Abstract
The photocatalytic degradation of formaldehyde by graphite-like C3N4 is one of the most attractive and environmentally friendly strategies to address the significant threat to human health posed by indoor air pollutants. Despite its potential, this degradation process still faces issues with suboptimal efficiency, which may be attributed to the rapid recombination of photogenerated excitons and the broad band gap. As a proof of concept, a series of graphite-like C3N4@C60 composites combining graphite-like C3N4 and C60 was developed via an in situ generation strategy. The obtained graphite-like C3N4@C60 composites exhibited a remarkable increase in the photocatalytic degradation efficiency of formaldehyde, of up to 99%, under visible light irradiation, outperforming pure graphite-like C3N4 and C60. This may be due to the composites’ enhanced built-in electric field. Additionally, the proposed composites maintained a formaldehyde removal efficiency of 84% even after six cycles, highlighting their potential for indoor air purification and paving the way for the development of efficient photocatalysts.
1. Introduction
As the atmospheric environment continues to deteriorate, formaldehyde has emerged as a critical indoor air pollutant that poses a serious threat to human health. It is commonly discharged by industrial activities and building materials, and its impact on human health is irreversible [1,2]. Even at low concentrations, long-term exposure to formaldehyde will extremely damage the human nervous system and respiratory system. Due to growing concern over the harmful effects of formaldehyde on human health, a variety of effective strategies have been developed to eliminate it rapidly. These methods, including adsorption purification [3], thermal catalysis [4,5], biofiltration, and condensation [6,7], are commonly used in indoor air purification. However, most of these approaches involve merely adsorbing formaldehyde onto a filter medium without degrading the pollutants. As a result, these air purifiers have several drawbacks, including low adsorption capacity, the rerelease of formaldehyde into the air, and difficulties in regenerating adsorbents. Thus, it is crucial to prioritize the exploration and development of effective solutions to eliminate formaldehyde at low concentration levels, especially in indoor environments.
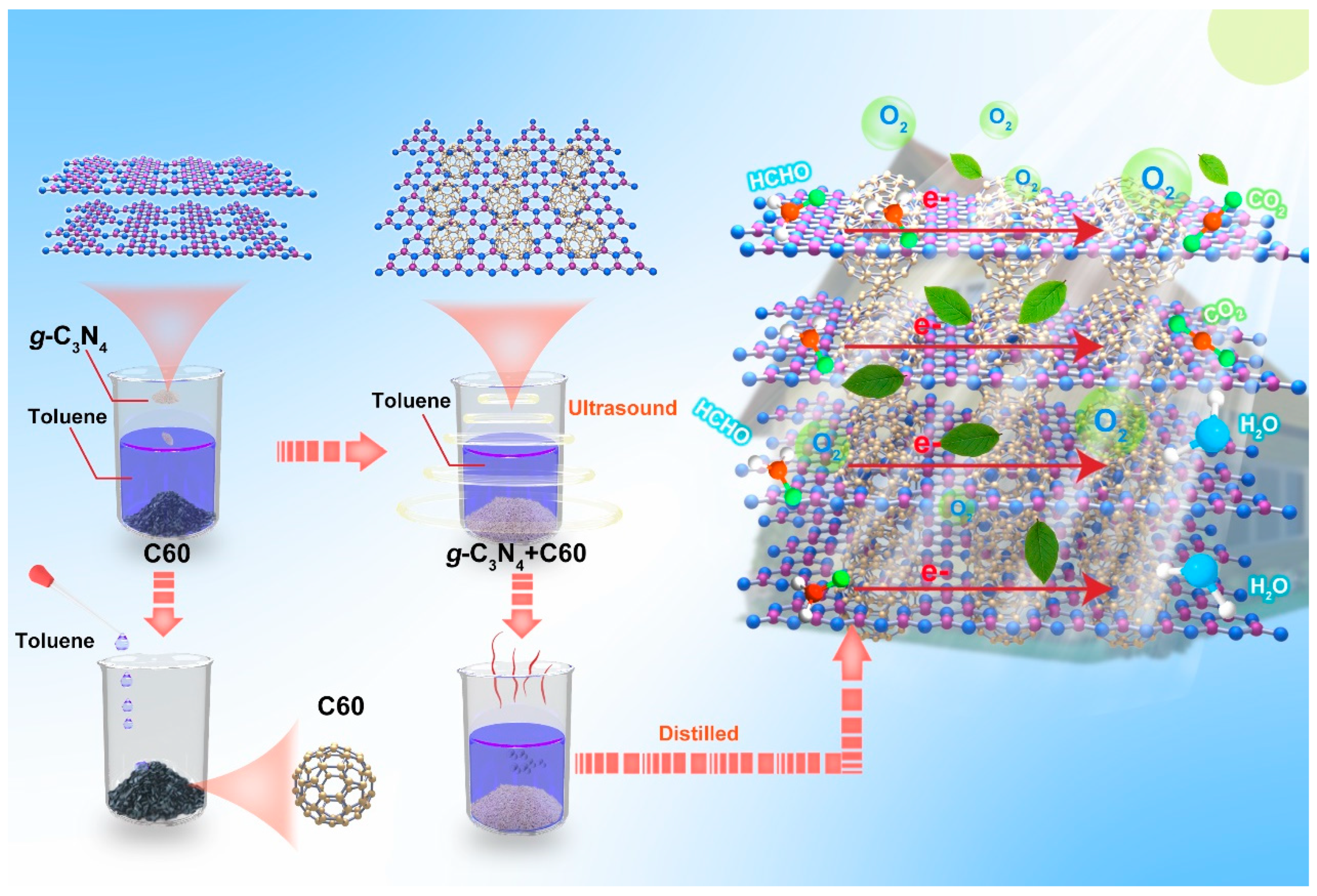
Photocatalytic oxidation is regarded as a versatile and promising strategy for air purification credited to its low energy consumption, high efficiency, and lack of secondary pollution; it can effectively mineralize formaldehyde to CO2 and H2O on inexpensive polymeric semiconductors under mild conditions [2,8,9,10,11,12,13]. The semiconductor graphite-like C3N4, which is considered one of the most advanced metal-free photocatalysts, has been widely applied for the photocatalytic degradation of formaldehyde due to its strong oxidation ability, low cost-effectiveness, and long-term stability [14,15,16,17,18]. However, its large band gap and limited light absorption capacity could result in the recombination of its internal excited carriers, which would undermine its photocatalytic efficiency [19,20]. To enhance the separation efficiency of photogenerated excitons, numerous strategies such as semiconductor composites [9], elemental doping [21,22], dye sensitization [23,24], surface engineering [25,26,27,28], and constructing heterojunctions [8] have been adopted to circumvent these obstacles. Despite efforts to enhance the photocatalytic efficiency of graphite-like C3N4, these strategies are limited by issues such as serious corrosion, high temperatures, and tedious preparation steps. Therefore, there is an utmost urgency to discover and develop a simple and efficient strategy for improving the photocatalytic efficiency of graphite-like C3N4 (Scheme 1).
Scheme 1.
Synthetic routes and notional structures of g-C3N4 and C60 composites.
Fullerenes, such as C60, are distinct forms of carbon with exceptional electronic characteristics [29,30,31,32,33,34]. C60 is considered favorable for efficient electron transfer reduction due to its closed-shell configuration [35,36]. The distinct structure of C60 makes it an outstanding electron acceptor that effectively induces quick photoinduced charge separation while experiencing comparatively slow charge recombination [37,38,39]. Wang et al. have provided a comprehensive overview of the recent notable progress in the realms of hydrogenation and oxidation facilitated by catalytic systems based on graphite-like C3N4 [40,41]. They also discovered that an amalgamation of carbon nitride and carbon nanotubes displayed a synergistic effect during optoelectronic conversion. Consequently, graphite-like C3N4 has become a category of 2D nanomaterials resembling graphite, and its distinct structure offers vast potential for utilization as a metal-free semiconductor that can govern photocatalytic reactions. Huang et al. reported that the electrical performance of covalent organic frameworks (COFs) can be enhanced by encapsulating fullerenes into the channels of COFs via a donor–acceptor interaction [42]. The improved electrical conductivity and carrier mobility can promote efficient charge transfer, which is highly desirable in photocatalytic processes.
