Mesosphaerum suaveolens Essential Oil Attenuates Inflammatory Response and Oxidative Stress in LPS-Stimulated RAW 264.7 Macrophages by Regulating NF-κB Signaling Pathway
Abstract
:1. Introduction
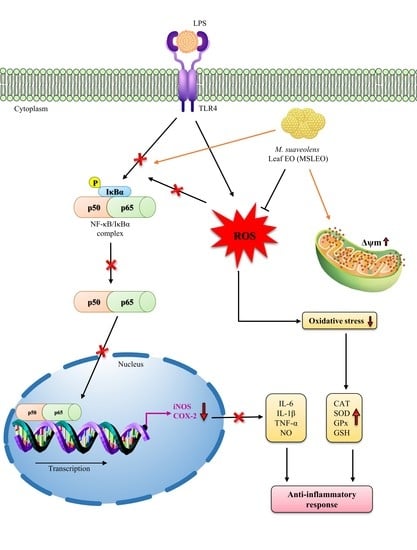
2. Results and Discussion
2.1. Chemical Composition of M. suaveolens Leaf Essential Oil (MSLEO)
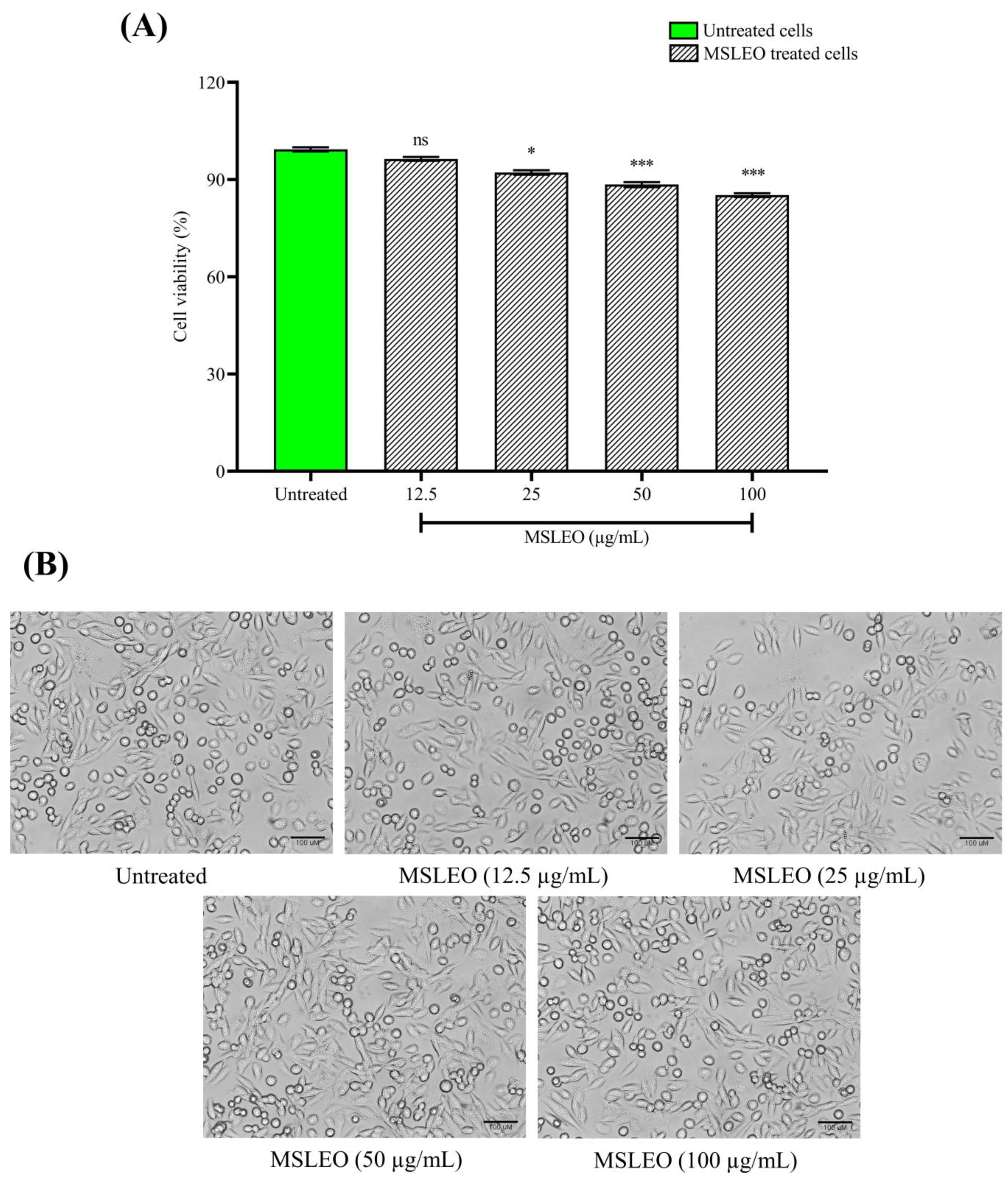
2.2. MSLEO Had No Impact on the Viability and Morphology of RAW 264.7 Cells
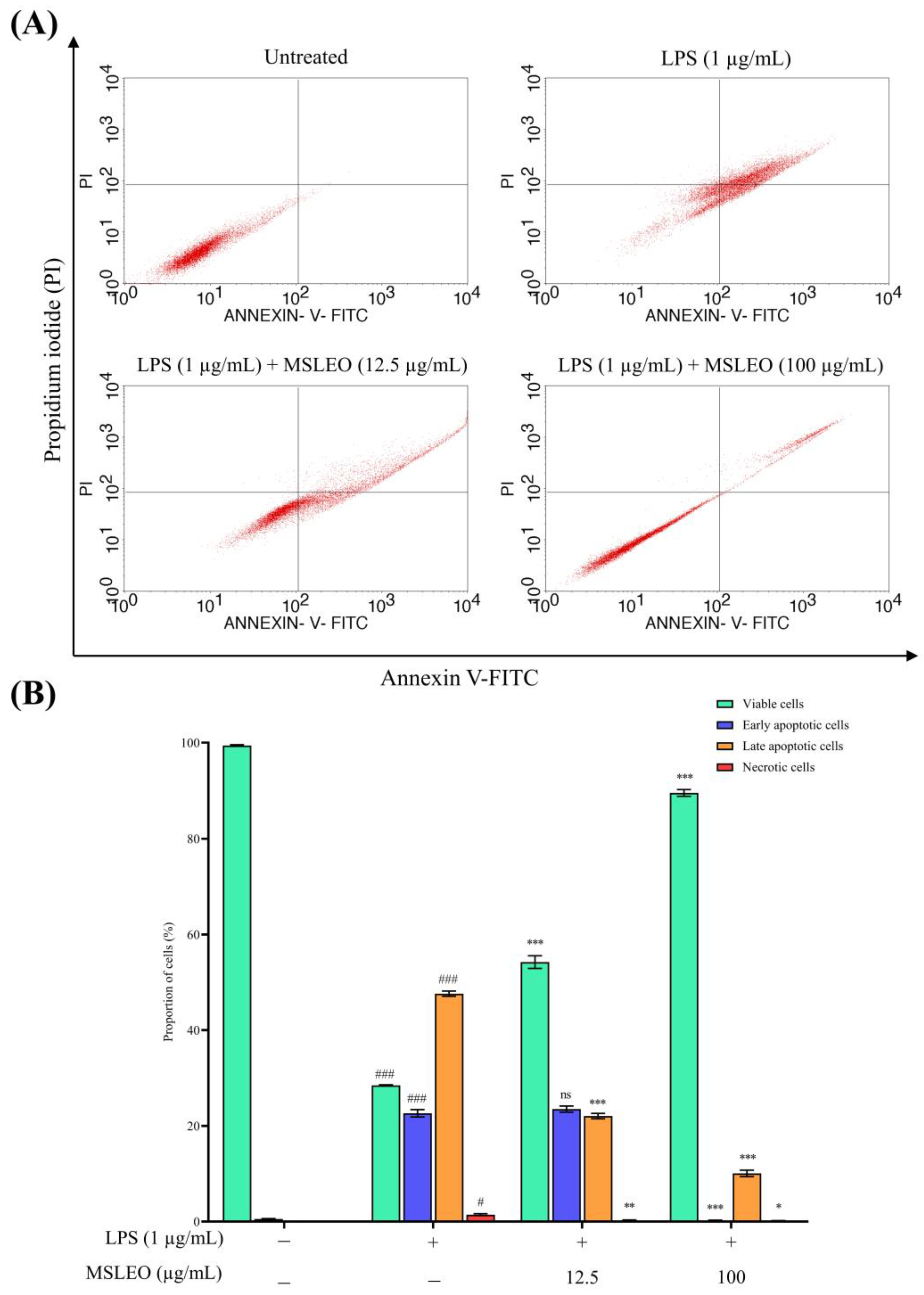
2.3. MSLEO Treatment Attenuates LPS-Induced Increase in Apoptosis
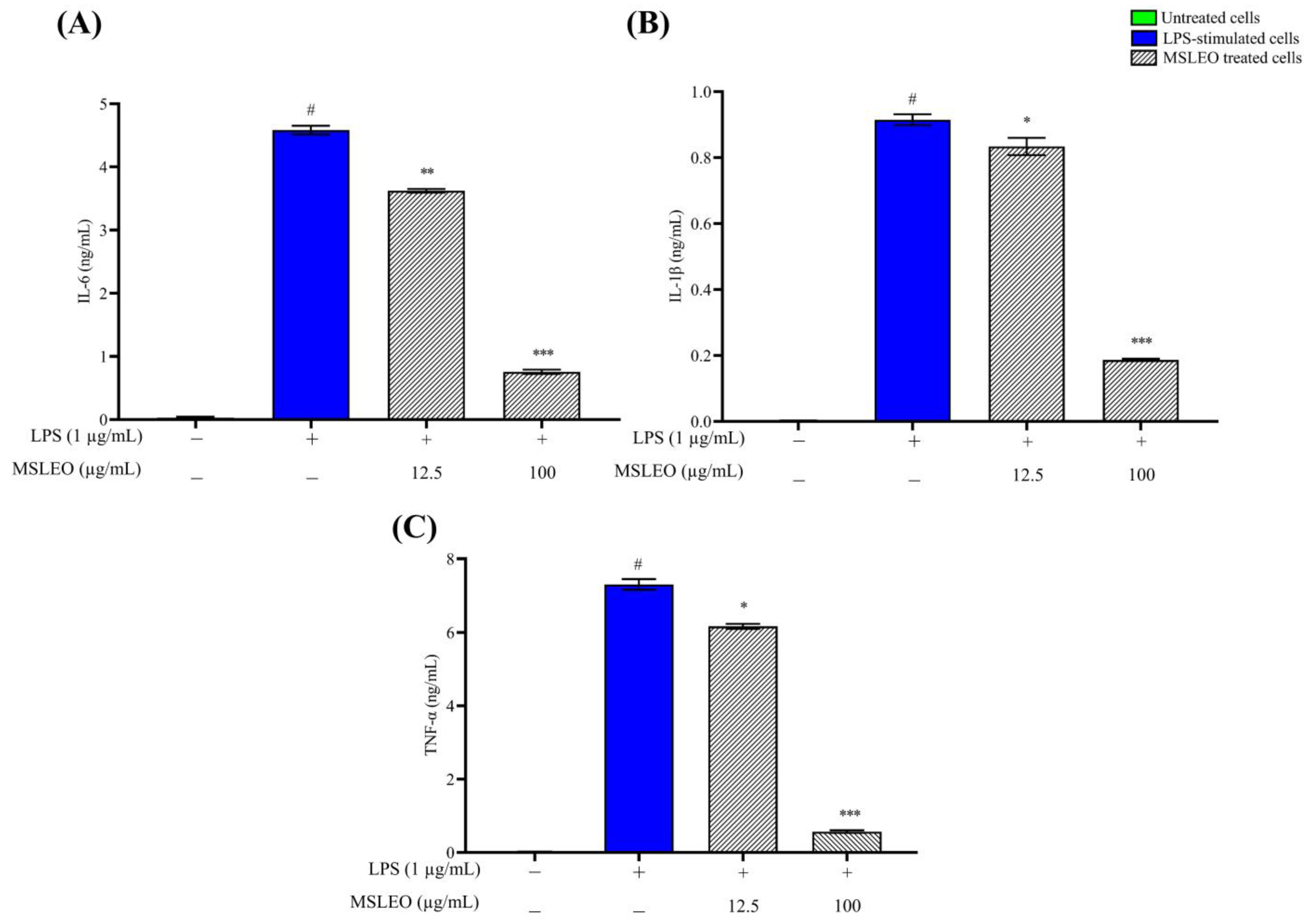
2.4. MSLEO Inhibits the LPS-Induced Increase in Expression of Proinflammatory Cytokines
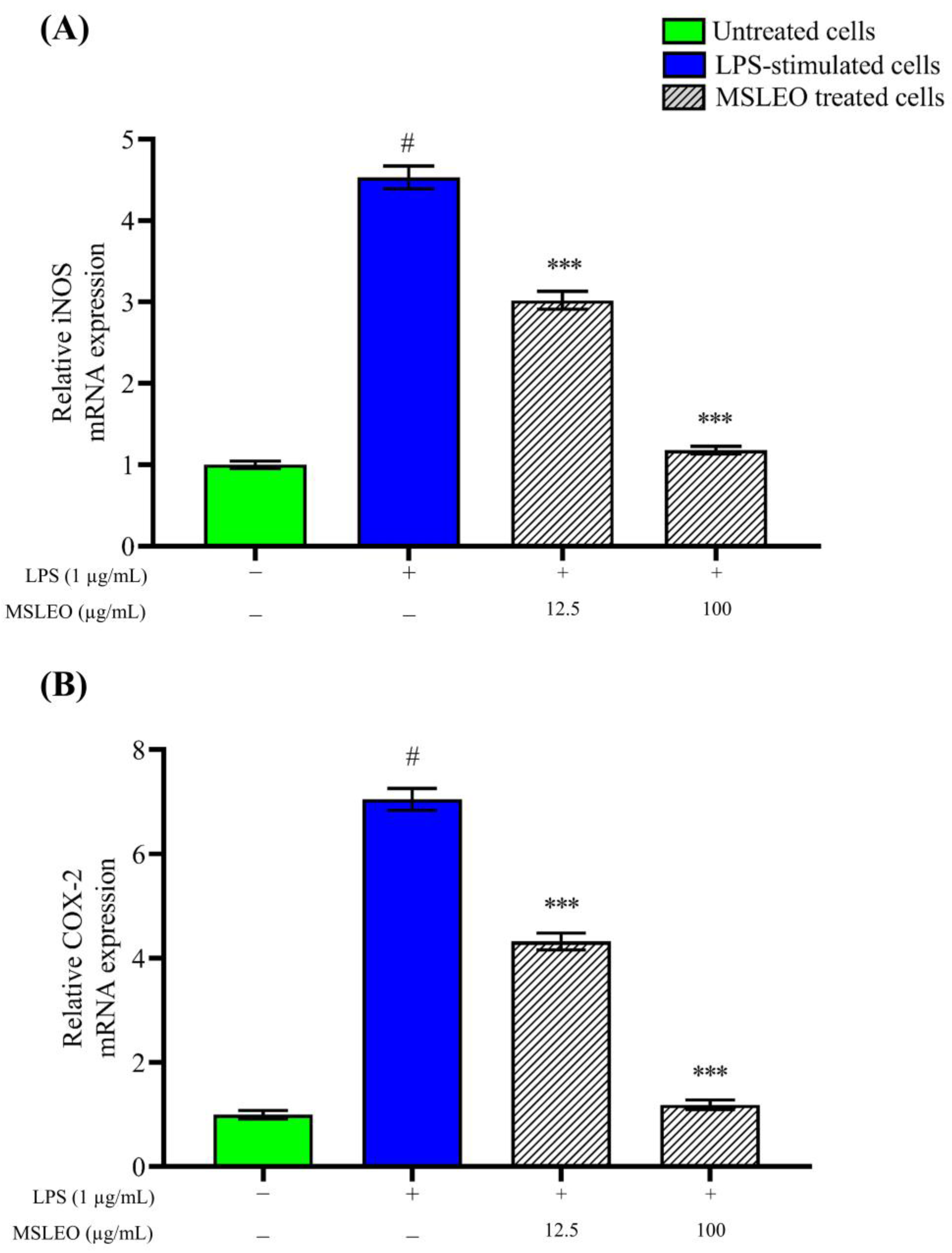
2.5. MSLEO Downregulates LPS-Induced Increase in iNOS and COX-2 mRNA Expression
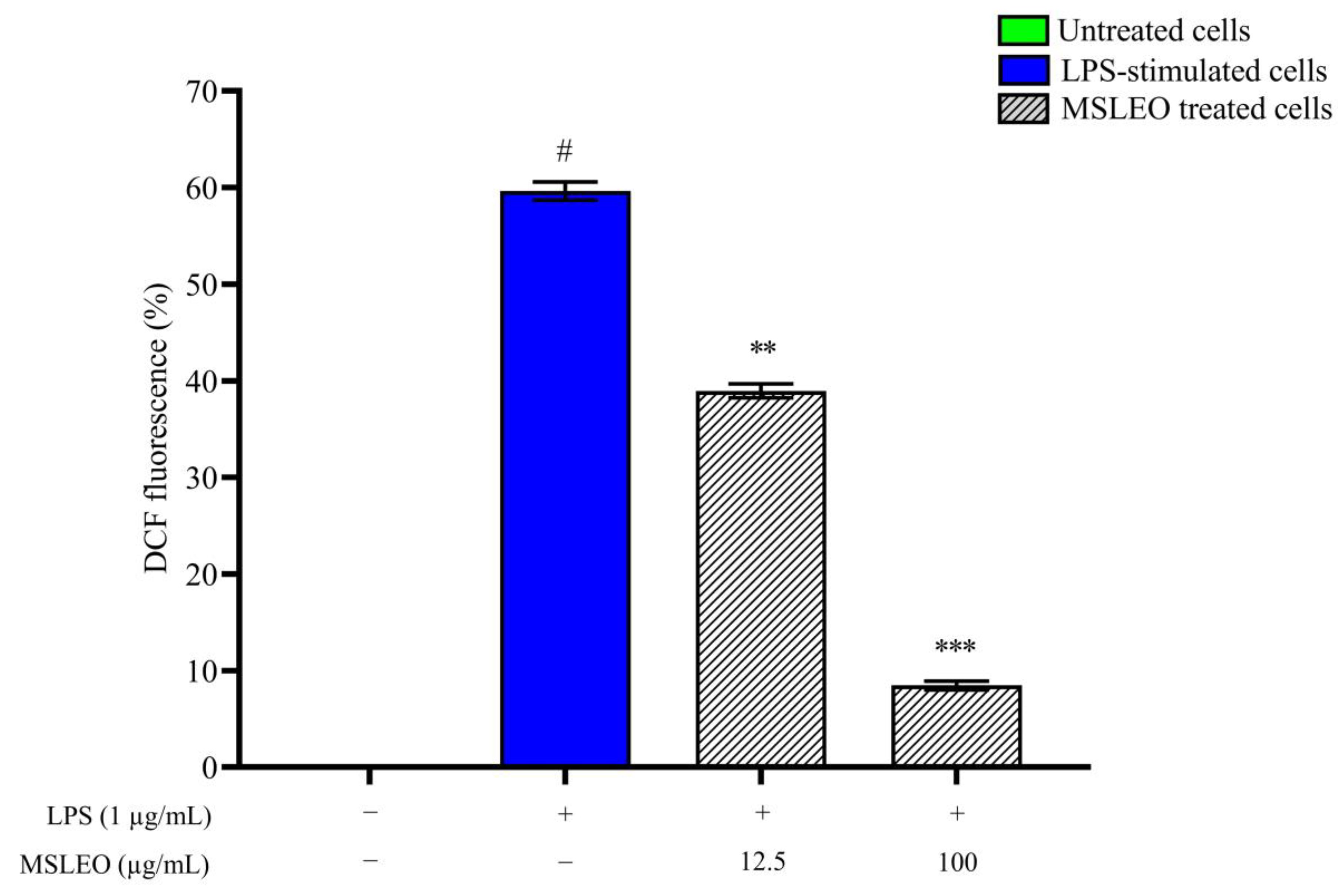
2.6. MSLEO Treatment Decreased LPS-Induced Increase in ROS Levels
2.7. MSLEO Enhances Endogenous Antioxidant Enzyme Activities
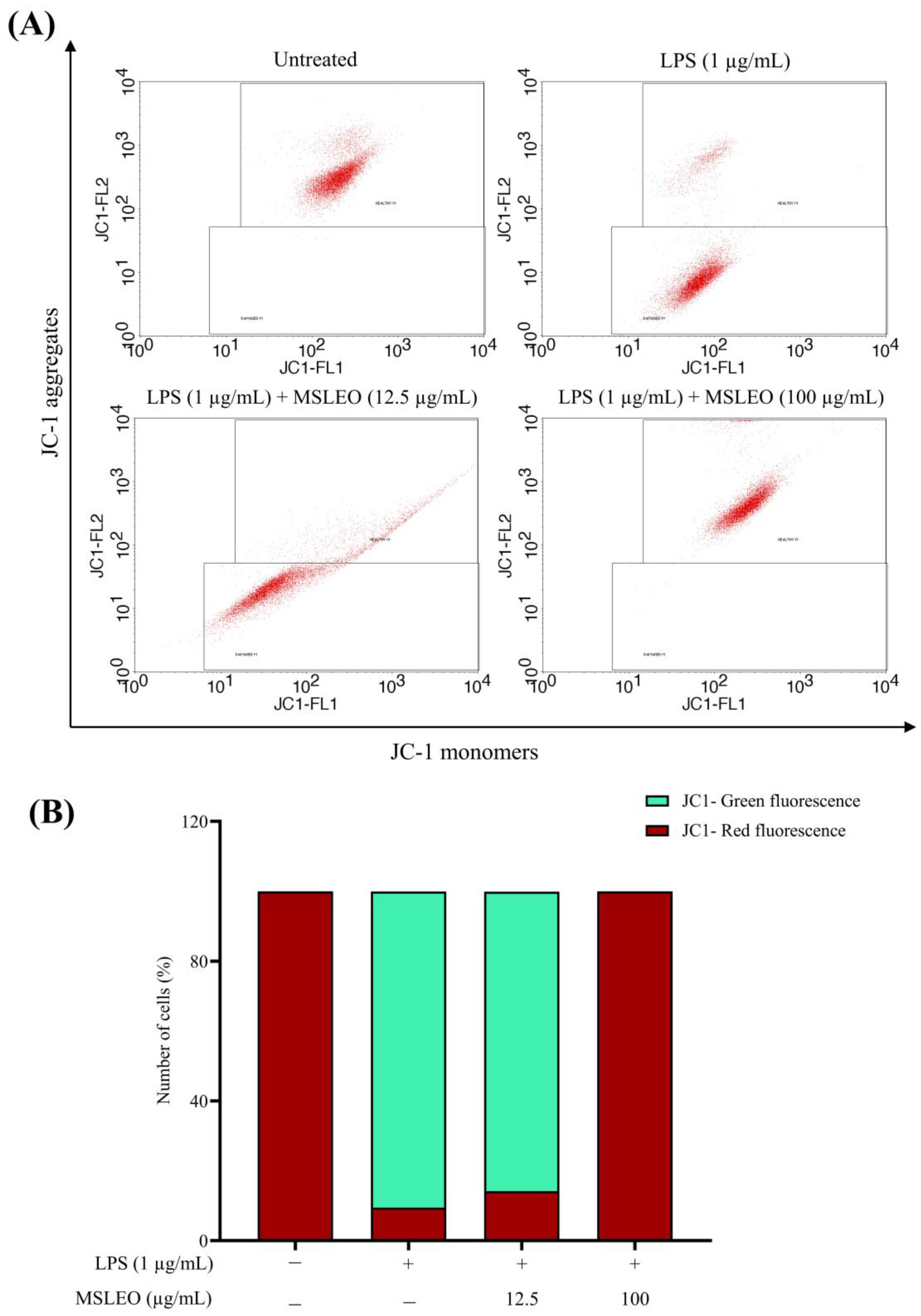
2.8. MSLEO Prevents Mitochondrial Membrane Potential (Δψm) from LPS-Stimulated Depolarization
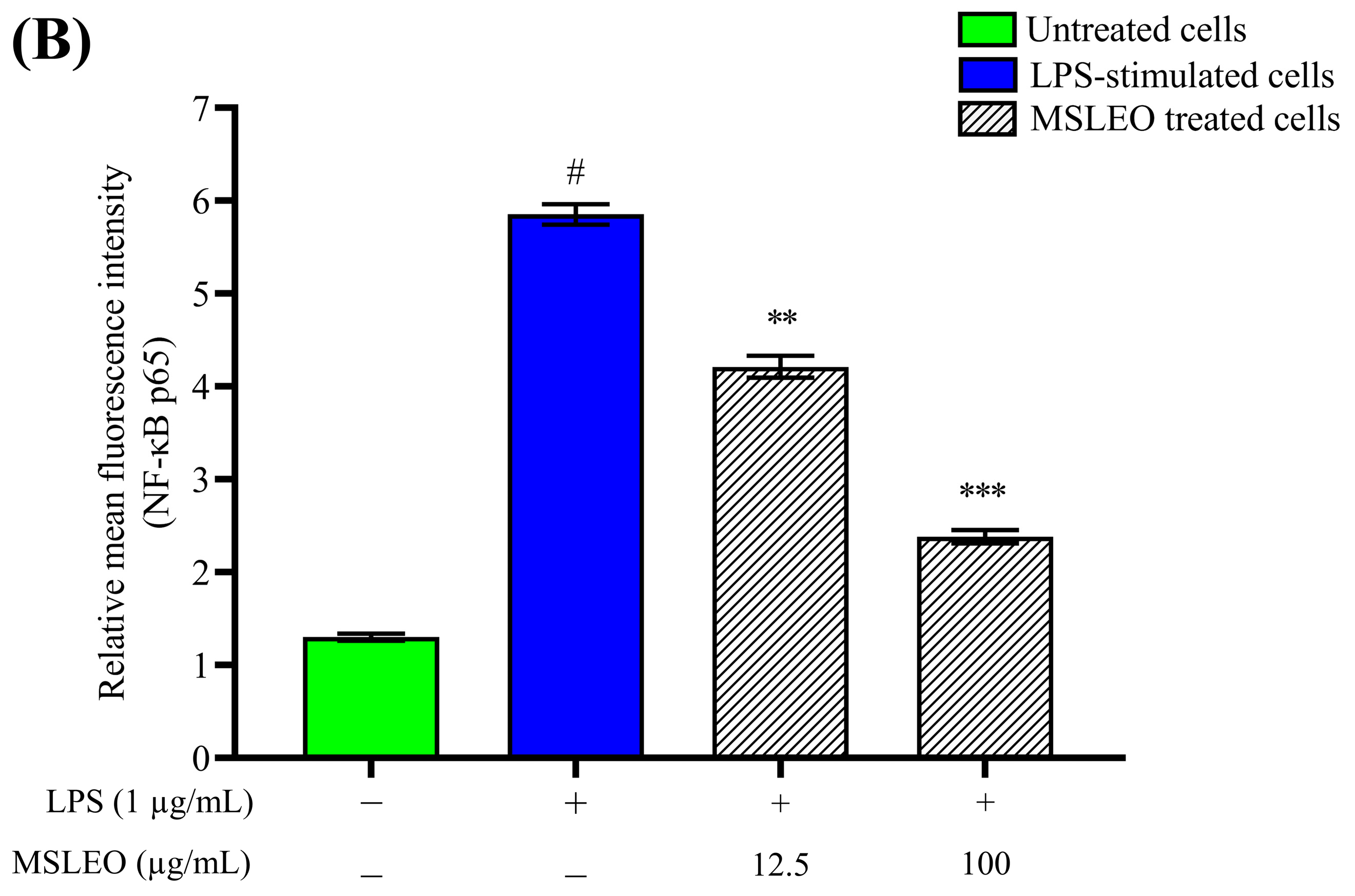
2.9. MSLEO Restricts NF-κB Nuclear Translocation in LPS-Stimulated RAW 264.7 Cells
3. Materials and Methods
3.1. Collection of Plant Samples and Essential Oil Isolation
3.2. Culture and Maintenance of Cells
3.3. GC-MS and GC-FID Analysis of MSLEO
3.4. Cytotoxicity Assay
3.5. Annexin V-FITC/PI Apoptosis Assay
3.6. Proinflammatory Cytokine (IL-6, IL-1β, and TNF-α) Detection by ELISA
3.7. RNA Extraction and Real-Time Quantitative PCR (RT-qPCR)
3.8. Intracellular ROS Assay
3.9. Measurement of Endogenous Antioxidant Enzyme Activities by ELISA
3.10. Mitochondrial Membrane Potential (MMP) Assay
3.11. NF-κB Nuclear Translocation Assay by Confocal Microscopy
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chou, S.T.; Lai, C.C.; Lai, C.P.; Chao, W.W. Chemical composition, antioxidant, anti-melanogenic and anti-inflammatory activities of Glechoma hederacea (Lamiaceae) essential oil. Ind. Crop. Prod. 2018, 122, 675–685. [Google Scholar] [CrossRef]
- Raka, R.N.; Zhiqian, D.; Yue, Y.; Luchang, Q.; Suyeon, P.; Junsong, X.; Hua, W. Pingyin rose essential oil alleviates LPS-induced inflammation in RAW 264.7 cells via the NF-κB pathway: An integrated in vitro and network pharmacology analysis. BMC Complement. Med. Ther. 2022, 22, 272. [Google Scholar] [CrossRef]
- Chen, Z.; Bozec, A.; Ramming, A.; Schett, G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat. Rev. Rheumatol. 2019, 15, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Sorci-Thomas, M.G.; Thomas, M.J. Microdomains, inflammation, and atherosclerosis. Circ. Res. 2016, 118, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121. [Google Scholar] [CrossRef]
- Polednik, K.M.; Koch, A.C.; Felzien, L.K. Effects of essential oil from Thymus vulgaris on viability and inflammation in zebrafish embryos. Zebrafish 2018, 15, 361–371. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627. [Google Scholar] [CrossRef] [Green Version]
- Billack, B. Macrophage activation: Role of toll-like receptors, nitric oxide, and nuclear factor kappa B. Am. J. Pharm. Educ. 2006, 70, 102. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [Green Version]
- Aviello, G.; Knaus, U.G. ROS in gastrointestinal inflammation: Rescue or sabotage? Br. J. Pharmacol. 2017, 174, 1704–1718. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Zhu, L.; Wang, S.; Gao, Y.; Jin, F. Molecular mechanism of the anti-inflammatory effects of plant essential oils: A systematic review. J. Ethnopharmacol. 2023, 307, 115829. [Google Scholar] [CrossRef]
- Huang, Z.; Xie, L.; Xu, Y.; Zhao, K.; Li, X.; Zhong, J.; Lu, Y.; Xu, X.; Goodin, S.; Zhang, K.; et al. Essential oils from Zingiber striolatum Diels attenuate inflammatory response and oxidative stress through regulation of MAPK and NF-κB signaling pathways. Antioxidants 2021, 10, 2019. [Google Scholar] [CrossRef] [PubMed]
- Zarghi, A.; Arfaei, S. Selective COX-2 inhibitors: A review of their structure-activity relationships. Iran. J. Pharm. Res. 2011, 10, 655. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.; Candelario, D.M.; Angelo, L.B. Nonsteroidal anti-inflammatory drugs: Updates on dosage formulations and adverse effects. Orthop. Nurs. 2020, 39, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.F.; Noakes, T.D.; Schwellnus, M.P.; Windt, A.; Bowerbank, P. Non-steroidal anti-inflammatory drugs fail to enhance healing of acute hamstring injuries treated with physiotherapy. S. Afr. Med. J. 1995, 85, 517–522. [Google Scholar] [PubMed]
- Maroon, J.C.; Bost, J.W.; Maroon, A. Natural anti-inflammatory agents for pain relief. Surg. Neurol. Int. 2010, 1, 80. [Google Scholar] [CrossRef] [Green Version]
