study on the supercooling degree and nucleation behavior of water-based graphene oxide nanofluids PCM. Int. J. Refrig. 2015, 50, 80–86. [Google Scholar] [CrossRef]
- Sandy, K.; Chantal, M.; Khalil, E.K.; Flavia, K. Examination and optimization of the design parameters for the thermal hysteresis phenomenon of the phase change material. In Proceedings of the 2021 IEEE 3rd International Multidisciplinary Conference on Engineering Technology (IMCET), Beirut, Lebanon, 8–10 December 2021; pp. 167–172. [Google Scholar]
- Moreles, E.; Huelsz, G.; Barrios, G. Hysteresis effects on the thermal performance of building envelope PCM-walls. Build. Simul. 2018, 11, 519–531. [Google Scholar] [CrossRef]
- Delcroix, B.; Kummert, M.; Daoud, A.; Bouchard, J. Influence of experimental conditions on measured thermal properties used to model phase change materials. Build. Simul. 2015, 8, 637–650. [Google Scholar] [CrossRef]
- Hsu, T.-H.; Chung, C.-H.; Chung, F.-J.; Chang, C.-C.; Lu, M.-C.; Chueh, Y.-L. Thermal hysteresis in phase-change materials: Encapsulated metal alloy core-shell microparticles. Nano Energy 2018, 51, 563–570. [Google Scholar] [CrossRef]
- Anand, A.; Shukla, A.; Kumar, A.; Buddhi, D.; Sharma, A. Cycle test stability and corrosion evaluation of phase change materials used in thermal energy storage systems. J. Energy Storage 2021, 39, 102664. [Google Scholar] [CrossRef]
- Yang, X.; Yuan, Y.; Zhang, N.; Cao, X.; Liu, C. Preparation and properties of myristic–palmitic–stearic acid/expanded graphite composites as phase change materials for energy storage. Sol. Energy 2014, 99, 259–266. [Google Scholar] [CrossRef]
- Pielichowska, K.; Bieda, J.; Szatkowski, P. Polyurethane/graphite nano-platelet composites for thermal energy storage. Renew. Energy 2016, 91, 456–465. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sarı, A. Preparation, thermal properties and thermal reliability of eutectic mixtures of fatty acids/expanded vermiculite as novel form-stable composites for energy storage. J. Ind. Eng. Chem. 2010, 16, 767–773. [Google Scholar] [CrossRef]
- Mullangi, D.; Dhavale, V.; Shalini, S.; Nandi, S.; Collins, S.; Woo, T.; Kurungot, S.; Vaidhyanathan, R. Low-Overpotential Electrocatalytic Water Splitting with Noble-Metal-Free Nanoparticles Supported in a sp3N-Rich Flexible COF. Adv. Energy Mater. 2016, 6, 1600110. [Google Scholar] [CrossRef]
- Mullangi, D.; Shalini, S.; Nandi, S.; Choksi, B.; Vaidhyanathan, R. Super-hydrophobic covalent organic frameworks for chemical resistant coatings and hydrophobic paper and textile composites. J. Mater. Chem. A 2017, 5, 8376–8384. [Google Scholar] [CrossRef]
- Evans, H.A.; Mullangi, D.; Deng, Z.; Wang, Y.; Peh, S.B.; Wei, F.; Wang, J.; Brown, C.M.; Zhao, D.; Canepa, P.; et al. Aluminum formate, Al(HCOO)3: An earth-abundant, scalable, and highly selective material for CO2 capture. Sci. Adv. 2022, 8, eade1473. [Google Scholar] [CrossRef]
- Khodadadi, J.; Hosseinizadeh, S. Nanoparticle-enhanced phase change materials (NEPCM) with great potential for improved thermal energy storage. Int. Commun. Heat Mass Transf. 2007, 34, 534–543. [Google Scholar] [CrossRef]
