Intensifying Electrochemical Activity of Ti3C2Tx MXene via Customized Interlayer Structure and Surface Chemistry
Abstract
:1. Introduction
2. Results and Discussion
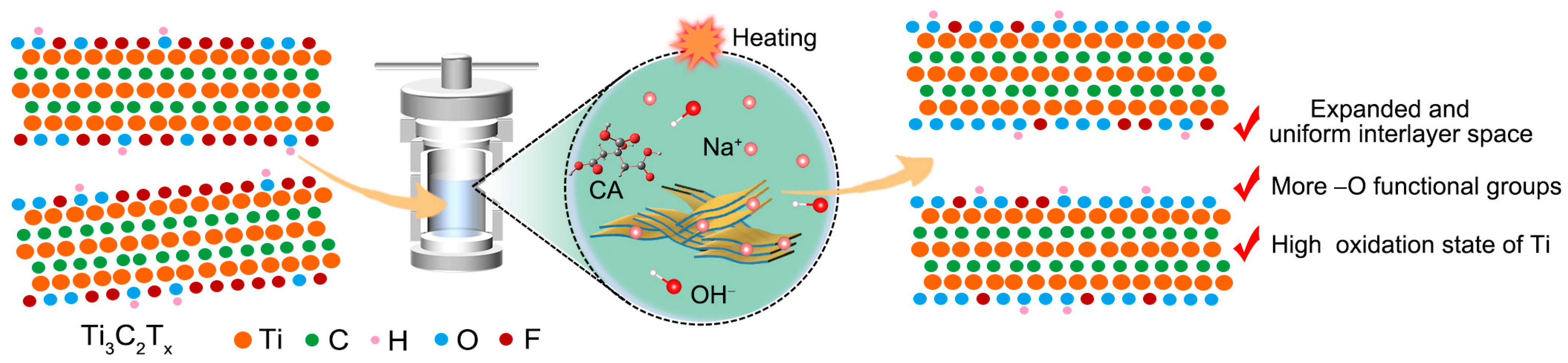
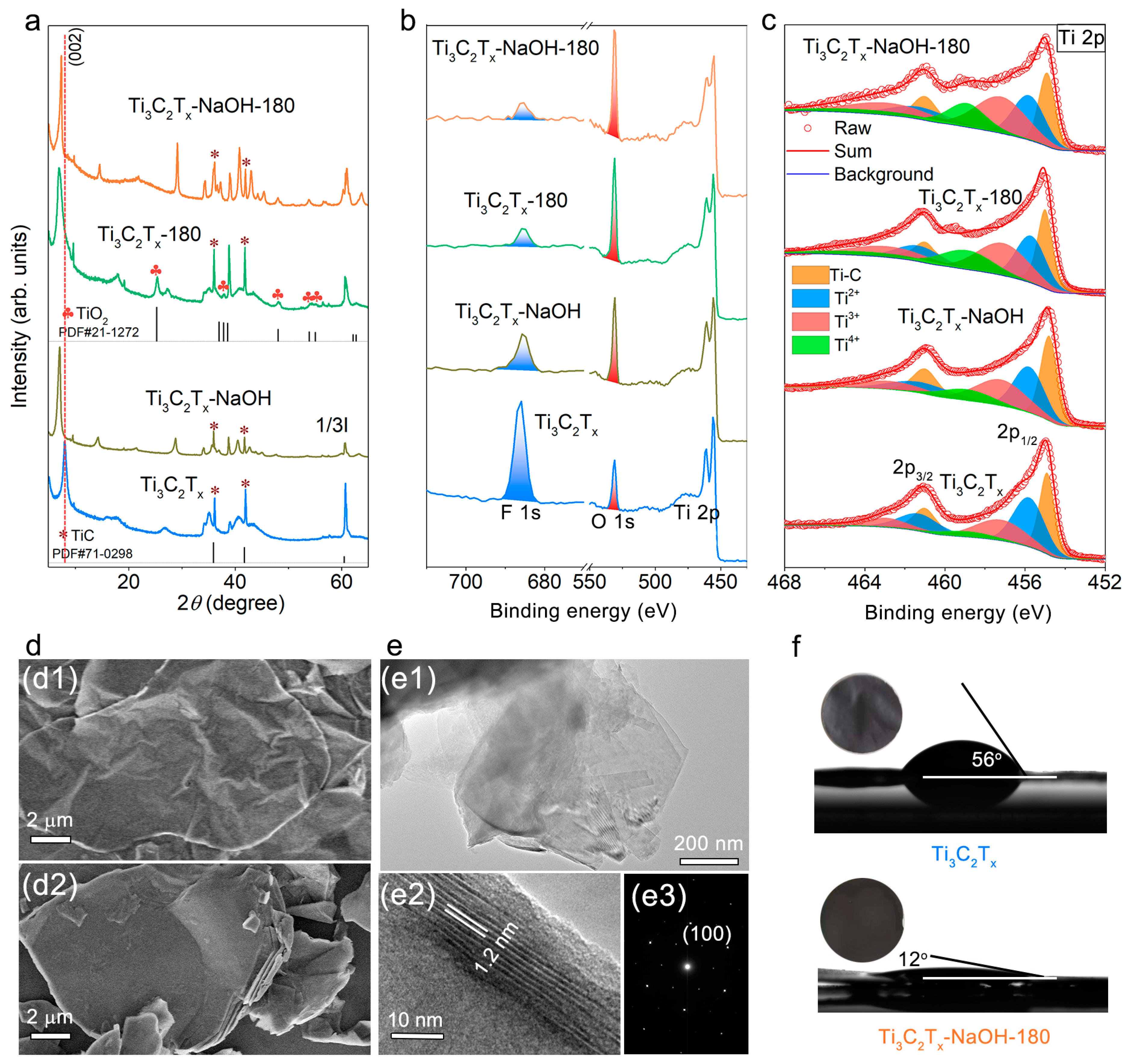
2.1. Material Characterization
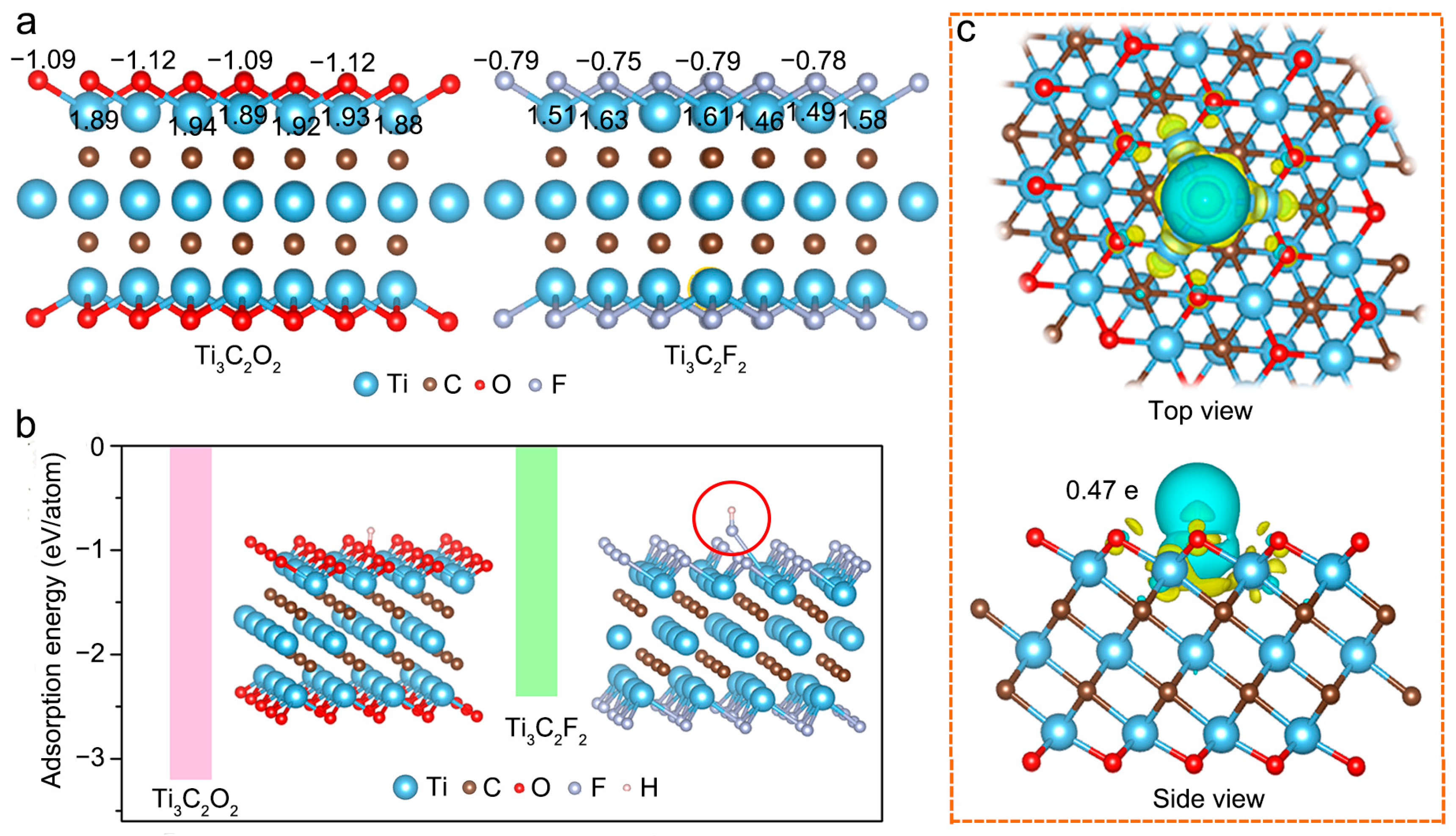
2.2. Density Functional Theory Calculations
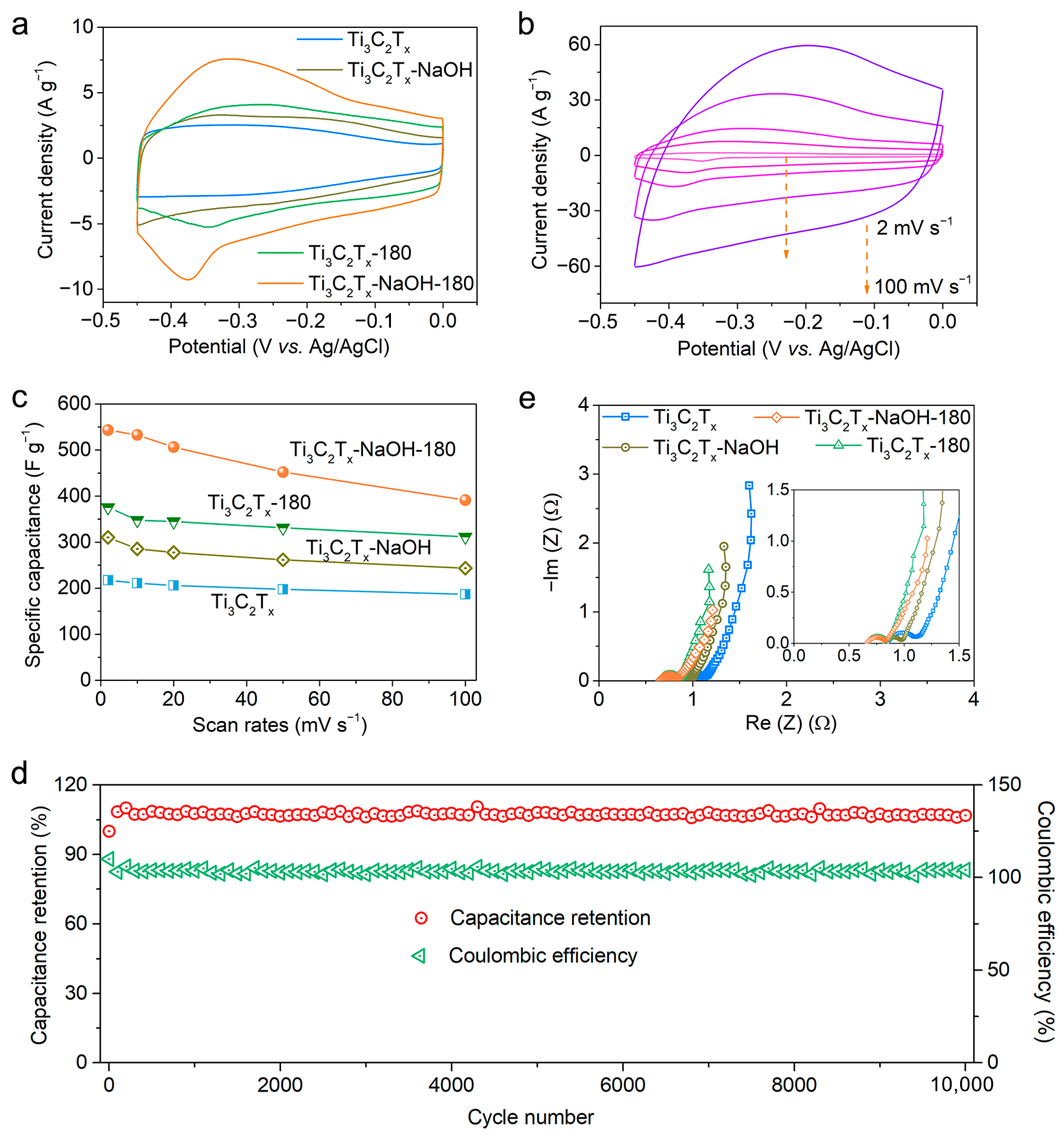
2.3. Electrochemical Performances of Electrodes
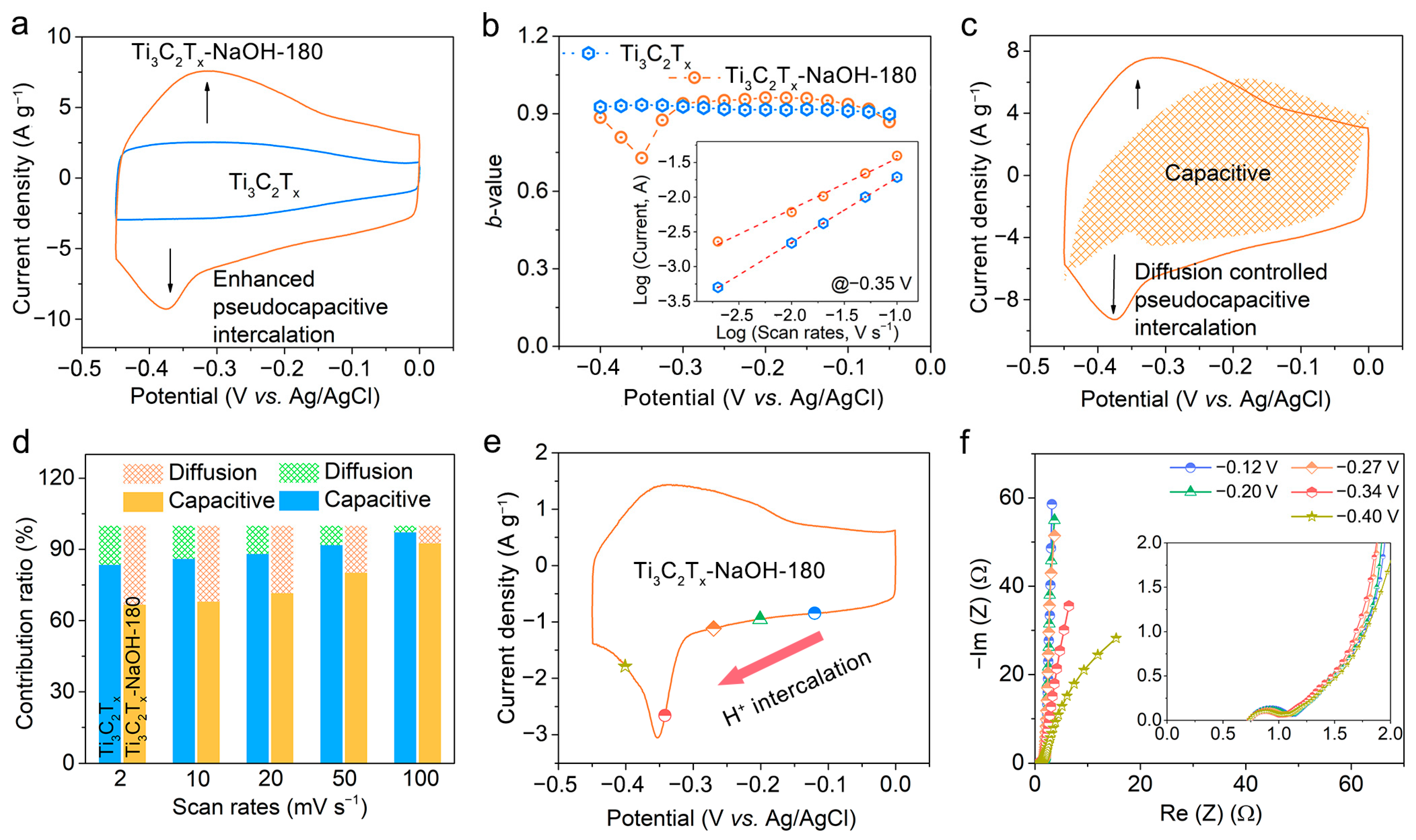
2.4. Kinetic and In Situ Electrochemical EIS Analysis
2.5. Ex Situ XRD Analysis
3. Experimental Section
3.1. Synthesis of Ti3C2Tx MXene
3.2. Modification of Interlayer Structure and Surface Chemical State
3.3. Material Characterizations
3.4. Electrochemical Measurements
3.5. Ex Situ Electrochemical XRD
3.6. Computational Detail
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, X.; Mathis, T.S.; Sun, Y.; Tsai, W.Y.; Shpigel, N.; Shao, H.; Zhang, D.; Hantanasirisakul, K.; Malchik, F.; Balke, N.; et al. Titanium carbide MXene shows an electrochemical anomaly in water-in-salt electrolytes. ACS Nano 2021, 15, 15274–15284. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Ashby, D.S.; Butts, D.M.; DeBlock, R.H.; Wei, Q.; Lau, J.; Dunn, B. Achieving high energy density and high power density with pseudocapacitive materials. Nat. Rev. Mater. 2020, 5, 5–19. [Google Scholar] [CrossRef]
- Pataniya, P.M.; Dabhi, S.; Patel, V.; Sumesh, C.K. Liquid phase exfoliated ReS2 nanocrystals on paper based electrodes for hydrogen evolution and supercapacitor applications. Surf. Interfaces 2022, 34, 102318. [Google Scholar] [CrossRef]
