Optimisation of Polyphenols Extraction from Wild Bilberry Leaves—Antimicrobial Properties and Stability Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Design of Experiments
2.2. Spectrophotometric Characterisation of the Extracts
2.3. Experiments Modelling and Analysis of Variance (ANOVA)
2.4. Numerical Optimisation
2.5. Extracts Composition Determined by HPLC–PDA
2.6. Evaluation of the Extract Chemical Stability
2.7. Biological Evaluation of the E7×3 Extract
2.7.1. Antibacterial Activity on Planktonic Growth
2.7.2. The Antibacterial Activity on Biofilm Formation
3. Materials and Methods
3.1. Materials
3.2. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, H.; Qin, S.; Sirohi, R.; Ahluwalia, V.; Zhou, Y.; Sindhu, R.; Binod, P.; Singhnia, R.R.; Patel, A.K.; Juneja, A.; et al. Sustainable blueberry waste recycling towards biorefinery strategy and circular bioeconomy: A review. Bioresour. Technol. 2021, 332, 125181. [Google Scholar] [CrossRef]
- Blueberries, Romania (2015–2020). Available online: http://data.un.org/Data.aspx?d=FAO&f=itemCode%3a552%3bcountryCode%3a183%3byear%3a2015%2c2016%2c2017%2c2018%2c2019%2c2020&c=2,4,5,6,7&s=countryName:asc,elementCode:asc,year:desc&v=1 (accessed on 28 September 2022).
- Pérez, R.; Laca, A.; Laca, A.; Díaz, M. Environmental behaviour of blueberry production at small-scale in Northern Spain and improvement opportunities. J. Clean. Prod. 2022, 339, 130594. [Google Scholar] [CrossRef]
- Debnath-Canning, M.; Unruh, S.; Vyas, P.; Daneshtalab, N.; Igamberdiev, A.U.; Weber, J.T. Fruits and leaves from wild blueberry plants contain diverse polyphenols and decrease neuroinflammatory responses in microglia. JFF 2020, 68, 103906. [Google Scholar] [CrossRef]
- Buda, V.; Brezoiu, A.-M.; Berger, D.; Pavel, I.Z.; Muntean, D.; Minda, D.; Dehelean, C.A.; Soica, C.; Diaconeasa, Z.; Folescu, R.; et al. Biological Evaluation of Black Chokeberry Extract Free and Embedded in Two Mesoporous Silica-Type Matrices. Pharmaceutics 2020, 12, 838. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Huang, Z.; Zhang, C.; Shi, C.; Lyu, L.; Li, W.; Wu, W. Comparative Analysis of the Morphological, Physiological, Proteomic, and Metabolic Mechanisms of the “Biloxi” Blueberry Response to Shade Stress. Front. Plant Sci. 2022, 13, 877789. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Jarillo, J.A.; Carrera, C.; Ferreiro-González, M.; Álvarez, J.; Palma, M.; Ayuso, J.; Barbero, G.F.; Espada-Bellido, E. Optimization of a Novel Method Based on Ultrasound-Assisted Extraction for the Quantification of Anthocyanins and Total Phenolic Compounds in Blueberry Samples (Vaccinium corymbosum L.). Foods 2020, 9, 1763. [Google Scholar] [CrossRef] [PubMed]
- Dinani, S.T.; van der Goot, A.J. Challenges and solutions of extracting value-added ingredients from fruit and vegetable by-products: A review. Crit. Rev. Food Sci. Nutr. 2022, 1–23. [Google Scholar] [CrossRef]
- Bujor, O.-C.; Tanase, C.; Popa, M.E. Phenolic Antioxidants in Aerial Parts of Wild Vaccinium Species: Towards Pharmaceutical and Biological Properties. Antioxidants 2019, 8, 649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Sanabria, A.; Kiesewetter, G.; Klimont, Z.; Schoepp, W.; Haberl, H. Potential for future reductions of global GHG and air pollutants from circular waste management systems. Nat. Commun. 2022, 13, 106. [Google Scholar] [CrossRef]
- Moges, A.; Barik, C.R.; Sahoo, L.; Goud, V.V. Optimization of polyphenol extraction from Hippophae salicifolia D. Don leaf using supercritical CO2 by response surface methodology. 3 Biotech 2022, 12, 292. [Google Scholar] [CrossRef]