In light of the above, using an in situ generation approach, we developed a series of photocatalytic composites, denoted as graphite-like C3N4@C60, wherein the guest molecule C60 was encapsulated into the host graphite-like C3N4 framework. The resulting graphite-like C3N4/C60@6:1 composite induced the formation of a strong built-in electric field, which accelerated charge transport kinetics. In addition, this composite achieved a formaldehyde degradation efficiency of up to 99% under visible-light irradiation, outperforming pure graphite-like C3N4 and C60. Our study presents a pioneering approach for designing photocatalysts based on graphite-like C3N4 and achieving efficient solar energy conversion.
2. Results and Discussion
Characterization of the Photocatalysts
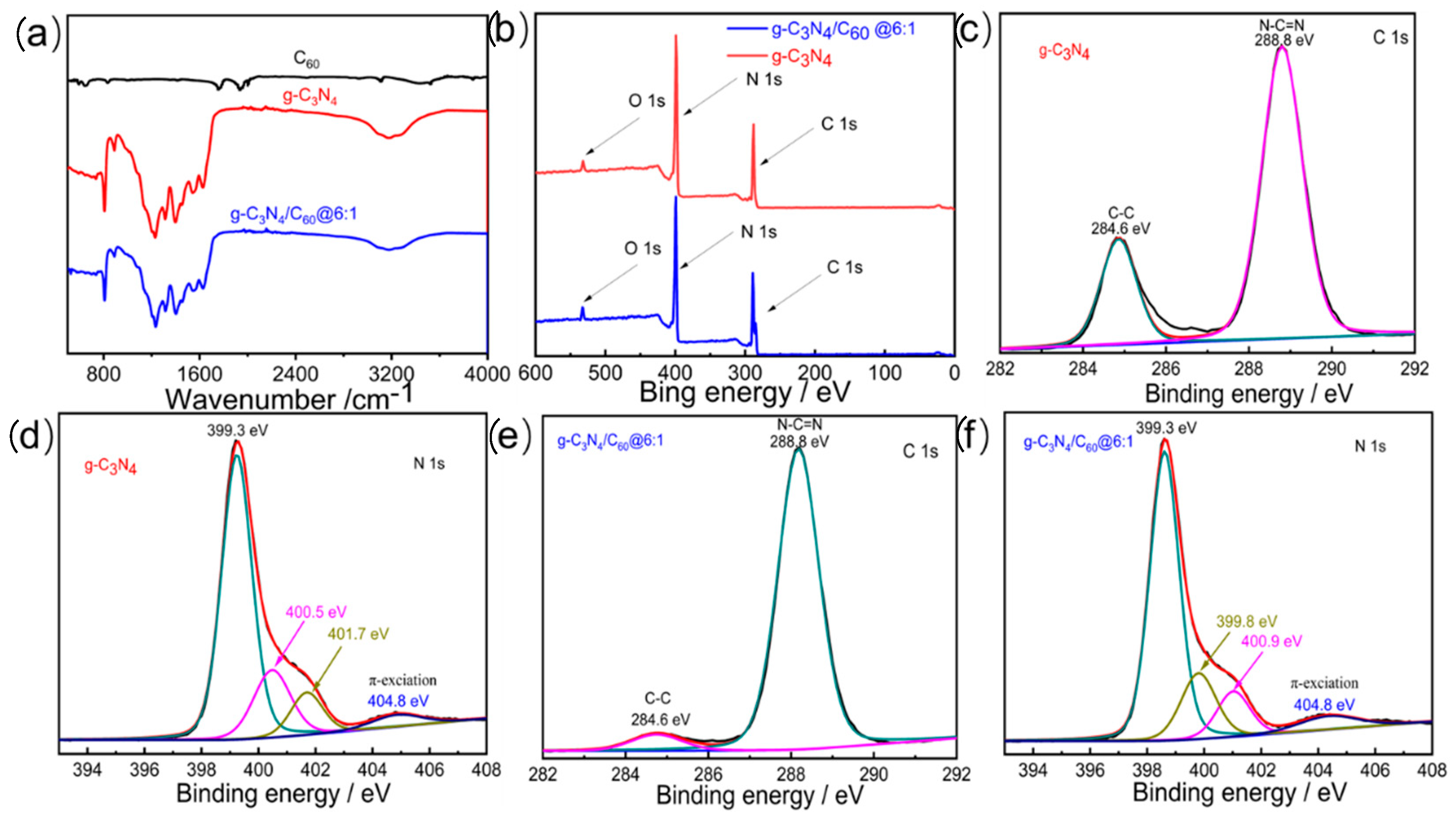
The as-prepared specimens were characterized by FT-IR spectra to confirm their structures (Figure 1a). The bands located at ~1610 cm−1 were ascribed to C=N stretching vibration, whereas the other strong bands, at ~1243 and ~1398 cm−1, were ascribed to C-N stretching vibration, matching the s-triazine ring in the graphite-like C3N4 well. The FT-IR spectrum of the C60 was relatively weak, but two peaks were observed at ~1760 and ~1940 cm−1, corresponding to the C60′s internal modes. There was no obvious structural variation between the graphite-like C3N4 and the 6 wt% C60/graphite-like C3N4 following the deposition of the C60. However, the characteristic peaks of the graphite-like C3N4 in the 6 wt% C60/graphite-like C3N4, ranging from ~1200 to 1700 cm−1, were shifted, indicating that there was a weak interaction between the C60 and the graphite-like C3N4. This may have facilitated electron transfer and improved the photocatalytic activity of the composites compared to the graphite-like C3N4.
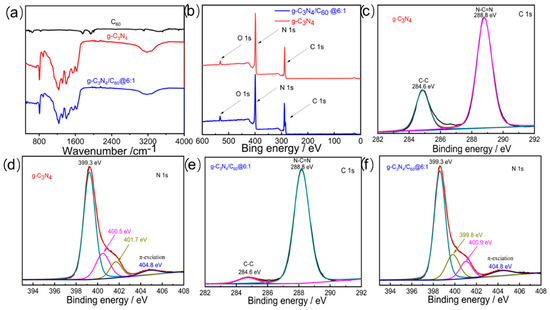
Figure 1.
(a) FT-IR spectra of C60, g-C3N4, and g-C3N4/C60@6:1, obtained in transmission mode; (b) XPS survey spectra of spectra of C60, g-C3N4, and g-C3N4/C60@6:1; (c) high-resolution XPS spectra of C 1s for g-C3N4; (d) high-resolution XPS spectra of N 1s for g-C3N4; (e) high-resolution XPS spectra of C 1s for g-C3N4/C60@6:1; and (f) high-resolution XPS spectra of N 1s for g-C3N4/C60@6:1.