- Wang, X.; Bak, S.-M.; Han, M.; Shuck, C.E.; McHugh, C.; Li, K.; Li, J.; Tang, J.; Gogotsi, Y. Surface redox pseudocapacitance of partially oxidized titanium carbide MXene in water-in-salt electrolyte. ACS Energy Lett. 2021, 7, 30–35. [Google Scholar] [CrossRef]
- Modi, K.H.; Pataniya, P.M.; Siraj, S.; Sahatiya, P.; Patel, V.; Sumesh, C.K. Synergistic effect from Ni2+ ions with SnS for all solid-state type symmetric supercapacitor. J. Energy Storage 2023, 63, 107040. [Google Scholar] [CrossRef]
- Lin, Z.; Goikolea, E.; Balducci, A.; Naoi, K.; Taberna, P.L.; Salanne, M.; Yushin, G.; Simon, P. Materials for supercapacitors: When Li-ion battery power is not enough. Mater. Today 2018, 21, 419–436. [Google Scholar] [CrossRef] [Green Version]
- Fleischmann, S.; Mitchell, J.B.; Wang, R.; Zhan, C.; Jiang, D.E.; Presser, V.; Augustyn, V. Pseudocapacitance: From fundamental understanding to high power energy storage materials. Chem. Rev. 2020, 120, 6738–6782. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Li, X.; Huang, Z.; Shuck, C.E.; Liang, G.; Gogotsi, Y.; Zhi, C. MXene chemistry, electrochemistry and energy storage applications. Nat. Rev. Chem. 2022, 6, 389–404. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Xiao, X. Intercalation in two-dimensional transition metal carbides and nitrides (MXenes) toward electrochemical capacitor and beyond. Energy Environ. Mater. 2020, 3, 306–322. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, J.; Das, P.; Wang, S.; Wu, Z.-S. High-voltage MXene-based supercapacitors: Present status and future perspectives. Small Methods 2023, 2201609. