Targeting Members of the Chemokine Family as a Novel Approach to Treating Neuropathic Pain
Abstract
:1. Chemokines and Neuropathic Pain
1.1. CC Chemokines in Neuropathic Pain
1.2. CXC Chemokines in Neuropathic Pain
1.3. XC Chemokines in Neuropathic Pain
1.4. CX3C Chemokine in Neuropathic Pain
2. Chemokine Receptors and Neuropathic Pain
2.1. Analgesic Potential of Targeting Single CC Chemokine Receptors
2.1.1. CCR1
2.1.2. CCR2
2.1.3. CCR3
2.1.4. CCR4
2.1.5. CCR5
2.1.6. CCR8
2.2. Analgesic Potential of Targeting Single CXC Chemokine Receptors
2.2.1. CXCR2
2.2.2. CXCR3
2.2.3. CXCR4
2.2.4. CXCR8
2.3. Analgesic Potential of Targeting the Single XCR Chemokine Receptor
2.4. Analgesic Potential of Targeting a Single CX3C Chemokine Receptor
2.5. Analgesic Potential of Targeting Multiple Chemokine Receptors
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic Pain in the General Population: A Systematic Review of Epidemiological Studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef]
- Scholz, J.; Finnerup, N.B.; Attal, N.; Aziz, Q.; Baron, R.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Cruccu, G.; Davis, K.D.; et al. The IASP Classification of Chronic Pain for ICD-11: Chronic Neuropathic Pain. Pain 2019, 160, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsikostas, D.-D.; Moka, E.; Orrillo, E.; Aurilio, C.; Vadalouca, A.; Paladini, A.; Varrassi, G. Neuropathic Pain in Neurologic Disorders: A Narrative Review. Cureus 2022, 14, e22419. [Google Scholar] [CrossRef]
- Cherif, F.; Zouari, H.G.; Cherif, W.; Hadded, M.; Cheour, M.; Damak, R. Depression Prevalence in Neuropathic Pain and Its Impact on the Quality of Life. Pain Res. Manag. 2020, 2020, 7408508. [Google Scholar] [CrossRef] [PubMed]
- Duo, L.; Yu, X.; Hu, R.; Duan, X.; Zhou, J.; Wang, K. Sleep Disorders in Chronic Pain and Its Neurochemical Mechanisms: A Narrative Review. Front. Psychiatry 2023, 14, 1157790. [Google Scholar] [CrossRef] [PubMed]
- Attal, N.; Bouhassira, D.; Colvin, L. Advances and Challenges in Neuropathic Pain: A Narrative Review and Future Directions. Br. J. Anaesth. 2023, 131, 79–92. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for Neuropathic Pain in Adults: A Systematic Review and Meta-Analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowski, K.; Mika, J. The Importance of Chemokines in Neuropathic Pain Development and Opioid Analgesic Potency. Pharmacol. Rep. 2018, 70, 821–830. [Google Scholar] [CrossRef]
- Gao, Y.; Ji, R. Chemokines, Neuronal-Glial Interactions, and Central Processing of Neuropathic Pain. Pharmacol. Ther. 2010, 126, 56–68. [Google Scholar] [CrossRef] [Green Version]
- Charo, I.F.; Ransohoff, R.M. The Many Roles of Chemokines and Chemokine Receptors in Inflammation. N. Engl. J. Med. 2006, 354, 610–621. [Google Scholar] [CrossRef]
- Laing, K.J.; Secombes, C.J. Chemokines. Dev. Comp. Immunol. 2004, 28, 443–460. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M. Chemokines and Chemokine Receptors: Standing at the Crossroads of Immunobiology and Neurobiology. Immunity 2009, 31, 711–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zychowska, M.; Rojewska, E.; Piotrowska, A.; Kreiner, G.; Nalepa, I.; Mika, J. Spinal CCL1/CCR8 Signaling Interplay as a Potential Therapeutic Target—Evidence from a Mouse Diabetic Neuropathy Model. Int. Immunopharmacol. 2017, 52, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, N.; Honda, K.; Uta, D.; Beppu, K.; Ushijima, Y.; Matsuzaki, Y.; Nakashima, S.; Kido, M.A.; Imoto, K.; Takano, Y.; et al. CCL-1 in the Spinal Cord Contributes to Neuropathic Pain Induced by Nerve Injury. Cell Death Dis. 2013, 4, e679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwiatkowski, K.; Popiolek-Barczyk, K.; Piotrowska, A.; Rojewska, E.; Ciapała, K.; Makuch, W.; Mika, J. Chemokines CCL2 and CCL7, but Not CCL12, Play a Significant Role in the Development of Pain-Related Behavior and Opioid-Induced Analgesia. Cytokine 2019, 119, 202–213. [Google Scholar] [CrossRef]
- Pawlik, K.; Ciapała, K.; Ciechanowska, A.; Kwiatkowski, K.; Mika, J. Pharmacological Evidence of the Important Roles of CCR1 and CCR3 and Their Endogenous Ligands CCL2/7/8 in Hypersensitivity Based on a Murine Model of Neuropathic Pain. Cells 2023, 12, 98. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, L.; Samad, O.A.; Suter, M.R.; Yasuhiko, K.; Xu, Z.-Z.; Park, J.-Y.; Lind, A.-L.; Ma, Q.; Ji, R.-R. JNK-Induced MCP-1 Production in Spinal Cord Astrocytes Contributes to Central Sensitization and Neuropathic Pain. J. Neurosci. 2009, 29, 4096–4108. [Google Scholar] [CrossRef] [Green Version]
- Illias, A.; Gist, A.C.; Zhang, H.; Kosturakis, A.K.; Dougherty, P.M. Chemokine CCL2 and Its Receptor CCR2 in the Dorsal Root Ganglion Contribute to Oxaliplatin-Induced Mechanical Hypersensitivity. Pain 2018, 159, 1308–1316. [Google Scholar] [CrossRef]
- Rojewska, E.; Zychowska, M.; Piotrowska, A.; Kreiner, G.; Nalepa, I.; Mika, J. Involvement of Macrophage Inflammatory Protein-1 Family Members in the Development of Diabetic Neuropathy and Their Contribution to Effectiveness of Morphine. Front. Immunol. 2018, 9, 494. [Google Scholar] [CrossRef] [Green Version]
- Matsushita, K.; Tozaki-Saitoh, H.; Kojima, C.; Masuda, T.; Tsuda, M.; Inoue, K.; Hoka, S. Chemokine (C-C Motif) Receptor 5 Is an Important Pathological Regulator in the Development and Maintenance of Neuropathic Pain. Anesthesiology 2014, 120, 1491–1503. [Google Scholar] [CrossRef] [Green Version]
- Kiguchi, N.; Kobayashi, Y.; Maeda, T.; Saika, F.; Kishioka, S. CC-Chemokine MIP-1α in the Spinal Cord Contributes to Nerve Injury-Induced Neuropathic Pain. Neurosci. Lett. 2010, 484, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Ochi-Ishi, R.; Nagata, K.; Inoue, T.; Tozaki-Saitoh, H.; Tsuda, M.; Inoue, K. Involvement of the Chemokine CCL3 and the Purinoceptor P2×7 in the Spinal Cord in Paclitaxel-Induced Mechanical Allodynia. Mol. Pain 2014, 10, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawlik, K.; Ciechanowska, A.; Ciapała, K.; Rojewska, E. Blockade of CC Chemokine Receptor Type 3 Diminishes Pain and Enhances Opioid Analgesic Potency in a Model of Neuropathic Pain. Front. Immunol. 2021, 12, 1–20. [Google Scholar] [CrossRef]
- Saika, F.; Kiguchi, N.; Kobayashi, Y.; Fukazawa, Y.; Kishioka, S. CC-Chemokine Ligand 4/Macrophage Inflammatory Protein-1beta Participates in the Induction of Neuropathic Pain after Peripheral Nerve Injury. Eur. J. Pain 2012, 16, 1271–1280. [Google Scholar] [CrossRef]
- Malon, J.T.; Cao, L. Calcitonin Gene-Related Peptide Contributes to Peripheral Nerve Injury-Induced Mechanical Hypersensitivity through CCL5 and P38 Pathways. J. Neuroimmunol. 2016, 297, 68–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Q.; Fan, Q.; Zhao, Y.; Cheng, M.-Y.; Liu, H.; Li, J.; Lu, F.-F.; Jia, J.-T.; Cheng, W.; Yan, C.-D. Spinal NF-ΚB and Chemokine Ligand 5 Expression during Spinal Glial Cell Activation in a Neuropathic Pain Model. PLoS ONE 2015, 10, e0115120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogacka, J.; Popiolek-Barczyk, K.; Pawlik, K.; Ciechanowska, A.; Makuch, W.; Rojewska, E.; Dobrogowski, J.; Przeklasa-Muszynska, A.; Mika, J. CCR4 Antagonist (C021) Influences the Level of Nociceptive Factors and Enhances the Analgesic Potency of Morphine in a Rat Model of Neuropathic Pain. Eur. J. Pharmacol. 2020, 880, 173166. [Google Scholar] [CrossRef]
- Piotrowska, A.; Rojewska, E.; Pawlik, K.; Kreiner, G.; Ciechanowska, A.; Makuch, W.; Zychowska, M.; Mika, J. Pharmacological Blockade of CXCR3 by (±)-NBI-74330 Reduces Neuropathic Pain and Enhances Opioid Effectiveness—Evidence from in Vivo and in Vitro Studies. BBA-Mol. Basis Dis. 2018, 1864, 3418–3437. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, Y.; Yang, Y.; Zhang, S.; Xu, T.; Xin, W.; Wu, S.; Zhang, X. GATA3-Dependent Epigenetic Upregulation of CCL21 Is Involved in the Development of Neuropathic Pain Induced by Bortezomib. Mol. Pain 2019, 15, 1744806919863292. [Google Scholar] [CrossRef]
- Biber, K.; Tsuda, M.; Tozaki-Saitoh, H.; Tsukamoto, K.; Toyomitsu, E.; Masuda, T.; Boddeke, H.; Inoue, K. Neuronal CCL21 Up-Regulates Microglia P2X4 Expression and Initiates Neuropathic Pain Development. EMBO J. 2011, 30, 1864–1873. [Google Scholar] [CrossRef]
- Piotrowska, A.; Rojewska, E.; Pawlik, K.; Kreiner, G.; Ciechanowska, A.; Makuch, W.; Nalepa, I.; Mika, J. Pharmacological Blockade of Spinal CXCL3/CXCR2 Signaling by NVP CXCR2 20, a Selective CXCR2 Antagonist, Reduces Neuropathic Pain Following Peripheral Nerve Injury. Front. Immunol. 2019, 10, 2198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.J.; Cao, D.L.; Zhang, X.; Ji, R.R.; Gao, Y.J. Chemokine Contribution to Neuropathic Pain: Respective Induction of CXCL1 and CXCR2 in Spinal Cord Astrocytes and Neurons. Pain 2013, 154, 2185–2197. [Google Scholar] [CrossRef] [Green Version]
- Kiguchi, N.; Kobayashi, Y.; Maeda, T.; Fukazawa, Y.; Tohya, K.; Kimura, M.; Kishioka, S. Epigenetic Augmentation of the Macrophage Inflammatory Protein 2/C-X-C Chemokine Receptor Type 2 Axis through Histone H3 Acetylation in Injured Peripheral Nerves Elicits Neuropathic Pain. J. Pharmacol. Exp. Ther. 2012, 340, 577–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Zhu, M.; Yuan, S.; Yu, W. Spinal CXCL5 Contributes to Nerve Injury-Induced Neuropathic Pain via Modulating GSK-3β Phosphorylation and Activity in Rats. Neurosci. Lett. 2016, 634, 52–59. [Google Scholar] [CrossRef]
- Li, H.L.; Huang, Y.; Zhou, Y.L.; Teng, R.H.; Zhou, S.Z.; Lin, J.P.; Yang, Y.; Zhu, S.M.; Xu, H.; Yao, Y.X. C-X-C Motif Chemokine 10 Contributes to the Development of Neuropathic Pain by Increasing the Permeability of the Blood–Spinal Cord Barrier. Front. Immunol. 2020, 11, 477. [Google Scholar] [CrossRef] [PubMed]
- Bu, H.; Shu, B.; Gao, F.; Liu, C.; Guan, X.; Ke, C.; Cao, F.; Hinton, A.O.; Xiang, H.; Yang, H.; et al. Spinal IFN-γ-Induced Protein-10 (CXCL10) Mediates Metastatic Breast Cancer-Induced Bone Pain by Activation of Microglia in Rat Models. Breast Cancer Res. Treat. 2014, 143, 255–263. [Google Scholar] [CrossRef]
- Luo, X.; Tai, W.L.; Sun, L.; Pan, Z.; Xia, Z.; Chung, S.K.; Cheung, C.W. Crosstalk between Astrocytic CXCL12 and Microglial CXCR4 Contributes to the Development of Neuropathic Pain. Mol. Pain 2016, 12, 1744806916636385. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Wang, X.; Li, Z.; Kong, C.; Zhao, Y.; Qian, J.-L.; Kan, Q.; Zhang, W.; Xu, J.-T. Upregulation of Chemokine CXCL12 in the Dorsal Root Ganglia and Spinal Cord Contributes to the Development and Maintenance of Neuropathic Pain Following Spared Nerve Injury in Rats. Neurosci. Bull. 2016, 32, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.Y.; Song, Z.W.; Guo, S.W.; He, J.S.; Wang, S.Y.; Zhu, J.G.; Yang, H.L.; Liu, J.B. CXCL12/CXCR4 Signaling Contributes to Neuropathic Pain via Central Sensitization Mechanisms in a Rat Spinal Nerve Ligation Model. CNS Neurosci. Ther. 2019, 25, 922–936. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.C.; Cao, D.L.; Zhang, X.; Zhang, Z.J.; He, L.N.; Li, C.H.; Zhang, W.W.; Wu, X.B.; Berta, T.; Ji, R.R.; et al. CXCL13 Drives Spinal Astrocyte Activation and Neuropathic Pain via CXCR5. J. Clin. Investig. 2016, 126, 745–761. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yin, C.; Pan, Y.; Yang, Y.; Li, W.; Ni, H.; Liu, B.; Nie, H.; Xu, R.; Wei, H.; et al. CXCL13 Contributes to Chronic Pain of a Mouse Model of CRPS-I via CXCR5-Mediated NF-ΚB Activation and pro-Inflammatory Cytokine Production in Spinal Cord Dorsal Horn. J. Neuroinflamm. 2023, 20, 109. [Google Scholar] [CrossRef] [PubMed]
- Rojewska, E.; Ciapała, K.; Mika, J. Kynurenic Acid and Zaprinast Diminished CXCL17-Evoked Pain-Related Behaviour and Enhanced Morphine Analgesia in a Mouse Neuropathic Pain Model. Pharmacol Rep. 2019, 71, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Ciechanowska, A.; Rojewska, E.; Piotrowska, A.; Barut, J.; Pawlik, K.; Ciapała, K.; Kreiner, G.; Mika, J. New Insights into the Analgesic Properties of the XCL1/XCR1 and XCL1/ITGA9 Axes Modulation under Neuropathic Pain Conditions—Evidence from Animal Studies. Front. Immunol. 2022, 13, 1058204. [Google Scholar] [CrossRef]
- Zychowska, M.; Rojewska, E.; Piotrowska, A.; Kreiner, G.; Mika, J. Microglial Inhibition Influences XCL1/XCR1 Expression and Causes Analgesic Effects in a Mouse Model of Diabetic Neuropathy. Anesthesiology 2016, 125, 573–589. [Google Scholar] [CrossRef] [Green Version]
- Milligan, E.D.; Zapata, V.; Chacur, M.; Schoeniger, D.; Biedenkapp, J.; O’Connor, K.A.; Verge, G.M.; Chapman, G.; Green, P.; Foster, A.C.; et al. Evidence That Exogenous and Endogenous Fractalkine Can Induce Spinal Nociceptive Facilitation in Rats. Eur. J. Neurosci. 2004, 20, 2294–2302. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.S.; Tao, R.; Zhang, J.; Liu, L.; Jiang, Y.H.; Ma, S.H.; Song, L.X.; Xia, L.J. Upregulation of CX3CL1 Mediated by NF-ΚB Activation in Dorsal Root Ganglion Contributes to Peripheral Sensitization and Chronic Pain Induced by Oxaliplatin Administration. Mol. Pain 2017, 13, 1744806917726256. [Google Scholar] [CrossRef] [Green Version]