- Jena, S.; Ray, A.; Mohanta, O.; Das, P.K.; Sahoo, A.; Nayak, S.; Panda, P.C. Neocinnamomum caudatum Essential oil ameliorates lipopolysaccharide-induced inflammation and oxidative stress in RAW 264.7 cells by inhibiting NF-κB activation and ROS production. Molecules 2022, 27, 8193. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef] [Green Version]
- Zuo, X.; Gu, Y.; Wang, C.; Zhang, J.; Zhang, J.; Wang, G.; Wang, F. A systematic review of the anti-inflammatory and immunomodulatory properties of 16 essential oils of herbs. Evid. Based Complement. Altern. Med. 2020, 2020, 8878927. [Google Scholar] [CrossRef]
- Bhargava, V.V.; Patel, S.C.; Desai, K.S. Importance of terpenoids and essential oils in chemotaxonomic approach. Int. J. Herb. Med. 2013, 1, 14–21. [Google Scholar]
- Nóbrega de Almeida, R.; Agra, M.D.F.; Negromonte Souto Maior, F.; De Sousa, D.P. Essential oils and their constituents: Anticonvulsant activity. Molecules 2011, 16, 2726–2742. [Google Scholar] [CrossRef]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org (accessed on 7 June 2023).
- Padalia, H.; Srivastava, V.; Kushwaha, S.P.S. Modeling potential invasion range of alien invasive species, Hyptis suaveolens (L.) Poit. in India: Comparison of MaxEnt and GARP. Ecol. Inform. 2014, 22, 36–43. [Google Scholar] [CrossRef]
- Afreen, T.; Srivastava, P.; Singh, H.; Singh, J.S. Effect of invasion by Hyptis suaveolens on plant diversity and selected soil properties of a constructed tropical grassland. J. Plant Ecol. 2018, 11, 751–760. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Tang, G.; Liu, X.; Li, J.; Wang, D.; Ji, S. An ethnopharmacological review of Hyptis suaveolens (L.) Poit. Trop. J. Pharm. Res. 2020, 19, 1541–1550. [Google Scholar] [CrossRef]
- Mishra, P.; Sohrab, S.; Mishra, S.K. A review on the phytochemical and pharmacological properties of Hyptis suaveolens (L.) Poit. Future J. Pharm. Sci. 2021, 7, 65. [Google Scholar] [CrossRef]
- Sastri, B.N. The Wealth of India—Raw Materials; Council of Scientific and Industrial Research (CSIR): New Delhi, India, 1959; Volume V (H–K). [Google Scholar]
- Adda, C.; Atachi, P.; Hell, K.; Tamò, M. Potential use of the bushmint, Hyptis suaveolens, for the control of infestation by the pink stalk borer, Sesamia calamistis on maize in southern Benin, West Africa. J. Insect Sci. 2011, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Seyoum, A.; Pålsson, K.; Kung’a, S.; Kabiru, E.W.; Lwande, W.; Killeen, G.F.; Hassanali, A.; Knots, B.G.J. Traditional use of mosquito-repellent plants in western Kenya and their evaluation in semi-field experimental huts against Anopheles gambiae: Ethnobotanical studies and application by thermal expulsion and direct burning. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, C.; Torres, I.; Mendoza-Hernández, G.; Garcia-Gasca, T.; Blanco-Labra, A. Analysis of protein fractions and some minerals present in Chan (Hyptis suaveolens L.) seeds. J. Food Sci. 2012, 77, 15–19. [Google Scholar] [CrossRef]
- Hsu, F.C.; Tsai, S.F.; Lee, S.S. Chemical investigation of Hyptis suaveolens seed, a potential antihyperuricemic nutraceutical, with assistance of HPLC-SPE-NMR. J. Food Drug Anal. 2019, 27, 897–905. [Google Scholar] [CrossRef] [PubMed]
- McNeil, M.; Facey, P.; Porter, R. Essential oils from the Hyptis genus—A review (1909–2009). Nat. Prod. Commun. 2011, 6, 1775–1796. [Google Scholar] [PubMed] [Green Version]
- Bezerra, J.W.A.; Rodrigues, F.C.; Lima Bezerra, J.J.; Vieira Pinheiro, A.A.; Almeida de Menezes, S.; Tavares, A.B.; Costa, A.R.; Augusta de Sousa Fernandes, P.; Bezerra da Silva, V.; Martins da Costa, J.G.; et al. Traditional uses, phytochemistry, and bioactivities of Mesosphaerum suaveolens (L.) Kuntze. Evid.-Based Complement. Altern. Med. 2022, 2022, 3829180. [Google Scholar] [CrossRef]
- Barbosa, L.C.A.; Martins, F.T.; Teixeira, R.R.; Polo, M.; Montanari, R.M. Chemical variability and biological activities of volatile oils from Hyptis suaveolens (L.) Poit. Agric. Conspec. Sci. 2013, 78, 1–10. [Google Scholar]
- Luz, T.R.S.A.; Leite, J.A.C.; de Mesquita, L.S.S.; Bezerra, S.A.; Silveira, D.P.B.; de Mesquita, J.W.C.; Gomes, R.E.C.; Vilanova, C.M.; de Sousa Ribeiro, M.N.; do Amaral, F.M.M.; et al. Seasonal variation in the chemical composition and biological activity of the essential oil of Mesosphaerum suaveolens (L.) Kuntze. Ind. Crop. Prod. 2020, 153, 112600. [Google Scholar] [CrossRef]
- Nantitanon, W.; Chowwanapoonpohn, S.; Okonogi, S. Antioxidant and antimicrobial activities of Hyptis suaveolens essential oil. Sci. Pharm. 2007, 75, 35–54. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.H.; Huang, Y.S.; Jiang, D.Q.; Yuan, K. The essential oils chemical compositions and antimicrobial, antioxidant activities and toxicity of three Hyptis species. Pharm. Biol. 2013, 51, 1125–1130. [Google Scholar] [CrossRef] [Green Version]