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.K.; Niu, Y.; Xu, M. MXenes for non-lithium-ion (Na, K, Ca, Mg, and Al) batteries and supercapacitors. Adv. Energy Mater. 2021, 11, 2000681. [Google Scholar] [CrossRef]
- Zang, X.; Wang, J.; Qin, Y.; Wang, T.; He, C.; Shao, Q.; Zhu, H.; Cao, N. Enhancing capacitance performance of Ti3C2Tx MXene as electrode materials of supercapacitor: From controlled preparation to composite structure construction. Nanomicro Lett. 2020, 12, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Ma, Y.; Zhang, X.; Abdolhosseinzadeh, S.; Sheng, H.; Lan, W.; Pakdel, A.; Heier, J.; Nüesch, F. Two-dimensional transition metal carbides and nitrides (MXenes): Synthesis, properties, and electrochemical energy storage applications. Energy Environ. Mater. 2019, 3, 29–55. [Google Scholar] [CrossRef]
- Yu, X.; Yun, S.; Yeon, J.S.; Bhattacharya, P.; Wang, L.; Lee, S.W.; Hu, X.; Park, H.S. Emergent pseudocapacitance of 2D nanomaterials. Adv. Energy Mater. 2018, 8, 1702930. [Google Scholar] [CrossRef]
- Hu, M.; Dai, J.; Chen, L.; Meng, A.; Wang, L.; Li, G.; Xie, H.; Li, Z. Selectivity for intercalated ions in MXene toward a high-performance capacitive electrode. Sci. China Mater. 2023, 66, 974–981. [Google Scholar] [CrossRef]
- Boota, M.; Pasini, M.; Galeotti, F.; Porzio, W.; Zhao, M.-Q.; Halim, J.; Gogotsi, Y. Interaction of polar and nonpolar polyfluorenes with layers of two-dimensional titanium carbide (MXene): Intercalation and pseudocapacitance. Chem. Mater. 2017, 29, 2731–2738. [Google Scholar] [CrossRef]