- Vajjha, R.S.; Das, D.K. Experimental determination of thermal conductivity of three nanofluids and development of new correlations. Int. J. Heat Mass Transf. 2009, 52, 4675–4682. [Google Scholar] [CrossRef]
- Gunjo, D.G.; Jena, S.R.; Mahanta, P.; Robi, P. Melting enhancement of a latent heat storage with dispersed Cu, CuO and Al2O3 nanoparticles for solar thermal application. Renew. Energy 2018, 121, 652–665. [Google Scholar] [CrossRef]
- Iachachene, F.; Haddad, Z.; Oztop, H.F.; Abu-Nada, E. Melting of phase change materials in a trapezoidal cavity: Orientation and nanoparticles effects. J. Mol. Liq. 2019, 292, 110592. [Google Scholar] [CrossRef]
- Gorzin, M.; Hosseini, M.J.; Rahimi, M.; Bahrampoury, R. Nano-enhancement of phase change material in a shell and multi-PCM-tube heat exchanger. J. Energy Storage 2019, 22, 88–97. [Google Scholar] [CrossRef]
- Keshteli, A.N.; Sheikholeslami, M. Solidification within a wavy triplex-tube heat storage unit utilizing numerical simulation considering Al2O3 nanoparticles. Phys. A Stat. Mech. Appl. 2019, 550, 123944. [Google Scholar] [CrossRef]
- Haddad, Z.; Abu-Nada, E.; Oztop, H.F.; Mataoui, A. Natural convection in nanofluids: Are the thermophoresis and Brownian motion effects significant in nanofluid heat transfer enhancement? Int. J. Term. Sci. 2012, 57, 152–162. [Google Scholar] [CrossRef]
- Buongiorno, J. Convective Transport in Nanofluids. J. Heat Transfer. 2006, 128, 240–250. [Google Scholar] [CrossRef]
- Riahi, M.K.; Ali, M.; Addad, Y.; Abu-Nada, E. Combined Newton–Raphson and streamlines-upwind Petrov–Galerkin it-erations for nanoparticles transport in buoyancy-driven flow. J. Eng. Math. 2022, 132, 22. [Google Scholar] [CrossRef]
- Amidu, M.A.; Addad, Y.; Riahi, M.K.; Abu-Nada, E. Numerical investigation of nanoparticles slip mechanisms impact on the natural convection heat transfer characteristics of nanofluids in an enclosure. Sci. Rep. 2021, 11, 15678. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2011, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Farha, O.K.; Wilmer, C.E.; Eryazici, I.; Hauser, B.G.; Parilla, P.A.; O’neill, K.; Sarjeant, A.A.; Nguyen, S.T.; Snurr, R.Q.; Hupp, J.T. Designing Higher Surface Area Metal–Organic Frameworks: Are Triple Bonds Better Than Phenyls? J. Am. Chem. Soc. 2012, 134, 9860–9863. [Google Scholar] [CrossRef] [Green Version]
- Dey, C.; Kundu, T.; Banerjee, R. Reversible phase transformation in proton conducting Strandberg-type POM based metal organic material. Chem. Commun. 2011, 48, 266–268. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Kim, J.; Ahn, W.-S. Synthesis of metal-organic frameworks: A mini review. Korean J. Chem. Eng. 2013, 30, 1667–1680. [Google Scholar] [CrossRef]
- Kim, J.; Yang, S.-T.; Choi, S.B.; Sim, J.; Kim, J.; Ahn, W.-S. Control of catenation in CuTATB-n metal–organic frameworks by sonochemical synthesis and its effect on CO2 adsorption. J. Mater. Chem. 2011, 21, 3070–3076. [Google Scholar] [CrossRef]
- Khan, N.A.; Haque, E.; Jhung, S.H. Rapid syntheses of a metal–organic framework material Cu3(BTC)2(H2O)3 under microwave: A quantitative analysis of accelerated syntheses. Phys. Chem. Chem. Phys. 2010, 12, 2625–2631. [Google Scholar] [CrossRef] [PubMed]