- Bezerra, J.W.A.; Costa, A.R.; Da Silva, M.A.P.; Rocha, M.I.; Boligon, A.A.; Da Rocha, J.B.T.; Barros, L.M.; Kamdem, J.P. Chemical composition and toxicological evaluation of Hyptis suaveolens (L.) Poiteau (LAMIACEAE) in Drosophila melanogaster and Artemia salina. S. Afr. J. Bot. 2017, 113, 437–442. [Google Scholar] [CrossRef]
- Bayala, B.; Zouré, A.A.; Zohoncon, T.M.; Tinguerie, B.L.; Baron, S.; Bakri, Y.; Simpore, J.; Lobaccaro, J.M.A. Effects of extracts and molecules derived from medicinal plants of West Africa in the prevention and treatment of gynecological cancers: A review. Am. J. Cancer Res. 2020, 10, 2730. [Google Scholar] [PubMed]
- Grassi, P.; Reyes, T.S.U.; Sosa, S.; Tubaro, A.; Hofer, O.; Zitterl-Eglseer, K. Anti-inflammatory activity of two diterpenes of Hyptis suaveolens from El Salvador. Z. Naturforsch. C 2006, 61, 165–170. [Google Scholar] [CrossRef]
- Shenoy, R.; Shirwaikar, A. Anti-inflammatory and free radical scavenging studies of Hyptis suaveolens (Labiatae). Indian Drugs 2002, 39, 574–577. [Google Scholar]
- Machado, F.D.F.; de Oliveira Formiga, R.; de Morais Lima, G.R.; de Jesus, N.Z.T.; Júnior, E.B.A.; Marinho, A.F.; Tavares, J.F.; Santos, F.A.; Viana, A.F.S.C.; Araújo, A.A.; et al. Hyptis suaveolens (L.) Poit protects colon from TNBS-induced inflammation via immunomodulatory, antioxidant and anti-proliferative mechanisms. J. Ethnopharmacol. 2021, 265, 113153. [Google Scholar] [CrossRef] [PubMed]
- Mallavarapu, G.R.; Ramesh, S.; Kaul, P.N.; Bhattacharya, A.K.; Rajeswara Rao, B.R. The essential oil of Hyptis suaveolens (L.) Poit. J. Essent. Oil Res. 1993, 5, 321–323. [Google Scholar] [CrossRef]
- Peerzada, N. Chemical composition of the essential oil of Hyptis suaveolens. Molecules 1997, 2, 165–168. [Google Scholar] [CrossRef] [Green Version]
- Malele, R.S.; Mutayabarwa, C.K.; Mwangi, J.W.; Thoithi, G.N.; Lopez, A.G.; Lucini, E.I.; Zygadlo, J.A. Essential oil of Hyptis suaveolens (L.) Poit. from Tanzania: Composition and antifungal activity. J. Essent. Oil Res. 2003, 15, 438–440. [Google Scholar] [CrossRef]
- Lohani, H.; Andola, H.C.; Chauhan, N. Variations in essential oil composition and biological activity of Hyptis suaveolens Poit: A high value aromatic plant of the Himalaya. Med. Plants Int. J. Phytomed. 2011, 3, 311–314. [Google Scholar] [CrossRef]
- Martins, F.T.; Santos, M.H.D.; Polo, M.; Barbosa, L.C.D.A. Chemical variation in the essential oil of Hyptis suaveolens (L.) Poit., under cultivation condition. Quim. Nova 2006, 29, 1203–1209. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.J.; Kim, J.M.; Lee, J.C.; Kim, W.K.; Chun, H.S. Protective effect of β-caryophyllene, a natural bicyclic sesquiterpene, against cerebral ischemic injury. J. Med. Food 2013, 16, 471–480. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Ahamed, M.B.K.; Majid, A.M.A. In vivo anti-inflammatory activity of β-caryophyllene, evaluated by molecular imaging. Mol. Med. Chem. 2015, 1, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Scandiffio, R.; Geddo, F.; Cottone, E.; Querio, G.; Antoniotti, S.; Gallo, M.P.; Maffei, M.E.; Bovolin, P. Protective effects of (E)-β-caryophyllene (BCP) in chronic inflammation. Nutrients 2020, 12, 3273. [Google Scholar] [CrossRef]
- Bell, S.J.; Gomez-Pinilla, F.; Ling, P.R. Beta-caryophyllene, an anti-inflammatory natural compound, improves cognition. J. Food Nutr. Sci. 2021, 3, 189–199. [Google Scholar]
- Brito, L.F.; Oliveira, H.B.M.; das Neves Selis, N.; e Souza, C.L.S.; Júnior, M.N.S.; de Souza, E.P.; Silva, L.S.C.D.; de Souza Nascimento, F.; Amorim, A.T.; Campos, G.B.; et al. Anti-inflammatory activity of β-caryophyllene combined with docosahexaenoic acid in a model of sepsis induced by Staphylococcus aureus in mice. J. Sci. Food Agric. 2019, 99, 5870–5880. [Google Scholar] [CrossRef] [PubMed]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A sesquiterpene with countless biological properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef] [Green Version]
- Jha, N.K.; Sharma, C.; Hashiesh, H.M.; Arunachalam, S.; Meeran, M.F.; Javed, H.; Patil, C.R.; Goyal, S.N.; Ojha, S. β-Caryophyllene, a natural dietary CB2 receptor selective cannabinoid can be a candidate to target the trinity of infection, immunity, and inflammation in Covid-19. Front. Pharmacol. 2021, 12, 590201. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- Apel, M.A.; Lima, M.E.; Sobral, M.; Young, M.C.M.; Cordeiro, I.; Schapoval, E.E.; Henriques, A.T.; Moreno, P.R.H. Anti-inflammatory activity of essential oil from leaves of Myrciaria tenella and Calycorectes sellowianus. Pharm. Biol. 2010, 48, 433–438. [Google Scholar] [CrossRef]