X-ray photoelectron spectroscopy (XPS) was conducted to verify the chemical compositions and determine the valence states of different species. The graphite-like C3N4 mainly comprised the elements C, N, and O. Among them, the presence of O was attributed to H2O and CO2 absorbed on the surface of the photocatalysts. All peak positions in the XPS spectra of the graphite-like C3N4 and the 6 wt% graphite-like C3N4/C60 composite were calibrated using C 1s at 284.6 eV as a reference (Figure 1b). The C 1s XPS spectrum exhibited two peaks, with distinct binding energies, at 284.6 and 288.8 eV, which corresponded to C-C and N-C=N, respectively. The N 1s XPS spectrum of the graphite-like C3N4 exhibited two distinct peaks at 400.5 and 401.7 eV, which might be attributed to C=N-C and N(C)3, respectively, upon deconvolution. A comparison of the N 1s XPS spectrum of the graphite-like C3N4 with that of the 6 wt% graphite-like C3N4/C60 indicated that the binding energies of N (N-C=N) peak-shifted from 400.5 eV (graphite-like C3N4) to 399.8 eV (6 wt% graphite-like C3N4/C60). This shift suggests that an interaction occurred between the graphite-like C3N4 and the C60 (Figure 1c–f). The extremely weak peak at 404.8 eV was ascribed to π excitation. Previous studies have demonstrated that a lower binding energy of N 1s for composites is indicative of an increased electronic cloud density around the N atoms of graphite-like C3N4. This effect is attributed to intermolecular electron diffusion from conjugated polymers to the N sites of graphite-like C3N4 through intermolecular π-π interactions.
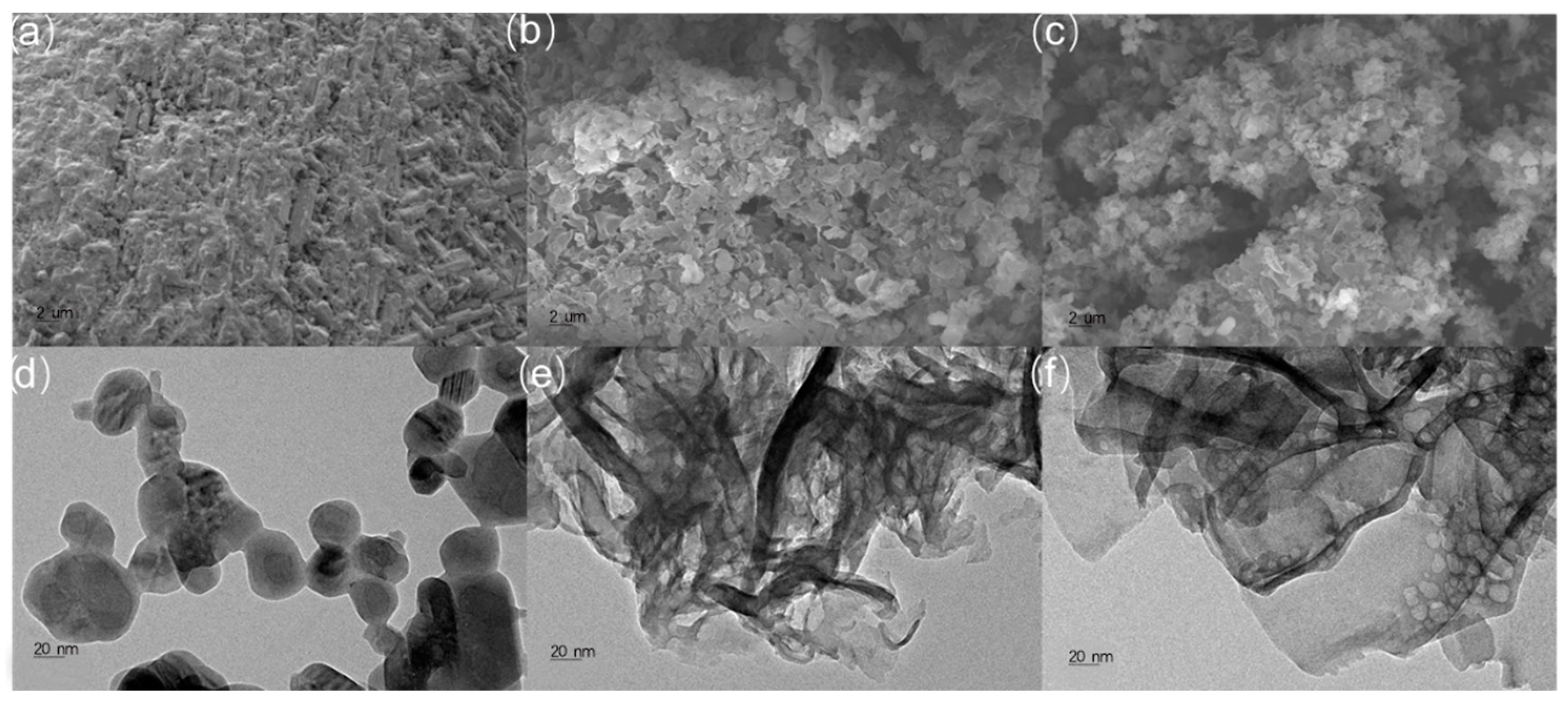
The crystallinities for C60, graphite-like C3N4, and graphite-like C3N4/C60@6:1 were characterized with X-ray diffraction. As can be clearly observed in Figure S1, the graphite-like C3N4 and the graphite-like C3N4/C60@6:1 showed similar, prominent strong-intensity peaks at 25.3°, which correspond to the in-plane repeated units and the structural packing motifs of the aromatic segments, respectively, indicating the successful preparation of the graphite-like C3N4. The C60 displayed a cubic phase and could be indexed based on its diffraction pattern, which demonstrated two peaks, at 10.7 and 15.5, attributed to the (111) and (220) crystal planes, respectively. The deposition of C60 on the graphite-like C3N4/C60@6:1 composite surface had no remarkable effects on the structure of the graphite-like C3N4, as evidenced by the fact that the positions and intensities of the characteristic peaks at 10.7° and 15.5°, corresponding to the (111) and (220) crystal planes, respectively, remained virtually unchanged compared with those of the bare graphite-like C3N4. These observations indicate that the C60 was successfully deposited onto the graphite-like C3N4 without changing the crystal structure (Figure S1). The specific surface areas of the graphite-like C3N4 and the graphite-like C3N4/C60@6:1 were explored using adsorption–desorption isotherms performed at 77 K (Figures S2 and S3). Accordingly, the BET surface areas of the graphite-like C3N4 and the graphite-like C3N4/C60@6:1 were determined to be 40.0 and 29.6 m2/g, respectively. However, the pore volume of graphite-like C3N4/C60@6:1 (0.082 cm3/g) was considerably lower than that of graphite-like C3N4 (0.093 cm3/g). This difference shows that the pores of the graphite-like C3N4 were blocked or occupied by C60 nanoparticles. The morphology of the graphite-like C3N4/C60@6:1 composite was similar to that of the graphite-like C3N4, and there was no specific structure of C60 in the graphite-like C3N4/C60@6:1 composite because of its limited concentration. Moreover, the presence of dark spots with the lower transmission in the graphite-like C3N4/C60@6:1 composite indicated that there might have been some perturbation or disruption of the C60 nanoparticles. However, this perturbation did not appear to have affected the overall porous structure of the graphite-like C3N4. The small sizes of the C60 nanoparticles may have allowed them to be easily incorporated into the graphite-like C3N4 nanosheets, resulting in a well-dispersed composite (Figure 2).
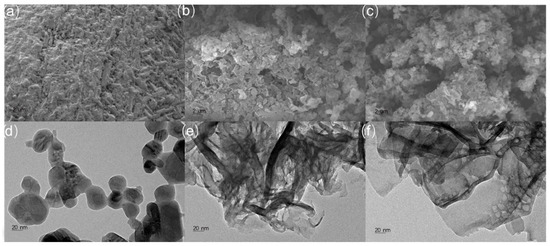
Figure 2.
SEM images of (a) C60, (b) g-C3N4, and (c) g-C3N4/C60@6:1 composite and TEM images of (d) C60, (e) g-C3N4 and (f) g-C3N4/C60@6:1 composite.