- Moh, P.Y.; Cubillas, P.; Anderson, M.W.; Attfield, M.P. Revelation of the Molecular Assembly of the Nanoporous Metal Organic Framework ZIF-8. J. Am. Chem. Soc. 2011, 133, 13304–13307. [Google Scholar] [CrossRef]
- Radhakrishnan, L.; Reboul, J.; Furukawa, S.; Srinivasu, P.; Kitagawa, S.; Yamauchi, Y. Preparation of Microporous Carbon Fibers through Carbonization of Al-Based Porous Coordination Polymer (Al-PCP) with Furfuryl Alcohol. Chem. Mater. 2011, 23, 1225–1231. [Google Scholar] [CrossRef]
- Xu, X.; Cao, R.; Jeong, S.; Cho, J. Spindle-like Mesoporous α-Fe2O3 Anode Material Prepared from MOF Template for High-Rate Lithium Batteries. Nano Lett. 2012, 12, 4988–4991. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Goenaga, G.A.; Call, A.V.; Liu, D.-J. Cobalt Imidazolate Framework as Precursor for Oxygen Reduction Reaction Electrocatalysts. Chem. Eur. J. 2011, 17, 2063–2067. [Google Scholar] [CrossRef]
- Maity, R.; Chakraborty, D.; Nandi, S.; Yadav, A.K.; Mullangi, D.; Vinod, C.P.; Vaidhyanathan, R. Aqueous-Phase Differentiation and Speciation of Fe3+ and Fe2+ Using Water-Stable Photoluminescent Lanthanide-Based Metal–Organic Framework. ACS Appl. Nano Mater. 2019, 2, 5169–5178. [Google Scholar] [CrossRef]
- Huang, N.; Zhai, L.; Coupry, D.E.; Addicoat, M.A.; Okushita, K.; Nishimura, K.; Heine, T.; Jiang, D. Multiple-component covalent organic frameworks. Nat. Commun. 2016, 7, 12325. [Google Scholar] [CrossRef]
- Guan, X.; Chen, F.; Fang, Q.; Qiu, S. Design and applications of three dimensional covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 1357–1384. [Google Scholar] [CrossRef]
- Mullangi, D.; Chakraborty, D.; Pradeep, A.; Koshti, V.; Vinod, C.P.; Panja, S.; Nair, S.; Vaidhyanathan, R. Highly Stable COF-Supported Co/Co(OH)2 Nanoparticles Heterogeneous Catalyst for Reduction of Nitrile/Nitro Compounds under Mild Conditions. Small 2018, 14, e1801233. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Mullangi, D.; Chandran, C.; Vaidhyanathan, R. Nanopores of a Covalent Organic Framework: A Customizable Vessel for Organocatalysis. ACS Omega 2022, 7, 15275–15295. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, W.; Zeng, Y.; Luo, Z. Simultaneous enhancement of latent heat and thermal conductivity of docosane-based phase change material in the presence of spongy graphene. Sol. Energy Mater. Sol. Cells 2014, 128, 48–51. [Google Scholar] [CrossRef]
- Al-Jethelah, M.; Ebadi, S.; Venkateshwar, K.; Tasnim, S.; Mahmud, S.; Dutta, A. Charging nanoparticle enhanced bio-based PCM in open cell metallic foams: An experimental investigation. Appl. Therm. Eng. 2018, 148, 1029–1042. [Google Scholar] [CrossRef]
- Masoumi, H.; Khoshkhoo, R.H.; Mirfendereski, S.M. Modification of physical and thermal characteristics of stearic acid as a phase change materials using TiO2-nanoparticles. Thermochim. Acta 2019, 675, 9–17. [Google Scholar] [CrossRef]
- Sivasamy, P.; Harikrishnan, S.; Hussain, S.I.; Kalaiselvam, S.; Babu, L.G. Improved thermal characteristics of Ag nanoparticles dispersed myristic acid as composite for low temperature thermal energy storage. Mater. Res. Express 2019, 6, 085066. [Google Scholar] [CrossRef]