- Zlotnik, A.; Yoshie, O. Chemokines: A New Classification System and Their Role in Immunity. Immunity 2000, 12, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Moser, B.; Wolf, M.; Walz, A.; Loetscher, P. Chemokines: Multiple Levels of Leukocyte Migration Control. Trends Immunol. 2004, 25, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Cartier, L.; Hartley, O.; Dubois-Dauphin, M.; Krause, K.H. Chemokine Receptors in the Central Nervous System: Role in Brain Inflammation and Neurodegenerative Diseases. Brain Res. Rev. 2005, 48, 16–42. [Google Scholar] [CrossRef]
- Zajaczkowska, R.; Kwiatkowski, K.; Pawlik, K.; Piotrowska, A.; Rojewska, E.; Makuch, W.; Wordliczek, J.; Mika, J. Metamizole Relieves Pain by Influencing Cytokine Levels in Dorsal Root Ganglia in a Rat Model of Neuropathic Pain. Pharmacol. Rep. 2020, 72, 1310–1322. [Google Scholar] [CrossRef]
- Michael, T.; Clark, A.K.; Bishop, T.; Grist, J.; Yip, P.K.; Moon, L.D.F.; Thompson, S.W.N.; Marchand, F.; McMahon, S.B. CCL2 Is a Key Mediator of Microglia Activation in Neuropathic Pain States. Eur. J. Pain 2009, 13, 263–272. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Piotrowska, A.; Rojewska, E.; Makuch, W.; Mika, J. The RS504393 Influences the Level of Nociceptive Factors and Enhances Opioid Analgesic Potency in Neuropathic Rats. J. Neuroimmune Pharmacol. 2017, 12, 402–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawlik, K.; Piotrowska, A.; Kwiatkowski, K.; Ciapała, K.; Popiolek-Barczyk, K.; Makuch, W.; Mika, J. The Blockade of CC Chemokine Receptor Type 1 Influences the Level of Nociceptive Factors and Enhances Opioid Analgesic Potency in a Rat Model of Neuropathic Pain. Immunology 2020, 159, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Stammers, A.T.; Liu, J.; Kwon, B.K. Expression of Inflammatory Cytokines Following Acute Spinal Cord Injury in a Rodent Model. J. Neurosci. Res. 2012, 90, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, K.; Pawlik, K.; Ciapała, K.; Piotrowska, A.; Makuch, W.; Mika, J. Bidirectional Action of Cenicriviroc, a CCR2/CCR5 Antagonist, Results in Alleviation of Pain-Related Behaviors and Potentiation of Opioid Analgesia in Rats With Peripheral Neuropathy. Front. Immunol. 2020, 11, 615327. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Piotrowska, A.; Rojewska, E.; Makuch, W.; Jurga, A.; Slusarczyk, J.; Trojan, E.; Basta-Kaim, A.; Mika, J. Beneficial Properties of Maraviroc on Neuropathic Pain Development and Opioid Effectiveness in Rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 68–78. [Google Scholar] [CrossRef]
- Ciechanowska, A.; Pawlik, K.; Ciapała, K.; Mika, J. Pharmacological Modulation of the MIP-1 Family and Their Receptors Reduces Neuropathic Pain Symptoms and Influences Morphine Analgesia: Evidence from a Mouse Model. Brain Sci. 2023, 13, 579. [Google Scholar] [CrossRef]
- Piotrowska, A.; Ciapała, K.; Pawlik, K.; Kwiatkowski, K.; Rojewska, E.; Mika, J. Comparison of the Effects of Chemokine Receptors CXCR2 and CXCR3 Pharmacological Modulation in Neuropathic Pain Model—In Vivo and In Vitro Study. Int. J. Mol. Sci. 2021, 22, 11074. [Google Scholar] [CrossRef]
- Imai, S.; Narita, M.M.; Ikegami, D.; Yamashita, A.; Shimizu, T.; Narita, M.M.; Niikura, K.; Furuya, M.; Kobayashi, Y.; Miyashita, K.; et al. Epigenetic Transcriptional Activation of Monocyte Chemotactic Protein 3 Contributes to Long-Lasting Neuropathic Pain. Brain 2013, 136, 828–843. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowski, K.; Ciapała, K.; Rojewska, E.; Makuch, W.; Mika, J. Comparison of the Beneficial Effects of RS504393, Maraviroc and Cenicriviroc on Neuropathic Pain-Related Symptoms in Rodents: Behavioral and Biochemical Analyses. Int. Immunopharmacol. 2020, 84, 106540. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, Y.; Zhao, L.; Wang, L. Expression Levels of CX3CL1 and CCL21 in the Spinal Cords of Rats with Neuropathic Pain and Correlation Levels with JNK/MCP-1 Signaling Pathways. Int. J. Clin. Exp. Med. 2020, 13, 3572–3579. [Google Scholar]
- Zychowska, M.; Rojewska, E.; Pilat, D.; Mika, J. The Role of Some Chemokines from the CXC Subfamily in a Mouse Model of Diabetic Neuropathy. J. Diabetes Res. 2015, 2015, 750182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Li, C.; Wang, Z.; Wang, T.; Zhou, Y.; Zheng, L. Blocking CXC Motif Chemokine Ligand 2 Ameliorates Diabetic Peripheral Neuropathy via Inhibiting Apoptosis and NLRP3 Inflammasome Activation. Biol. Pharm. Bull. 2023, 46, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xue, Z.; Yang, B.; Liu, L.; Zhang, P.; Shi, J.; Fu, X.; Xue, Y.; Hao, Y.; Ji, G. Effects of Intrathecally Administered Interferon α on Chronic Constriction Injury Model Rats’ Mechanical Pain Threshold and G Protein Expression in the Spinal Cord. Folia Neuropathol. 2023, 61, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, S.M.; Cho, Y.W.; Jung, Y.J.; Lee, D.G.; Jang, S.H.; Park, H.W.; Hwang, S.J.; Ahn, S.H. Changes in Expression of MRNA for Interleukin-8 and Effects of Interleukin-8 Receptor Inhibitor in the Spinal Dorsal Horn in a Rat Model of Lumbar Disc Herniation. Spine 2011, 36, 2139–2146. [Google Scholar] [CrossRef]
- Khan, J.; Hassun, H.; Zusman, T.; Korczeniewska, O.; Eliav, E. Interleukin-8 Levels in Rat Models of Nerve Damage and Neuropathic Pain. Neurosci. Lett. 2017, 657, 106–112. [Google Scholar] [CrossRef]
- Kong, Y.F.; Sha, W.L.; Wu, X.B.; Zhao, L.X.; Ma, L.J.; Gao, Y.J. CXCL10/CXCR3 Signaling in the DRG Exacerbates Neuropathic Pain in Mice. Neurosci. Bull. 2021, 37, 339–352. [Google Scholar] [CrossRef]
- Strong, J.A.; Xie, W.; Coyle, D.E.; Zhang, J.M. Microarray Analysis of Rat Sensory Ganglia after Local Inflammation Implicates Novel Cytokines in Pain. PLoS ONE 2012, 7, e40779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Huang, X.; Di, Y.; Qu, L.; Fan, N. Effect of CXCL12/CXCR4 Signaling on Neuropathic Pain after Chronic Compression of Dorsal Root Ganglion. Sci. Rep. 2017, 7, 570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Z.-H.; Song, X.-J.; Yang, C.-L.; Cao, P.; Mao, Y.; Jin, Y.; Xu, M.-Y.; Wang, H.-T.; Zhu, X.; Wang, W.; et al. Up-Regulation of Microglial Chemokine CXCL12 in Anterior Cingulate Cortex Mediates Neuropathic Pain in Diabetic Mice. Acta Pharmacol. Sin. 2023, 44, 1337–1349. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Xiong, H.; Wang, W.; Liu, Y.; Yin, L.; Tu, C.; Wang, H.; Xiang, X.; Xu, J.; et al. CXCL13/CXCR5 Signaling Contributes to Diabetes-Induced Tactile Allodynia via Activating PERK, PSTAT3, PAKT Pathways and pro-Inflammatory Cytokines Production in the Spinal Cord of Male Mice. Brain. Behav. Immun. 2019, 80, 711–724. [Google Scholar] [CrossRef]
- Ma, L.; Yu, L.; Jiang, B.C.; Wang, J.; Guo, X.; Huang, Y.; Ren, J.; Sun, N.; Gao, D.S.; Ding, H.; et al. Znf382 Controls Mouse Neuropathic Pain via Silencer-Based Epigenetic Inhibition of Cxcl13 in Drg Neurons. J. Exp. Med. 2021, 218, e20210920. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yi, D.; Yu, Z.; Zhu, B.; Li, S.; Liu, X. Identification of the Hub Genes Related to Nerve Injury-Induced Neuropathic Pain. Front. Neurosci. 2020, 14, 488. [Google Scholar] [CrossRef]
- Zhang, T.; Liang, W.; Zhang, M.; Cui, S.; Huang, X.; Ou, W.; Huang, R.; Gao, J.; Jia, Z.; Zhang, S. Daphnetin Improves Neuropathic Pain by Inhibiting the Expression of Chemokines and Inflammatory Factors in the Spinal Cord and Interfering with Glial Cell Polarization. Pharmaceuticals 2023, 16, 243. [Google Scholar] [CrossRef]
- Bu, H.; Jiao, P.; Fan, X.; Gao, Y.; Zhang, L.; Guo, H. The Role of Botulinum Toxin Type A Related Axon Transport in Neuropathic Pain Induced by Chronic Constriction Injury. Korean J. Pain 2022, 35, 391–402. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, S.B.; Luo, Y.X.; Yang, Y.L.; Zhang, X.Z.; Li, B.; Meng, Y.; Chen, Y.J.; Guo, R.X.; Xiong, Y.C.; et al. NFATc2-Dependent Epigenetic Upregulation of CXCL14 Is Involved in the Development of Neuropathic Pain Induced by Paclitaxel. J. Neuroinflamm. 2020, 17, 310. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Song, C.; Huang, Y.; Lei, W.; Sun, J. MMP-9 Regulates CX3CL1/CX3CR1 in the Early Phase of Neuropathic Pain in Chronic Sciatic Nerve Constriction Injury (CCI) Rats. Ann. Palliat. Med. 2020, 9, 2020027. [Google Scholar] [CrossRef]
- Savarin-Vuaillat, C.; Ransohoff, R.M. Chemokines and Chemokine Receptors in Neurological Disease: Raise, Retain, or Reduce? Neurotherapeutics 2007, 4, 590–601. [Google Scholar] [CrossRef] [Green Version]
- Bogacka, J.; Ciapała, K.; Pawlik, K.; Dobrogowski, J.; Przeklasa-Muszynska, A.; Mika, J. Blockade of CCR4 Diminishes Hypersensitivity and Enhances Opioid Analgesia—Evidence from a Mouse Model of Diabetic Neuropathy. Neuroscience 2020, 441, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Bogacka, J.; Ciapała, K.; Pawlik, K.; Kwiatkowski, K.; Dobrogowski, J.; Przeklasa-Muszynska, A.; Mika, J. CCR4 Antagonist (C021) Administration Diminishes Hypersensitivity and Enhances the Analgesic Potency of Morphine and Buprenorphine in a Mouse Model of Neuropathic Pain. Front. Immunol. 2020, 11, 1241. [Google Scholar] [CrossRef]
- Kwon, M.J.; Shin, H.Y.; Cui, Y.; Kim, H.; Le Thi, A.H.; Choi, J.Y.; Kim, E.Y.; Hwang, D.H.; Kim, B.G. CCL2 Mediates Neuron–Macrophage Interactions to Drive Proregenerative Macrophage Activation Following Preconditioning Injury. J. Neurosci. 2015, 35, 15934–15947. [Google Scholar] [CrossRef] [Green Version]
- Kiguchi, N.; Kobayashi, Y.; Saika, F.; Kishioka, S. Epigenetic Upregulation of CCL2 and CCL3 via Histone Modifications in Infiltrating Macrophages after Peripheral Nerve Injury. Cytokine 2013, 64, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiang, Q.S.; Echeverry, S.; Mogil, J.S.; De Koninck, Y.; Rivest, S. Expression of CCR2 in Both Resident and Bone Marrow-Derived Microglia Plays a Critical Role in Neuropathic Pain. J. Neurosci. 2007, 27, 12396–12406. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.; Gruol, D.L. The Chemokine CCL2 Activates P38 Mitogen-Activated Protein Kinase Pathway in Cultured Rat Hippocampal Cells. J. Neuroimmunol. 2008, 199, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, R.; Suter, M.R. P38 MAPK, Microglial Signaling, and Neuropathic Pain. Mol. Pain 2007, 3, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, S.; Kotani, T.; Kuwabara, H.; Suzuka, T.; Kiboshi, T.; Fukui, K.; Ishida, T.; Fujiki, Y.; Shiba, H.; Hata, K.; et al. CCL2 Produced by CD68+/CD163+ Macrophages as a Promising Clinical Biomarker of Microscopic Polyangiitis-Interstitial Lung Disease. Rheumatology 2021, 60, 4643–4653. [Google Scholar] [CrossRef]
- Tecchio, C.; Cassatella, M.A. Neutrophil-Derived Chemokines on the Road to Immunity. Semin. Immunol. 2016, 28, 119. [Google Scholar] [CrossRef] [PubMed]
- Errede, M.; Annese, T.; Petrosino, V.; Longo, G.; Girolamo, F.; de Trizio, I.; d’Amati, A.; Uccelli, A.; Kerlero de Rosbo, N.; Virgintino, D. Microglia-Derived CCL2 Has a Prime Role in Neocortex Neuroinflammation. Fluids Barriers CNS 2022, 19, 68. [Google Scholar] [CrossRef]
- Zhu, X.; Xie, W.; Zhang, J.; Strong, J.A.; Zhang, J.M. Sympathectomy Decreases Pain Behaviors and Nerve Regeneration by Downregulating Monocyte Chemokine CCL2 in Dorsal Root Ganglia in the Rat Tibial Nerve Crush Model. Pain 2022, 163, E106–E120. [Google Scholar] [CrossRef]
- Yang, F.; Jing, J.J.; Fu, S.Y.; Su, X.Z.; Zhong, Y.L.; Chen, D.S.; Wu, X.Z.; Zou, Y.Q. Spinal MCP-1 Contributes to Central Post-Stroke Pain by Inducing Central Sensitization in Rats. Mol. Neurobiol. 2023, 60, 2086–2098. [Google Scholar] [CrossRef]
- De Haas, A.H.; Van Weering, H.R.J.; De Jong, E.K.; Boddeke, H.W.G.M.; Biber, K.P.H. Neuronal Chemokines: Versatile Messengers in Central Nervous System Cell Interaction. Mol. Neurobiol. 2007, 36, 137–151. [Google Scholar] [CrossRef] [Green Version]
- Hanisch, U.K. Microglia as a Source and Target of Cytokines. Glia 2002, 40, 140–155. [Google Scholar] [CrossRef]
- Wallace, C.A.; Moir, G.; Malone, D.F.G.; Duncan, L.; Devarajan, G.; Crane, I.J. Regulation of T-Lymphocyte CCL3 and CCL4 Production by Retinal Pigment Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 722–730. [Google Scholar] [CrossRef] [Green Version]
- Blidberg, K.; Palmberg, L.; Dahlén, B.; Lantz, A.S.; Larsson, K. Chemokine Release by Neutrophils in Chronic Obstructive Pulmonary Disease. Innate Immun. 2012, 18, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Sałat, K. Chemotherapy-Induced Peripheral Neuropathy: Part 1—Current State of Knowledge and Perspectives for Pharmacotherapy. Pharmacol. Rep. 2020, 72, 486–507. [Google Scholar] [CrossRef] [PubMed]