- Wani, S.M.; Shah, A.G.; Masoodi, F.A. Optimizing conditions for antioxidant extraction from sea buckthorn leaf (Hippophae rhamnoides L.) as herbal tea using response surface methodology (RSM). IFRJ 2013, 20, 1677–1681. [Google Scholar]
- Isopencu, G.; Stroescu, M.; Brosteanu, A.; Chira, N.; Pârvulescu, O.C.; Busuioc, C.; Stoica-Guzun, A. Optimization of ultrasound and microwave assisted oil extraction from sea buckthorn seeds by response surface methodology. J. Food Process Eng. 2019, 42, e12947. [Google Scholar] [CrossRef] [Green Version]
- Ćućuz, V.; Cvejić, J.; Torović, L.; Gojković-Bukarica, L.; Acevska, J.; Dimitrovska, A.; Aldawoud, T.M.S.; Galanakis, C.M. Design of experiments (DoE) to model phenolic compounds recovery from grape pomace using ultrasounds. J. Food Sci. Technol. 2022, 59, 2913–2924. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, M.L.; Iurian, S.; Puscas, C.; Silaghi-Dumitrescu, R.; Hanganu, D.; Bogdan, C.; Vlase, L.; Oniga, I.; Benedec, D. A Design of Experiments Strategy to Enhance the Recovery of Polyphenolic Compounds from Vitis vinifera By-Products through Heat Reflux Extraction. Biomolecules 2019, 9, 529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrypnik, L.; Novikova, A. Response Surface Modeling and Optimization of Polyphenols Extraction from Apple Pomace Based on Nonionic Emulsifiers. Agronomy 2020, 10, 92. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Lin, A.H.-M.; Han, Q.; Xu, Q. Evaluation of Direct Ultrasound-Assisted Extraction of Phenolic Compounds from Potato Peels. Processes 2020, 8, 165. [Google Scholar] [CrossRef]
- Anastácio, A.; Silva, R.; Carvalho, I.S. Phenolics extraction from sweet potato peels: Modelling and optimization by response surface modelling and artificial neural network. J. Food Sci. Technol. 2016, 53, 4117–4125. [Google Scholar] [CrossRef]
- Anastácio, A.; Carvalho, I.S. Phenolics extraction from sweet potato peels: Key factors screening through a Placket–Burman design. Ind. Crop. Prod. 2013, 43, 99–105. [Google Scholar] [CrossRef]
- Syrpas, M.; Valanciene, E.; Augustiniene, E.; Malys, N. Valorization of Bilberry (Vaccinium myrtillus L.) Pomace by Enzyme-Assisted Extraction: Process Optimization and Comparison with Conventional Solid-Liquid Extraction. Antioxidants 2021, 10, 773. [Google Scholar] [CrossRef]
- Marzullo, L.; Ochkur, O.; Orlandini, S.; Renai, L.; Gotti, R.; Koshovyi, O.; Furlanetto, S.; Del Bubba, M. Quality by Design in optimizing the extraction of (poly)phenolic compounds from Vaccinium myrtillus berries. J. Chromatogr. A 2022, 1677, 463329. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V.; Lefsrud, M. Effect of Postharvest LED Application on Phenolic and Antioxidant Components of Blueberry Leaves. ChemEngineering 2018, 2, 56. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, S.; Sanchez, G.; Alves, M.J.; Fraqueza, M.J. Editorial: Natural Compounds in Food Safety and Preservation. Frontr. Nutr. 2021, 8, 676. [Google Scholar] [CrossRef]
- Harish, K.; Neha, C.; Varsha, N.; Naveen, K.; Raman, S. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef] [Green Version]
- Ștefănescu, B.-E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crișan, G. The Chemical and Biological Profiles of Leaves from Commercial Blueberry Varieties. Plants 2020, 9, 1193. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chai, Z.; Hutabarat, R.P.; Zeng, Q.; Niu, L.; Li, D.; Yu, H.; Huang, W. Blueberry leaves from 73 different cultivars in southeastern China as nutraceutical supplements rich in antioxidants. Food Res. Int. 2019, 122, 548–560. [Google Scholar] [CrossRef]