- Nascimento, K.F.; Moreira, F.M.F.; Santos, J.A.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; do Carmo Vieira, M.; Ruiz, A.L.T.G.; Foglio, M.A.; de Carvalho, J.E.; et al. Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef]
- Santos, D.E.; Radai, J.A.S.; do Nascimento, K.F.; Formagio, A.S.N.; de Matos Balsalobre, N.; Ziff, E.B.; Castelon Konkiewitz, E.; Kassuya, C.A.L. Contribution of spathulenol to the anti-nociceptive effects of Psidium guineense. Nutr. Neurosci. 2022, 25, 812–822. [Google Scholar] [CrossRef]
- Fernandez, M.A.; Tornos, M.P.; Garcia, M.D.; De las Heras, B.; Villar, A.M.; Saenz, M.T. Anti-inflammatory activity of abietic acid, a diterpene isolated from Pimenta racemosa var. grissea. J. Pharm. Pharmacol. 2001, 53, 867–872. [Google Scholar] [CrossRef]
- González, M.A. Aromatic abietane diterpenoids: Their biological activity and synthesis. Nat. Prod. Rep. 2014, 32, 684–704. [Google Scholar] [CrossRef]
- Silva, R.O.; Sousa, F.B.M.; Damasceno, S.R.; Carvalho, N.S.; Silva, V.G.; Oliveira, F.R.M.; Sousa, D.P.; Aragão, K.S.; Barbosa, A.L.; Freitas, R.M.; et al. Phytol, a diterpene alcohol, inhibits the inflammatory response by reducing cytokine production and oxidative stress. Fundam. Clin. Pharmacol. 2014, 28, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Martins, H.B.; Selis, N.D.N.; Nascimento, F.S.; Carvalho, S.P.D.; Gusmão, L.D.O.; Nascimento, J.D.S.; Brito, A.K.P.; Souza, S.I.D.; Oliveira, M.V.D.; Timenetsky, J.; et al. Anti-inflammatory activity of the essential oil citral in experimental infection with Staphylococcus aureus in a model air pouch. Evid.-Based Complement. Altern. Med. 2017, 2017, 2505610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sitarek, P.; Rijo, P.; Garcia, C.; Skała, E.; Kalemba, D.; Białas, A.J.; Szemraj, J.; Pytel, D.; Toma, M.; Wysokińska, H.; et al. Antibacterial, anti-inflammatory, antioxidant, and antiproliferative properties of essential oils from hairy and normal roots of Leonurus sibiricus L. and their chemical composition. Oxid. Med. Cell Longev. 2017, 2017, 7384061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salminen, A.; Lehtonen, M.; Suuronen, T.; Kaarniranta, K.; Huuskonen, J. Terpenoids: Natural inhibitors of NF-κB signaling with anti-inflammatory and anticancer potential. Cell. Mol. Life Sci. 2008, 65, 2979–2999. [Google Scholar] [CrossRef] [PubMed]
- De Cássia da Silveira e Sá, R.; Andrade, L.N.; de Sousa, D.P. A review on anti-inflammatory activity of monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef] [Green Version]
- De Santana Souza, M.T.; Almeida, J.R.G.D.S.; de Souza Araujo, A.A.; Duarte, M.C.; Gelain, D.P.; Moreira, J.C.F.; Dos Santos, M.R.V.; Quintans-Júnior, L.J. Structure-activity relationship of terpenes with anti-inflammatory profile—A systematic review. Basic Clin. Pharmacol. Toxicol. 2014, 115, 244–256. [Google Scholar] [CrossRef]
- Jain, H.; Dhingra, N.; Narsinghani, T.; Sharma, R. Insights into the mechanism of natural terpenoids as NF-κB inhibitors: An overview on their anticancer potential. Exp. Oncol. 2016, 38, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.J.; Fischer, N.; Efferth, T. Phytochemicals as inhibitors of NF-kB for treatment of Alzheimer’s disease. Pharmacol. Res. 2018, 129, 262–273. [Google Scholar] [CrossRef]
- Chen, B.; Li, H.; Ou, G.; Ren, L.; Yang, X.; Zeng, M. Curcumin attenuates MSU crystal-induced inflammation by inhibiting the degradation of IkappaB alpha and blocking mitochondrial damage. Arthritis Res. Ther. 2019, 21, 193. [Google Scholar] [CrossRef] [Green Version]
- Li, W.Y.; Yang, F.; Chen, J.H.; Ren, G.F. β-caryophyllene ameliorates MSU-induced gouty arthritis and inflammation through inhibiting NLRP3 and NF-κB signal pathway: In silico and in vivo. Front. Pharmacol. 2021, 12, 651305. [Google Scholar] [CrossRef]
- Liu, M.; Niu, W.; Oub, L. β-Caryophyllene ameliorates the Mycoplasmal pneumonia through the inhibition of NF-κB signal transduction in mice. Saudi J. Biol. Sci. 2021, 28, 4240–4246. [Google Scholar] [CrossRef]
- Younis, N.S. β-Caryophyllene ameliorates cyclophosphamide induced cardiac injury: The association of TLR4/NFκB and Nrf2/HO1/NQO1 pathways. J. Cardiovasc. Dev. Dis. 2022, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Chiu, L.C.; Wang, J.Y.; Lin, C.H.; Hsu, C.H.; Lin, L.C.; Fu, S.L. Diterpenoid compounds isolated from Chloranthus oldhamii Solms exert anti-inflammatory effects by inhibiting the ikk/nf-κb pathway. Molecules 2021, 26, 6540. [Google Scholar] [CrossRef] [PubMed]
- Claro-Cala, C.M.; Grao-Cruces, E.; Toscano, R.; Millan-Linares, M.C.; Montserrat-de la Paz, S.; Martin, M.E. Acyclic diterpene phytol from hemp seed oil (Cannabis sativa L.) exerts anti-inflammatory activity on primary human monocytes-macrophages. Foods 2022, 11, 2366. [Google Scholar] [CrossRef] [PubMed]