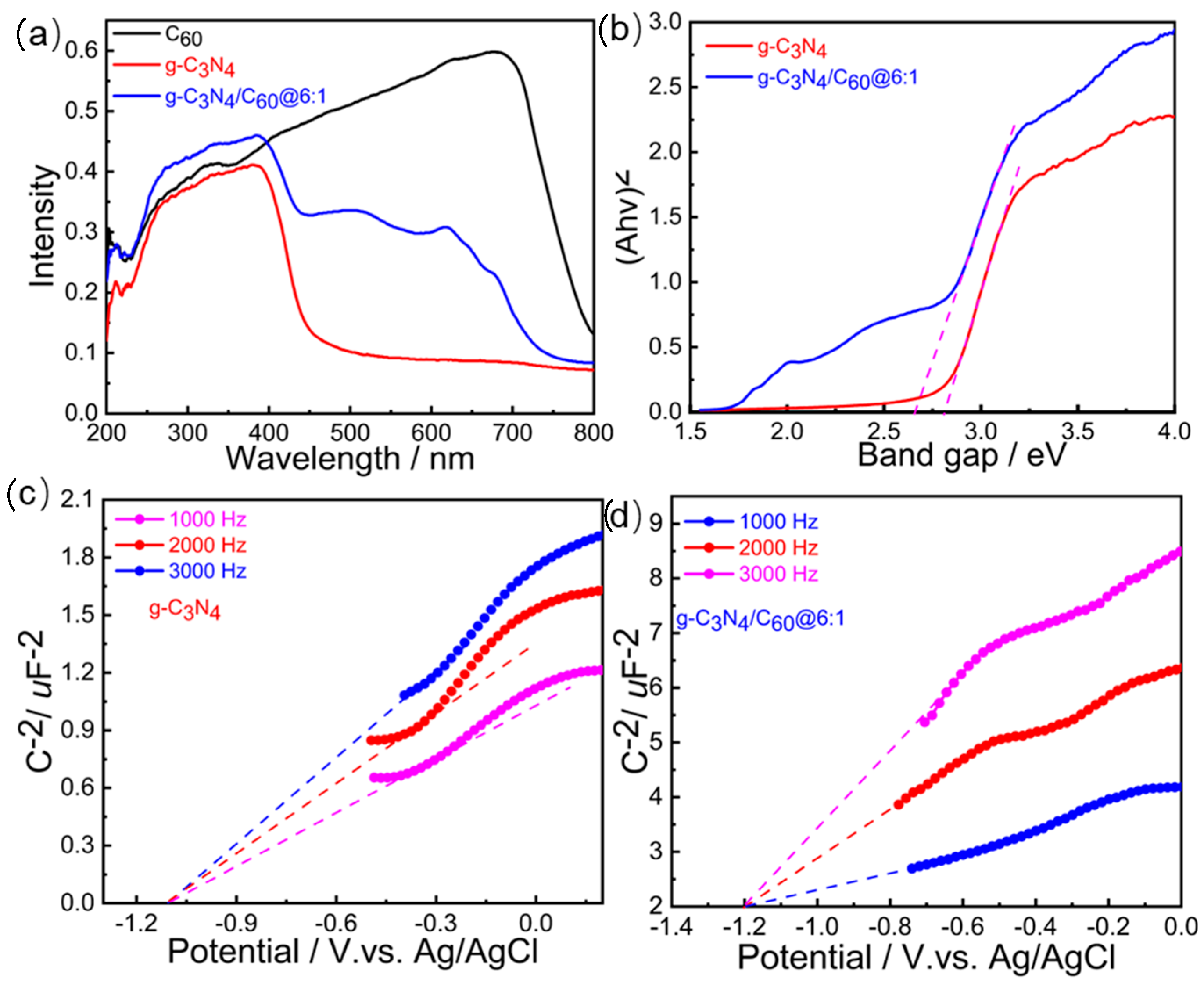
UV-vis diffuse reflectance spectroscopy was utilized to characterize the optical properties of the C60, the graphite-like C3N4, and the graphite-like C3N4/C60@6:1. Both the C60 and the graphite-like C3N4/C60@6:1 composite exhibited wide absorption edges, at ~700 nm, ascribing to the intrinsic band gap absorption of the C60. (Figure 3a). The graphite-like C3N4/C60@6:1 composite showed obvious red-shift edges after the introduction of C60, indicating that the graphite-like C3N4/C60@6:1 composite could broaden the spectrum to the visible region compared to the bare graphite-like C3N4. Based on the equation Eg = 1240/λ, the band gaps of the graphite-like C3N4/C60@6:1 and the graphite-like C3N4 were determined to be 2.62 and 2.85 eV, respectively (Figure 3b). The smaller band gap for the graphite-like C3N4/C60@6:1 indicates that it is more easily excited to generate free holes and electrons. The lowest unoccupied molecular orbital (LUMO) levels of graphite-like C3N4 and graphite-like C3N4/C60@6:1 were calculated using the Mott−Schottky method to be −1.10 and −1.20 V versus those of Ag/AgCl, indicating that the as-prepared graphite-like C3N4/C60@6:1 featured a more sufficient driving force for the reduction of O2 to O2•− (−0.48 V versus Ag/AgCl) compared to the graphite-like C3N4 (Figure 3c,d).
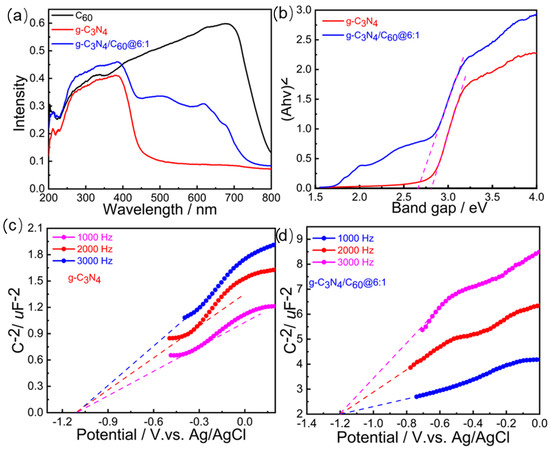
Figure 3.
(a) UV-vis diffuse reflectance spectra of C60, g-C3N4, and g-C3N4/C60@6:1. (b) Band gaps of C60, g-C3N4, and g-C3N4/C60@6:1 determined with Kubelka-Munk-transformed reflectance. (c) Mott-Schottky plots of g-C3N4 in 0.2 M Na2SO4 aqueous solution at 1000 Hz, 2000 Hz, and 3000 Hz. (d) Mott-Schottky plots of g-C3N4/C60@6:1 in 0.2 M of Na2SO4 aqueous solution at 1000 Hz, 2000 Hz, and 3000 Hz.