- Shehadeh, N.; Pollack, S.; Wildbaum, G.; Zohar, Y.; Shafat, I.; Makhoul, R.; Daod, E.; Hakim, F.; Perlman, R.; Karin, N. Selective Autoantibody Production against CCL3 Is Associated with Human Type 1 Diabetes Mellitus and Serves As a Novel Biomarker for Its Diagnosis. J. Immunol. 2009, 182, 8104–8109. [Google Scholar] [CrossRef] [Green Version]
- Makker, P.G.S.; Duffy, S.S.; Lees, J.G.; Perera, C.J.; Tonkin, R.S.; Butovsky, O.; Park, S.B.; Goldstein, D.; Moalem-Taylor, G. Characterisation of Immune and Neuroinflammatory Changes Associated with Chemotherapy-Induced Peripheral Neuropathy. PLoS ONE 2017, 12, e0170814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajuebor, M.N.; Hogaboam, C.M.; Kunkel, S.L.; Proudfoot, A.E.I.; Wallace, J.L. The Chemokine RANTES Is a Crucial Mediator of the Progression from Acute to Chronic Colitis in the Rat. J. Immunol. 2001, 166, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Schall, T.J.; Bacon, K.; Toy, K.J.; Goeddel, D.V. Selective Attraction of Monocytes and T Lymphocytes of the Memory Phenotype by Cytokine RANTES. Nature 1990, 347, 669–671. [Google Scholar] [CrossRef]
- Hang, L.; Shao, D.-H.; Chen, Z.; Chen, Y.-F.; Shu, W.-W.; Zhao, Z.-G. Involvement of Spinal CC Chemokine Ligand 5 in the Development of Bone Cancer Pain in Rats. Basic Clin. Pharmacol. Toxicol. 2013, 113, 325–328. [Google Scholar] [CrossRef]
- Liou, J.T.; Yuan, H.B.; Mao, C.C.; Lai, Y.S.; Day, Y.J. Absence of C-C Motif Chemokine Ligand 5 in Mice Leads to Decreased Local Macrophage Recruitment and Behavioral Hypersensitivity in a Murine Neuropathic Pain Model. Pain 2012, 153, 1283–1291. [Google Scholar] [CrossRef]
- Liou, J.; Mao, C.-C.; Ching-Wah Sum, D.; Liu, F.-C.; Lai, Y.-S.; Li, J.-C.; Day, Y.-J. Peritoneal Administration of Met-RANTES Attenuates Inflammatory and Nociceptive Responses in a Murine Neuropathic Pain Model. J. Pain 2013, 14, 24–35. [Google Scholar] [CrossRef]
- Oh, S.; Tran, P.B.; Gillard, S.E.; Hurley, R.W.; Hammond, D.L.; Miller, R.J. Chemokines and Glycoprotein120 Produce Pain Hypersensitivity by Directly Exciting Primary Nociceptive Neurons. J. Neurosci. 2001, 21, 5027–5035. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Du, X.; Lan, F.; Li, N.; Zhang, C.; Zhu, C.; Wang, X.; He, Y.; Shao, Z.; Chen, H.; et al. Eosinophilic Inflammation Promotes CCL6-Dependent Metastatic Tumor Growth. Sci. Adv. 2021, 7, 5943–5969. [Google Scholar] [CrossRef]
- Kanno, M.; Suzuki, S.; Fujiwara, T.; Yokoyama, A.; Sakamoto, A.; Takahashi, H.; Imai, Y.; Tanaka, J. Functional Expression of CCL6 by Rat Microglia: A Possible Role of CCL6 in Cell–cell Communication. J. Neuroimmunol. 2005, 167, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, F.; Zhang, C.; Li, N.; Huang, H.; Shao, Z.; Zhang, M.; Zhan, X.; He, Y.; Ju, Z.; et al. Eosinophil-Derived Chemokine (HCCL15/23, MCCL6) Interacts with CCR1 to Promote Eosinophilic Airway Inflammation. Signal Transduct. Target. Ther. 2021, 6, 91. [Google Scholar] [CrossRef]
- Bäckryd, E.; Lind, A.-L.; Thulin, M.; Larsson, A.; Gerdle, B.; Gordh, T. High Levels of Cerebrospinal Fluid Chemokines Point to the Presence of Neuroinflammation in Peripheral Neuropathic Pain: A Cross-Sectional Study of 2 Cohorts of Patients Compared with Healthy Controls. Pain 2017, 158, 2487–2495. [Google Scholar] [CrossRef]
- Ke, B.; Huang, X.X.; Li, Y.; Li, L.Y.; Xu, Q.X.; Gao, Y.; Liu, Y.; Luo, J. Neuronal-Derived Ccl7 Drives Neuropathic Pain by Promoting Astrocyte Proliferation. Neuroreport 2016, 27, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Thirion, S.; Nys, G.; Fiten, P.; Masure, S.; Van Damme, J.; Opdenakker, G. Mouse Macrophage Derived Monocyte Chemotactic Protein-3: CDNA Cloning and Identification as MARC/FIC. Biochem. Biophys. Res. Commun. 1994, 201, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhang, Y.; Zhang, J.; Zhu, Z.; Lv, Q.; Su, J. Astrocyte-Derived CCL7 Promotes Microglia-Mediated Inflammation Following Traumatic Brain Injury. Int. Immunopharmacol. 2021, 99, 107975. [Google Scholar] [CrossRef]
- Ali, S.; Robertson, H.; Wain, J.H.; Isaacs, J.D.; Malik, G.; Kirby, J.A. A Non-Glycosaminoglycan-Binding Variant of CC Chemokine Ligand 7 (Monocyte Chemoattractant Protein-3) Antagonizes Chemokine-Mediated Inflammation. J. Immunol. 2005, 175, 1257–1266. [Google Scholar] [CrossRef]
- Xuan, W.; Qu, Q.; Zheng, B.; Xiong, S.; Fan, G.-H. The Chemotaxis of M1 and M2 Macrophages Is Regulated by Different Chemokines. J. Leukoc. Biol. 2015, 97, 61–69. [Google Scholar] [CrossRef]
- Li, J.; Deng, G.; Wang, H.; Yang, M.; Yang, R.; Li, X.; Zhang, X.; Yuan, H. Interleukin-1β Pre-Treated Bone Marrow Stromal Cells Alleviate Neuropathic Pain through CCL7-Mediated Inhibition of Microglial Activation in the Spinal Cord. Sci. Rep. 2017, 7, 42260. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Jiang, B.C.; Cao, D.L.; Zhao, L.X.; Zhang, Y.L. Chemokine CCL8 and Its Receptor CCR5 in the Spinal Cord Are Involved in Visceral Pain Induced by Experimental Colitis in Mice. Brain Res. Bull. 2017, 135, 170–178. [Google Scholar] [CrossRef]
- Yang, P.; Chen, W.; Xu, H.; Yang, J.; Jiang, J.; Jiang, Y.; Xu, G. Correlation of CCL8 Expression with Immune Cell Infiltration of Skin Cutaneous Melanoma: Potential as a Prognostic Indicator and Therapeutic Pathway. Cancer Cell Int. 2021, 21, 635. [Google Scholar] [CrossRef]
- Denk, F.; Crow, M.; Didangelos, A.; Lopes, D.M.; McMahon, S.B. Persistent Alterations in Microglial Enhancers in a Model of Chronic Pain. Cell Rep. 2016, 15, 1771–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longobardi, L.; Temple, J.D.; Tagliafierro, L.; Willcockson, H.; Esposito, A.; D’Onofrio, N.; Stein, E.; Li, T.; Myers, T.J.; Ozkan, H.; et al. Role of the C-C Chemokine Receptor-2 in a Murine Model of Injury-Induced Osteoarthritis. Osteoarthr. Cartil. 2017, 25, 914–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provost, V.; Larose, M.-C.; Langlois, A.; Rola-Pleszczynski, M.; Flamand, N.; Laviolette, M. CCL26/Eotaxin-3 Is More Effective to Induce the Migration of Eosinophils of Asthmatics than CCL11/Eotaxin-1 and CCL24/Eotaxin-2. J. Leukoc. Biol. 2013, 94, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Menzies-Gow, A.; Ying, S.; Sabroe, I.; Stubbs, V.L.; Soler, D.; Williams, T.J.; Kay, A.B. Eotaxin (CCL11) and Eotaxin-2 (CCL24) Induce Recruitment of Eosinophils, Basophils, Neutrophils, and Macrophages as Well as Features of Early- and Late-Phase Allergic Reactions Following Cutaneous Injection in Human Atopic and Nonatopic Volunteers. J. Immunol. 2002, 169, 2712–2718. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.K.; Giles, D.A.; Segal, B.M.; Irani, D.N. An Emerging Role for Eotaxins in Neurodegenerative Disease. Clin. Immunol. 2018, 189, 29–33. [Google Scholar] [CrossRef]
- Nazarinia, D.; Behzadifard, M.; Gholampour, J.; Karimi, R.; Gholampour, M. Eotaxin-1 (CCL11) in Neuroinflammatory Disorders and Possible Role in COVID-19 Neurologic Complications. Acta Neurol. Belg. 2022, 122, 865–869. [Google Scholar] [CrossRef]
- García, J.J.; Cidoncha, A.; Bote, M.E.; Hinchado, M.D.; Ortega, E. Altered Profile of Chemokines in Fibromyalgia Patients. Ann. Clin. Biochem. 2014, 51, 576–581. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.L.; Yu, S.Y. Synovial Fluid Eotaxin-1 Levels May Reflect Disease Progression in Primary Knee Osteoarthritis Among Elderly Han Chinese: A Cross-Sectional Study. Cartilage 2019, 10, 408. [Google Scholar] [CrossRef]
- Izumi, K.; Bieber, K.; Ludwig, R.J. Current Clinical Trials in Pemphigus and Pemphigoid. Front. Immunol. 2019, 10, 978. [Google Scholar] [CrossRef] [Green Version]
- Pelletier, J.P.R.; Mukhtar, F. Passive Monoclonal and Polyclonal Antibody Therapies. In Immunologic Concepts in Transfusion Medicine; Elsevier: Amsterdam, The Netherlands, 2020; pp. 251–348. [Google Scholar] [CrossRef]
- Furer, V.; Hazan, E.; Mor, A.; Segal, M.; Katav, A.; Aloush, V.; Elkayam, O.; George, J.; Ablin, J.N. Elevated Levels of Eotaxin-2 in Serum of Fibromyalgia Patients. Pain Res. Manag. 2018, 2018, 7257681. [Google Scholar] [CrossRef]
- Yoshie, O.; Matsushima, K. CCR4 and Its Ligands: From Bench to Bedside. Int. Immunol. 2015, 27, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Scheu, S.; Ali, S.; Ruland, C.; Arolt, V.; Alferink, J. The C-C Chemokines CCL17 and CCL22 and Their Receptor CCR4 in CNS Autoimmunity. Int. J. Mol. Sci. 2017, 18, 2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogacka, J.; Pawlik, K.; Ciapała, K.; Ciechanowska, A.; Mika, J. CC Chemokine Receptor 4 (CCR4) as a Possible New Target for Therapy. Int. J. Mol. Sci. 2022, 23, 15638. [Google Scholar] [CrossRef] [PubMed]
- Rapp, M.; Wintergerst, M.W.M.; Kunz, W.G.; Vetter, V.K.; Knott, M.M.L.; Lisowski, D.; Haubner, S.; Moder, S.; Thaler, R.; Eiber, S.; et al. CCL22 Controls Immunity by Promoting Regulatory T Cell Communication with Dendritic Cells in Lymph Nodes. J. Exp. Med. 2019, 216, 1170. [Google Scholar] [CrossRef]
- Dai, Y.; Wu, Z.; Wang, F.; Zhang, Z.; Yu, M. Identification of Chemokines and Growth Factors in Proliferative Diabetic Retinopathy Vitreous. Biomed Res. Int. 2014, 2014, 486386. [Google Scholar] [CrossRef] [Green Version]
- Barros, J.F.; Waclawiak, I.; Pecli, C.; Borges, P.A.; Georgii, J.L.; Ramos-Junior, E.S.; Canetti, C.; Courau, T.; Klatzmann, D.; Kunkel, S.L.; et al. Role of Chemokine Receptor CCR4 and Regulatory T Cells in Wound Healing of Diabetic Mice. J. Investig. Dermatol. 2019, 139, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wang, Y.; Lin, S.; Liu, D.; Mo, G.; Zhang, H.; Dou, Y. Identification of Potential Biomarkers for Abdominal Pain in IBS Patients by Bioinformatics Approach. BMC Gastroenterol. 2021, 21, 48. [Google Scholar] [CrossRef] [PubMed]
- Paish, H.L.; Baldock, T.E.; Gillespie, C.S.; del Carpio Pons, A.; Mann, D.A.; Deehan, D.J.; Borthwick, L.A.; Kalson, N.S. Chronic, Active Inflammation in Patients With Failed Total Knee Replacements Undergoing Revision Surgery. J. Orthop. Res. 2019, 37, 2316–2324. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.Y.S.; Reimer, D.; Zehrer, A.; Lu, M.; Mielenz, D.; Körner, H. Expression of Membrane-Bound CC Chemokine Ligand 20 on Follicular T Helper Cells in T-B-Cell Conjugates. Front. Immunol. 2017, 8, 1871. [Google Scholar] [CrossRef] [Green Version]
- Lötsch, J.; Mustonen, L.; Harno, H.; Kalso, E. Machine-Learning Analysis of Serum Proteomics in Neuropathic Pain after Nerve Injury in Breast Cancer Surgery Points at Chemokine Signaling via SIRT2 Regulation. Int. J. Mol. Sci. 2022, 23, 3488. [Google Scholar] [CrossRef] [PubMed]
- Miclescu, A.A.; Granlund, P.; Butler, S.; Gordh, T. Association between Systemic Inflammation and Experimental Pain Sensitivity in Subjects with Pain and Painless Neuropathy after Traumatic Nerve Injuries. Scand. J. Pain 2023, 23, 184–199. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, R.; Wang, X.; Hu, K.; Huang, L.; Lu, M.; Hu, Q. CCL19 and CCR7 Expression, Signaling Pathways, and Adjuvant Functions in Viral Infection and Prevention. Front. Cell Dev. Biol. 2019, 7, 212. [Google Scholar] [CrossRef] [Green Version]
- Jönsson, M.; Gerdle, B.; Ghafouri, B.; Bäckryd, E. The Inflammatory Profile of Cerebrospinal Fluid, Plasma, and Saliva from Patients with Severe Neuropathic Pain and Healthy Controls-a Pilot Study. BMC Neurosci. 2021, 22, 6. [Google Scholar] [CrossRef]
- Guo, R.; Chen, Y.; Liu, L.; Wen, J.; Yang, H.; Zhu, Y.; Gao, M.; Liang, H.; Lai, W.; Long, H. Nerve Growth Factor Enhances Tooth Mechanical Hyperalgesia Through C-C Chemokine Ligand 19 in Rats. Front. Neurol. 2021, 12, 596. [Google Scholar] [CrossRef] [PubMed]
- Rappert, A.; Biber, K.; Nolte, C.; Lipp, M.; Schubel, A.; Lu, B.; Gerard, N.P.; Gerard, C.; Boddeke, H.W.G.M.; Kettenmann, H. Secondary Lymphoid Tissue Chemokine (CCL21) Activates CXCR3 to Trigger a Cl− Current and Chemotaxis in Murine Microglia. J. Immunol. 2002, 168, 3221–3226. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Waxman, S.G.; Hains, B.C. Modulation of Thalamic Nociceptive Processing after Spinal Cord Injury through Remote Activation of Thalamic Microglia by Cysteine–Cysteine Chemokine Ligand 21. J. Neurosci. 2007, 27, 8893–8902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, K.; Pickert, G.; Wijnvoord, N.; Häussler, A.; Tegeder, I. Dichotomy of CCL21 and CXCR3 in Nerve Injury-Evoked and Autoimmunity-Evoked Hyperalgesia. Brain. Behav. Immun. 2013, 32, 186–200. [Google Scholar] [CrossRef]
- Jun, K.J.; Lee, M.J.; Shin, D.C.; Woo, M.Y.; Kim, K.; Park, S. Identification of CCL1 as a Gene Differentially Expressed in CD4+ T Cells Expressing TIM-3. Immune Netw. 2011, 11, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, L.; Xiao, W.; Wang, F.; Liu, J.; Zhi, L.J. MiR-21-5p Inhibits Neuropathic Pain Development via Directly Targeting C-C Motif Ligand 1 and Tissue Inhibitor of Metalloproteinase-3. J. Cell. Biochem. 2019, 120, 16614–16623. [Google Scholar] [CrossRef]
- The Effects of Bindarit in Diabetic Nephropathy. Available online: https://clinicaltrials.gov/ct2/show/NCT01109212 (accessed on 1 June 2023).