- Song, S.; Qiu, F.; Zhu, W.; Guo, Y.; Zhang, Y.; Ju, Y.; Feng, R.; Liu, Y.; Chen, Z.; Zhou, J.; et al. Polyethylene glycol/halloysite@Ag nanocomposite PCM for thermal energy storage: Simultaneously high latent heat and enhanced thermal conductivity. Sol. Energy Mater. Sol. Cells 2019, 193, 237–245. [Google Scholar] [CrossRef]
- Li, T.; Wu, D.; He, F.; Wang, R. Experimental investigation on copper foam/hydrated salt composite phase change material for thermal energy storage. Int. J. Heat Mass Transf. 2017, 115, 148–157. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, M.; Fan, J.; Li, L.; Xu, T.; Yuan, W. Thermal conductivity enhancement of hydrated salt phase change materials employing copper foam as the supporting material. Sol. Energy Mater. Sol. Cells 2019, 199, 91–98. [Google Scholar] [CrossRef]
- Sheng, N.; Dong, K.; Zhu, C.; Akiyama, T.; Nomura, T. Thermal conductivity enhancement of erythritol phase change material with percolated aluminum filler. Mater. Chem. Phys. 2019, 229, 87–91. [Google Scholar] [CrossRef]
- Wei, H.; Li, X. Preparation and characterization of a lauric-myristic-stearic acid/Al2O3-loaded expanded vermiculite composite phase change material with enhanced thermal conductivity. Sol. Energy Mater. Sol. Cells 2017, 166, 1–8. [Google Scholar] [CrossRef]
- Singh, D.; Salyan, S. Thermo-chemical stability of nano composite myo- inositol for solar thermal energy storage. In Proceedings of the International Conference on Recent Trends in Engineering & Technology, CUSAT, Kerala, India, 18–20 January 2016. [Google Scholar]
- Abdelrahman, H.; Wahba, M.; Refaey, H.; Moawad, M.; Berbish, N. Performance enhancement of photovoltaic cells by changing configuration and using PCM (RT35HC) with nanoparticles Al2O3. Sol. Energy 2019, 177, 665–671. [Google Scholar] [CrossRef]
- Zarma, I.; Ahmed, M.A.; Ookawara, S. Enhancing the performance of concentrator photovoltaic systems using Nanoparticle-phase change material heat sinks. Energy Convers. Manag. 2019, 179, 229–242. [Google Scholar] [CrossRef]
- Zeng, Y.; Fan, L.W.; Xiao, Y.Q.; Yu, Z.T.; Cen, K.F. An experimental investigation of melting of nanoparticle-enhanced phase change materials (NePCMs) in a bottom-heated vertical cylindrical cavity. Int. J. Heat Mass Transf. 2013, 66, 111–117. [Google Scholar] [CrossRef]
- Altohamy, A.A.; Rabbo, M.A.; Sakr, R.; Attia, A.A. Effect of water based Al2O3 nanoparticle PCM on cool storage performance. Appl. Therm. Eng. 2015, 84, 331–338. [Google Scholar] [CrossRef]
- Hasan, A.; McCormack, S.; Huang, M.; Norton, B. Evaluation of phase change materials for thermal regulation enhancement of building integrated photovoltaics. Sol. Energy 2010, 84, 1601–1612. [Google Scholar] [CrossRef] [Green Version]
- Nada, S.; El-Nagar, D. Possibility of using PCMs in temperature control and performance enhancements of free stand and building integrated PV modules. Renew. Energy 2018, 127, 630–641. [Google Scholar] [CrossRef]
- Karimi, G.; Azizi, M.; Babapoor, A. Experimental study of a cylindrical lithium ion battery thermal management using phase change material composites. J. Energy Storage 2016, 8, 168–174. [Google Scholar] [CrossRef]
- Temel, U.N. Passive thermal management of a simulated battery pack at different climate conditions. Appl. Therm. Eng. 2019, 158, 113796. [Google Scholar] [CrossRef]