- Valente, J.; Zuzarte, M.; Gonçalves, M.J.; Lopes, M.C.; Cavaleiro, C.; Salgueiro, L.; Cruz, M.T. Antifungal, antioxidant and anti-inflammatory activities of Oenanthe crocata L. essential oil. Food Chem. Toxicol. 2013, 62, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.E.; Elsayed, S.A.; Madkor, H.R.; Eldien, H.M.S.; Mohafez, O.M. Yarrow oil ameliorates ulcerative colitis in mice model via regulating the NF-κB and PPAR-γ pathways. Intest. Res. 2021, 19, 194. [Google Scholar] [CrossRef]
- Yoon, W.J.; Moon, J.Y.; Kang, J.Y.; Kim, G.O.; Lee, N.H.; Hyun, C.G. Neolitsea sericea essential oil attenuates LPS-induced inflammation in RAW 264.7 macrophages by suppressing NF-κB and MAPK activation. Nat. Prod. Commun. 2010, 5, 1934578X1000500835. [Google Scholar] [CrossRef] [PubMed]
- Fraternale, D.; Teodori, L.; Rudov, A.; Prattichizzo, F.; Olivieri, F.; Guidarelli, A.; Albertini, M.C. The in vitro activity of Angelica archangelica L. essential oil on inflammation. J. Med. Food 2018, 21, 1238–1243. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Xu, X.; Lai, Y.; Hua, Z.C. Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci. Rep. 2019, 39, BSR20180992. [Google Scholar] [CrossRef] [Green Version]
- Xaus, J.; Comalada, M.; Valledor, A.F.; Lloberas, J.; López-Soriano, F.; Argilés, J.M.; Bogdan, C.; Celada, A. LPS induces apoptosis in macrophages mostly through the autocrine production of TNF-α. Blood 2000, 95, 3823–3831. [Google Scholar] [CrossRef]
- George, L.; Ramasamy, T.; Sirajudeen, K.N.S.; Manickam, V. LPS-induced apoptosis is partially mediated by hydrogen sulphide in RAW 264.7 murine macrophages. Immunol. Investig. 2019, 48, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.; Zhang, X.; Bi, Y.; Miao, R.; Zhang, Z.; Su, H. Anti-inflammatory effects of cumin essential oil by blocking JNK, ERK, and NF-κB signaling pathways in LPS-stimulated RAW 264.7 cells. Evid. Based Complement. Alternat. Med. 2015, 2015, 474509. [Google Scholar] [CrossRef]
- Raetz, C.R.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, X.; Sun, W.; Ke, C.; Wang, F.; Xue, Y.; Luo, Z.; Wang, X.; Zhang, J.; Zhang, Y. Anti-inflammatory activities of leaf oil from Cinnamomum subavenium in vitro and in vivo. Biomed. Res. Int. 2019, 2019, 1823149. [Google Scholar] [CrossRef] [Green Version]
- Budiastuti, B.; Sukardiman, S.; Primaharinastiti, R.; Nurcholida, R. Anti-inflammatory activity of Cinnamon bark oil (Cinnamomum Burmannii (Nees & T. Nees) Blume) from blume from Lombok, Timur, Indonesia. Pharmacogn. J. 2021, 13, 1005–1013. [Google Scholar] [CrossRef]
- Youssef, D.A.; El-Fayoumi, H.M.; Mahmoud, M.F. Beta-caryophyllene protects against diet-induced dyslipidemia and vascular inflammation in rats: Involvement of CB2 and PPAR-γ receptors. Chem. Biol. Interact. 2019, 297, 16–24. [Google Scholar] [CrossRef]
- Machado, K.D.C.; Islam, M.T.; Ali, E.S.; Rouf, R.; Uddin, S.J.; Dev, S.; Shilpi, J.A.; Shill, M.C.; Reza, H.M.; Das, A.K.; et al. A systematic review on the neuroprotective perspectives of beta-caryophyllene. Phytother. Res. 2018, 32, 2376–2388. [Google Scholar] [CrossRef]
- Chao, L.K.; Hua, K.F.; Hsu, H.Y.; Cheng, S.S.; Liu, J.Y.; Chang, S.T. Study on the anti-inflammatory activity of essential oil from leaves of Cinnamomum osmophloeum. J. Agric. Food Chem. 2005, 53, 7274–7278. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Webb, J.L.; Harvey, M.W.; Holden, D.W.; Evans, T.J. Macrophage nitric oxide synthase associates with cortical actin but is not recruited to phagosomes. Infect. Immun. 2001, 69, 6391–6400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, P.; Xie, M.; Chen, G.; Dai, Z.; Hu, B.; Zeng, X.; Sun, Y. Anti-inflammatory effects of dicaffeoylquinic acids from Ilex kudingcha on lipopolysaccharide-treated RAW264.7 macrophages and potential mechanisms. Food Chem. Toxicol. 2019, 126, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Kadiiska, M.B.; Jiang, J.J.; Stadler, K.; Mason, R.P. Free radical production requires both inducible nitric oxide synthase and xanthine oxidase in LPS-treated skin. Proc. Natl. Acad. Sci. USA 2006, 103, 4616–4621. [Google Scholar] [CrossRef] [PubMed]
- Liou, H.C. Regulation of the immune system by NF-κB and IκB. BMB Rep. 2002, 35, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Han, S.D.; Kim, M.; Mony, T.J.; Lee, E.S.; Kim, K.M.; Choi, S.H.; Hong, S.H.; Choi, J.W.; Park, S.J. Mentha arvensis essential oil exerts anti-inflammatory in LPS-stimulated inflammatory responses via inhibition of ERK/NF-κB signaling pathway and anti-atopic dermatitis-like effects in 2, 4-dinitrochlorobezene-induced BALB/c mice. Antioxidants 2021, 10, 1941. [Google Scholar] [CrossRef]