- Ge, S.; Shrestha, B.; Paul, D.; Keating, C.; Cone, R.; Guglielmotti, A.; Pachter, J.S. The CCL2 Synthesis Inhibitor Bindarit Targets Cells of the Neurovascular Unit, and Suppresses Experimental Autoimmune Encephalomyelitis. J. Neuroinflamm. 2012, 9, 171. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Zhao, B.; Xu, L.; Wang, Y.; Liu, B.; Xu, M.; He, Q.; Ni, C.; Fu, J.; Kong, M.; et al. CXCR1 Participates in Bone Cancer Pain Induced by Walker 256 Breast Cancer Cells in Female Rats. Mol. Pain 2022, 18, 17448069221135743. [Google Scholar] [CrossRef] [PubMed]
- Bie, Y.; Ge, W.; Yang, Z.; Cheng, X.; Zhao, Z.; Li, S.; Wang, W.; Wang, Y.; Zhao, X.; Yin, Z.; et al. The Crucial Role of CXCL8 and Its Receptors in Colorectal Liver Metastasis. Dis. Markers 2019, 2019, 8023460. [Google Scholar] [CrossRef]
- Langjahr, M.; Schubert, A.L.; Sommer, C.; Üçeyler, N. Increased Pro-Inflammatory Cytokine Gene Expression in Peripheral Blood Mononuclear Cells of Patients with Polyneuropathies. J. Neurol. 2018, 265, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Staats Pires, A.; Heng, B.; Tan, V.X.; Latini, A.; Russo, M.A.; Santarelli, D.M.; Bailey, D.; Wynne, K.; O’Brien, J.A.; Guillemin, G.J.; et al. Kynurenine, Tetrahydrobiopterin, and Cytokine Inflammatory Biomarkers in Individuals Affected by Diabetic Neuropathic Pain. Front. Neurosci. 2020, 14, 890. [Google Scholar] [CrossRef]
- Gulati, K.; Gangele, K.; Agarwal, N.; Jamsandekar, M.; Kumar, D.; Poluri, K.M. Molecular Cloning and Biophysical Characterization of CXCL3 Chemokine. Int. J. Biol. Macromol. 2018, 107, 575–584. [Google Scholar] [CrossRef]
- Shibata, F. The Role of Rat Cytokine-Induced Neutrophil Chemoattractants (CINCs) in Inflammation. Yakugaku Zasshi 2002, 122, 263–268. [Google Scholar] [CrossRef]
- Lv, Q.-Y.; Zou, H.-Z.; Xu, Y.-Y.; Shao, Z.-Y.; Wu, R.-Q.; Li, K.-J.; Deng, X.; Gu, D.-N.; Jiang, H.-X.; Su, M.; et al. Expression Levels of Chemokine (C-X-C Motif) Ligands CXCL1 and CXCL3 as Prognostic Biomarkers in Rectal Adenocarcinoma: Evidence from Gene Expression Omnibus (GEO) Analyses. Bioengineered 2021, 12, 3711. [Google Scholar] [CrossRef]
- Li, H.; Xie, W.; Strong, J.A.; Zhang, J.M. Systemic Antiinflammatory Corticosteroid Reduces Mechanical Pain Behavior, Sympathetic Sprouting, and Elevation of Proinflammatory Cytokines in a Rat Model of Neuropathic Pain. Anesthesiology 2007, 107, 469–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, J.; Xin, P.; Kong, Y.; Wang, Z.; Wang, X. Resolvin D2 Reduces Chronic Neuropathic Pain and Bone Cancer Pain via Spinal Inhibition of IL-17 Secretion, CXCL1 Release and Astrocyte Activation in Mice. Brain Sci. 2023, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Al-Alwan, L.A.; Chang, Y.; Mogas, A.; Halayko, A.J.; Baglole, C.J.; Martin, J.G.; Rousseau, S.; Eidelman, D.H.; Hamid, Q. Differential Roles of CXCL2 and CXCL3 and Their Receptors in Regulating Normal and Asthmatic Airway Smooth Muscle Cell Migration. J. Immunol. 2013, 191, 2731–2741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zhang, L.; Li, H.; Ge, C.; Zhao, F.; Tian, H.; Chen, T.; Jiang, G.; Xie, H.; Cui, Y.; et al. CXCL3 Contributes to CD133+CSCs Maintenance and Forms a Positive Feedback Regulation Loop with CD133 in HCC via Erk1/2 Phosphorylation. Sci. Rep. 2016, 6, 27426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popiolek-Barczyk, K.; Mika, J. Targeting the Microglial Signaling Pathways: New Insights in the Modulation of Neuropathic Pain. Curr. Med. Chem. 2016, 23, 2908–2928. [Google Scholar] [CrossRef] [Green Version]
- Furuichi, K.; Wada, T.; Yokoyama, H.; Kobayashi, K. ichi Role of Cytokines and Chemokines in Renal Ischemia-Reperfusion Injury. Drug News Perspect. 2002, 15, 477–482. [Google Scholar] [CrossRef]
- De Jong, E.K.; De Haas, A.H.; Brouwer, N.; Van Weering, H.R.J.; Hensens, M.; Bechmann, I.; Pratley, P.; Wesseling, E.; Boddeke, H.W.G.M.; Biber, K. Expression of CXCL4 in Microglia in Vitro and in Vivo and Its Possible Signaling through CXCR3. J. Neurochem. 2008, 105, 1726–1736. [Google Scholar] [CrossRef]
- Turbic, A.; Leong, S.Y.; Turnley, A.M. Chemokines and Inflammatory Mediators Interact to Regulate Adult Murine Neural Precursor Cell Proliferation, Survival and Differentiation. PLoS ONE 2011, 6, e25406. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.C.; He, L.N.; Wu, X.B.; Shi, H.; Zhang, W.W.; Zhang, Z.J.; Cao, D.L.; Li, C.H.; Gu, J.; Gao, Y.J. Promoted Interaction of C/EBPα with Demethylated Cxcr3 Gene Promoter Contributes to Neuropathic Pain in Mice. J. Neurosci. 2017, 37, 685–700. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Song, C.Y.; Yuan, Y.; Eber, A.; Rodriguez, Y.; Levitt, R.C.; Takacs, P.; Yang, Z.; Goldberg, R.; Candiotti, K.A. Diabetic Neuropathic Pain Development in Type 2 Diabetic Mouse Model and the Prophylactic and Therapeutic Effects of Coenzyme Q10. Neurobiol. Dis. 2013, 58, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.Y.; Jiang, M.; Xu, F.; Wang, D.; Ding, B.; Ma, L.J.; Wu, H. CXCL10 and CXCR3 in the Trigeminal Ganglion Contribute to Trigeminal Neuropathic Pain in Mice. J. Pain Res. 2021, 14, 41–51. [Google Scholar] [CrossRef]
- Ascaso, P.; Palanca, A.; Martinez-Hervás, S.; Sanz, M.J.; Ascaso, J.F.; Piqueras, L.; Real, J.T. Peripheral Blood Levels of CXCL10 Are a Useful Marker for Diabetic Polyneuropathy in Subjects with Type 2 Diabetes. Int. J. Clin. Pract. 2021, 75, e14302. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Tan, Y.H.; Feng, S.Y.; Fu, K.Y. CXCR3 Signalling Partially Contributes to the Pathogenesis of Neuropathic Pain in Male Rodents. J. Oral Rehabil. 2022, 49, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Kim, S.H.; Arifuzzaman, S.; Yoon, T.; Chai, J.C.; Lee, Y.S.; Park, K.S.; Jung, K.H.; Chai, Y.G. Transcriptome Sequencing Reveals That LPS-Triggered Transcriptional Responses in Established Microglia BV2 Cell Lines Are Poorly Representative of Primary Microglia. J. Neuroinflamm. 2016, 13, 182. [Google Scholar] [CrossRef] [Green Version]
- Biber, K.; Boddeke, E. Neuronal CC Chemokines: The Distinct Roles of CCL21 and CCL2 in Neuropathic Pain. Front. Cell. Neurosci. 2014, 8, 210. [Google Scholar] [CrossRef] [Green Version]
- Biber, K.; Sauter, A.; Brouwer, N.; Copray, S.C.V.M.; Boddeke, H.W.G.M. Ischemia-Induced Neuronal Expression of the Microglia Attracting Chemokine Secondary Lymphoid-Tissue Chemokine (SLC). Glia 2001, 34, 121–133. [Google Scholar] [CrossRef]
- Jong, E.K.; Vinet, J.; Stanulovic, V.S.; Meijer, M.; Wesseling, E.; Sjollema, K.; Boddeke, H.W.G.M.; Biber, K.; de Jong, E.K. Expression, Transport, and Axonal Sorting of Neuronal CCL21 in Large Dense-Core Vesicles. FASEB J. 2008, 22, 4136–4145. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Hu, X.-M.; Liu, Y.-N.; Han, Y.; Chen, L.-P.; Wang, C.-C.; Song, C. CXCL12 in Astrocytes Contributes to Bone Cancer Pain through CXCR4-Mediated Neuronal Sensitization and Glial Activation in Rat Spinal Cord. J. Neuroinflamm. 2014, 11, 75. [Google Scholar] [CrossRef] [Green Version]
- Cheng, K.I.; Chen, S.L.; Hsu, J.H.; Cheng, Y.C.; Chang, Y.C.; Lee, C.H.; Yeh, J.L.; Dai, Z.K.; Wu, B.N. Loganin Prevents CXCL12/CXCR4-Regulated Neuropathic Pain via the NLRP3 Inflammasome Axis in Nerve-Injured Rats. Phytomedicine 2021, 92, 153734. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.J.; McCully, M.L.; Martínez-Muñoz, L.; Santiago, C.; Wheeldon, J.; Caucheteux, S.; Thelen, S.; Cecchinato, V.; Laufer, J.M.; Purvanov, V.; et al. Epithelial Chemokine CXCL14 Synergizes with CXCL12 via Allosteric Modulation of CXCR4. FASEB J. 2017, 31, 3084–3097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, J.L.; Holman, D.W.; Klein, R.S. Chemokines in the Balance: Maintenance of Homeostasis and Protection at CNS Barriers. Front. Cell. Neurosci. 2014, 8, 154. [Google Scholar] [CrossRef] [Green Version]
- Barbaria, E.M.; Kohl, B.; Buhren, B.A.; Hasenpusch-Theil, K.; Kruse, F.; Küry, P.; Martini, R.; Müller, H.W. The α-Chemokine CXCL14 Is up-Regulated in the Sciatic Nerve of a Mouse Model of Charcot–Marie–Tooth Disease Type 1A and Alters Myelin Gene Expression in Cultured Schwann Cells. Neurobiol. Dis. 2009, 33, 448–458. [Google Scholar] [CrossRef]
- Yamamoto, T.; Sasaguri, K.; Mizumoto, N.; Suzuki, H. The Chemokine CXCL14-like Immunoreactivity Co-Exists with Somatostatin, but Not NPY in the Rat Dorsal Horn and Has Intimate Association with GABAergic Neurons in the Lateral Spinal Nucleus. Acta Histochem. Cytochem. 2020, 53, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.B.; Cao, D.L.; Zhang, X.; Jiang, B.C.; Zhao, L.X.; Qian, B.; Gao, Y.J. CXCL13/CXCR5 Enhances Sodium Channel Nav1.8 Current Density via P38 MAP Kinase in Primary Sensory Neurons Following Inflammatory Pain. Sci. Rep. 2016, 6, 34836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Cao, D.L.; Zhang, Z.J.; Jiang, B.C.; Gao, Y.J. Chemokine CXCL13 Mediates Orofacial Neuropathic Pain via CXCR5/ERK Pathway in the Trigeminal Ganglion of Mice. J. Neuroinflamm. 2016, 13, 183. [Google Scholar] [CrossRef] [Green Version]
- Matloubian, M.; David, A.; Engel, S.; Ryan, J.E.; Cyster, J.G. A Transmembrane CXC Chemokine Is a Ligand for HIV-Coreceptor Bonzo. Nat. Immunol. 2000, 1, 298–304. [Google Scholar] [CrossRef]
- Abel, S.; Hundhausen, C.; Mentlein, R.; Schulte, A.; Berkhout, T.A.; Broadway, N.; Hartmann, D.; Sedlacek, R.; Dietrich, S.; Muetze, B.; et al. The Transmembrane CXC-Chemokine Ligand 16 Is Induced by IFN-γ and TNF-α and Shed by the Activity of the Disintegrin-Like Metalloproteinase ADAM10. J. Immunol. 2004, 172, 6362–6372. [Google Scholar] [CrossRef] [Green Version]
- Vallejo, R.; Tilley, D.M.; Cedeño, D.L.; Kelley, C.A.; DeMaegd, M.; Benyamin, R. Genomics of the Effect of Spinal Cord Stimulation on an Animal Model of Neuropathic Pain. Neuromodulation 2016, 19, 576–586. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, S.; Liao, F.; Huang, Z.; Yang, X.; Zou, Y.; He, X.; Guo, Q.; Huang, C. A Transcriptomic Analysis of Neuropathic Pain in the Anterior Cingulate Cortex after Nerve Injury. Bioengineered 2022, 13, 2058–2075. [Google Scholar] [CrossRef] [PubMed]
- Maravillas-Montero, J.L.; Burkhardt, A.M.; Hevezi, P.A.; Carnevale, C.D.; Smit, M.J.; Zlotnik, A. Cutting Edge: GPR35/CXCR8 Is the Receptor of the Mucosal Chemokine CXCL17. J. Immunol. 2015, 194, 29–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binti Mohd Amir, N.A.S.; Mackenzie, A.E.; Jenkins, L.; Boustani, K.; Hillier, M.C.; Tsuchiya, T.; Milligan, G.; Pease, J.E. Evidence for the Existence of a CXCL17 Receptor Distinct from GPR35. J. Immunol. 2018, 201, 714–724. [Google Scholar] [CrossRef] [Green Version]
- Pisabarro, M.T.; Leung, B.; Kwong, M.; Corpuz, R.; Frantz, G.D.; Chiang, N.; Vandlen, R.; Diehl, L.J.; Skelton, N.; Kim, H.S.; et al. Cutting Edge: Novel Human Dendritic Cell- and Monocyte-Attracting Chemokine-Like Protein Identified by Fold Recognition Methods. J. Immunol. 2006, 176, 2069–2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.Y.; Wang, C.J.; Lin, T.Y.; Hsiao, C.L.; Luo, C.W. CXCL17, an Orphan Chemokine, Acts as a Novel Angiogenic and Anti-Inflammatory Factor. Am. J. Physiol. Endocrinol. Metab. 2013, 304, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.J.; Zhou, Y.J.; Yang, X.L.; Shao, Z.M.; Ou, Z.L. The Role and Clinical Significance of the CXCL17-CXCR8 (GPR35) Axis in Breast Cancer. Biochem. Biophys. Res. Commun. 2017, 493, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.M.; McCracken, V.J.; Dimmitt, R.A.; Lorenz, R.G. Expression of CXCL15 (Lungkine) in Murine Gastrointestinal, Urogenital and Endocrine Organs. J. Histochem. Cytochem. 2007, 55, 515–524. [Google Scholar] [CrossRef]
- Fox, J.C.; Nakayama, T.; Tyler, R.C.; Sander, T.L.; Yoshie, O.; Volkman, B.F. Structural and Agonist Properties of XCL2, the Other Member of the C-Chemokine Subfamily. Cytokine 2015, 71, 302–311. [Google Scholar] [CrossRef]
- Ni, L.Y.; Zhou, L.; Wang, H.Q.; Luo, X.C.; Dan, X.M.; Li, Y.W. Identification and Expression Analysis of Three XCR1-like Receptors from Epinephelus Coioides after Cryptocaryon Irritans Infection. Fish Shellfish Immunol. 2017, 67, 95–102. [Google Scholar] [CrossRef]
- Lei, Y.; Takahama, Y. XCL1 and XCR1 in the Immune System. Microbes Infect. 2012, 14, 262–267. [Google Scholar] [CrossRef]
- Matsumoto, N.; Kon, S.; Nakatsuru, T.; Miyashita, T.; Inui, K.; Saitoh, K.; Kitai, Y.; Muromoto, R.; Kashiwakura, J.-I.; Uede, T.; et al. A Novel A9 Integrin Ligand, XCL1/Lymphotactin, Is Involved in the Development of Murine Models of Autoimmune Diseases. J. Immunol. 2017, 199, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Islam, B.; Stephenson, J.; Young, B.; Manca, M.; Buckley, D.A.; Radford, H.; Zis, P.; Johnson, M.I.; Finn, D.P.; McHugh, P.C. The Identification of Blood Biomarkers of Chronic Neuropathic Pain by Comparative Transcriptomics. NeuroMolecular Med. 2021, 24, 320–338. [Google Scholar] [CrossRef]