- Mashtalir, O.; Lukatskaya, M.R.; Kolesnikov, A.I.; Raymundo-Pinero, E.; Naguib, M.; Barsoum, M.W.; Gogotsi, Y. Effect of hydrazine intercalation on structure and capacitance of 2D titanium carbide (MXene). Nanoscale 2016, 8, 9128–9133. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Lin, C.; Yang, Y.; Xu, L.; Du, X.; Xie, J.; Lin, J.; Sun, J. Achieving high pseudocapacitance of 2D titanium carbide (MXene) by cation intercalation and surface modification. Adv. Energy Mater. 2017, 7, 1602725. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Dall'Agnese, Y.; Cook, K.M.; Taberna, P.-L.; Gogotsi, Y.; Simon, P. High capacitance of surface-modified 2D titanium carbide in acidic electrolyte. Electrochem. Commun. 2014, 48, 118–122. [Google Scholar]
- Hu, M.; Li, Z.; Zhang, H.; Hu, T.; Zhang, C.; Wu, Z.; Wang, X. Self-assembled Ti3C2Tx MXene film with high gravimetric capacitance. Chem. Commun. 2015, 51, 13531–13533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Y.; Rufford, T.E.; Chen, X.; Li, N.; Lyu, M.; Dai, L.; Wang, L. Nitrogen-doped Ti3C2Tx MXene electrodes for high-performance supercapacitors. Nano Energy 2017, 38, 368–376. [Google Scholar] [CrossRef]
- Yang, C.; Tang, Y.; Tian, Y.; Luo, Y.; Faraz Ud Din, M.; Yin, X.; Que, W. Flexible nitrogen-doped 2D titanium carbides (MXene) films constructed by an ex situ solvothermal method with extraordinary volumetric capacitance. Adv. Energy Mater. 2018, 8, 1802087. [Google Scholar] [CrossRef]