- Li, D.; Li, B.; Ma, Y.; Sun, X.; Lin, Y.; Meng, X. Polyphenols, anthocyanins, and flavonoids contents and the antioxidant capacity of various cultivars of highbush and half-high blueberries. J. Food Compos. 2017, 62, 84–93. [Google Scholar] [CrossRef]
- Guo, X.; Li, S.; Wang, D.; Huang, Z.; Sarwar, N.; Mubeen, K.; Shakeel, M.; Hussain, M. Effects of water and fertilizer coupling on the physiological characteristics and growth of rabbiteye blueberry. PLoS ONE 2021, 16, e0254013. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Laib, I.; Barkat, M. Optimization of Conditions for Extraction of Polyphenols and the Determination of the Impact of Cooking on Total Polyphenolic, Antioxidant, and Anticholinesterase Activities of Potato. Foods 2018, 7, 36. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.Y.; Holt, R.R.; Lazarus, S.A.; Ensunsa, J.L.; Hammerstone, J.F.; Schmitz, H.H.; Keen, C.L. Stability of the Flavan-3-ols Epicatechin and Catechin and Related Dimeric Procyanidins Derived from Cocoa. J. Agric. Food Chem. 2002, 50, 1700–1705. [Google Scholar] [CrossRef]
- Satoh, Y.; Ishihara, K. Investigation of the antimicrobial activity of Bilberry (Vaccinium myrtillus L.) extract against periodontopathic bacteria. J. Oral. Biosci. 2020, 62, 169–174. [Google Scholar] [CrossRef]
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.-S.; Liu, Z.; Kumar, V. Exploring Phytochemicals for Combating Antibiotic Resistance in Microbial Pathogens. Front. Pharmacol. 2021, 12, 720726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhai, T.; Haider, S.; Liu, Y.; Huang, Z.J. Synergistic Effect of Chlorogenic Acid and Caffeic Acid with Fosfomycin on Growth Inhibition of a Resistant Listeria monocytogenes Strain. ACS Omega 2020, 5, 7537–7544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brezoiu, A.M.; Matei, C.; Deaconu, M.; Stanciuc, A.-M.; Trifan, A.; Gaspar, A.; Berger, D. Polyphenols extract from grape pomace. Characterization and valorisation through encapsulation into mesoporous silica-type matrices. Food Chem. Toxicol. 2019, 133, 110787. [Google Scholar] [CrossRef] [PubMed]
- Brezoiu, A.-M.; Bajenaru, L.; Berger, D.; Mitran, R.-A.; Deaconu, M.; Lincu, D.; Guzun, A.S.; Matei, C.; Moisescu, M.G.; Negreanu-Pirjol, T. Effect of Nanoconfinement of Polyphenolic Extract from Grape Pomace into Functionalized Mesoporous Silica on Its Biocompatibility and Radical Scavenging Activity. Antioxidants 2020, 9, 696. [Google Scholar] [CrossRef]
- Brezoiu, A.-M.; Prundeanu, M.; Beger, D.; Deaconu, M.; Matei, C.; Oprea, O.; Vasile, E.; Negreanu, T.; Muntean, D.; Danciu, C. Properties of Salvia officinalis L. and Thymus serpyllum L. Extracts Free and Embedded into Mesopores of Silica and Titania Nanomaterials. Nanomaterials 2020, 10, 820. [Google Scholar] [CrossRef] [PubMed]
- Caesar, J.; Tamm, A.; Ruckteschler, N.; Leifke, A.L.; Weber, B. Revisiting chlorophyll extraction methods in biological soil crusts—Methodology for determination of chlorophyll a and chlorophyll a Cb as compared to previous methods. Biogeosciences 2018, 15, 1415–1424. [Google Scholar] [CrossRef] [Green Version]
- Guidelines for Stability Testing of Pharmaceutical Products Containing Well Established Drug Substances in Conventional Dosage Forms, World Health Organization. Available online: https://www.paho.org/hq/dmdocuments/2008/6_Annex_5_report_34.pdf (accessed on 30 January 2023).