- Winter, A.N.; Subbarayan, M.S.; Grimmig, B.; Weesner, J.A.; Moss, L.; Peters, M.; Weeber, E.; Nash, K.; Bickford, P.C. Two Forms of CX3CL1 Display Differential Activity and Rescue Cognitive Deficits in CX3CL1 Knockout Mice. J. Neuroinflamm. 2020, 17, 157. [Google Scholar] [CrossRef]
- Clark, A.K.; Yip, P.K.; Grist, J.; Gentry, C.; Staniland, A.A.; Marchand, F.; Dehvari, M.; Wotherspoon, G.; Winter, J.; Ullah, J.; et al. Inhibition of Spinal Microglial Cathepsin S for the Reversal of Neuropathic Pain. Proc. Natl. Acad. Sci. USA 2007, 104, 10655–10660. [Google Scholar] [CrossRef]
- Paul, D.; Basavan, D. Implications of Fractalkine on Glial Function, Ablation and Glial Proteins/Receptors/Markers—Understanding Its Therapeutic Usefulness in Neurological Settings: A Narrative Review. Futur. J. Pharm. Sci. 2022, 8, 56. [Google Scholar] [CrossRef]
- Atta, A.A.; Ibrahim, W.W.; Mohamed, A.F.; Abdelkader, N.F. Microglia Polarization in Nociplastic Pain: Mechanisms and Perspectives. Inflammopharmacology 2023, 31, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.L.; Xiao, C.; Lu, B.; Zhang, J.; Yuan, X.Z.; Chen, W.; Yu, L.N.; Zhang, F.J.; Chen, G.; Yan, M. CX3CL1/CX3CR1 Regulates Nerve Injury-Induced Pain Hypersensitivity through the ERK5 Signaling Pathway. J. Neurosci. Res. 2013, 91, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Malcangio, M. Fractalkine/CX3CR1 Pathway in Neuropathic Pain: An Update. Front. Pain Res. 2021, 2, 35. [Google Scholar] [CrossRef]
- White, F.; Feldman, P.; Miller, R.J. Chemokine Signaling and the Management of Neuropathic Pain. Mol. Interv. 2009, 9, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Piotrowska, A.; Kwiatkowski, K.; Rojewska, E.; Slusarczyk, J.; Makuch, W.; Basta-Kaim, A.; Przewlocka, B.; Mika, J. Direct and Indirect Pharmacological Modulation of CCL2/CCR2 Pathway Results in Attenuation of Neuropathic Pain—In Vivo and in Vitro Evidence. J. Neuroimmunol. 2016, 297, 9–19. [Google Scholar] [CrossRef]
- Pevida, M.; Lastra, A.; Hidalgo, A.; Baamonde, A.; Menéndez, L. Spinal CCL2 and Microglial Activation Are Involved in Paclitaxel-Evoked Cold Hyperalgesia. Brain Res. Bull. 2013, 95, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Dong, Y.L.; Lu, Y.; Cao, S.; Zhao, Z.Q.; Gao, Y.J. Chemokine CCL2 and Its Receptor CCR2 in the Medullary Dorsal Horn Are Involved in Trigeminal Neuropathic Pain. J. Neuroinflamm. 2012, 9, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, A.; Paré, M.; Mcintosh, F.; Jr Elmes, S.; Martino, G.; Jomphe, C.; Lessard, E.; Lembo, P.M.; Vaillancourt, F.; Perkins, M.N.; et al. Blocking Spinal CCR2 with AZ889 Reversed Hyperalgesia in a Model of Neuropathic Pain. Mol. Pain 2010, 6, 1744–8069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piotrowska, A.; Kwiatkowski, K.; Rojewska, E.; Makuch, W.; Mika, J. Maraviroc Reduces Neuropathic Pain through Polarization of Microglia and Astroglia—Evidence from in Vivo and in Vitro Studies. Neuropharmacology 2016, 108, 207–219. [Google Scholar] [CrossRef]
- Pevida, M.; Lastra, A.; Meana, Á.; Hidalgo, A.; Baamonde, A.; Menéndez, L. The Chemokine CCL5 Induces CCR1-Mediated Hyperalgesia in Mice Inoculated with NCTC 2472 Tumoral Cells. Neuroscience 2014, 259, 113–125. [Google Scholar] [CrossRef]
- Lewis, N.D.; Muthukumarana, A.; Fogal, S.E.; Corradini, L.; Stefanopoulos, D.E.; Adusumalli, P.; Pelletier, J.; Panzenbeck, M.; Berg, K.; Canfield, M.; et al. CCR1 Plays a Critical Role in Modulating Pain through Hematopoietic and Non-Hematopoietic Cells. PLoS ONE 2014, 9, e105883. [Google Scholar] [CrossRef]
- Ambrosini, E.; Aloisi, F. Chemokines and Glial Cells: A Complex Network in the Central Nervous System. Neurochem. Res. 2004, 29, 1017–1038. [Google Scholar] [CrossRef]
- Amat, M.; Benjamim, C.F.; Williams, L.M.; Prats, N.; Terricabras, E.; Beleta, J.; Kunkel, S.L.; Godessart, N. Pharmacological Blockade of CCR1 Ameliorates Murine Arthritis and Alters Cytokine Networks in Vivo. Br. J. Pharmacol. 2006, 149, 666–675. [Google Scholar] [CrossRef] [Green Version]
- Futosi, K.; Fodor, S.; Mócsai, A. Neutrophil Cell Surface Receptors and Their Intracellular Signal Transduction Pathways. Int. Immunopharmacol. 2013, 17, 638–650. [Google Scholar] [CrossRef] [Green Version]
- Henc, I.; Rodzinnej, E.B.-F.M. Chemokiny Jako Ważne Mediatory Stanu Zapalnego. Forum Med. Rodz. 2013, 7, 251–262. [Google Scholar]
- Strazza, M.; Mor, A. Consider the Chemokines: A Review of the Interplay between Chemokines and T Cell Subset Function. Discov. Med. 2017, 24, 31–39. [Google Scholar] [PubMed]
- White, F.; Bhangoo, S.K.; Miller, R.J. Chemokines: Integrators of Pain and Inflammation. Nat. Rev. Drug Discov. 2005, 4, 834–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.Y.; Lee, D.H.; Lee, J.; Choi, C.; Kim, J.-Y.; Nam, J.-S.; Lim, Y.; Lee, Y.H. C-C Motif Chemokine Receptor 1 (CCR1) Is a Target of the EGF-AKT-MTOR-STAT3 Signaling Axis in Breast Cancer Cells. Oncotarget 2017, 8, 94591–94605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwiatkowski, K.; Mika, J. Chemokines under Neuropathic Pain. Ból 2014, 15, 19–35. [Google Scholar] [CrossRef]
- Kan, A.A.; van der Hel, W.S.; Kolk, S.M.; Bos, I.W.M.; Verlinde, S.A.M.W.; van Nieuwenhuizen, O.; de Graan, P.N.E. Prolonged Increase in Rat Hippocampal Chemokine Signalling after Status Epilepticus. J. Neuroimmunol. 2012, 245, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Nakamoto, Y.; Nemoto-Sasaki, Y.; Fujii, C.; Wang, H.; Hashii, M.; Ohmoto, Y.; Kaneko, S.; Kobayashi, K.; Mukaida, N. Potential Interaction between CCR1 and Its Ligand, CCL3, Induced by Endogenously Produced Interleukin-1 in Human Hepatomas. Am. J. Pathol. 2003, 162, 1249–1258. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Zheng, X.-Y.; Yang, J.-P.; Wang, L.-N.; Ji, F.-H. Involvement of Spinal Monocyte Chemoattractant Protein-1 (MCP-1) in Cancer-Induced Bone Pain in Rats. Neurosci. Lett. 2012, 517, 60–63. [Google Scholar] [CrossRef]
- Li, M.; Jiang, H.; Gu, K.; Sun, X.; Gu, J.; Li, C.; Wang, G. Lidocaine Alleviates Neuropathic Pain and Neuroinflammation by Inhibiting HMGB1 Expression to Mediate MIP-1α/CCR1 Pathway. J. Neuroimmune Pharmacol. 2021, 16, 318–333. [Google Scholar] [CrossRef]
- Shi, C.; Jin, J.; Xu, H.; Ma, J.; Li, T.; Xie, Y.; Li, Z. CCR1 Enhances SUMOylation of DGCR8 by Up-Regulating ERK Phosphorylation to Promote Spinal Nerve Ligation-Induced Neuropathic Pain. Gene Ther. 2021, 29, 379–389. [Google Scholar] [CrossRef]
- Mika, J.; Zychowska, M.; Popiolek-Barczyk, K.; Rojewska, E.; Przewlocka, B. Importance of Glial Activation in Neuropathic Pain. Eur. J. Pharmacol. 2013, 716, 106–119. [Google Scholar] [CrossRef]
- Mika, J. Modulation of Microglia Can Attenuate Neuropathic Pain Symptoms and Enhance Morphine Effectiveness. Pharmacol Rep. 2008, 60, 297–307. [Google Scholar] [PubMed]
- Pilat, D.; Piotrowska, A.; Rojewska, E.; Jurga, A.; Ślusarczyk, J.; Makuch, W.; Basta-Kaim, A.; Przewlocka, B.; Mika, J. Blockade of IL-18 Signaling Diminished Neuropathic Pain and Enhanced the Efficacy of Morphine and Buprenorphine. Mol. Cell. Neurosci. 2016, 71, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Pilat, D.; Rojewska, E.; Jurga, A.M.; Piotrowska, A.; Makuch, W.; Przewlocka, B.; Mika, J. IL-1 Receptor Antagonist Improves Morphine and Buprenorphine Efficacy in a Rat Neuropathic Pain Model. Eur. J. Pharmacol. 2015, 764, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Arnatt, C.K.; Falls, B.A.; Yuan, Y.; Raborg, T.J.; Masvekar, R.R.; El-Hage, N.; Selley, D.E.; Nicola, A.V.; Knapp, P.E.; Hauser, K.F.; et al. Exploration of Bivalent Ligands Targeting Putative Mu Opioid Receptor and Chemokine Receptor CCR5 Dimerization. Bioorg. Med. Chem. 2016, 24, 5969–5987. [Google Scholar] [CrossRef] [Green Version]
- Kramp, B.K.; Megens, R.T.A.; Sarabi, A.; Winkler, S.; Projahn, D.; Weber, C.; Koenen, R.R.; von Hundelshausen, P. Exchange of Extracellular Domains of CCR1 and CCR5 Reveals Confined Functions in CCL5-Mediated Cell Recruitment. Thromb. Haemost. 2013, 110, 795–806. [Google Scholar] [CrossRef]
- Di Prisco, S.; Summa, M.; Chellakudam, V.; Rossi, P.I.A.; Pittaluga, A. RANTES-Mediated Control of Excitatory Amino Acid Release in Mouse Spinal Cord. J. Neurochem. 2012, 121, 428–437. [Google Scholar] [CrossRef]
- Liu, J.; Robert Merritt, J. CC Chemokine Receptor Small Molecule Antagonists in the Treatment of Rheumatoid Arthritis and Other Diseases: A Current View. Curr. Top. Med. Chem. 2010, 10, 1250–1267. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.H.; Chee, A.; Shi, P.; Lin, C.L.; Zhao, Y.; Zhang, L.; An, H.S. Small Molecule Antagonist of C-C Chemokine Receptor 1 (CCR1) Reduces Disc Inflammation in the Rabbit Model. Spine J. 2020, 20, 2025–2036. [Google Scholar] [CrossRef]
- Ansari, M.A.; Nadeem, A.; Attia, S.M.; Bakheet, S.A.; Shahid, M.; Rehman, M.U.; Alanazi, M.M.; Alhamed, A.S.; Ibrahim, K.E.; Albekairi, N.A.; et al. CCR1 Antagonist J-113863 Corrects the Imbalance of pro- and Anti-Inflammatory Cytokines in a SJL/J Mouse Model of Relapsing-Remitting Multiple Sclerosis. Immunobiology 2022, 227, 152245. [Google Scholar] [CrossRef]
- Al-Mazroua, H.A.; Nadeem, A.; Ansari, M.A.; Attia, S.M.; Bakheet, S.A.; Albekairi, T.H.; Ali, N.; Alasmari, F.; Algahtani, M.; Alsaad, A.M.S.; et al. CCR1 Antagonist Ameliorates Experimental Autoimmune Encephalomyelitis by Inhibition of Th9/Th22-Related Markers in the Brain and Periphery. Mol. Immunol. 2022, 144, 127–137. [Google Scholar] [CrossRef]
- Gladue, R.P.; Brown, M.F.; Zwillich, S.H. CCR1 Antagonists: What Have We Learned From Clinical Trials. Curr. Top. Med. Chem. 2010, 10, 1268–1277. [Google Scholar] [CrossRef]
- Trummer, D.; Walzer, A.; Groettrup-Wolfers, E.; Schmitz, H. Efficacy, Safety and Tolerability of the CCR1 Antagonist BAY 86-5047 for the Treatment of Endometriosis-Associated Pelvic Pain: A Randomized Controlled Trial. Acta Obstet. Gynecol. Scand. 2017, 96, 694–701. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Cao, S.; Zhu, M.-D.; Liu, J.-Q.; Chen, J.-J.; Gao, Y.-J. Contribution of Chemokine CCL2/CCR2 Signaling in the Dorsal Root Ganglion and Spinal Cord to the Maintenance of Neuropathic Pain in a Rat Model of Lumbar Disc Herniation. J. Pain 2014, 15, 516–526. [Google Scholar] [CrossRef]
- Kurihara, T.; Bravo, R. Cloning and Functional Expression of MCCR2, a Murine Receptor for the C-C Chemokines JE and FIC. J. Biol. Chem. 1996, 271, 11603–11606. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Bhangoo, S.; Banisadr, G.; Freitag, C.; Ren, D.; White, F.A.; Miller, R.J. Visualization of Chemokine Receptor Activation in Transgenic Mice Reveals Peripheral Activation of CCR2 Receptors in States of Neuropathic Pain. J. Neurosci. 2009, 29, 8051–8062. [Google Scholar] [CrossRef] [Green Version]
- Abbadie, C.; Lindia, J.A.; Cumiskey, A.M.; Peterson, L.B.; Mudgett, J.S.; Bayne, E.K.; DeMartino, J.A.; MacIntyre, D.E.; Forrest, M.J. Impaired Neuropathic Pain Responses in Mice Lacking the Chemokine Receptor CCR2. Proc. Natl. Acad. Sci. USA 2003, 100, 7947–7952. [Google Scholar] [CrossRef]
- BMS-741672 for Diabetic Neuropathic Pain—No Study Results Posted—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/results/NCT00683423 (accessed on 25 May 2023).
- Liu, S.; Lan, X.B.; Tian, M.M.; Zhu, C.H.; Ma, L.; Yang, J.M.; Du, J.; Zheng, P.; Yu, J.Q.; Liu, N. Targeting the Chemokine Ligand 2–chemokine Receptor 2 Axis Provides the Possibility of Immunotherapy in Chronic Pain. Eur. J. Pharmacol. 2023, 947, 175646. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Delarasse, C. Complex Role of Chemokine Mediators in Animal Models of Alzheimer’s Disease. Biomed. J. 2018, 41, 34–40. [Google Scholar] [CrossRef]
- Albright, A.V.; Shieh, J.T.C.; Itoh, T.; Lee, B.; Pleasure, D.; O’Connor, M.J.; Doms, R.W.; González-Scarano, F. Microglia Express CCR5, CXCR4, and CCR3, but of These, CCR5 Is the Principal Coreceptor for Human Immunodeficiency Virus Type 1 Dementia Isolates. J. Virol. 1999, 73, 205–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, J.N.; Lloyd, C.M.; Sabroe, I.; Durham, S.R.; Till, S.J. T Lymphocytes Expressing CCR3 Are Increased in Allergic Rhinitis Compared with Non-Allergic Controls and Following Allergen Immunotherapy. Allergy Eur. J. Allergy Clin. Immunol. 2007, 62, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flynn, G.; Maru, S.; Loughlin, J.; Romero, I.A.; Male, D. Regulation of Chemokine Receptor Expression in Human Microglia and Astrocytes. J. Neuroimmunol. 2003, 136, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Huaux, F.; Gharaee-Kermani, M.; Liu, T.; Morel, V.; McGarry, B.; Ullenbruch, M.; Kunkel, S.L.; Wang, J.; Xing, Z.; Phan, S.H. Role of Eotaxin-1 (CCL11) and CC Chemokine Receptor 3 (CCR3) in Bleomycin-Induced Lung Injury and Fibrosis. Am. J. Pathol. 2005, 167, 1485–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humbles, A.A.; Lu, B.; Friend, D.S.; Okinaga, S.; Lora, J.; Al-garawi, A.; Martin, T.R.; Gerard, N.P.; Gerard, C. The Murine CCR3 Receptor Regulates Both the Role of Eosinophils and Mast Cells in Allergen-Induced Airway Inflammation and Hyperresponsiveness. Proc. Natl. Acad. Sci. USA 2002, 99, 1479–1484. [Google Scholar] [CrossRef]