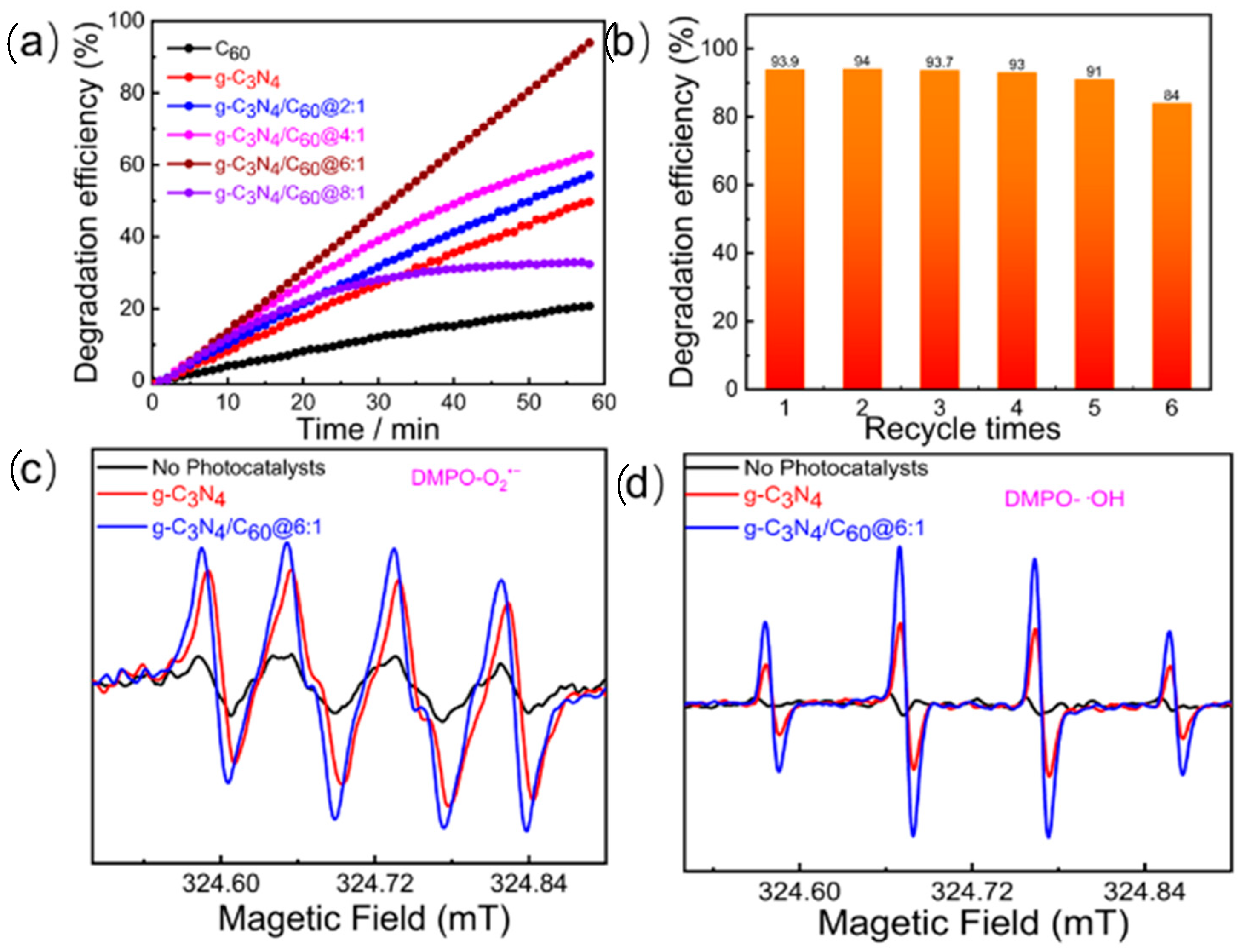
The photocatalytic oxidation of formaldehyde was employed to assess the photocatalytic activity via the IR multi-gas monitor under UV-vis light irradiation for 1 h at a wavelength range of 310–800 nm and an intensity of 2.9 mW/cm2. As displayed in Figure 4a, the photocatalytic performance of the graphite-like C3N4/C60@6:1 composite in HCHO degradation varied with different mass ratios of the composite material. With an increase in the loading of the C60 on the graphite-like C3N4, the photocatalytic efficiency increased from 40.2% to 96.4%, indicating that the incorporation of C60 can remarkably enhance graphite-like C3N4 during photocatalytic HCHO degradation. As the amount of C60 increases in a graphite-like C3N4/C60 composite, it could lead to a decrease in photocatalytic activity due to shading effects that would reduce the amount of light that would reach the graphite-like C3N4. Under the experimental conditions, the photocatalytic efficiency of the C60 alone was found to be relatively low (14%). This observation suggests that the incorporation of C60 into g-C3N4 will enhance photocatalytic activity for HCHO degradation and can act as an efficient photocatalyst. Apart from its photocatalytic activity, photochemical stability is also a crucial consideration for photocatalytic materials. In the case of graphite-like C3N4/C60@6:1, the results of recycling experiments showed that the composite material maintained its photocatalytic activity for the oxidation of the HCHO over multiple cycles without any significant decline in activity (Figure 4b). Furthermore, the control photocatalysis experiment showed that the catalyst was needed for formaldehyde oxidation, and light irradiation alone was not sufficient to degrade the formaldehyde (not shown).
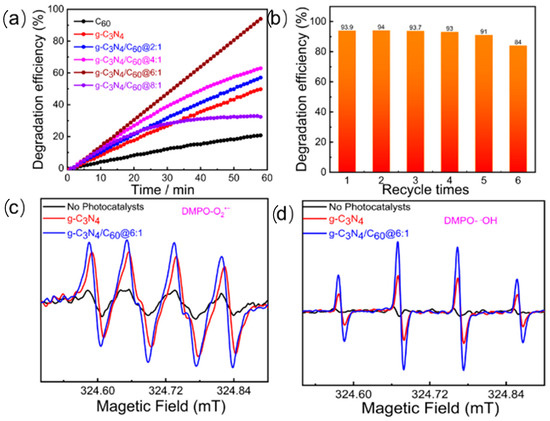
Figure 4.
(a) Photocatalytic degradation efficiency of gaseous HCHO (120 ppm); (b) reusability of g-C3N4/C60@6:1 in gaseous HCHO; (c) ESR spectra of g-C3N4/C60@6:1 for detecting O2•−; and (d) ESR spectra of g-C3N4/C60@6:1 for detecting •OH.
To explore the photocatalytic mechanisms of formaldehyde degradation by graphite-like C3N4/C60@6:1, radical detection was conducted using 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as an active species-trapping agent (Figure 4c). Under visible light and dark conditions, absolute methanol was employed as a solvent to capture •OH and O2•−, respectively. Under the dark conditions, there were no characteristic EPR signals observed. However, when the sample was irradiated with the visible light, characteristic DMPO-•OH (hydroxyl radical) and DMPO-O2•− (superoxide radical) peaks appeared. This suggests that the interaction between photoinduced electrons and holes may result in the generation of active •OH and O2•− species. Based on the EPR experiments, the main reactive oxygen species involved in the photocatalysis were •OH and O2•−. These reactive species were produced when photoinduced charge carriers interacted with the H2O and O2 adsorbed on the surface of the catalyst. When exposed to the visible light, the photocatalyst generated photoinduced electrons, which reacted with the adsorbed O2 molecules to form O2•−. This radical then reacted with the H2O to produce •OH. Meanwhile, the surface •OH oxidized formaldehyde into formate species. The valence band holes in the catalyst directly oxidized the H2O and/or the −OH to form additional •OH. Eventually, the •OH further oxidized the formate species into H2O and CO2.