- Raha, S.; Kim, S.M.; Lee, H.J.; Lee, S.J.; Heo, J.D.; Venkatarame Gowda Saralamma, V.; Ha, S.E.; Kim, E.H.; Mun, S.P.; Kim, G.S. Essential oil from Korean Chamaecyparis obtusa leaf ameliorates respiratory activity in Sprague-Dawley rats and exhibits protection from NF-κB-induced inflammation in WI38 fibroblast cells. Int. J. Mol. Med. 2019, 43, 393–403. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, Y.; Huang, J.; Gu, H.; Li, C.; Zhang, L.; Liu, G.; Zhou, W.; Du, Z. The essential oil derived from Perilla frutescens (L.) Britt. attenuates imiquimod–induced psoriasis-like skin lesions in BALB/c mice. Molecules 2022, 27, 2996. [Google Scholar] [CrossRef]
- Rodrigues, R.D.O.; Yaochite, J.N.U.; Braga, M.A.; Sousa, A.R.D.; Sasahara, G.L.; Fonseca, S.G.D.C.; Araújo, T.D.D.V.; Santiago, G.M.P.; Sousa, L.M.D.; Carvalho, J.L.D.; et al. Antioxidant and anti-inflammatory activities of Bauhinia ungulata L. (Fabaceae) on LPS-stimulated RAW 264.7 cells. Pharmacogn. J. 2019, 11, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Zhou, S.; Takeda, R.; Okazaki, K.; Sekita, M.; Sakamoto, K. Anti-inflammatory activities of amber extract in lipopolysaccharide-induced RAW 264.7 macrophages. Biomed. Pharmacother. 2021, 141, 111854. [Google Scholar] [CrossRef]
- Nogueira, V.; Hay, N. Molecular pathways: Reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin. Cancer Res. 2013, 19, 4309–4314. [Google Scholar] [CrossRef] [Green Version]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chelombitko, M.A. Role of reactive oxygen species in inflammation: A minireview. Mosc. Univ. Biol. Sci. Bull. 2018, 73, 199–202. [Google Scholar] [CrossRef] [Green Version]
- Poyton, R.O.; Ball, K.A.; Castello, P.R. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol. Metab. 2009, 20, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Bognar, E.; Sarszegi, Z.; Szabo, A.; Debreceni, B.; Kalman, N.; Tucsek, Z.; Sumegi, B.; Gallyas, F., Jr. Antioxidant and anti-inflammatory effects in RAW264.7 macrophages of malvidin, a major red wine polyphenol. PLoS ONE 2013, 8, e65355. [Google Scholar] [CrossRef]
- Hsouna, A.B.; Dhibi, S.; Dhifi, W.; Saad, R.B.; Brini, F.; Hfaidh, N.; Mnif, W. Essential oil from halophyte Lobularia maritima: Protective effects against CCl 4-induced hepatic oxidative damage in rats and inhibition of the production of proinflammatory gene expression by lipopolysaccharide-stimulated RAW 264.7 macrophages. RSC Adv. 2019, 9, 36758–36770. [Google Scholar] [CrossRef]
- Avola, R.; Granata, G.; Geraci, C.; Napoli, E.; Graziano, A.C.E.; Cardile, V. Oregano (Origanum vulgare L.) essential oil provides anti-inflammatory activity and facilitates wound healing in a human keratinocytes cell model. Food Chem. Toxicol. 2020, 144, 111586. [Google Scholar] [CrossRef]
- Castellani, P.; Balza, E.; Rubartelli, A. Inflammation, DAMPs, tumor development, and progression: A vicious circle orchestrated by redox signaling. Antioxid. Redox Signal. 2014, 20, 1086–1097. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Arrate, M.P.; Rodriguez, J.M.; Tran, T.M.; Brock, T.A.; Cunningham, S.A. Cloning of human junctional adhesion molecule 3 (JAM3) and its identification as the JAM2 counter-receptor. J. Biol. Chem. 2001, 276, 45826–45832. [Google Scholar] [CrossRef] [Green Version]
- Aronis, A.; Melendez, J.A.; Golan, O.; Shilo, S.; Dicter, N.; Tirosh, O. Potentiation of Fas-mediated apoptosis by attenuated production of mitochondria-derived reactive oxygen species. Cell Death Differ. 2003, 10, 335–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, S.; Xu, Y.; Nie, Z.; Liu, H.; Gao, W.; Li, C.; Zhao, P. Metabolomic and antioxidant enzyme activity changes in response to cadmium stress under boron application of wheat (Triticum aestivum). Environ. Sci. Pollut. Res. 2022, 29, 34701–34713. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [Green Version]
- Paiva, C.N.; Bozza, M.T. Are reactive oxygen species always detrimental to pathogens? Antioxid. Redox. Signal. 2014, 20, 1000–1037. [Google Scholar] [CrossRef] [Green Version]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Grzybowski, A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [Green Version]
- Maestri, D.M.; Nepote, V.; Lamarque, A.L.; Zygadlo, J.A. Natural products as antioxidants. In Phytochemistry: Advances in Research; Imperato, F., Ed.; Research Signopost: Kerala, India, 2006; pp. 105–135. [Google Scholar]
- Miguel, M.G. Antioxidant activity of medicinal and aromatic plants. A review. Flavour Fragr. J. 2010, 25, 291–312. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, J.; Peng, J.; Wei, H. Oregano essential oil induces SOD1 and GSH expression through Nrf2 activation and alleviates hydrogen peroxide-induced oxidative damage in IPEC-J2 cells. Oxid. Med. Cell. Longev. 2016, 2016, 13. [Google Scholar] [CrossRef] [Green Version]