- Bertrand, C.P.; Ponath, P.D. CCR3 Blockade as a New Therapy for Asthma. Expert Opin. Investig. Drugs 2000, 9, 43–52. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, S.Y.; Song, S.J.; Hong, H.K.; Lee, Y.; Oh, B.Y.; Lee, W.Y.; Cho, Y.B. Crosstalk between CCL7 and CCR3 Promotes Metastasis of Colon Cancer Cells via ERK-JNK Signaling Pathways. Oncotarget 2016, 7, 36842–36853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaspar, K.; Kukova, G.; Bunemann, E.; Buhren, B.A.; Sonkoly, E.; Szollosi, A.G.; Muller, A.; Savinko, T.; Lauerma, A.I.; Alenius, H.; et al. The Chemokine Receptor CCR3 Participates in Tissue Remodeling during Atopic Skin Inflammation. J. Dermatol. Sci. 2013, 71, 12–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyoda, H.; Honda, Y.; Tanaka, S.; Miyagawa, T.; Honda, M.; Honda, K.; Tokunaga, K.; Kodama, T. Narcolepsy Susceptibility Gene CCR3 Modulates Sleep-Wake Patterns in Mice. PLoS ONE 2017, 12, e0187888. [Google Scholar] [CrossRef] [Green Version]
- Kindstedt, E.; Holm, C.K.; Sulniute, R.; Martinez-Carrasco, I.; Lundmark, R.; Lundberg, P. CCL11, a Novel Mediator of Inflammatory Bone Resorption. Sci. Rep. 2017, 7, 5334. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.-P.; Xu, M.-L.; Yuan, B.-T.; Ma, L.-J.; Gao, Y.-J. Chemokine CCL7 Mediates Trigeminal Neuropathic Pain via CCR2/CCR3-ERK Pathway in the Trigeminal Ganglion of Mice. Mol. Pain 2023, 174480692311693. [Google Scholar] [CrossRef]
- Ugur, M.; Derouiche, L.; Massotte, D. Heteromerization Modulates Mu Opioid Receptor Functional Properties in Vivo. Front. Pharmacol. 2018, 9, 1240. [Google Scholar] [CrossRef] [Green Version]
- TJ, R. Bidirectional Regulation of Opioid and Chemokine Function. Front. Immunol. 2020, 11, 94. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.K.; Choi, D.Y.; Jung, Y.Y.; Yun, Y.W.; Lee, B.J.; Han, S.B.; Hong, J.T. Decreased Pain Responses of C-C Chemokine Receptor 5 Knockout Mice to Chemical or Inflammatory Stimuli. Neuropharmacology 2013, 67, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Y.; Hong, T.; Cheng, B.; Gan, S.; Chen, L.; Zhang, J.; Zuo, L.; Li, J.; Cui, X. Blocking the Autocrine Regulatory Loop of Gankyrin/STAT3/CCL24/CCR3 Impairs the Progression and Pazopanib Resistance of Clear Cell Renal Cell Carcinoma. Cell Death Dis. 2020, 11, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, S.A.; Kanabar, V.; Riffo-Vasquez, Y.; Mohamed, Z.; Cleary, S.J.; Corrigan, C.; James, A.L.; Elliot, J.G.; Shute, J.K.; Page, C.P.; et al. Platelets Independently Recruit into Asthmatic Lungs and Models of Allergic Inflammation via CCR3. Am. J. Respir. Cell Mol. Biol. 2021, 64, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Shen, J.; Yang, H.; Xu, Y.; Gao, W.; Wang, J.; Zhang, H.; He, S. Upregulated Expression of CCR3 in Osteoarthritis and CCR3 Mediated Activation of Fibroblast-like Synoviocytes. Cytokine 2016, 77, 211–219. [Google Scholar] [CrossRef]
- Salanga, C.L.; Handel, T.M. Chemokine Oligomerization and Interactions with Receptors and Glycosaminoglycans: The Role of Structural Dynamics in Function. Exp. Cell Res. 2011, 317, 590–601. [Google Scholar] [CrossRef] [Green Version]
- Meucci, O.; Fatatis, A.; Simen, A.A.; Bushell, T.J.; Gray, P.W.; Miller, R.J. Chemokines Regulate Hippocampal Neuronal Signaling and Gp120 Neurotoxicity. Proc. Natl. Acad. Sci. USA 1998, 95, 14500–14505. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Arabi, Z.; Ahangar-Parvin, R.; Mohammadi-Kordkhayli, M.; Nemati, M. Ginger Extract Modulates the Expression of Chemokines CCL20 and CCL22 and Their Receptors (CCR6 and CCR4) in the Central Nervous System of Mice with Experimental Autoimmune Encephalomyelitis. Drug Res. 2017, 67, 632–639. [Google Scholar] [CrossRef]
- Bajetto, A.; Bonavia, R.; Barbero, S.; Schettini, G. Characterization of Chemokines and Their Receptors in the Central Nervous System: Physiopathological Implications. J. Neurochem. 2002, 82, 1311–1329. [Google Scholar] [CrossRef]
- Purandare, A.; Somerville, J. Antagonists of CCR4 as Immunomodulatory Agents. Curr. Top. Med. Chem. 2012, 6, 1335–1344. [Google Scholar] [CrossRef]
- Purandare, A.V.; Gao, A.; Wan, H.; Somerville, J.; Burke, C.; Seachord, C.; Vaccaro, W.; Wityak, J.; Poss, M.A. Identification of Chemokine Receptor CCR4 Antagonist. Bioorganic Med. Chem. Lett. 2005, 15, 2669–2672. [Google Scholar] [CrossRef] [PubMed]
- Pfützner, J.; Hellhammer, J.; Musholt, P.; Pfützner, A.H.; Böhnke, J.; Hero, T.; Amann-Zalan, I.; Ganz, M.; Forst, T.; Pfützner, A. Evaluation of Dexterity in Insulin-Treated Patients with Type 1 and Type 2 Diabetes Mellitus. J. Diabetes Sci. Technol. 2011, 5, 158–165. [Google Scholar] [CrossRef]
- Mueller, M.J.; Minor, S.D.; Sahrmann, S.A.; Schaaf, J.A.; Strube, M.J. Differences in the Gait Characteristics of Patients with Diabetes and Peripheral Neuropathy Compared with Age-Matched Controls. Phys. Ther. 1994, 74, 299–313. [Google Scholar] [CrossRef]
- Remer, M.; Al-Shamkhani, A.; Glennie, M.; Johnson, P. Mogamulizumab and the Treatment of CCR4-Positive T-Cell Lymphomas. Immunotherapy 2014, 6, 1187–1206. [Google Scholar] [CrossRef]
- Doi, T.; Muro, K.; Ishii, H.; Kato, T.; Tsushima, T.; Takenoyama, M.; Oizumi, S.; Gemmoto, K.; Suna, H.; Enokitani, K.; et al. A Phase I Study of the Anti-CC Chemokine Receptor 4 Antibody, Mogamulizumab, in Combination with Nivolumab in Patients with Advanced or Metastatic Solid Tumors. Clin. Cancer Res. 2019, 25, 6614–6622. [Google Scholar] [CrossRef] [Green Version]
- Kaul, M.; Ma, Q.; Medders, K.E.; Desai, M.K.; Lipton, S.A. HIV-1 Coreceptors CCR5 and CXCR4 Both Mediate Neuronal Cell Death but CCR5 Paradoxically Can Also Contribute to Protection. Cell Death Differ. 2007, 14, 296–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rottman, J.B.; Ganley, K.P.; Williams, K.; Wu, L.; Mackay, C.R.; Ringler, D.J. Cellular Localization of the Chemokine Receptor CCR5: Correlation to Cellular Targets of HIV-1 Infection. Am. J. Pathol. 1997, 151, 1341–1351. [Google Scholar]
- Carbonell, W.S.; Murase, S.I.; Horwitz, A.F.; Mandell, J.W. Migration of Perilesional Microglia after Focal Brain Injury and Modulation by CC Chemokine Receptor 5: An in Situ Time-Lapse Confocal Imaging Study. J. Neurosci. 2005, 25, 7040–7047. [Google Scholar] [CrossRef] [Green Version]
- Marella, M.; Chabry, J. Neurons and Astrocytes Respond to Prion Infection by Inducing Microglia Recruitment. J. Neurosci. 2004, 24, 620–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyagi, T.; Chuang, L.F.; Doi, R.H.; Carlos, M.P.; Torres, J.V.; Chuang, R.Y. Morphine Induces Gene Expression of CCR5 in Human CEM X174 Lymphocytes. J. Biol. Chem. 2000, 275, 31305–31310. [Google Scholar] [CrossRef] [Green Version]
- Bidlack, J.M. Detection and Function of Opioid Receptors on Cells from the Immune System. Clin. Diagn. Lab. Immunol. 2000, 7, 719–723. [Google Scholar] [CrossRef] [Green Version]
- Mika, J.; Popiolek-Barczyk, K.; Rojewska, E.; Makuch, W.; Starowicz, K.; Przewlocka, B. Delta-Opioid Receptor Analgesia Is Independent of Microglial Activation in a Rat Model of Neuropathic Pain. PLoS ONE 2014, 9, e104420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Li, J.; Bot, G.; Szabo, I.; Rogers, T.J.; Liu-Chen, L.-Y. Heterodimerization and Cross-Desensitization between the μ-Opioid Receptor and the Chemokine CCR5 Receptor. Eur. J. Pharmacol. 2004, 483, 175–186. [Google Scholar] [CrossRef]
- Szabo, I.; Chen, X.-H.; Xin, L.; Adler, M.W.; Howard, O.M.Z.; Oppenheim, J.J.; Rogers, T.J. Heterologous Desensitization of Opioid Receptors by Chemokines Inhibits Chemotaxis and Enhances the Perception of Pain. Proc. Natl. Acad. Sci. USA 2002, 99, 10276–10281. [Google Scholar] [CrossRef]
- Lisi, L.; Tramutola, A.; De Luca, A.; Navarra, P.; Dello Russo, C. Modulatory Effects of the CCR5 Antagonist Maraviroc on Microglial Pro-Inflammatory Activation Elicited by Gp120. J. Neurochem. 2012, 120, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Pease, J.E.; Horuk, R. Chemokine Receptor Antagonists: Part 2. Expert Opin. Ther. Pat. 2009, 19, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Manjavachi, M.N.; Passos, G.F.; Trevisan, G.; Araújo, S.B.; Pontes, J.P.; Fernandes, E.S.; Costa, R.; Calixto, J.B. Spinal Blockage of CXCL1 and Its Receptor CXCR2 Inhibits Paclitaxel-Induced Peripheral Neuropathy in Mice. Neuropharmacology 2019, 151, 136–143. [Google Scholar] [CrossRef]
- Yang, J.; Liu, F.; Zhang, Y.Y.; Lin, J.; Li, Y.L.; Zhou, C.; Li, C.J.; Shen, J.F. C-X-C Motif Chemokine Ligand 1 and Its Receptor C-X-C Motif Chemokine Receptor 2 in Trigeminal Ganglion Contribute to Nerve Injury-Induced Orofacial Mechanical Allodynia. J. Oral Rehabil. 2022, 49, 195–206. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, Y.; Wang, M.; Qian, C.; Wang, C.; Tang, J.; Cai, Z.; Dai, W.; Zhu, X. Pharmacological Inhibition of CXCR2 Alleviates Neuropathic Pain by Inactivating Microglia in a Rat L5 Spinal Nerve Ligation Model. Am. J. Transl. Res. 2020, 12, 3803–3812. [Google Scholar]
- Qin, J.; Li, A.; Huang, Y.; Teng, R.H.; Yang, Y.; Yao, Y.X. CXCR3 Contributes to Neuropathic Pain via ERK Activation in the Anterior Cingulate Cortex. Biochem. Biophys. Res. Commun. 2020, 531, 166–171. [Google Scholar] [CrossRef]
- Xie, F.; Wang, Y.; Li, X.; Chao, Y.-C.; Yue, Y. Early Repeated Administration of CXCR4 Antagonist AMD3100 Dose-Dependently Improves Neuropathic Pain in Rats After L5 Spinal Nerve Ligation. Neurochem. Res. 2016, 41, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zou, Y.Q.; Li, M.; Luo, W.J.; Chen, G.Z.; Wu, X.Z. Intervertebral Foramen Injection of Plerixafor Attenuates Neuropathic Pain after Chronic Compression of the Dorsal Root Ganglion: Possible Involvement of the down-Regulation of Nav1.8 and Nav1.9. Eur. J. Pharmacol. 2021, 908, 174322. [Google Scholar] [CrossRef]
- Rojewska, E.; Piotrowska, A.; Jurga, A.; Makuch, W.; Mika, J. Zaprinast Diminished Pain and Enhanced Opioid Analgesia in a Rat Neuropathic Pain Model. Eur. J. Pharmacol. 2018, 839, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Horuk, R.; Martin, A.W.; Wang, Z.; Schweitzer, L.; Gerassimides, A.; Guo, H.; Lu, Z.; Hesselgesser, J.; Perez, H.D.; Kim, J.; et al. Expression of Chemokine Receptors by Subsets of Neurons in the Central Nervous System. J. Immunol. 1997, 158, 2882–2890. [Google Scholar] [CrossRef]
- Sun, Y.; Sahbaie, P.; Liang, D.Y.; Li, W.W.; Li, X.Q.; Shi, X.Y.; Clark, J.D. Epigenetic Regulation of Spinal Cxcr2 Signaling in Incisional Hypersensitivity in Mice. Anesthesiology 2013, 119, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.Y.; Shi, X.; Liu, P.; Sun, Y.; Sahbaie, P.; Li, W.W.; Yeomans, D.C.; Clark, J.D. The Chemokine Receptor CXCR2 Supports Nociceptive Sensitization after Traumatic Brain Injury. Mol. Pain 2017, 13, 1744806917730212. [Google Scholar] [CrossRef] [Green Version]
- Moraes, T.R.; Elisei, L.S.; Malta, I.H.; Galdino, G. Participation of CXCL1 in the Glial Cells during Neuropathic Pain. Eur. J. Pharmacol. 2020, 875, 173039. [Google Scholar] [CrossRef]
- Zhou, L.; Hu, Y.; Li, C.; Yan, Y.; Ao, L.; Yu, B.; Fang, W.; Liu, J.; Li, Y. Levo-Corydalmine Alleviates Vincristine-Induced Neuropathic Pain in Mice by Inhibiting an NF-Kappa B-Dependent CXCL1/CXCR2 Signaling Pathway. Neuropharmacology 2018, 135, 34–47. [Google Scholar] [CrossRef]
- Jiang, S.; Liang, J.; Li, W.; Wang, L.; Song, M.; Xu, S.; Liu, G.; Du, Q.; Zhai, D.; Tang, L.; et al. The Role of CXCL1/CXCR2 Axis in Neurological Diseases. Int. Immunopharmacol. 2023, 120, 110330. [Google Scholar] [CrossRef]
- Old, E.A.; Malcangio, M. Chemokine Mediated Neuron-Glia Communication and Aberrant Signalling in Neuropathic Pain States. Curr. Opin. Pharmacol. 2012, 12, 67–73. [Google Scholar] [CrossRef]
- Lasagni, L.; Francalanci, M.; Annunziato, F.; Lazzeri, E.; Giannini, S.; Cosmi, L.; Sagrinati, C.; Mazzinghi, B.; Orlando, C.; Maggi, E.; et al. An Alternatively Spliced Variant of CXCR3 Mediates the Inhibition of Endothelial Cell Growth Induced by IP-10, Mig, and I-TAC, and Acts as Functional Receptor for Platelet Factor 4. J. Exp. Med. 2003, 197, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Clark-Lewis, I.; Mattioli, I.; Gong, J.H.; Loetscher, P. Structure-Function Relationship between the Human Chemokine Receptor CXCR3 and Its Ligands. J. Biol. Chem. 2003, 278, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Smit, M.J.; Verdijk, P.; Van der Raaij-Helmer, E.M.H.; Navis, M.; Hensbergen, P.J.; Leurs, R.; Tensen, C.P. CXCR3-Mediated Chemotaxis of Human T Cells Is Regulated by a G i-and Phospholipase C-Dependent Pathway and Not via Activation of MEK/P44/P42 MAPK nor Akt/PI-3 Kinase. Blood 2003, 102, 1959–1965. [Google Scholar] [CrossRef]