- Shimada, T.; Takenaka, N.; Ando, Y.; Otani, M.; Okubo, M.; Yamada, A. Relationship between electric double-layer structure of MXene electrode and its surface functional groups. Chem. Mater. 2022, 34, 2069–2075. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Y.; Jing, Y.; Ma, J.; Du, C.F.; Yan, Q. Surface modified MXene-based nanocomposites for electrochemical energy conversion and storage. Small 2019, 15, e1901503. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Bak, S.-M.; Yu, X.; Yang, X.-Q.; Barsoum, M.W.; Gogotsi, Y. Probing the mechanism of high capacitance in 2D titanium carbide using in situ X-ray absorption spectroscopy. Adv. Energy Mater. 2015, 5, 1500589. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Kota, S.; Lin, Z.; Zhao, M.-Q.; Shpigel, N.; Levi, M.D.; Halim, J.; Taberna, P.-L.; Barsoum, M.W.; Simon, P.; et al. Ultra-high-rate pseudocapacitive energy storage in two-dimensional transition metal carbides. Nat. Energy 2017, 6, 17105. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhu, Y.; Zhang, M.; Sui, J.; Peng, W.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. N-butyllithium-treated Ti3C2Tx MXene with excellent pseudocapacitor performance. ACS Nano 2019, 13, 9449–9456. [Google Scholar] [CrossRef] [PubMed]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Tang, J.; Mathis, T.S.; Kurra, N.; Sarycheva, A.; Xiao, X.; Hedhili, M.N.; Jiang, Q.; Alshareef, H.N.; Xu, B.; Pan, F.; et al. Tuning the electrochemical performance of titanium carbide MXene by controllable in situ anodic oxidation. Angew. Chem. Int. Ed. 2019, 58, 17849–17855. [Google Scholar] [CrossRef] [Green Version]