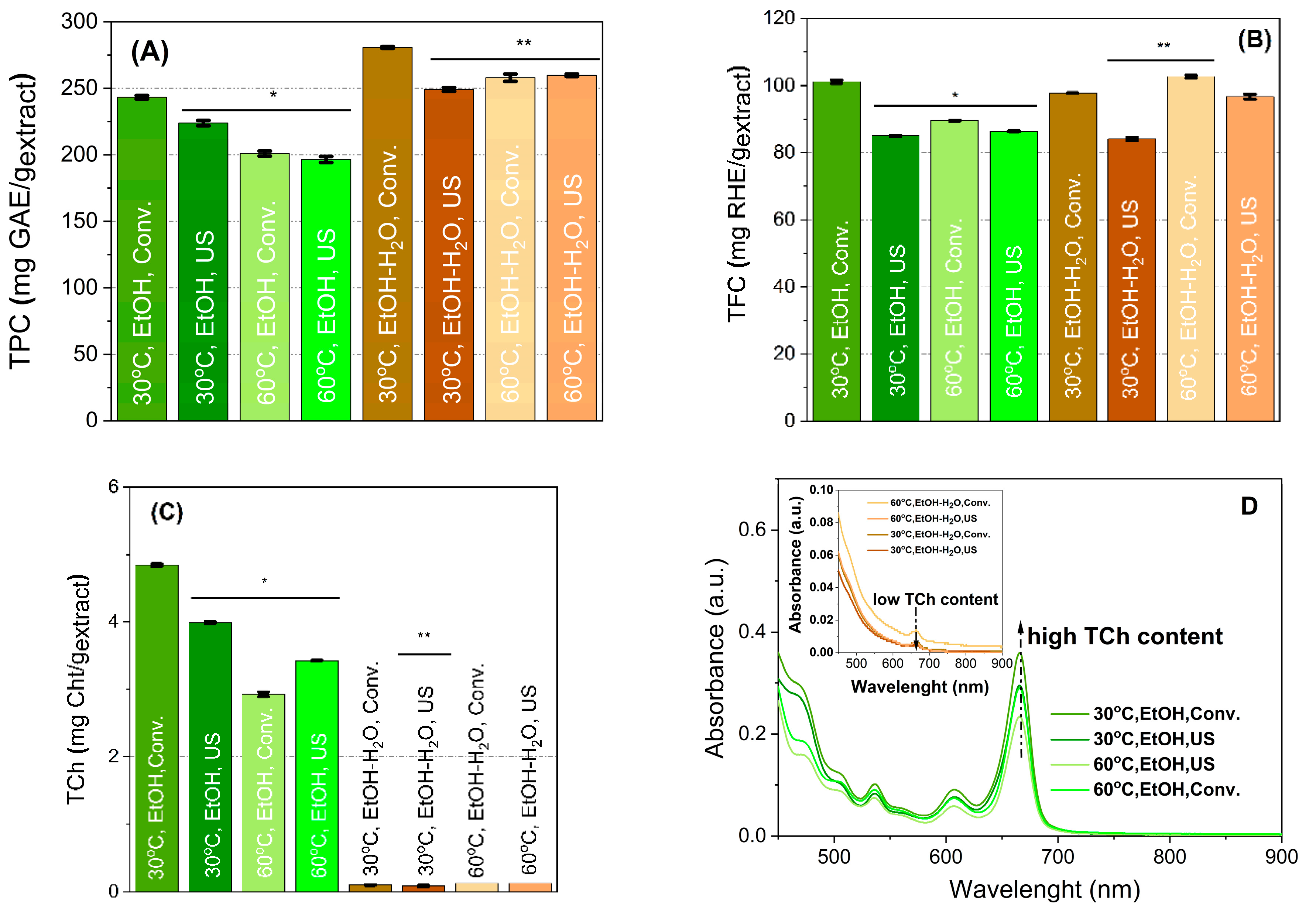

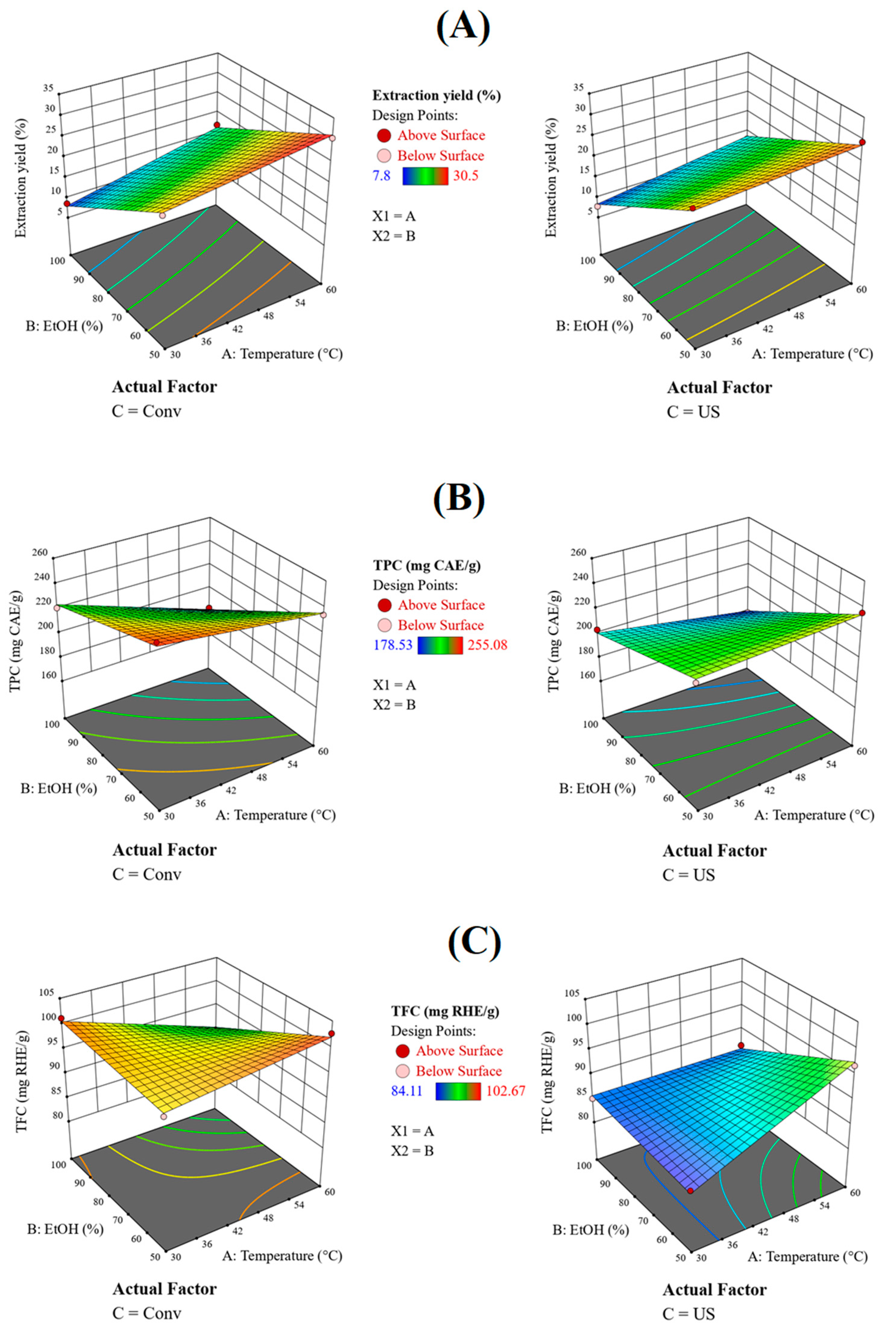


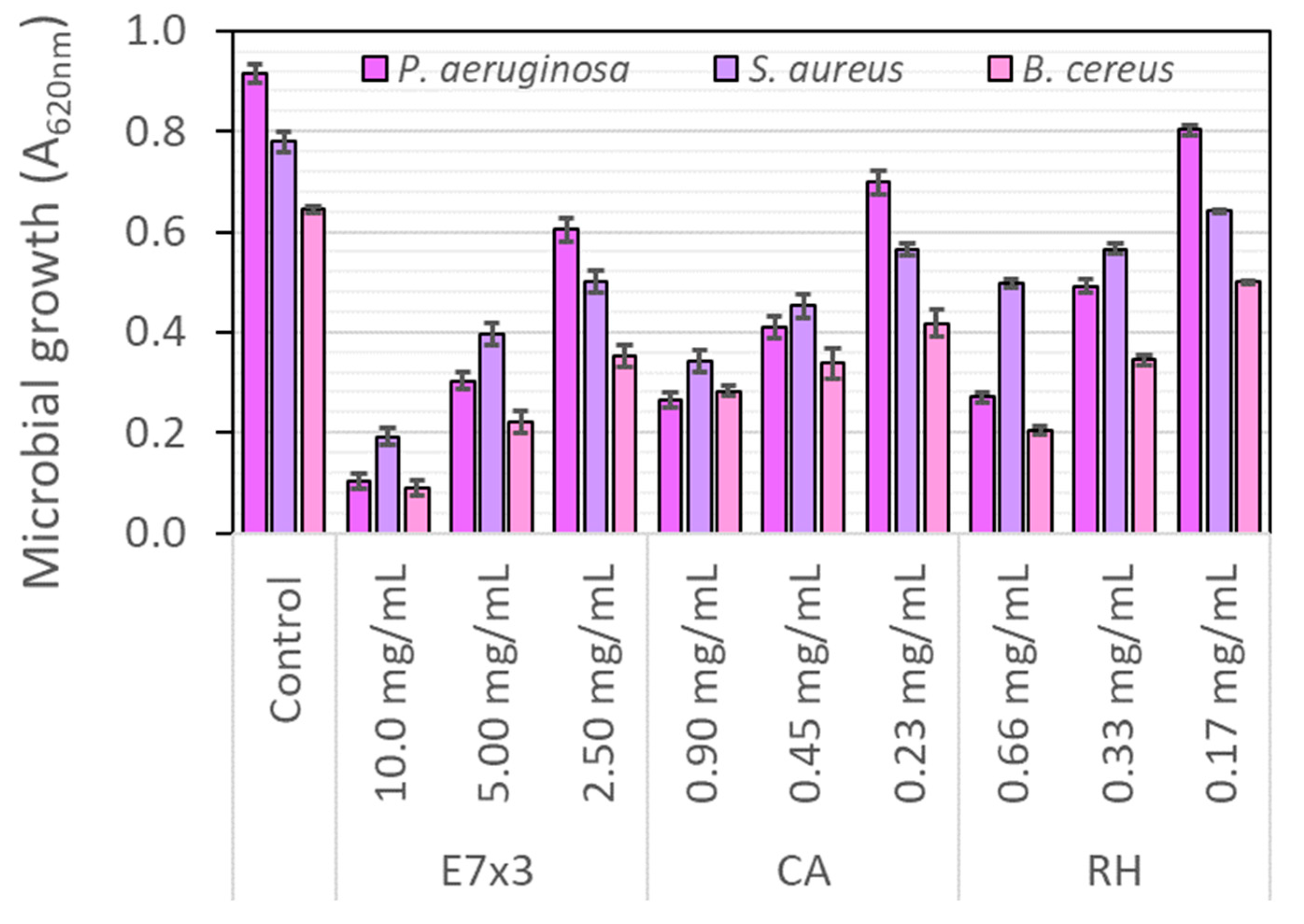

| Factor | Factor Code | Coded Level | Level |
|---|---|---|---|
| Temperature | A | −1 | 30 °C |
| +1 | 60 °C | ||
| Solvent | B | −1 | EtOH 100% |
| +1 | EtOH 50% | ||
| Extraction method | C | −1 | Conv. |
| +1 | US |
| Run | A | B | C |
|---|---|---|---|
| 1 | −1 | −1 | −1 |
| 2 | −1 | −1 | +1 |
| 3 | −1 | +1 | −1 |
| 4 | −1 | +1 | +1 |
| 5 | +1 | −1 | −1 |
| 6 | +1 | −1 | +1 |
| 7 | +1 | +1 | −1 |
| 8 | +1 | +1 | +1 |
| (R2 = 0.9948, p = 0.0130) | |
| Conventional extraction | Extraction yield (%) = 44.05 + 0.035833·A − 0.442·B + 0.002367·AB |
| Ultrasound extraction | Extraction yield (%) = 47.15 − 0.055833·A − 0.442·B + 0.002367·AB |
| (R2 = 0.9924, p = 0.0188) | |
| Conventional extraction | TPC (mgCAE/g) = 271.15750 + 0.322167·A − 0.0469·B − 0.017443·AB |
| Ultrasound extraction | TPC (mgCAE/g) = 226.10250 + 1.05217·A − 0.0469·B − 0.017443·AB |
| (R2·=·0.9912, p = 0.0218) | |
| Conventional extraction | TFC (mgRHE/g) = 78.815 − 0.58075·A + 0.32·B − 0.00923 AB |