- Green, M.V.; Thayer, S.A. HIV Gp120 Upregulates Tonic Inhibition through A5-Containing GABA A Rs. Neuropharmacology 2019, 149, 161–168. [Google Scholar] [CrossRef]
- Zhang, X.; Han, J.; Man, K.; Li, X.; Du, J.; Chu, E.S.H.; Go, M.Y.Y.; Sung, J.J.Y.; Yu, J. CXC Chemokine Receptor 3 Promotes Steatohepatitis in Mice through Mediating Inflammatory Cytokines, Macrophages and Autophagy. J. Hepatol. 2016, 64, 160–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berchiche, Y.A.; Sakmar, T.P. CXC Chemokine Receptor 3 Alternative Splice Variants Selectively Activate Different Signaling Pathways. Mol. Pharmacol. 2016, 90, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, M.R.; Northcutt, A.L.; Chao, L.W.; Kearney, J.J.; Zhang, Y.; Berkelhammer, D.L.; Loram, L.C.; Rozeske, R.R.; Bland, S.T.; Maier, S.F.; et al. Minocycline Suppresses Morphine-Induced Respiratory Depression, Suppresses Morphine-Induced Reward, and Enhances Systemic Morphine-Induced Analgesia. Brain. Behav. Immun. 2008, 22, 1248–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Clercq, E. Mozobil® (Plerixafor, AMD3100), 10 Years after Its Approval by the US Food and Drug Administration. Chem. Chemother. 2019, 27, 2040206619829382. [Google Scholar] [CrossRef] [Green Version]
- Zuk, A.; Gershenovich, M.; Ivanova, Y.; MacFarland, R.T.; Fricker, S.P.; Ledbetter, S. CXCR4 Antagonism as a Therapeutic Approach to Prevent Acute Kidney Injury. Am. J. Physiol.-Ren. Physiol. 2014, 307, F783–F797. [Google Scholar] [CrossRef] [Green Version]
- Jujo, K.; Ii, M.; Sekiguchi, H.; Klyachko, E.; Misener, S.; Tanaka, T.; Tongers, J.; Roncalli, J.; Renault, M.A.; Thorne, T.; et al. CXC-Chemokine Receptor 4 Antagonist AMD3100 Promotes Cardiac Functional Recovery after Ischemia/Reperfusion Injury via Endothelial Nitric Oxide Synthase-Dependent Mechanism. Circulation 2013, 127, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Hendrix, C.W.; Flexner, C.; Macfarland, R.T.; Giandomenico, C.; Fuchs, E.J.; Redpath, E.; Bridger, G.; Henson, G.W. Pharmacokinetics and Safety of AMD-3100, a Novel Antagonist of the CXCR- 4 Chemokine Receptor, in Human Volunteers. Antimicrob. Agents Chemother. 2000, 44, 1667–1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhangoo, S.K.; Ripsch, M.S.; Buchanan, D.J.; Miller, R.J.; White, F.A. Increased Chemokine Signaling in a Model of HIV1-Associated Peripheral Neuropathy. Mol. Pain 2009, 5, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.-M.; Liu, Y.-N.; Zhang, H.-L.; Cao, S.-B.; Zhang, T.; Chen, L.-P.; Shen, W. CXCL12/CXCR4 Chemokine Signaling in Spinal Glia Induces Pain Hypersensitivity through MAPKs-Mediated Neuroinflammation in Bone Cancer Rats. J. Neurochem. 2015, 132, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Menichella, D.M.; Abdelhak, B.; Ren, D.; Shum, A.; Frietag, C.; Miller, R.J. CXCR4 Chemokine Receptor Signaling Mediates Pain in Diabetic Neuropathy. Mol. Pain 2014, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Ashjari, D.; Karamali, N.; Rajabinejad, M.; Hassani, S.S.; Afshar Hezarkhani, L.; Afshari, D.; Gorgin Karaji, A.; Salari, F.; Rezaiemanesh, A. The Axis of Long Non-Coding RNA MALAT1/MiR-1-3p/CXCR4 Is Dysregulated in Patients with Diabetic Neuropathy. Heliyon 2022, 8, e09178. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Tai, W.L.; Sun, L.; Qiu, Q.; Xia, Z.; Chung, S.K.; Cheung, C.W. Central Administration of C-X-C Chemokine Receptor Type 4 Antagonist Alleviates the Development and Maintenance of Peripheral Neuropathic Pain in Mice. PLoS ONE 2014, 9, e104860. [Google Scholar] [CrossRef] [Green Version]
- Wilson, N.M.; Jung, H.; Ripsch, M.S.; Miller, R.J.; White, F.A. CXCR4 Signaling Mediates Morphine-Induced Tactile Hyperalgesia. Brain. Behav. Immun. 2011, 25, 565–573. [Google Scholar] [CrossRef] [Green Version]
- Alkondon, M.; Pereira, E.F.R.; Todd, S.W.; Randall, W.R.; Lane, M.V.; Albuquerque, E.X. Functional G-Protein-Coupled Receptor 35 Is Expressed by Neurons in the CA1 Field of the Hippocampus. Biochem. Pharmacol. 2015, 93, 506–518. [Google Scholar] [CrossRef]
- Berlinguer-Palmini, R.; Masi, A.; Narducci, R.; Cavone, L.; Maratea, D.; Cozzi, A.; Sili, M.; Moroni, F.; Mannaioni, G. GPR35 Activation Reduces Ca2+ Transients and Contributes to the Kynurenic Acid-Dependent Reduction of Synaptic Activity at CA3-CA1 Synapses. PLoS ONE 2013, 8, e82180. [Google Scholar] [CrossRef] [Green Version]
- Cosi, C.; Mannaioni, G.; Cozzi, A.; Carl, V.; Sili, M.; Cavone, L.; Maratea, D.; Moroni, F. G-Protein Coupled Receptor 35 (GPR35) Activation and Inflammatory Pain: Studies on the Antinociceptive Effects of Kynurenic Acid and Zaprinast. Neuropharmacology 2011, 60, 1227–1231. [Google Scholar] [CrossRef]
- Wang, J.; Simonavicius, N.; Wu, X.; Swaminath, G.; Reagan, J.; Tian, H.; Ling, L. Kynurenic Acid as a Ligand for Orphan G Protein-Coupled Receptor GPR35. J. Biol. Chem. 2006, 281, 22021–22028. [Google Scholar] [CrossRef] [Green Version]
- Ohshiro, H.; Tonai-Kachi, H.; Ichikawa, K. GPR35 Is a Functional Receptor in Rat Dorsal Root Ganglion Neurons. Biochem. Biophys. Res. Commun. 2008, 365, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Tonai-Kachi, H.; Shinjo, K. Zaprinast, a Well-Known Cyclic Guanosine Monophosphate-Specific Phosphodiesterase Inhibitor, Is an Agonist for GPR35. FEBS Lett. 2006, 580, 5003–5008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackenzie, A.E.; Milligan, G. The Emerging Pharmacology and Function of GPR35 in the Nervous System. Neuropharmacology 2017, 113, 661–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, P.; Lane, T.R.; Gao, H.G.L.; Hurst, D.P.; Kotsikorou, E.; Le, L.; Brailoiu, E.; Reggio, P.H.; Abood, M.E. Crucial Positively Charged Residues for Ligand Activation of the GPR35 Receptor. J. Biol. Chem. 2014, 289, 3625–3638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resta, F.; Masi, A.; Sili, M.; Laurino, A.; Moroni, F.; Mannaioni, G. Kynurenic Acid and Zaprinast Induce Analgesia by Modulating HCN Channels through GPR35 Activation. Neuropharmacology 2016, 108, 136–143. [Google Scholar] [CrossRef]
- Chess, A.C.; Simoni, M.K.; Alling, T.E.; Bucci, D.J. Elevations of Endogenous Kynurenic Acid Produce Spatial Working Memory Deficits. Schizophr. Bull. 2007, 33, 797–804. [Google Scholar] [CrossRef] [Green Version]
- Koola, M.M. Galantamine-Memantine Combination for Cognitive Impairments Due to Electroconvulsive Therapy, Traumatic Brain Injury, and Neurologic and Psychiatric Disorders: Kynurenic Acid and Mismatch Negativity Target Engagement. Prim. Care Companion J. Clin. Psychiatry 2018, 20, 27296. [Google Scholar] [CrossRef]
- Banerjee, J.; Alkondon, M.; Pereira, E.F.R.; Albuquerque, E.X. Regulation of GABAergic Inputs to CA1 Pyramidal Neurons by Nicotinic Receptors and Kynurenic Acid. J. Pharmacol. Exp. Ther. 2012, 341, 500–509. [Google Scholar] [CrossRef] [Green Version]
- Elmslie, K.S.; Yoshikami, D. Effects of Kynurenate on Root Potentials Evoked by Synaptic Activity and Amino Acids in the Frog Spinal Cord. Brain Res. 1985, 330, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Näsström, J.; Karlsson, U.; Post, C. Antinociceptive Actions of Different Classes of Excitatory Amino Acid Receptor Antagonists in Mice. Eur. J. Pharmacol. 1992, 212, 21–29. [Google Scholar] [CrossRef]
- Chapman, V.; Dickenson, A.H. Time-Related Roles of Excitatory Amino Acid Receptors during Persistent Noxiously Evoked Responses of Rat Dorsal Horn Neurones. Brain Res. 1995, 703, 45–50. [Google Scholar] [CrossRef]
- Yoon, M.H.; Jeong, I.C.; Hong, B.B.; Seong, W.J.; Sung, S.C.; Kyung, Y.Y.; Chang, Y.J.; Seok, J.K.; Sung, T.C.; Chang, M.K. Lack of the Nitric Oxide-Cyclic GMP-Potassium Channel Pathway for the Antinociceptive Effect of Intrathecal Zaprinast in a Rat Formalin Test. Neurosci. Lett. 2005, 390, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Southern, C.; Cook, J.M.; Neetoo-Isseljee, Z.; Taylor, D.L.; Kettleborough, C.A.; Merritt, A.; Bassoni, D.L.; Raab, W.J.; Quinn, E.; Wehrman, T.S.; et al. Screening β-Arrestin Recruitment for the Identification of Natural Ligands for Orphan G-Protein-Coupled Receptors. J. Biomol. Screen. 2013, 18, 599–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.J.; Lee, S.J.; Nam, S.Y.; Im, D.S. GPR35 Mediates Lodoxamide-Induced Migration Inhibitory Response but Not CXCL17-Induced Migration Stimulatory Response in THP-1 Cells; Is GPR35 a Receptor for CXCL17? Br. J. Pharmacol. 2018, 175, 154–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, R.; Liu, J.; Li, Z.; Zhang, W.; Wang, F.; Zhang, B. Recent Advances in CXCL12/CXCR4 Antagonists and Nano-Based Drug Delivery Systems for Cancer Therapy. Pharmaceutics 2022, 14, 1541. [Google Scholar] [CrossRef] [PubMed]
- Bird, E.; Iannitti, T.; Christmas, C.R.; Obara, I.; Andreev, V.I.; King, A.E.; Boissonade, F.M. A Novel Role for Lymphotactin (XCL1) Signaling in the Nervous System: XCL1 Acts via Its Receptor XCR1 to Increase Trigeminal Neuronal Excitability. Neuroscience 2018, 379, 334–349. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, F.; Cairns, C.M.; Gordon, J.R.; Xiang, J. Neutrophils and B Cells Express XCR1 Receptor and Chemotactically Respond to Lymphotactin. Biochem. Biophys. Res. Commun. 2001, 281, 378–382. [Google Scholar] [CrossRef]
- Qin, M.; Chen, C.; Wang, N.; Yu, D.; Yu, S.; Wang, X.; Liu, T.; Lv, L.; Guan, Q. Total Saponins of Panax Ginseng via the CX3CL1/CX3CR1 Axis Attenuates Neuroinflammation and Exerted Antidepressant-like Effects in Chronic Unpredictable Mild Stress in Rats. Phyther. Res. 2022, 37, 1823–1838. [Google Scholar] [CrossRef]
- Verge, G.M.; Milligan, E.D.; Maier, S.F.; Watkins, L.R.; Naeve, G.S.; Foster, A.C. Fractalkine (CX3CL1) and Fractalkine Receptor (CX3CR1) Distribution in Spinal Cord and Dorsal Root Ganglia under Basal and Neuropathic Pain Conditions. Eur. J. Neurosci. 2004, 20, 1150–1160. [Google Scholar] [CrossRef]
- Zhuang, Z.; Kawasaki, Y.; Tan, P.H.; Wen, Y.R.; Huang, J.; Ji, R.R. Role of the CX3CR1/P38 MAPK Pathway in Spinal Microglia for the Development of Neuropathic Pain Following Nerve Injury-Induced Cleavage of Fractalkine. Brain Behav. Immun. 2007, 21, 642–651. [Google Scholar] [CrossRef] [Green Version]
- Milligan, E.; Zapata, V.; Schoeniger, D.; Chacur, M.; Green, P.; Poole, S.; Martin, D.; Maier, S.F.; Watkins, L.R. An Initial Investigation of Spinal Mechanisms Underlying Pain Enhancement Induced by Fractalkine, a Neuronally Released Chemokine. Eur. J. Neurosci. 2005, 22, 2775–2782. [Google Scholar] [CrossRef] [PubMed]
- Staniland, A.A.; Clark, A.K.; Wodarski, R.; Sasso, O.; Maione, F.; D’Acquisto, F.; Malcangio, M. Reduced Inflammatory and Neuropathic Pain and Decreased Spinal Microglial Response in Fractalkine Receptor (CX3CR1) Knockout Mice. J. Neurochem. 2010, 114, 1143–1157. [Google Scholar] [CrossRef]
- Sessler, K.; Blechschmidt, V.; Hoheisel, U.; Mense, S.; Schirmer, L.; Treede, R.D. Spinal Cord Fractalkine (CX3CL1) Signaling Is Critical for Neuronal Sensitization in Experimental Nonspecific, Myofascial Low Back Pain. J. Neurophysiol. 2021, 125, 1598–1611. [Google Scholar] [CrossRef] [PubMed]
- Holmes, F.E.; Arnott, N.; Vanderplank, P.; Kerr, N.C.H.; Longbrake, E.E.; Popovich, P.G.; Imai, T.; Combadière, C.; Murphy, P.M.; Wynick, D. Intra-Neural Administration of Fractalkine Attenuates Neuropathic Pain-Related Behaviour. J. Neurochem. 2008, 106, 640–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noh, C.; Shin, H.J.; Lee, S.; Kim, S.I.; Kim, Y.H.; Lee, W.H.; Kim, D.W.; Lee, S.Y.; Ko, Y.K. CX3CR1-Targeted PLGA Nanoparticles Reduce Microglia Activation and Pain Behavior in Rats with Spinal Nerve Ligation. Int. J. Mol. Sci. 2020, 21, 3469. [Google Scholar] [CrossRef]
- Ruff, M.R.; Inan, S.; Shi, X.Q.; Meissler, J.J.; Adler, M.W.; Eisenstein, T.K.; Zhang, J. Potentiation of Morphine Antinociception and Inhibition of Diabetic Neuropathic Pain by the Multi-Chemokine Receptor Antagonist Peptide RAP-103. Life Sci. 2022, 306, 120788. [Google Scholar] [CrossRef]
- Padi, S.S.V.; Shi, X.Q.; Zhao, Y.Q.; Ruff, M.R.; Baichoo, N.; Pert, C.B.; Zhang, J. Attenuation of Rodent Neuropathic Pain by an Orally Active Peptide, RAP-103, Which Potently Blocks CCR2- and CCR5-Mediated Monocyte Chemotaxis and Inflammation. Pain 2012, 153, 95–106. [Google Scholar] [CrossRef]