- Halim, J.; Cook, K.M.; Naguib, M.; Eklund, P.; Gogotsi, Y.; Rosen, J.; Barsoum, M.W. X-ray photoelectron spectroscopy of select multi-layered transition metal carbides (MXenes). Appl. Surf. Sci. 2015, 362, 406–417. [Google Scholar] [CrossRef] [Green Version]
- Osti, N.C.; Naguib, M.; Ostadhossein, A.; Xie, Y.; Kent, P.R.C.; Dyatkin, B.; Rother, G.; Heller, W.T.; van Duin, A.C.T.; Gogotsi, Y.; et al. Effect of metal ion intercalation on the structure of MXene and water dynamics on its internal surfaces. ACS Appl. Mater. Interfaces 2016, 8, 8859–8863. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Dall’Agnese, Y.; Naguib, M.; Gogotsi, Y.; Barsoum, M.W.; Zhuang, H.L.; Kent, P.R.C. Prediction and characterization of MXene nanosheet anodes for non-lithium-ion batteries. ACS Nano 2014, 8, 9606–9615. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, Y.; Zhu, X.; Huang, C.; Kai, J.-J.; Fan, J. Theoretical investigation of the structure-property correlation of MXenes as anode materials for alkali metal ion batteries. J. Phys. Chem. C 2020, 124, 14978–14986. [Google Scholar] [CrossRef]
- Xie, Y.; Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y.; Yu, X.; Nam, K.-W.; Yang, X.-Q.; Kolesnikov, A.I.; Kent, P.R.C. Role of surface structure on Li-ion energy storage capacity of two-dimensional transition-metal carbides. J. Am. Chem. Soc. 2014, 136, 6385–6394. [Google Scholar] [CrossRef]
- Béguin, F.; Frąckowiak, E. Supercapacitors: Materials, Systems, and Applications; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (Anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Levi, M.D.; Lukatskaya, M.R.; Sigalov, S.; Beidaghi, M.; Shpigel, N.; Daikhin, L.; Aurbach, D.; Barsoum, M.W.; Gogotsi, Y. Solving the capacitive paradox of 2D MXene using electrochemical quartz-crystal admittance and in situ electronic conductance measurements. Adv. Energy Mater. 