- Alimohammadi, M.; Aghli, Y.; Alavi, E.S.; Sardarabadi, M.; Passandideh-Fard, M. Experimental investigation of the effects of using nano/phase change materials (NPCM) as coolant of electronic chipsets, under free and forced convection. Appl. Therm. Eng. 2017, 111, 271–279. [Google Scholar] [CrossRef]
- Krishna, J.; Kishore, P.; Solomon, A.B. Heat pipe with nano enhanced-PCM for electronic cooling application. Exp. Therm. Fluid Sci. 2017, 81, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Fayyaz, H.; Hussain, A.; Ali, I.; Shahid, H.; Ali, H.M. Experimental Analysis of Nano-Enhanced Phase-Change Material with Different Configurations of Heat Sinks. Materials 2022, 15, 8244. [Google Scholar] [CrossRef]
- Chaichan, M.T.; Kazem, H.A. Single slope solar distillator productivity improvement using phase change material and Al2O3 nanoparticle. Sol. Energy 2018, 164, 370–381. [Google Scholar] [CrossRef]
- Wilson, J.; Singh, A.; Singh, A.; Ganapathy, S. Waste heat recovery from diesel engine using custom designed heat exchanger and thermal storage system with nanoenhanced phase change material. Therm. Sci. 2017, 21, 715–727. [Google Scholar] [CrossRef]
- Teamah, H. Comprehensive review of the application of phase change materials in residential heating applications. Alex. Eng. J. 2021, 60, 3829–3843. [Google Scholar] [CrossRef]
- Qiu, L.; Ouyang, Y.; Feng, Y.; Zhang, X. Review on micro/nano phase change materials for solar thermal applications. Renew. Energy 2019, 140, 513–538. [Google Scholar] [CrossRef]
- Narayanan, S.S.; Kardam, A.; Kumar, V.; Bhardwaj, N.; Madhwal, D.; Shukla, P.; Kumar, A.; Verma, A.; Jain, V. Development of sunlight-driven eutectic phase change material nanocomposite for applications in solar water heating. Resour. Technol. 2017, 3, 272–279. [Google Scholar] [CrossRef]
- Al-Kayiem, H.H.; Lin, S.C. Performance evaluation of a solar water heater integrated with a PCM nanocomposite TES at various inclinations. Sol. Energy 2014, 109, 82–92. [Google Scholar] [CrossRef]
- Alshukri, M.J.; Eidan, A.A.; Najim, S.I. The influence of integrated Micro-ZnO and Nano-CuO particles/paraffin wax as a thermal booster on the performance of heat pipe evacuated solar tube collector. J. Energy Storage 2021, 37, 102506. [Google Scholar] [CrossRef]
- Der, J.P.; Kostiuk, L.W.; McDonald, A.G. Analysis of the performance of a tankless water heating combo system: Simultaneous space heating and domestic hot water operation. Energy Build. 2017, 135, 50–61. [Google Scholar] [CrossRef]
- Xie, B.; Li, C.; Zhang, B.; Yang, L.; Xiao, G.; Chen, J. Evaluation of stearic acid/coconut shell charcoal composite phase change thermal energy storage materials for tankless solar water heater. Energy Built Environ. 2020, 1, 187–198. [Google Scholar] [CrossRef]
- Babiak, J.; Olesen, B.W.; Petras, D. Low Temperature Heating and High Temperature Cooling: Embedded Water Based Surface Heating and Cooling Systems. Rehva 2009, 1–106. [Google Scholar]
- Jeon, J.; Jeong, S.-G.; Lee, J.-H.; Seo, J.; Kim, S. High thermal performance composite PCMs loading xGnP for application to building using radiant floor heating system. Sol. Energy Mater. Sol. Cells 2012, 101, 51–56. [Google Scholar] [CrossRef]