- Tavares, L.A.; Rezende, A.A.; Santos, J.L.; Estevam, C.S.; Silva, A.M.; Schneider, J.K.; Cunha, J.L.; Droppa-Almeida, D.; Correia-Neto, I.J.; Cardoso, J.C.; et al. Cymbopogon winterianus essential oil attenuates bleomycin-induced pulmonary fibrosis in a murine model. Pharmaceutics 2021, 13, 679. [Google Scholar] [CrossRef]
- Kamatou, G.P.; Viljoen, A.M. A review of the application and pharmacological properties of α-bisabolol and α-bisabolol-rich oils. J. Am. Oil Chem. Soc. 2010, 87, 1–7. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Yin, J.Y.; Li, D.F.; Zhu, C.T.; Ye, J.P.; Pan, Y.Q. Effects of inflammatory and anti-inflammatory environments on the macrophage mitochondrial function. Sci. Rep. 2020, 10, 20324. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, N.R.; Rodrigues, N.R.; Macedo, G.E.; Bristot, I.J.; Boligon, A.A.; de Campos, M.M.; Cunha, F.A.; Coutinho, H.D.; Klamt, F.; Merritt, T.J.; et al. Eugenia uniflora leaf essential oil promotes mitochondrial dysfunction in Drosophila melanogaster through the inhibition of oxidative phosphorylation. Toxicol. Res. 2017, 6, 526–534. [Google Scholar] [CrossRef] [Green Version]
- Ledrhem, M.; Nakamura, M.; Obitsu, M.; Hirae, K.; Kameyama, J.; Bouamama, H.; Gadhi, C.; Katakura, Y. Essential oils derived from Cistus species activate mitochondria by inducing SIRT1 expression in human keratinocytes, leading to senescence inhibition. Molecules 2022, 27, 2053. [Google Scholar] [CrossRef]
- Ullah, H.; Di Minno, A.; Santarcangelo, C.; Khan, H.; Daglia, M. Improvement of oxidative stress and mitochondrial dysfunction by β-caryophyllene: A focus on the nervous system. Antioxidants 2021, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ye, M.; Shi, Y.; Zhu, H.; Chi, L.; Pan, C.; Xu, Y.; Zheng, X.; Xiang, H.; Li, C. Phytochemical components and biological activities of essential oils from three selected medicinal plants. Ind. Crop. Prod. 2021, 160, 113127. [Google Scholar] [CrossRef]
- Bottex-Gauthier, C.; Pollet, S.; Favier, A.; Vidal, D.R. The Rel/NF-kappa-B transcription factors: Complex role in cell regulation. Pathol. Biol. 2002, 50, 204–211. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Wan, F.; Lenardo, M.J. The nuclear signaling of NF-κB: Current knowledge, new insights, and future perspectives. Cell Res. 2010, 20, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Nam, N.H. Naturally occurring NF-κB inhibitors. Mini Rev. Med. Chem. 2006, 6, 945–951. [Google Scholar] [CrossRef]
- Shih, R.H.; Wang, C.Y.; Yang, C.M. NF-kappaB signaling pathways in neurological inflammation: A mini review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labbozzetta, M.; Notarbartolo, M.; Poma, P. Can NF-κB be considered a valid drug target in neoplastic diseases? Our point of view. Int. J. Mol. Sci. 2020, 21, 3070. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.L.; Li, L.H.; Weng, Y.C.; Hua, K.F.; Ju, T.C. Eucalyptus essential oils inhibit the lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages through reducing MAPK and NF-κB pathways. BMC Complement. Med. Ther. 2020, 20, 200. [Google Scholar] [CrossRef] [PubMed]
- Van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [CrossRef]
- Reutelingsperger, C.P.M.; Van Heerde, W.L. Annexin V, the regulator of phosphatidylserine-catalyzed inflammation and coagulation during apoptosis. Cell. Mol. Life Sci. 1997, 53, 527–532. [Google Scholar] [CrossRef]
- Komoriya, A.; Packard, B.Z.; Brown, M.J.; Wu, M.L.; Henkart, P.A. Assessment of caspase activities in intact apoptotic thymocytes using cell-permeable fluorogenic caspase substrates. J. Exp. Med. 2000, 191, 1819–1828. [Google Scholar] [CrossRef]
- Syam, S.; Bustamam, A.; Abdullah, R.; Sukari, M.A.; Hashim, N.M.; Mohan, S.; Looi, C.Y.; Wong, W.F.; Yahayu, M.A.; Abdelwahab, S.I. β Mangostin suppress LPS-induced inflammatory response in RAW 264.7 macrophages in vitro and carrageenan-induced peritonitis in vivo. J. Ethnopharmacol. 2014, 153, 435–445. [Google Scholar] [CrossRef]
- Baek, S.H.; Park, T.; Kang, M.G.; Park, D. Anti-inflammatory activity and ROS regulation effect of sinapaldehyde in LPS-stimulated RAW 264.7 macrophages. Molecules 2020, 25, 4089. [Google Scholar] [CrossRef]
- Yuan, Z.H.; Liang, Z.E.; Wu, J.; Yi, J.E.; Chen, X.J.; Sun, Z.L. A potential mechanism for the anti-apoptotic property of koumine involving mitochondrial pathway in LPS-mediated RAW 264.7 macrophages. Molecules 2016, 21, 1317. [Google Scholar] [CrossRef] [Green Version]


| S. No. | Compound a | Ri b | Ri c | Peak Area % | RF d |
|---|---|---|---|---|---|
| 1 | Sabinene | 966 | 975 | 0.21 ± 0.01 | 1.0 |
| 2 | 1-Octen-3-ol | 973 | 979 | 0.34 ± 0.01 | 1.5 |
| 3 | Eucalyptol | 1028 | 1031 | 1.70 ± 0.04 | 1.3 |
| 4 | Fenchone | 1083 | 1086 | 0.14 ± 0.01 | 1.3 |
| 5 | Terpinen-4-ol | 1175 | 1177 | 0.76 ± 0.02 | 1.3 |
| 6 | α-Cubebene | 1337 | 1351 | 0.17 ± 0.01 | 1.0 |
| 7 | α-Copaene | 1367 | 1376 | 0.87 ± 0.03 | 1.0 |