2015, 5, 1400815. [Google Scholar] [CrossRef]
- Ando, Y.; Okubo, M.; Yamada, A.; Otani, M. Capacitive versus pseudocapacitive storage in MXene. Adv. Funct. Mater. 2020, 30, 2000820. [Google Scholar] [CrossRef]
- Okubo, M.; Sugahara, A.; Kajiyama, S.; Yamada, A. MXene as a charge storage host. Acc. Chem. Res. 2018, 51, 591–599. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Xu, H.; Fan, J.; Su, H.; Liu, C.; Chen, G.; Dall’ Agnese, Y.; Gao, Y. Metal ion-induced porous MXene for all-solid-state flexible supercapacitors. Nano Lett. 2023, 23, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Bi, Y.; Jing, Y.; Dai, J.; Li, Z.; Sun, C.; Meng, A.; Xie, H.; Hu, M. Phosphorus doping strategy-induced synergistic modification of interlayer structure and chemical state in Ti3C2Tx toward enhancing capacitance. Molecules 2023, 28, 4892. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dai, J.; Li, Y.; Sun, C.; Meng, A.; Cheng, R.; Zhao, J.; Hu, M.; Wang, X. Intercalation-deintercalation design in MXenes for high performance supercapacitors. Nano Res. 2022, 15, 3213–3221. [Google Scholar] [CrossRef]
- Gupta, N.; Sahu, R.K.; Mishra, T.; Bhattacharya, P. Microwave-assisted rapid synthesis of titanium phosphate free phosphorus doped Ti3C2 MXene with boosted pseudocapacitance. J. Mater. Chem. A 2022, 10, 15794–15810. [Google Scholar] [CrossRef]