- Li, D.; Wang, B.; Li, Q.; Liu, C.; Arici, M.; Wu, Y. A numerical model to investigate non-gray photothermal characteristics of paraffin-containing glazed windows. Sol. Energy 2019, 194, 225–238. [Google Scholar] [CrossRef]
- Goia, F.; Perino, M.; Haase, M. A numerical model to evaluate the thermal behaviour of PCM glazing system configurations. Energy Build. 2012, 54, 141–153. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Z.; Li, D.; Wu, Y.; Arıcı, M. Seasonal thermal performance analysis of glazed window filled with paraffin including various nanoparticles. Int. J. Energy Res. 2020, 44, 3008–3019. [Google Scholar] [CrossRef]
- Violidakis, I.; Atsonios, K.; Iliadis, P.; Nikolopoulos, N. Dynamic modeling and energy analysis of renewable heating and electricity systems at residential buildings using phase change material based heat storage technologies. J. Energy Storage 2020, 32, 101942. [Google Scholar] [CrossRef]
- Jilte, R.; Afzal, A.; Panchal, S. A novel battery thermal management system using nano-enhanced phase change materials. Energy 2021, 219, 119564. [Google Scholar] [CrossRef]
- Ferrante, A.; Semprini, G. Building energy retrofitting in urban areas. Procedia Eng. 2011, 21, 968–975. [Google Scholar] [CrossRef] [Green Version]
- Sardari, P.T.; Babaei-Mahani, R.; Giddings, D.; Yasseri, S.; A Moghimi, M.; Bahai, H. Energy recovery from domestic radiators using a compact composite metal Foam/PCM latent heat storage. J. Clean. Prod. 2020, 257, 120504. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Munyalo, J.M.; Tian, Z.; Ji, J. Preparation and thermophysical properties of low temperature composite phase change material octanoic-lauric acid/expanded graphite. J. Mol. Liq. 2019, 277, 577–583. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Zhang, Y.-F.; Bai, S.-L. High thermal conductivity of flexible polymer composites due to synergistic effect of multilayer graphene flakes and graphene foam. Compos. Part A Appl. Sci. Manuf. 2016, 85, 148–155. [Google Scholar] [CrossRef]
- Gu, X.; Liu, P.; Bian, L.; He, H. Enhanced thermal conductivity of palmitic acid/mullite phase change composite with graphite powder for thermal energy storage. Renew. Energy 2019, 138, 833–841. [Google Scholar] [CrossRef]
- Han, X.; Zhang, X.; Hua, W.; Yuan, W.; Jia, X.; Wang, Z.F. Preparation and application of composite EG/Ba(OH)2 ·8H2O form-stable phase change material for solar thermal storage. Int. J. Energy Res. 2019, 43, 2227–2240. [Google Scholar] [CrossRef]
- Fu, W.; Zou, T.; Liang, X.; Wang, S.; Gao, X.; Zhang, Z.; Fang, Y. Thermal properties and thermal conductivity enhancement of composite phase change material using sodium acetate trihydrate–urea/expanded graphite for radiant floor heating system. Appl. Therm. Eng. 2018, 138, 618–626. [Google Scholar] [CrossRef]
- Wang, J.; Han, W.; Ge, C.; Guan, H.; Yang, H.; Zhang, X. Form-stable oxalic acid dihydrate/glycolic acid-based composite PCMs for thermal energy storage. Renew. Energy 2019, 136, 657–663. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, X.; Huang, Z.; Liu, Y.; Fang, M.; Wu, X.; Min, X. Thermal conductivity enhanced polyethylene glycol/expanded perlite shape-stabilized composite phase change materials with Cu powder for thermal energy storage. Mater. Res. Express 2018, 5, 095503. [Google Scholar] [CrossRef]