| 8 | β-Elemene | 1380 | 1390 | 0.41 ± 0.02 | 1.0 |
| 9 | α-Gurjunene | 1396 | 1409 | 0.16 ± 0.01 | 1.0 |
| 10 | β-Caryophyllene | 1418 | 1419 | 16.17 ± 0.62 | 1.0 |
| 11 | trans-α-Bergamotene | 1426 | 1434 | 1.82 ± 0.07 | 1.0 |
| 12 | α-Humulene | 1446 | 1454 | 1.40 ± 0.05 | 1.0 |
| 13 | allo-Aromadendrene | 1450 | 1460 | 0.28 ± 0.01 | 1.0 |
| 14 | β-Chamigrene | 1462 | 1477 | 0.15 ± 0.01 | 1.0 |
| 15 | γ-Muurolene | 1470 | 1479 | 0.51 ± 0.02 | 1.0 |
| 16 | β-Selinene | 1479 | 1490 | 1.25 ± 0.03 | 1.0 |
| 17 | Viridiflorene | 1481 | 1496 | 0.17 ± 0.01 | 1.0 |
| 18 | α-Selinene | 1487 | 1498 | 1.86 ± 0.04 | 1.0 |
| 19 | δ-Cadinene | 1508 | 1523 | 0.54 ± 0.02 | 1.0 |
| 20 | Isocaryophyllene oxide | 1539 | 1527 | 0.20 ± 0.01 | 1.5 |
| 21 | Spathulenol | 1575 | 1578 | 7.89 ± 0.28 | 1.3 |
| 22 | Caryophyllene oxide | 1577 | 1583 | 2.04 ± 0.08 | 1.5 |
| 23 | Viridiflorol | 1584 | 1592 | 0.23 ± 0.01 | 1.3 |
| 24 | Humulene epoxide II | 1598 | 1608 | 0.46 ± 0.01 | 1.5 |
| 25 | 10-epi-γ-Eudesmol | 1606 | 1623 | 0.38 ± 0.02 | 1.3 |
| 26 | β-Atlantol | 1623 | 1608 | 0.46 ± 0.02 | 1.3 |
| 27 | Caryophylla-4(12),8(13)-dien-5β-ol | 1627 | 1640 | 0.24 ± 0.01 | 1.3 |
| 28 | Selin-11-en-4-α-ol | 1649 | 1659 | 2.10 ± 0.07 | 1.3 |
| 29 | 14-Hydroxy-9-epi-(E)-caryophyllene | 1662 | 1669 | 1.02 ± 0.03 | 1.3 |
| 30 | (Z)-α-trans-Bergamotol | 1681 | 1690 | 1.46 ± 0.05 | 1.3 |
| 31 | Hexahydrofarnesyl acetone | 1829 | 1836 | 0.42 ± 0.02 | 1.3 |
| 32 | Rimuene | 1894 | 1896 | 0.52 ± 0.02 | 1.4 |
| 33 | (3Z)-Cembrene A | 1935 | 1966 | 0.18 ± 0.01 | 1.4 |
| 34 | Manoyl oxide | 1976 | 1977 | 1.90 ± 0.06 | 1.4 |
| 35 | Phyllocladene | 2004 | 2017 | 11.85 ± 0.43 | 1.4 |
| 36 | Abieta-8,12-diene | 2015 | 2022 | 0.16 ± 0.01 | 1.4 |
| 37 | Abietatriene | 2054 | 2056 | 11.46 ± 0.52 | 1.4 |
| 38 | Kaur-16-ene | 2059 | 2061 | 0.46 ± 0.03 | 1.4 |
| 39 | Abietadiene | 2085 | 2087 | 3.79 ± 0.18 | 1.4 |
| 40 | Phytol | 2114 | 2114 | 1.70 ± 0.08 | 1.3 |
| 41 | Abieta-8(14),13(15)-diene | 2140 | 2154 | 0.41 ± 0.02 | 1.4 |
| 42 | Isopimara-7,15-dien-3-one | 2225 | 2227 | 0.61 ± 0.02 | 1.4 |
| 43 | Dehydroabietal | 2256 | 2275 | 0.19 ± 0.01 | 1.4 |
| 44 | Isopimarol | 2303 | 2305 | 0.34 ± 0.03 | 1.3 |
| 45 | 8,13-Abietadien-18-ol | 2318 | 2324 | 6.65 ± 0.32 | 1.4 |
| 46 | 4-Epiabietol | 2349 | 2344 | 0.43 ± 0.01 | 1.4 |
| 47 | Dehydroabietol | 2358 | 2368 | 3.58 ± 0.16 | 1.4 |
| 48 | Abietol | 2392 | 2401 | 0.57 ± 0.02 | 1.3 |
| Ether | 1.70 ± 0.04 | ||||
| Fatty alcohol | 0.34 ± 0.01 | ||||
| Monoterpene alcohol | 0.76 ± 0.02 | ||||
| Monoterpene hydrocarbon | 0.21 ± 0.01 | ||||
| Monoterpene ketone | 0.14 ± 0.01 | ||||
| Sesquiterpene hydrocarbons | 25.74 ± 0.54 | ||||
| Sesquiterpene alcohols | 13.76 ± 0.29 | ||||
| Sesquiterpene oxides | 2.70 ± 0.06 | ||||
| Oxygenated sesquiterpene | 0.42 ± 0.02 | ||||
| Diterpene hydrocarbons | 28.82 ± 0.87 | ||||
| Diterpene alcohols | 2.62 ± 0.08 | ||||
| Oxygenated diterpenes | 13.34 ± 0.38 | ||||
| Total identified | 90.55 ± 1.89 | ||||
| Target Gene | Primer Sequence | |
|---|---|---|
| iNOS | Forward | 5′-CTTCAACACCAAGGTTGTCTGCA-3′ |
| Reverse | 5′-ATGTCATGAGCAAAGGCGCAGAA-3′ | |
| COX-2 | Forward | 5′-CACTACATCCTGACCCACTT-3′ |
| Reverse | 5′-ATGCTCCTGCTTGAGTATGT-3′ | |
| GAPDH | Forward | 5′-GCAAAGTGGAGATTGTTGCCATC-3′ |
| Reverse | 5′-CATATTTCTCGTGGTTCACACCC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohanta, O.; Ray, A.; Jena, S.; Sahoo, A.; Panda, S.S.; Das, P.K.; Nayak, S.; Panda, P.C. Mesosphaerum suaveolens Essential Oil Attenuates Inflammatory Response and Oxidative Stress in LPS-Stimulated RAW 264.7 Macrophages by Regulating NF-κB Signaling Pathway. Molecules 2023, 28, 5817. https://doi.org/10.3390/molecules28155817
Mohanta O, Ray A, Jena S, Sahoo A, Panda SS, Das PK, Nayak S, Panda PC. Mesosphaerum suaveolens Essential Oil Attenuates Inflammatory Response and Oxidative Stress in LPS-Stimulated RAW 264.7 Macrophages by Regulating NF-κB Signaling Pathway. Molecules. 2023; 28(15):5817. https://doi.org/10.3390/molecules28155817
Chicago/Turabian StyleMohanta, Omprakash, Asit Ray, Sudipta Jena, Ambika Sahoo, Soumya Swarup Panda, Prabhat Kumar Das, Sanghamitra Nayak, and Pratap Chandra Panda. 2023. "Mesosphaerum suaveolens Essential Oil Attenuates Inflammatory Response and Oxidative Stress in LPS-Stimulated RAW 264.7 Macrophages by Regulating NF-κB Signaling Pathway" Molecules 28, no. 15: 5817. https://doi.org/10.3390/molecules28155817
APA StyleMohanta, O., Ray, A., Jena, S., Sahoo, A., Panda, S. S., Das, P. K., Nayak, S., & Panda, P. C. (2023). Mesosphaerum suaveolens Essential Oil Attenuates Inflammatory Response and Oxidative Stress in LPS-Stimulated RAW 264.7 Macrophages by Regulating NF-κB Signaling Pathway. Molecules, 28(15), 5817. https://doi.org/10.3390/molecules28155817