- Zhang, T.; Xiao, J.; Li, L.; Zhao, J.; Gao, H. A high-performance supercapacitor electrode based on freestanding N-doped Ti3C2Tx film. Ceram. Int. 2020, 46, 21482–21488. [Google Scholar] [CrossRef]
- Tian, Y.; Que, W.; Luo, Y.; Yang, C.; Yin, X.; Kong, L.B. Surface nitrogen-modified 2D titanium carbide (MXene) with high energy density for aqueous supercapacitor applications. J. Mater. Chem. A 2019, 7, 5416–5425. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, M.; Chen, L.; Jing, Y.; Zhu, Y.; Dai, J.; Meng, A.; Sun, C.; Jia, J.; Li, Z. Intensifying Electrochemical Activity of Ti3C2Tx MXene via Customized Interlayer Structure and Surface Chemistry. Molecules 2023, 28, 5776. https://doi.org/10.3390/molecules28155776
Hu M, Chen L, Jing Y, Zhu Y, Dai J, Meng A, Sun C, Jia J, Li Z. Intensifying Electrochemical Activity of Ti3C2Tx MXene via Customized Interlayer Structure and Surface Chemistry. Molecules. 2023; 28(15):5776. https://doi.org/10.3390/molecules28155776
Chicago/Turabian StyleHu, Minmin, Lihong Chen, Yunqi Jing, Yuanyuan Zhu, Jun Dai, Alan Meng, Changlong Sun, Jin Jia, and Zhenjiang Li. 2023. "Intensifying Electrochemical Activity of Ti3C2Tx MXene via Customized Interlayer Structure and Surface Chemistry" Molecules 28, no. 15: 5776. https://doi.org/10.3390/molecules28155776
APA StyleHu, M., Chen, L., Jing, Y., Zhu, Y., Dai, J., Meng, A., Sun, C., Jia, J., & Li, Z. (2023). Intensifying Electrochemical Activity of Ti3C2Tx MXene via Customized Interlayer Structure and Surface Chemistry. Molecules, 28(15), 5776. https://doi.org/10.3390/molecules28155776