- Gong, S.; Cheng, X.; Li, Y.; Shi, D.; Wang, X.; Zhong, H. Enhancement of ceramic foam modified hierarchical Al2O3@expanded graphite on thermal properties of 1-octadecanol phase change materials. J. Energy Storage 2019, 26, 101025. [Google Scholar] [CrossRef]
- Hamada, A.T.; Sharaf, O.Z.; Orhan, M.F. A novel photovoltaic/thermal (PV/T) solar collector based on a multi-functional nano-encapsulated phase-change material (nano-ePCM) dispersion. Energy Convers. Manag. 2023, 280, 116797. [Google Scholar] [CrossRef]
- Sivashankar, M.; Selvam, C.; Manikandan, S.; Harish, S. Performance improvement in concentrated photovoltaics using nano-enhanced phase change material with graphene nanoplatelets. Energy 2020, 208, 118408. [Google Scholar] [CrossRef]
- Kumaresan, V.; Velraj, R.; Das, S.K. The effect of carbon nanotubes in enhancing the thermal transport properties of PCM during solidification. Heat Mass Transf. 2012, 48, 1345–1355. [Google Scholar] [CrossRef]
- Constantinescu, M.; Dumitrache, L.; Constantinescu, D.; Anghel, E.; Popa, V.; Stoica, A.; Olteanu, M. Latent heat nano composite building materials. Eur. Polym. J. 2010, 46, 2247–2254. [Google Scholar] [CrossRef]
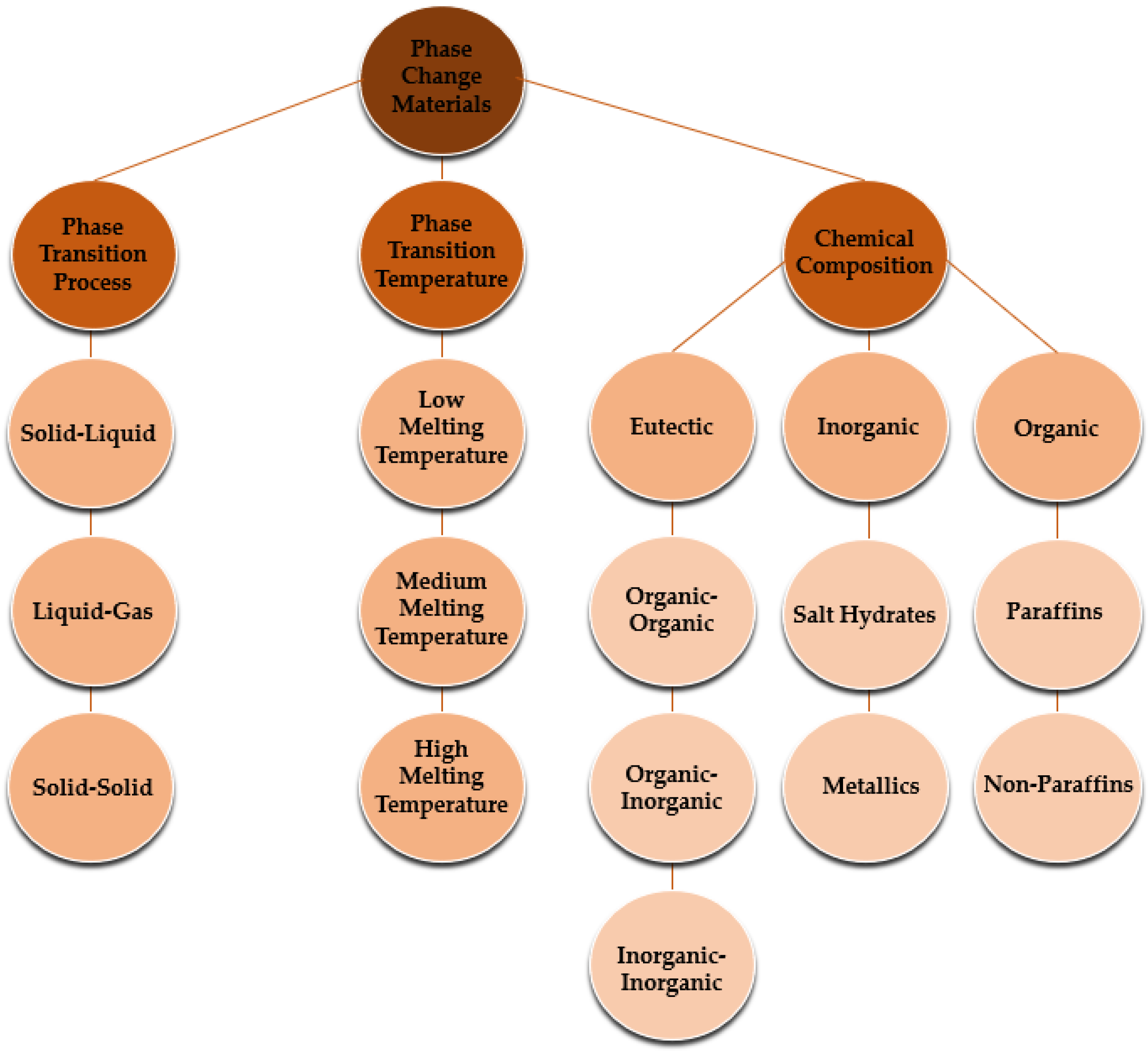








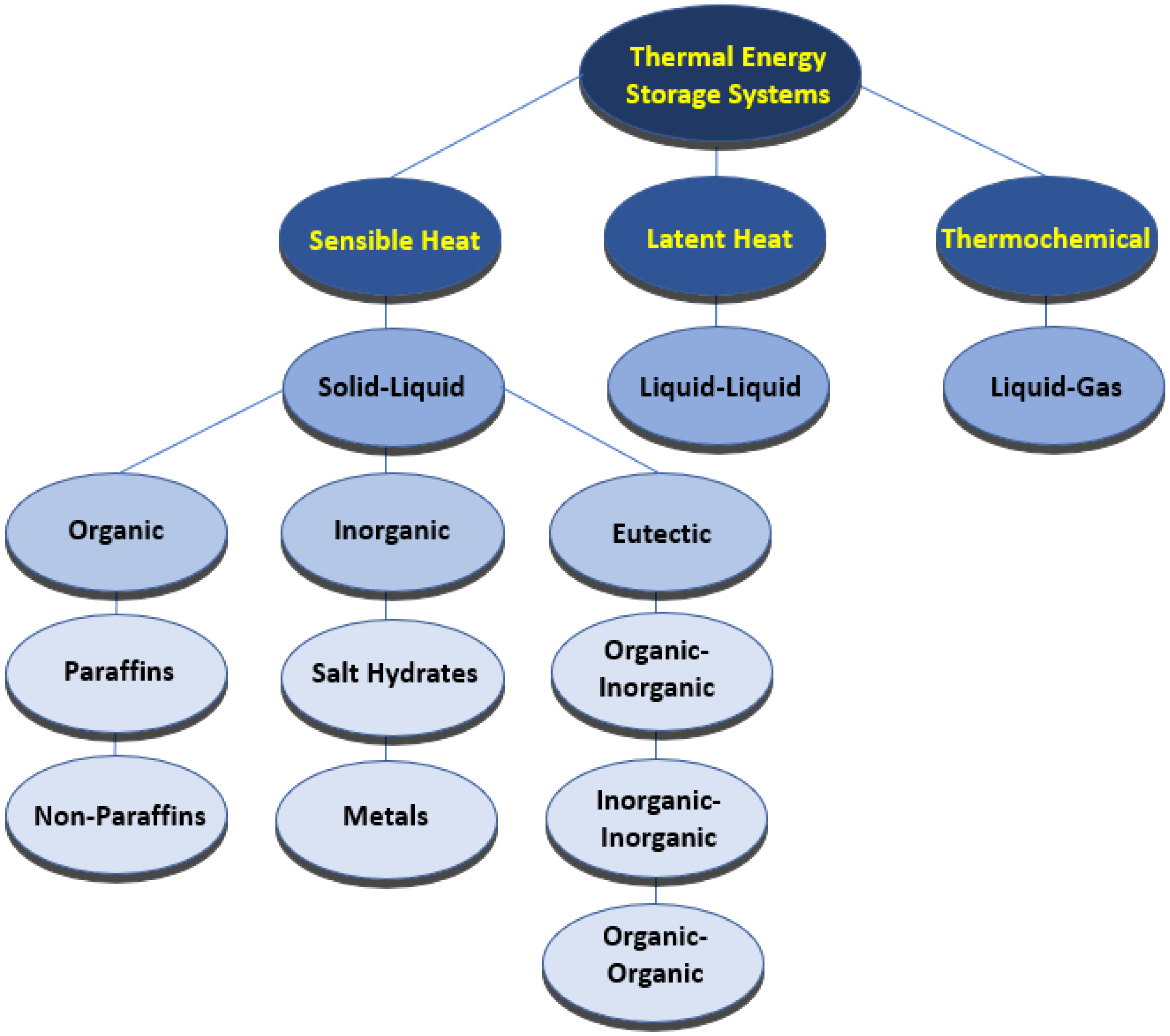

| Base Phase Change Materials | Nanoparticles | Remarks on the Thermophysical Properties | Authors | Reference |
|---|---|---|---|---|
| Sodium Acetate Trihydrate | Copper 10–30 nm | 20% thermal conductivity increase at 0.5% wt. Nearly 0.5 °C decrease in the subcooling degree. | Cui et al. | [83] |
| Erythritol | Aluminum, copper, silica, titanium oxide | 8% thermal conductivity increase at copper 2.5% wt. Aluminum had the lowest decrease in the specific heat capacity. | Soni et al. | [84] |
| Paraffin | Silica 11–14 nm, alumina and iron oxide 20 nm, zinc oxide 50 nm | Nearly 222% increase in the thermal diffusivity at iron oxide 8% wt. The thermal conductivity increased by up to nearly 0.92 w/m.K at alumina 4% wt. | Babapoor and Karimi | [85] |
| Paraffin | Titanium oxide 160 nm, copper oxide 190 nm, graphene oxide 450 nm | Around 101% thermal conductivity increase at 0.3% wt. graphene oxide. Maximum latent heat capacity increase of nearly 65% with copper oxide. Decrease in the specific heat with all nanoparticles. | Rufuss et al. | [80] |
| Eicosane | Graphite | 4.5-fold thermal conductivity increase at graphite 3.5% wt. 12.5-fold viscosity increase at graphite 3.5% wt. | Srinivasan et al. | [86] |
| RT50 | Copper oxide | Thermal conductivity and viscosity increase. Nearly 11% melting time decrease at 4% wt. | Pahamli et al. | [87] |
| Paraffin (IGI-1230A) | Helical-form graphene nanofibers, exfoliated graphene nanoplatelets, multi-walled carbon nanotubes | Helical-form graphene nanofibers, exfoliated graphene nanoplatelets did not alter the viscosity. Up to 75% viscosity increase with the multi-walled carbon nanotubes. | Weigand et al. | [88] |
| RT35 | Copper oxide, alumina | 2-fold thermal conductivity increase with alumina–copper oxide 75–25%. | Kumar and Krishna | [89] |
| Paraffin | Hybrid graphene–silver nanostructures | 6.7% latent heat increase at 0.3% wt. 90% thermal conductivity increase at 0.3% wt. | Paul et al. | [90] |