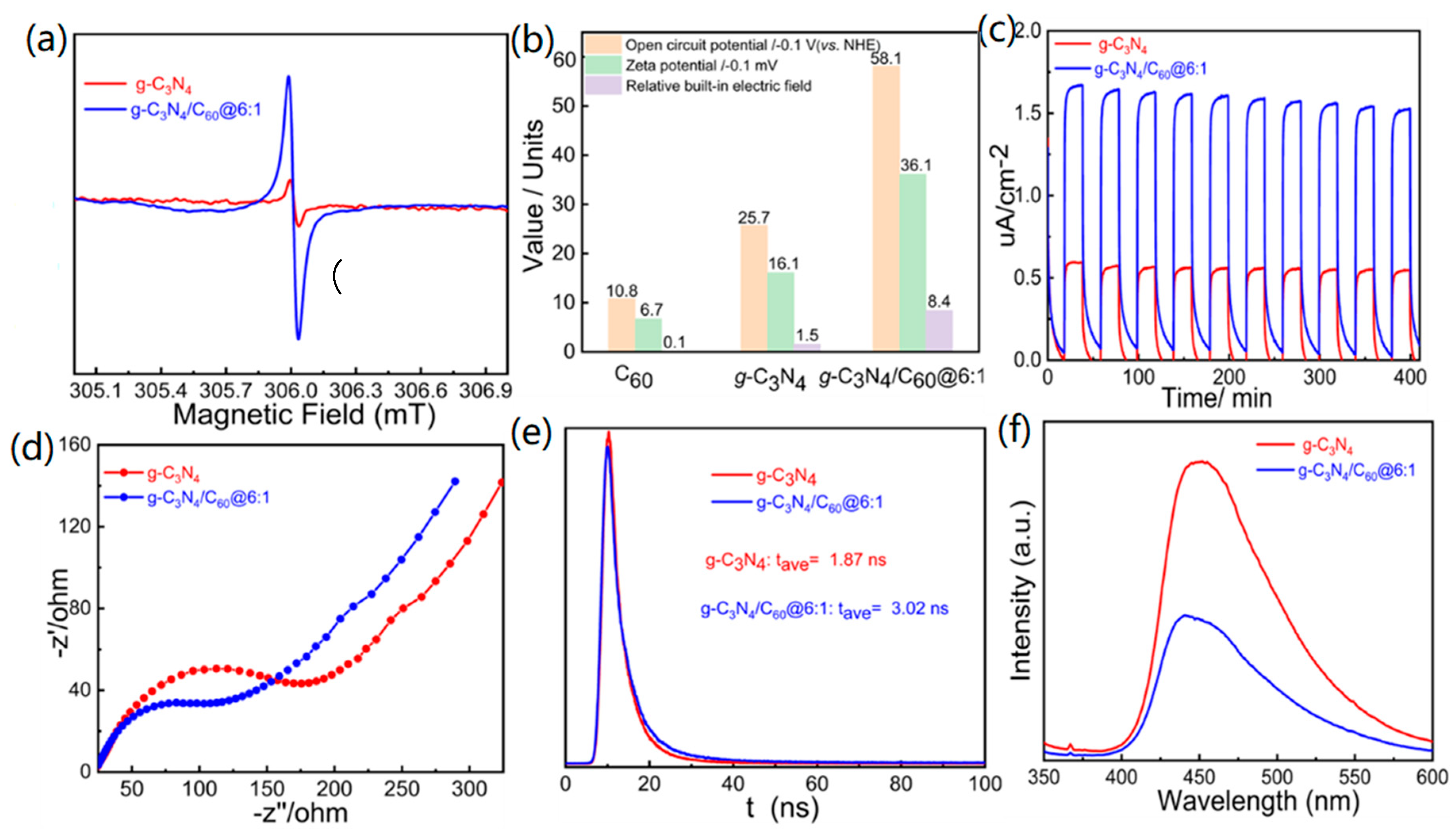
In order to better understand how composites can better enhance photocatalytic activity compared to pure graphite-like C3N4, a range of photoelectrochemical properties was examined. The EPR signals of the graphite-like C3N4/C60@6:1 composite showed a significant increase when exposed to visible light compared with those of pure graphite-like C3N4. This indicates that these composites enable more effective production of unpaired electrons and photoinduced charge carrier pairs in graphite-like C3N4. Thus, charge migration and separation are facilitated through the encapsulation of C60 into pure graphite-like C3N4 (Figure 5a). The built-in electrical field is a critical factor for driving photogenerated holes and electrons to drift in reverse directions in photocatalysts, which, in turn, dramatically accelerates exciton separation. The BIEF intensities of C60, graphite-like C3N4, and graphite-like C3N4/C60@6:1 were estimated by employing the model established by Kanata, and the results indicated that BIEF strength could be assessed with both surface charge density and surface potential. The surface potentials were determined to be 10.8, 25.7, and 58.7 mV for the C60, the graphite-like C3N4, and the graphite-like C3N4/C60@6:1, respectively. Moreover, the corresponding zeta potentials of the C60, the graphite-like C3N4, and the graphite-like C3N4/C60@6:1 were −6.7, −16.1, and −36.1 V, respectively. According to the open-circuit potential and zeta potential, the graphite-like C3N4/C60@6:1 (8.4) had the strongest built-in electric field, which exceeded those of the C60 (0.1) and the graphite-like C3N4 (1.5). This significant increase in the built-in electric field indicates a strong driving force for achieving the efficient separation of excitons (Figure 5b).
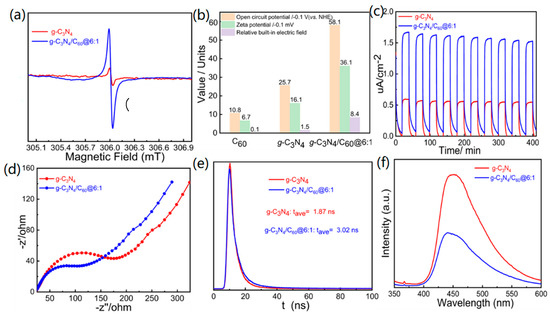
Figure 5.
(a) EPR spectra for g-C3N4 and g-C3N4/C60@6:1; (b) characterization of the BEF values for C60, g-C3N4, and g-C3N4/C60@6:1; (c) transient photocurrent responses for g-C3N4 and g-C3N4/C60@6:1; (d) Nyquist plots for g-C3N4 and g-C3N4/C60@6:1; (e) photoluminescence decay traces for g-C3N4 and g-C3N4/C60@6:1; and (f) photoluminescence spectra of g-C3N4 and g-C3N4/C60@6:1.
The vital role of encapsulated C60 in the charge mobility was further explored. As expected, the graphite-like C3N4/C60@6:1 exhibited a higher photocurrent intensity than that of the pure graphite-like C3N4, which implied accelerated production and separation of photoinduced electron-and-hole pairs at the graphite-like C3N4/C60@6:1 interface (Figure 5c). Electrochemical impedance spectra were employed to explore the transfer of photogenerated charge carriers. Semicircular Nyquist plots showed a remarkable decrease in radii upon the deposition of C60 on graphite-like C3N4, indicating a significant enhancement in the rate of charge transfer (Figure 5d). These findings have demonstrated that the electrochemical impedance of graphite-like C3N4 is optimized when it is combined with C60.
Transient fluorescence was conducted to illustrate the separation behaviors of photoinduced electron-and-hole pairs by calculating the excited-state lifetimes of graphite-like C3N4 and graphite-like C3N4/C60@6:1. The graphite-like C3N4/C60@6:1 composite demonstrated a longer transient fluorescence lifetime (3.02 ns) compared to the pure graphite-like C3N4 (1.87 ns), indicating that graphite-like C3N4/C60@6:1 has a higher potential for efficiently achieving the photocatalytic degradation of gaseous HCHO (Figure 5e). A significantly lower photoluminescence intensity of the graphite-like C3N4/C60@6:1 could be observed compared with that of the graphite-like C3N4, which, in principle, suggests that graphite-like C3N4/C60@6:1 is more effective in the separation and transfer of photogenerated charge carriers and the suppression of photogenerated exciton recombination (Figure 5f).
3. Materials and Methods
3.1. Materials
Urea (98%) and C60 (98%) were supplied by Aladdin. Toluene and other conventional reagents were obtained from d from Beijing HWRK Chem Co., Ltd., (Beijing, China). All solvents and chemicals were used directly without any further purification.
3.2. Preparation of Graphite-like C3N4
With reference to the prior literature, 20 g of urea was placed in a crucible and heated to 550 °C for 5 h. The graphite-like C3N4 was obtained as powdery yellow granules and was used directly without further treatment.
3.3. Preparation of Graphite-like C3N4@C60 Composites
To prepare a mixture of C60 in the toluene (50 mL), the as-prepared graphite-like C3N4 (200 mg) was added. After stirring, the mixture was treated ultrasonically for 1 h. After the removal of the toluene under vacuum, the residues were rinsed with ethanol and dried to obtain gray graphite-like C3N4@C60 composites. Different graphite-like C3N4@C60 composites were synthesized, with the weight ratios of C60:graphite-like C3N4 ranging from 0 to 8 wt%.