| Ultrasound extraction | TFC (mgRHE/g) = 53.58 + 0.92525·A + 0.32 B − 0.00923 AB |
| (R2 = 0.9915, p = 0.0212) | |
| Conventional extraction | TCh (mgCht/g) = −4.9575 + 0.0315·A + 0.1117·B − 0.00084·AB |
| Ultrasound extraction | TCh (mgCht/g) = −6.0625 + 0.05383·A + 0.1117·B − 0.00084·AB |
| (R2 = 0.9827, p = 0.0426) | |
| Conventional extraction | RSADPPH (mg/TE/g) = 474.5125 + 1.82183·A − 0.9277·B − 0.038793·AB |
| Ultrasound extraction | RSADPPH (mg/TE/g) = 389.8575 + 3.22917·A − 0.9277·B − 0.038793·AB |
| (R2 = 0.9898, p = 0.0252) | |
| Conventional extraction | RSAABTS (mg/TE/g) = 936.08 + 0.52833·A − 2.5778·B − 0.034867·AB |
| Ultrasound extraction | RSAABTS (mg/TE/g) = 825.96 + 1.50317·A − 2.5778·B − 0.034867·AB |
| Optimal Extraction Conditions | Temperature (°C) | Solvent | Extraction Method | Desirability |
|---|---|---|---|---|
| Extraction yield (%) | 60 | EtOH–H2O | Conv. | 1.000 |
| TPC | 30 | EtOH–H2O | Conv. | 0.964 |
| TFC | 60 | EtOH–H2O | Conv. | 0.962 |
| TCh | 30 | EtOH | Conv | 0.998 |
| RSAABTS | 60 | EtOH–H2O | Conv. | 1.000 |