| Polyethylene Glycol | Graphene nanoplatelets | 23% thermal conductivity increase at 0.5% wt. 4 K crystallization temperature decrease at 0.5% wt. | Marcos et al. | [91] |
| Paraffin | Polyaniline and copper oxide | 8.2% latent heat capacity increase at 1% wt. polyaniline and 7.8% latent heat capacity increase with copper oxide. Nearly 47% thermal conductivity increase at 1% wt. polyaniline and nearly 64% thermal conductivity increase at 1% polyaniline | George et al. | [92] |
| Phase Change Material | Nanoparticles | Application | Remarks | Authors | Reference |
|---|---|---|---|---|---|
| Octanoic acid | Expanded graphite | Medical refrigeration and air conditioning | Good thermal conductivity and low melting point | Li et al. | [174] |
| PDMS | Graphene flakes and graphene foam | Thermal management | Improved latent heat | Zhao et al. | [175] |
| Palmitic acid | Mullite | Solar energy storing and solar heating | Good shape stability and avoids leakages | Gu et al. | [176] |
| Ba (OH)2 8H2O | Expanded graphite | Industrial Waste Heat Recovery | Enhanced thermal conductivity | Han et al. | [177] |
| Sodium acetate trihydrate–urea | Expanded graphite | Thermal management | Improved thermal conductivity | Fu et al. | [178] |
| Oxalic acid dihydrate/glycolic acid binary eutectic | Hydrothermal carbon and polyacrylamide-co-acrylic acid copolymer | Low thermal architectural applications | Improved thermal conductivity | Wang et al. | [179] |
| Polyethylene glycol | Copper | Building and industrial waste heat recovery | Improved stability and optimal melting temperature | Xu et al. | [180] |
| 1-Octadecanol | Alumina-expanded graphite | Solar energy storage | Supercooling | Gong et al. | [181] |
| Paraffin | Silica shell/encapsulate | Photovoltaic/thermal systems | The cell temperature decreased by 5 °C and 10 °C Thermal exergy increases of 66% and 208% | Hamada et al. | [182] |
| OM35 | Graphene nanoplatelets | Concentrated photovoltaic cells | The maximum increase in the power output and efficiency were 7% and 6%, respectively, at 0.5% wt. | Sivashankar et al. | [183] |
| Deionized water | Multi-walled carbon nanotubes | Building cooling and thermal management of intermittently operated electronic devices | The solidification time was reduced by 14–20% at 0.6% wt. | Kumaresan et al. | [184] |
| Epoxy resin | Aluminum | Building thermal management | Improved latent heat Adequate phase change temperature Good stability | Constantinescu et al. | [185] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, J.; Moita, A.; Moreira, A. An Overview of the Nano-Enhanced Phase Change Materials for Energy Harvesting and Conversion. Molecules 2023, 28, 5763. https://doi.org/10.3390/molecules28155763
Pereira J, Moita A, Moreira A. An Overview of the Nano-Enhanced Phase Change Materials for Energy Harvesting and Conversion. Molecules. 2023; 28(15):5763. https://doi.org/10.3390/molecules28155763
Chicago/Turabian StylePereira, José, Ana Moita, and António Moreira. 2023. "An Overview of the Nano-Enhanced Phase Change Materials for Energy Harvesting and Conversion" Molecules 28, no. 15: 5763. https://doi.org/10.3390/molecules28155763