3.4. Photocatalytic Degradation of Gaseous Formaldehyde
Approximately 300 mg of composites was evenly dispersed in 30 mL of distilled water, followed by sonication for 30 min. The resulting suspension was transferred into three surface dishes with 6 cm diameters, followed by drying at 60 °C for 12 h. The surface dishes were placed in a reactor, and the reactor was left in a closed system. Then, formaldehyde was injected using a microsyringe and the initial concentration of formaldehyde was set at about 120 ± 20 ppm. The concentrations of formaldehyde were measured online using an IR multi-gas monitor (INNOVA Air Tech Instruments Model 1412) under UV-vis light irradiation for 1 h at a wavelength range of 310–800 nm and an intensity of 2.9 mW/cm2.
4. Conclusions
In summary, various graphite-like C3N4 and C60 composites were developed via an in situ generation strategy. The efficiency charge separation and photocatalysis for the resultant composites were tuned by simply varying the weight ratios between the C60 and the graphite-like C3N4. It was found that the graphite-like C3N4/C60@6:1 composite exhibited the strongest built-in electric field, thus realizing efficient charge separation and rendering superior photocatalytic efficiency for formaldehyde degradation compared to the graphite-like C3N4 and the C60. Overall, this research has offered valuable insights into the design and development of novel synergistic systems for practical applications, particularly in the field of photocatalysis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28155815/s1. Figure S1. XRD for C60, g-C3N4, and g-C3N4/C60@6:1. Figure S2. Nitrogen adsorption and desorption isotherms of g-C3N4, and g-C3N4/C60@6:1 at 77 K. Figure S3. pore size distribution for g-C3N4 and g-C3N4/C60@6:1.
Author Contributions
D.P.: resources, investigation, methods and forms, Analysis, writing the first draft, verification, final revision of the paper, etc. Z.Z.: capital, conceptualization, methodology, first draft supervision, etc. J.Z.: review of the first draft, analysis of the opinions on the revision of the paper, etc. Y.Y.: Concept, data statistics and analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been strongly supported by the Hunan Province Key R&D (2022NK2043) Hebei Provincial Scientific Research Project for Introducing of National High-level Innovative Talents (2021HBQZYCXY011) and the Hunan Provincial Science and Technology Innovation Leaders (2021RC4033).
Institutional Review Board Statement
No applicable.
Informed Consent Statement
No applicable.
Data Availability Statement
No applicable.
Acknowledgments
Thanks go out to all research partners in this project for their cooperation and collaboration.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Han, K.H.; Zhang, J.S.; Guo, B. Toward effective design and adoption of catalyst-based filter for indoor hazards: Formaldehyde abatement under realistic conditions. J. Hazard. Mater. 2017, 331, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Shi, M.; Li, Y.; Wu, Z.; Yang, L.; Zhang, S.; Xiao, X.; Liu, C.; Dai, W.; Chen, C.; et al. Congregated-electrons-strengthened anchoring and mineralization of gaseous formaldehyde on a novel self-supporting Cu2−xSe/Cu2O heterojunction photocatalyst under visible lights: A viable mesh for designing air purifier. Appl. Catal. B Environ. 2022, 312, 121427. [Google Scholar] [CrossRef]
- Suresh, S.; Bandosz, T.J. Removal of formaldehyde on carbon -based materials: A review of the recent approaches and findings. Carbon 2018, 137, 207–221. [Google Scholar] [CrossRef]
- Yusuf, A.; Snape, C.; He, J.; Xu, H.; Liu, C.; Zhao, M.; Chen, G.Z.; Tang, B.; Wang, C.; Wang, J.; et al. Advances on transition metal oxides catalysts for formaldehyde oxidation: A review. Catal. Rev. 2017, 59, 189–233. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Z.; Shangguan, W. Low-temperature catalysis for VOCs removal in technology and application: A state-of-the-art review. Catal. Today 2016, 264, 270–278. [Google Scholar] [CrossRef]
- Xu, Z.; Qin, N.; Wang, J.; Tong, H. Formaldehyde biofiltration as affected by spider plant. Bioresour. Technol. 2010, 101, 6930–6934. [Google Scholar] [CrossRef]
- Dingle, P.; Tapsell, P.; Hu, S. Reducing Formaldehyde Exposure in Office Environments Using Plants. Bull. Environ. Contam. Toxicol. 2000, 64, 302–308. [Google Scholar] [CrossRef]
- Liu, S.-H.; Lin, W.-X. A simple method to prepare g-C3N4-TiO2/waste zeolites as visible-light-responsive photocatalytic coatings for degradation of indoor formaldehyde. J. Hazard. Mater. 2019, 368, 468–476. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, W.; Yu, J.; Tian, X.; Li, X.; Deng, Z.; Lin, F.; Zhang, Y. Enhanced photocatalytic degradation efficiency of formaldehyde by in-situ fabricated TiO2/C/CaCO3 heterojunction photocatalyst from mussel shell extract. J. Solid State Chem. 2022, 311, 123110. [Google Scholar] [CrossRef]
- Li, J.; Cui, W.; Chen, P.; Dong, X.; Chu, Y.; Sheng, J.; Zhang, Y.; Wang, Z.; Dong, F. Unraveling the mechanism of binary channel reactions in photocatalytic formaldehyde decomposition for promoted mineralization. Appl. Catal. B Environ. 2020, 260, 118130. [Google Scholar] [CrossRef]
- Sheng, C.; Wang, C.; Wang, H.; Jin, C.; Sun, Q.; Li, S. Self-photodegradation of formaldehyde under visible-light by solid wood modified via nanostructured Fe-doped WO3 accompanied with superior dimensional stability. J. Hazard. Mater. 2017, 328, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Shayegan, Z.; Lee, C.-S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Zhong, L.; Haghighat, F.; Lee, C.-S.; Lakdawala, N. Performance of ultraviolet photocatalytic oxidation for indoor air applications: Systematic experimental evaluation. J. Hazard. Mater. 2013, 261, 130–138. [Google Scholar] [CrossRef]
- Xiang, N.; Bai, Y.; Li, Q.; Han, X.; Zheng, J.; Zhao, Q.; Hou, Y.; Huang, Z. ZIF-67-derived hierarchical hollow Co3O4@CoMn2O4 nanocages for efficient catalytic oxidation of formaldehyde at low temperature. Mol. Catal. 2022, 528, 112519. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, J.; Peng, K.; Qin, G.; Yang, Y.; Huang, Z. The intrinsic effects of oxygen vacancy and doped non-noble metal in TiO2(B) on photocatalytic oxidation VOCs by visible light driving. J. Environ. Chem. Eng. 2022, 10, 107390. [Google Scholar] [CrossRef]
- Zuo, S.; Zan, J.; Li, D.; Guan, Z.; Yang, F.; Xu, H.; Huang, M.; Xia, D. Efficient peroxymonosulfate nonradical activity of Zn-Mn-Al2O3@g-C3N4 via synergism of Zn, Mn doping and g-C3N4 composite. Sep. Purif. Technol. 2021, 272, 118965. [Google Scholar] [CrossRef]