| RSADPPH | 30 | EtOH–H2O | Conv. | 1.000 |
| All responses | 60 | EtOH–H2O | Conv. | 0.784 |
| Catechin Hydrate | Chlorogenic Acid | Caffeic Acid | (-) Epicatechin | Rutin Hydrate | |
|---|---|---|---|---|---|
| RT (min) | 12.751 ± 0.009 | 13.469 ± 0.011 | 15.521 ± 0.006 | 17.686 ± 0.011 | 27.435 ± 0.012 |
| Run 1 | 4.462 ± 0.044 | 75.207 ± 0.222 | nd | 14.851 ± 0.094 | 16.372 ± 0.016 |
| Run 2 | 4.156 ± 0.025 | 61.849 ± 0.233 | nd | 14.110 ± 0.098 | 14.739 ± 0.083 |
| Run 3 | 4.918 ± 0.004 | 65.866 ± 0.457 | nd | 14.824 ± 0.095 | 15.454 ± 0.104 |
| Run 4 | 5.517 ± 0.022 | 74.039 ± 0.017 | nd | 15.498 ± 0.008 | 14.814 ± 0.007 |
| Run 5 | 4.456 ± 0.010 | 69.304 ± 0.036 | 0.744 ± 0.000 | 14.002 ± 0.082 | 38.232 ± 0.023 |
| Run 6 | 3.683 ± 0.064 | 60.901 ± 0.068 | 0.575 ± 0.004 | 10.988 ± 0.036 | 32.271 ± 0.102 |
| Run 7 | 5.799 ± 0.057 | 87.980 ± 0.012 | 1.882 ± 0.009 | 12.482 ± 0.045 | 46.650 ± 0.096 |
| Run 8 | 4.002 ± 0.089 | 62.521 ± 0.007 | 1.429 ± 0.003 | 10.362 ± 0.019 | 33.901 ± 0.010 |
| Extract | Time at 40 °C at 75% RHu (Days) | Volatile Components | Organics Combustion | Dried Residue |