- Laura, B.; Elisabetta, B.; Adelchi, R.P.; Roberto, R.; Loredana, C.; Andrea, A.; d’Angelo Michele; Vanessa, C.; Antonio, G.; Marcello, A.; et al. CXCR1/2 Pathways in Paclitaxel-Induced Neuropathic Pain. Oncotarget 2017, 8, 23188–23201. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.S.; Choi, Y.I.; Park, S.U.; Han, Y.S.; Kwon, J.; Jung, S.J. Prevention of Chemotherapy-Induced Peripheral Neuropathy by Inhibiting C-X-C Motif Chemokine Receptor 2. Int. J. Mol. Sci. 2023, 24, 1855. [Google Scholar] [CrossRef]
- Wise, E.L.; Duchesnes, C.; Da Fonseca, P.C.A.; Allen, R.A.; Williams, T.J.; Pease, J.E. Small Molecule Receptor Agonists and Antagonists of CCR3 Provide Insight into Mechanisms of Chemokine Receptor Activation. J. Biol. Chem. 2007, 282, 27935–27943. [Google Scholar] [CrossRef] [Green Version]
- Cherney, R.J.; Anjanappa, P.; Selvakumar, K.; Batt, D.G.; Brown, G.D.; Rose, A.V.; Vuppugalla, R.; Chen, J.; Pang, J.; Xu, S.; et al. BMS-813160: A Potent CCR2 and CCR5 Dual Antagonist Selected as a Clinical Candidate. ACS Med. Chem. Lett. 2021, 12, 1753–1758. [Google Scholar] [CrossRef] [PubMed]
- Gale, J.D.; Gilbert, S.; Blumenthal, S.; Elliott, T.; Pergola, P.E.; Goteti, K.; Scheele, W.; Perros-Huguet, C. Effect of PF-04634817, an Oral CCR2/5 Chemokine Receptor Antagonist, on Albuminuria in Adults with Overt Diabetic Nephropathy. Kidney Int. Rep. 2018, 3, 1316–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salanga, C.L.; O’Hayre, M.; Handel, T. Modulation of Chemokine Receptor Activity through Dimerization and Crosstalk. Cell. Mol. Life Sci. 2009, 66, 1370–1386. [Google Scholar] [CrossRef] [Green Version]
- Pranzatelli, M.R. Advances in Biomarker-Guided Therapy for Pediatric- and Adult-Onset Neuroinflammatory Disorders: Targeting Chemokines/Cytokines. Front. Immunol. 2018, 9, 557. [Google Scholar] [CrossRef]
- Kiguchi, N.; Maeda, T.; Kobayashi, Y.; Fukazawa, Y.; Kishioka, S. Macrophage Inflammatory Protein-1alpha Mediates the Development of Neuropathic Pain Following Peripheral Nerve Injury through Interleukin-1beta up-Regulation. Pain 2010, 149, 305–315. [Google Scholar] [CrossRef]
- Thompson, M.; Saag, M.; Dejesus, E.; Gathe, J.; Lalezari, J.; Landay, A.L.; Cade, J.; Enejosa, J.; Lefebvre, E.; Feinberg, J. A 48-Week Randomized Phase 2b Study Evaluating Cenicriviroc versus Efavirenz in Treatment-Naive HIV-Infected Adults with C-C Chemokine Receptor Type 5-Tropic Virus. AIDS 2016, 30, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Bertini, R.; Allegretti, M.; Bizzarri, C.; Moriconi, A.; Locati, M.; Zampella, G.; Cervellera, M.N.; Di Cioccio, V.; Cesta, M.C.; Galliera, E.; et al. Noncompetitive Allosteric Inhibitors of the Inflammatory Chemokine Receptors CXCR1 and CXCR2: Prevention of Reperfusion Injury. Proc. Natl. Acad. Sci. USA 2004, 101, 11791–11796. [Google Scholar] [CrossRef]
- Meesawatsom, P.; Hathway, G.; Bennett, A.; Constantin-Teodosiu, D.; Chapman, V. Spinal Neuronal Excitability and Neuroinflammation in a Model of Chemotherapeutic Neuropathic Pain: Targeting the Resolution Pathways. J. Neuroinflamm. 2020, 17, 316. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Tomonaga, D.; Kitazono, K.; Yoshioka, Y.; Liu, J.; Rousseau, J.P.; Kinkead, R.; Ruff, M.R.; Pert, C.B. Neuropathic Pain Inhibitor, RAP-103, Is a Potent Inhibitor of Microglial CCL1/CCR8. Neurochem. Int. 2018, 119, 184–189. [Google Scholar] [CrossRef]
- Bongiovanni, A.R.; Zhao, P.; Inan, S.; Wiah, S.; Shekarabi, A.; Farkas, D.J.; Watson, M.N.; Wimmer, M.E.; Ruff, M.R.; Rawls, S.M. Multi-Chemokine Receptor Antagonist RAP-103 Inhibits Opioid-Derived Respiratory Depression, Reduces Opioid Reinforcement and Physical Dependence, and Normalizes Opioid-Induced Dysregulation of Mesolimbic Chemokine Receptors in Rats. Drug Alcohol Depend. 2022, 238, 109556. [Google Scholar] [CrossRef] [PubMed]

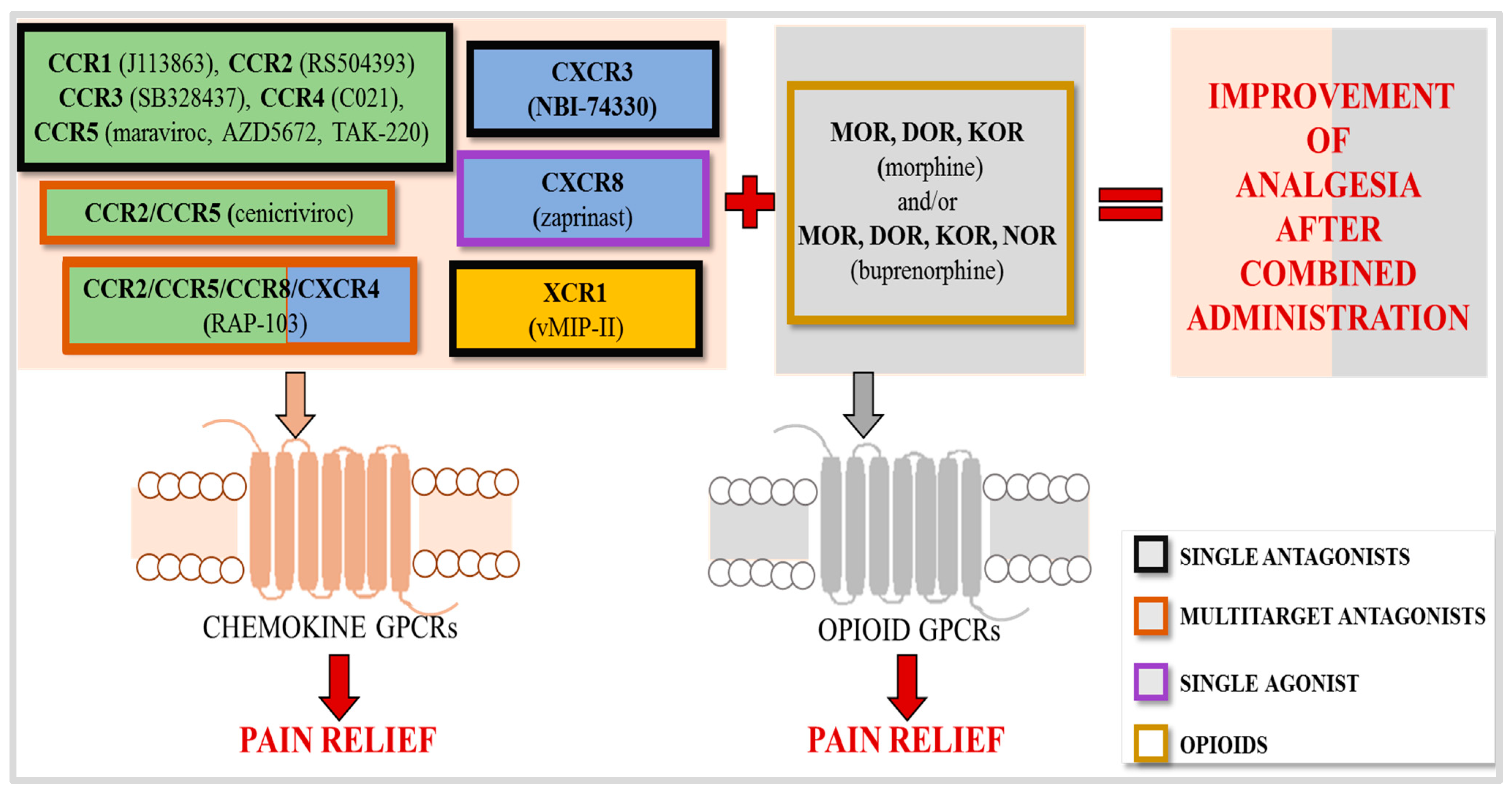
| Chemokine | Chemokine Receptors | Chemokine-Evoked Hypersensitivity | nAb against Chemokine-Evoked Analgesia |
|---|---|---|---|
| CCL1, I-309 | CCR8 | YES [13,14] | PSNL [14], STZ [13] |
| CCL2, MCP-1 | CCR1, CCR2, CCR4 | YES [15,16,17] | CCI [15], OX [18] |
| CCL3, MIP-1α | CCR1, CCR5 | YES [16,19,20,21] | STZ [19], PTX [22], CCI [15,23], PSNL [21] |
| CCL4, MIP-1β | CCR1, CCR5 | YES [19] | PSNL [24] |
| CCL5, RA—NTES | CCR1, CCR3, CCR5 | YES [16] | L5Tx [25], CCI [26] |
| CCL6, - | CCR1 | ND | ND |
| CCL7, MCP3 | CCR1, CCR2, CCR3, CCR5 | YES [15,16] | CCI [15] |
| CCL8, MCP-2 | CCR1, CCR2, CCR3, CCR5 | YES [16] | ND |
| CCL9/, MIP-1γ, CCL10 | CCR1 | YES [16,19] | STZ [19] |
| CCL11, Eotaxin | CXCR3, CCR3, CCR5 | ND | ND |
| CCL12, MCP-5 | CCR2 | NO [15] | ND |
| CCL13, MCP-4 | CCR1, CCR2, CCR3, CCR5 | NOT PRESENT IN MICE/RATS | |
| CCL14, HCC-1 | CCR1 | NOT PRESENT IN MICE/RATS | |
| CCL15, HCC-2 | CCR1, CCR3 | NOT PRESENT IN MICE/RATS | |
| CCL16, HCC-4 | CCR1 | NOT PRESENT IN MICE/RATS | |
| CCL17, TARC | CCR4 | YES [27] | ND |
| CCL18, DC-CK1 | CCR8 | NOT PRESENT IN MICE/RATS | |
| CCL19, MIP-3β | CCR7 | ND | ND |
| CCL20, MIP-3α | CCR6 | ND | ND |
| CCL21, 6Ckine | CXCR3, CCR7 | YES [28,29] | CCI [28] SNI [30] BTZ [29] |
| CCL22, MDC | CCR4 | YES [27] | ND |
| CCL23, MPIF-1 | CCR1 | NOT PRESENT IN MICE/RATS | |
| CCL24, Eotaxin-2 | CCR3 | ND | ND |
| CCL25, TECK | CCR9, CCR10 | ND | ND |
| CCL26, Eotaxin-3 | CCR3, CCR10 | ND | ND |
| CCL27, CTACK | CCR10 | ND | ND |
| CCL28, MEC | CCR3, CCR10 | ND | ND |
| CXCL1, GROα, CINC-1 | CXCR2 | YES [31] | SNL [32] |
| CXCL2, GROβ, CINC-3 | CXCR2 | YES [31] | PSNL [33] |
| CXCL3, GROγ, CINC-2 | CXCR2 | YES [31] | CCI [31] |
| CXCL4, PF4 | CXCR3 | YES [28] | ND |
| CXCL5, ENA-78 | CXCR1, CXCR2 | YES [34] | CCI [34] |
| CXCL6, GCP-2 | CXCR1, CXCR2 | ND | ND |
| CXCL7, NAP-2 | CXCR1, CXCR2 | ND | ND |
| CXCL8, IL-8 | CXCR1, CXCR2 | ND | ND |
| CXCL9, Mig | CXCR3 | YES [28] | CCI [28] |
| CXCL10, IP-10 | CXCR3 | YES [28,35] | CCI [28], CIBP [36] |
| CXCL11, I-TAC | CXCR3 | YES [28] | ND |
| CXCL12, SDF-1α/β | CXCR4 | YES [37] | SpNI [38], SNL [39] |
| CXCL13, BCA-1 | CXCR5 | YES [40] | CPIP [41] |
| CXCL14, BRAK | CXCR4 | ND | ND |
| CXCL15, Lungkine | Unknown | ND | ND |
| CXCL16, SCYB16 | CXCR6 | ND | ND |
| CXCL17, VCC-1 | CXCR8 (GPR35) | YES [42] | ND |
| XCL1, SCM-1α | XCR1 | YES [43,44] | CCI [43] STZ [44] |
| CX3CL1, Fractalkine | CX3CR1 | YES [45] | OX [46] |
| Chemokine | Upregulation in Neuropathic Pain Models | |||
|---|---|---|---|---|
| mRNA | Structures/Models | Protein | Structures/Models | |
| CCL1 | YES | SC, DRG/PSNL [14], DRG/CCI [50] | YES | SC/PSNL [14], SC/STZ [13] |
| CCL2 | YES | DRG/CCI [50,51], SC, DRG/CCI [52,53], SC/CCI [16] | YES | SC, DRG/CCI [52], SC/CCI [16], DRG/CCI [51], DRG/OX [18], SC/SCI [54] |
| CCL3 | YES | SC, DRG/CCI [55,56], SC/CCI [53,57] | YES | SC/CCI [16,57], SC/STZ [19] |
| CCL4 | YES | SC/CCI [16,53,57] | NO | SC/CCI [16] |
| CCL5 | YES | SC/CCI [16] | YES | SC/CCI [16,26] |
| CCL6 | YES | SC, DRG/CCI [53,58], SC/CCI [16] | NO | SC/CCI [16] |
| CCL7 | YES | SC, DRG/CCI [23,53,58], SC/CCI [16], SC/PSNL [59] | YES | SC, DRG/CCI [23], SC/CCI [16] |
| CCL8 | YES | SC/CCI [16] | YES | SC/CCI [16] |
| CCL9 | YES | SC, DRG/CCI [53], SC/CCI [16,57] | YES | SC/CCI [16,57], SC/STZ [19] |
| CCL11 | YES | SC, DRG/CCI [23] | YES | DRG/CCI [23] |
| CCL12 | YES | SC, DRG/CCI [60] SC [15] | ND | ND |
| CCL13 | NOT PRESENT IN MICE/RATS | |||
| CCL14 | NOT PRESENT IN MICE/RATS | |||
| CCL15 | NOT PRESENT IN MICE/RATS | |||
| CCL16 | NOT PRESENT IN MICE/RATS | |||
| CCL17 | NO | SC/CCI [27] | ND | ND |
| CCL18 | NOT PRESENT IN MICE/RATS | |||
| CCL19 | ND | ND | ND | ND |
| CCL20 | ND | ND | ND | ND |
| CCL21 | YES | SC/BTZ [29] | YES | SC/SNI [61], DRG/SNI [30], SC/BTZ [29] |
| CCL22 | NO | SC/CCI [27] | ND | ND |
| CCL23 | NOT PRESENT IN MICE/RATS | |||
| CCL24 | NO | SC, DRG/CCI [23], SC/CCI [16] | ND | ND |
| CCL25 | ND | ND | ND | ND |
| CCL26 | YES | SC/CCI [23] | ND | ND |
| CCL27 | ND | ND | ND | ND |
| CCL28 | NO | SC/CCI [16] | ND | ND |
| CXCL1 | YES | SC/SNL [32], SC/STZ [62], SC/CCI [31] | YES | SC/SNL [32], SC/STZ [62] |
| CXCL2 | YES | SC/CCI [31], SCN/PSNL [33], SN/STZ [63] | YES | SN/STZ [63] |
| CXCL3 | YES | SC/CCI [31] | YES | SC, DRG/CCI [31] |
| CXCL4 | YES | SC, DRG/CCI [28] | YES | SC, DRG/CCI [28] |
| CXCL5 | YES | SC/CCI [34] | YES | SC/STZ [62] |
| CXCL6 | ND | ND | NO | CSF/CCI [64] |
| CXCL7 | ND | ND | ND | ND |
| CXCL8 | YES | DRG/LDH [65] | YES | SN/CCI [66] |
| CXCL9 | YES | DRG/CCI [28] SC/STZ [62], DRG/SNL [67] | YES | SC, DRG/CCI [28], SC/STZ [62] |
| CXCL10 | YES | SC/CCI [28], DRG/SNL [67] | YES | SC/CCI [28,35] |
| CXCL11 | YES | SC, DRG/CCI [28], DRG/SNL [67,68], SC/STZ [62] | YES | SC/CCI [28] |
| CXCL12 | YES | DRG/CCD [69] | YES | SC/STZ [62], SC/SNL [39], SC/PSNL/CPIP [37], SC/SpNI [38], ACC/STZ [70] |
| CXCL13 | YES | SC/SNL [40], SC/DB [71], DRG/SNL [68,72], DRG/SpNI [73], SC/TNFα [74] | YES | SC/SNL [40], SC/DB [71], SC, DRG,PS/CCI [75], SC/TNFα [74] |
| CXCL14 | YES | SC/PTX [76], DRG/SNL [68] | YES | SC/PTX [76] |
| CXCL15 | ND | ND | ND | ND |
| CXCL16 | ND | ND | ND | ND |
| CXCL17 | ND | ND | ND | ND |
| XCL1 | YES | SC/CCI [43] | YES | SC/CCI [43], SC/STZ [44] |
| CX3CL1 | YES | DRG/OX [46], SC/TNFα [74] | YES | DRG/OX [46,77], DRG/CCI [77], SC/TNFα [74] |
| Receptor | Antagonist | Neuropathic Pain Relief in Mouse/Rat Models/Route of Drug Administration | Enhancement of Opioid Effectiveness in Neuropathy |
|---|---|---|---|
| The CC Receptors | |||
| CCR1 | J113863 | CCI/i.t. [16,53] STZ/i.t [19] | YES [19,53] ND |
| BX513 | SSNL/i.t. [20] | ND | |
| BX471 | ND | ND | |
| BAY86-5047 | ND | ND | |
| CCR2 | RS504393 | CCI/i.t. [52,202] PTX/s.c. [203] IAMNT/ic. [204] | YES [52] ND ND |
| AZ889 | CCI/ p.o. [205] | ND | |
| INCB 3284 | ND | ND | |
| RS 102895 | ND | ND | |
| MK-0812 | ND | ND | |
| PF-4136309 | ND | ND | |
| CCR3 | SB328437 | CCI/i.t. [16,23] | YES [23] |
| SB 297006 | ND | ND | |
| GW 766994 | ND | ND | |
| INCB 3284 | ND | ND | |
| CCR4 | C021 | CCI/it./i.p. [27,80] STZ/i.t/i.p. [79] | YES [27,80] YES [79] |
| AZD 2098 | ND | ND | |
| GSK 2239633A | ND | ND | |
| CCR5 | Maraviroc | CCI/i.t./i.p. [56,60,206] | YES [56,60] |
| AZD5672 | CCI/i.t. [57] | YES [57] | |
| TAK-220 | CCI/i.t. [57] | YES [57] | |
| Vicriviroc | ND | ND | |
| CCR6 | PF-07054894 | ND | ND |
| CCR7 | - | ND | ND |
| CCR8 | AZ084 | ND | ND |
| R243 | ND | ND | |
| CCR9 | Vercirnon | ND | ND |
| MLN3126 | ND | ND | |
| CCR10 | BI-6901 | ND | ND |
| Receptor | Antagonist/ Agonist | Neuropathic Pain Relief in Mouse/Rat Models | Enhancement of Opioid Effectiveness in Neuropathic Pain |
|---|---|---|---|
| The CXC Receptors | |||
| CXCR1 | - | ND | ND |
| CXCR2 | NVP CXCR2 20 | CCI/i.t. [31,58] | NO [31,58] |
| SB225002 | PTX/i.t/i.p. [280] SNL/i.t [32] OFP/tg [281] PSL/pn [33] L5-SNL/ns [282] | ND ND ND ND ND | |
| SB 332235 | ND | ND | |
| SB 265610 | ND | ND | |
| AZ10397767 | ND | ND | |
| AZD 5069 | ND | ND | |
| Danirixin | ND | ND | |
| CXCR3 | NBI-74330 | CCI/i.t. [28,58] | YES [28] |
| AMG487 | CCI/acc [283] | ND | |
| CXCR4 | AMD 3100 (Plerixafor) | L5-SNL/i.p. [284] CCD/ive [285] CPIP/i.t. [37] | ND ND ND |
| AMD 3465 | SNL/i.t. [37] CPIP/i.t. [37] | ND ND | |
| CTCE 9908 | ND | ND | |
| KRH 3955 | ND | ND | |
| IT1t | ND | ND | |
| WZ811 | ND | ND | |
| Mavorixafor | ND | ND | |
| BKT 140 | ND | ND | |
| MSX-122 | ND | ND | |
| CXCR5 | - | ND | ND |
| CXCR6 | ML-339 | ND | ND |
| CXCR7 | - | ND | ND |
| CXCR8 | Zaprinast | CCI/i.t. [42,286] | YES [42,286] |
| THE XC RECEPTOR | |||
| XCR1 | vMIP-II | CCI/i.t. [43] | YES [43] |
| THE CX3C RECEPTOR | |||
| CX3CR1 | JMS 17-2 | ND | ND |
| Receptor | Multitarget Antagonist/ Inhibitor | Neuropathic Pain Relief in Mouse/Rat Models | Enhancement of Opioid Effectiveness in Neuropathic Pain |
|---|---|---|---|
| CCR1/CCR3 | UCB 35625 | CCI/i.t. [16] | ND |
| CCR2/CCR5 | Cenicriviroc | CCI/i.t./i.p. [55,60] | YES [55] |
| BMS-813160 | ND | ND | |
| PF-04634817 | ND | ND | |
| CCR2/CCR5/CXCR3 | TAK 779 | ND | ND |
| CCR2/CCR5/CCR8/ CXCR4 | RAP-103 | STZ/i.p. [340] PSNI/p.o. [341] | YES [340] |
| CXCR1/CXCR2 | Reparixin | PTX/m.o.p. [342] OX/i.t./i.p. [343] | ND |
| Navarixin | ND | ND | |
| SCH 563705 | ND | ND | |
| Ladarixin | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlik, K.; Mika, J. Targeting Members of the Chemokine Family as a Novel Approach to Treating Neuropathic Pain. Molecules 2023, 28, 5766. https://doi.org/10.3390/molecules28155766
Pawlik K, Mika J. Targeting Members of the Chemokine Family as a Novel Approach to Treating Neuropathic Pain. Molecules. 2023; 28(15):5766. https://doi.org/10.3390/molecules28155766
Chicago/Turabian StylePawlik, Katarzyna, and Joanna Mika. 2023. "Targeting Members of the Chemokine Family as a Novel Approach to Treating Neuropathic Pain" Molecules 28, no. 15: 5766. https://doi.org/10.3390/molecules28155766