- Liu, M.; Wei, C.; Zhuzhang, H.; Zhou, J.; Pan, Z.; Lin, W.; Yu, Z.; Zhang, G.; Wang, X. Fully Condensed Poly (Triazine Imide) Crystals: Extended π-Conjugation and Structural Defects for Overall Water Splitting. Angew. Chem. Int. Ed. 2021, 61, e202113389. [Google Scholar] [CrossRef]
- Fang, Y.; Hou, Y.; Fu, X.; Wang, X. Semiconducting Polymers for Oxygen Evolution Reaction under Light Illumination. Chem. Rev. 2022, 122, 4204–4256. [Google Scholar] [CrossRef]
- Chen, X.; Weng, M.; Lan, M.; Weng, Z.; Wang, J.; Guo, L.; Lin, Z.; Qiu, B. Superior antibacterial activity of sulfur-doped g-C3N4 nanosheets dispersed by Tetrastigma hemsleyanum Diels & Gilg’s polysaccharides-3 solution. Int. J. Biol. Macromol. 2021, 168, 453–463. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Yang, D.; Liu, J.; He, L.; Tang, M.; Feng, W.; Wu, X. Fabrication, characterization and high photocatalytic activity of Ag–ZnO heterojunctions under UV-visible light. RSC Adv. 2021, 11, 27257–27266. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, Q.; Xia, Y.; Wang, J.; Chen, H.; Xu, Q.; Liu, J.; Feng, W.; Chen, S. Preparation and characterization of Cu-doped TiO2 nanomaterials with anatase/rutile/brookite triphasic structure and their photocatalytic activity. J. Mater. Sci. Mater. Electron. 2021, 32, 21511–21524. [Google Scholar] [CrossRef]
- Patnaik, S.; Sahoo, D.P.; Parida, K. Recent advances in anion doped g-C3N4 photocatalysts: A review. Carbon 2021, 172, 682–711. [Google Scholar] [CrossRef]
- Qin, J.; Huo, J.; Zhang, P.; Zeng, J.; Wang, T.; Zeng, H. Improving the photocatalytic hydrogen production of Ag/g-C3N4 nanocomposites by dye-sensitization under visible light irradiation. Nanoscale 2016, 8, 2249–2259. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Bai, X.; Shi, J.; Du, X.; Xu, L.; Jin, P. Quasi-full-visible-light absorption by D35-TiO2/g-C3N4 for synergistic persulfate activation towards efficient photodegradation of micropollutants. Appl. Catal. B Environ. 2019, 256, 117759. [Google Scholar] [CrossRef]
- Yu, Y.; Yan, W.; Wang, X.; Li, P.; Gao, W.; Zou, H.; Wu, S.; Ding, K. Surface Engineering for Extremely Enhanced Charge Separation and Photocatalytic Hydrogen Evolution on g-C3N4. Adv. Mater. 2018, 30, 1705060. [Google Scholar] [CrossRef]
- Liu, D.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J.-M. Surface Engineering of g-C3N4 by Stacked BiOBr Sheets Rich in Oxygen Vacancies for Boosting Photocatalytic Performance. Angew. Chem. Int. Ed. 2020, 59, 4519–4524. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Xu, X.; Zhou, Y.; Liu, J.; Yang, H. Rational design via surface engineering on dual 2-dimensional ZnSe/g-C3N4 heterojunction for efficient sequestration of elemental mercury. Chem. Eng. J. 2022, 448, 137606. [Google Scholar] [CrossRef]
- Talukdar, M.; Behera, S.K.; Bhattacharya, K.; Deb, P. Surface modified mesoporous g-C3N4@FeNi3 as prompt and proficient magnetic adsorbent for crude oil recovery. Appl. Surf. Sci. 2019, 473, 275–281. [Google Scholar] [CrossRef]
- Clancy, A.; Bayazit, M.K.; Hodge, S.A.; Skipper, N.T.; Howard, C.A.; Shaffer, M.S.P. Charged Carbon Nanomaterials: Redox Chemistries of Fullerenes, Carbon Nanotubes, and Graphenes. Chem. Rev. 2018, 118, 7363–7408. [Google Scholar] [CrossRef]
- Paquin, F.; Rivnay, J.; Salleo, A.; Stingelin, N.; Silva, C. Multi-phase semicrystalline microstructures drive exciton dissociation in neat plastic semiconductors. J. Mater. Chem. C 2015, 3, 10715–10722. [Google Scholar] [CrossRef]
- Tezuka, N.; Umeyama, T.; Matano, Y.; Shishido, T.; Yoshida, K.; Ogawa, T.; Isoda, S.; Stranius, K.; Chukharev, V.; Tkachenko, N.V.; et al. Photophysics and photoelectrochemical properties of nanohybrids consisting of fullerene-encapsulated single-walled carbon nanotubes and poly(3-hexylthiophene). Energy Environ. Sci. 2011, 4, 741–750. [Google Scholar] [CrossRef]
- Huber, R.C.; Ferreira, A.S.; Thompson, R.; Kilbride, D.; Knutson, N.S.; Devi, L.S.; Toso, D.B.; Challa, J.R.; Zhou, Z.H.; Rubin, Y.; et al. Long-lived photoinduced polaron formation in conjugated polyelectrolyte-fullerene assemblies. Science 2015, 348, 1340–1343. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Chen, C.-H.; Chen, N.; Echegoyen, L. Fullerenes as Nanocontainers That Stabilize Unique Actinide Species Inside: Structures, Formation, and Reactivity. Accounts Chem. Res. 2019, 52, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Zieleniewska, A.; Lodermeyer, F.; Roth, A.; Guldi, D.M. Fullerenes—How 25 years of charge transfer chemistry have shaped our understanding of (interfacial) interactions. Chem. Soc. Rev. 2018, 47, 702–714. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Guan, J.; Zhen, J.; Sun, Z.; Du, P.; Lu, Y.; Yang, S. A facile mechanochemical route to a covalently bonded graphitic carbon nitride (g-C3N4) and fullerene hybrid toward enhanced visible light photocatalytic hydrogen production. Nanoscale 2017, 9, 5615–5623. [Google Scholar] [CrossRef]
- Lee, H.K.H.; Telford, A.M.; Röhr, J.A.; Wyatt, M.F.; Rice, B.; Wu, J.; Maciel, A.d.C.; Tuladhar, S.M.; Speller, E.; McGettrick, J.; et al. The role of fullerenes in the environmental stability of polymer:fullerene solar cells. Energy Environ. Sci. 2018, 11, 417–428. [Google Scholar] [CrossRef]
- Li, P.; Fang, J.; Wang, Y.; Manzhos, S.; Cai, L.; Song, Z.; Li, Y.; Song, T.; Wang, X.; Guo, X.; et al. Synergistic Effect of Dielectric Property and Energy Transfer on Charge Separation in Non-Fullerene-Based Solar Cells. Angew. Chem. Int. Ed. 2021, 60, 15054–15062. [Google Scholar] [CrossRef]
- Huang, Q.; Zhuang, G.; Jia, H.; Qian, M.; Cui, S.; Yang, S.; Du, P. Photoconductive Curved-Nanographene/Fullerene Supramolecular Heterojunctions. Angew. Chem. 2019, 131, 6310–6315. [Google Scholar] [CrossRef]
- Eom, T.; Barát, V.; Khan, A.; Stuparu, M.C. Aggregation-free and high stability core–shell polymer nanoparticles with high fullerene loading capacity, variable fullerene type, and compatibility towards biological conditions. Chem. Sci. 2021, 12, 4949–49577. [Google Scholar] [CrossRef]
- Zhang, P.; Deng, J.; Mao, J.; Li, H.; Wang, Y. Selective aerobic oxidation of alcohols by a mesoporous graphitic carbon nitride/N-hydroxyphthalimide system under visible-light illumination at room temperature. Chin. J. Catal. 2015, 36, 1580–1586. [Google Scholar] [CrossRef]
- Gong, Y.; Li, M.; Li, H.; Wang, Y. Graphitic carbon nitride polymers: Promising catalysts or catalyst supports for heterogeneous oxidation and hydrogenation. Green Chem. 2015, 17, 715–736 . [Google Scholar] [CrossRef]
- Xu, X.; Yue, Y.; Xin, G.; Huang, N. Rational Construction of Electrically Conductive Covalent Organic Frameworks through Encapsulating Fullerene via Donor–Acceptor Interaction. Macromol. Rapid Commun. 2022, 44, 2200715. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).