|---|---|---|---|---|
| (% wt vs. Dry Mass) | ||||
| E7×3 | 0 | 44.4 | 51.1 | 2.1 |
| 7 | 34.4 | 49.2 | 1.6 | |
| 14 | 33.5 | 46.8 | 2.0 | |
| 28 | 34.9 | 45.0 | 3.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brezoiu, A.-M.; Deaconu, M.; Mitran, R.-A.; Prelipcean, A.-M.; Matei, C.; Berger, D. Optimisation of Polyphenols Extraction from Wild Bilberry Leaves—Antimicrobial Properties and Stability Studies. Molecules 2023, 28, 5795. https://doi.org/10.3390/molecules28155795
Brezoiu A-M, Deaconu M, Mitran R-A, Prelipcean A-M, Matei C, Berger D. Optimisation of Polyphenols Extraction from Wild Bilberry Leaves—Antimicrobial Properties and Stability Studies. Molecules. 2023; 28(15):5795. https://doi.org/10.3390/molecules28155795
Chicago/Turabian StyleBrezoiu, Ana-Maria, Mihaela Deaconu, Raul-Augustin Mitran, Ana-Maria Prelipcean, Cristian Matei, and Daniela Berger. 2023. "Optimisation of Polyphenols Extraction from Wild Bilberry Leaves—Antimicrobial Properties and Stability Studies" Molecules 28, no. 15: 5795. https://doi.org/10.3390/molecules28155795







