Abstract
Propolis is a natural bee-produced substance with antimicrobial, anti-inflammatory, and wound-healing properties, containing some components from the leaves, buds and resins of plants. It has been used for centuries for various health benefits. In this manuscript, our group reviewed the radioprotective effect of propolis using PubMed and Embase, and our review was conducted according to the PRISMA statement. Finally, 27 articles were included in this review, which includes the radioprotective effect of propolis from cell-based studies (n = 8), animal models (n = 14), and human trials (n = 5). Results reflected that the dosage forms of propolis extracted in the scientific literature were ethanolic extracts of propolis, a water-soluble derivate of propolis, or capsules. The efficacy of the radioprotective properties from propolis is extracted from the bibliography, as several compounds of this resinous mixture individually or synergistically are possible candidates that have the radioprotective effect. In fact, studies prior to 2011 lacked a comprehensive characterization of propolis due to the variability in active compounds among different batches of propolis and were limited to analytical techniques. Furthermore, in this manuscript, we have selected studies to include primarily propolis types from Brazil, Croatia, Egypt, European countries, and those commercialized in Spain. They all contained ethanolic extract of propolis (EEP) and were influenced by different dosage forms. EEP showed a significant presence of lipophilic bioactive compounds like flavones, flavonols, and flavanones.
1. Introduction
Propolis is a resinous mixture that bees collect from various botanical sources, including buds, flowers, and plant exudates. Bees modify the collected resins by adding their salivary secretions, wax, and pollen, resulting in the creation of propolis. The collection of resins by bees typically takes place during the hottest hours of the day when the resins are more malleable and easier to gather [1]. Due to its balsamic and resinous nature, propolis has a highly sticky consistency. It encompasses a diverse array of components, including terpenes, polysaccharides, aromatic acids, polyphenols, esters of phenolic acids, vitamins, and amino acids [2]. However, propolis can vary in composition and effectiveness depending on its botanical sources and geographical origin [3]. Propolis has long been associated with various medicinal properties and has been utilized for its potential health benefits. Some of the attributed medicinal properties of propolis include binder, immunomodulator, antimicrobial, antifungal, anti-inflammatory, hepatoprotective, antioxidant, anti-hemorrhagic, anti-parasitic, anti-tumor, anti-edema, stimulating epithelial regeneration, cholesterol reducer, revitalizing, detoxifying and tonic [4,5,6]. The objective of this review was to assess the radioprotective effect of propolis in different experimental settings, including studies involving cells, animals, and humans.
2. Results and Discussion
Table 1, Table 2 and Table 3 show the radioprotective effect of the propolis including cell-based studies, animal models, and human trials, respectively.

Table 1.
Radioprotective effect of propolis in cell-based studies.

Table 2.
Studies of radioprotective effect of propolis in non-human animals.

Table 3.
Studies of radioprotective effect of propolis in humans.
For cell-based studies, six out of eight articles [7,8,9,10,11,12] reflected the use of ionizing radiation in their research, while the other two referenced the application of UV radiation, including UV-B [13] and UV-A [14]. Furthermore, six out of eight articles [7,8,9,10,11,13] indicated the use of ethanolic extract of propolis (EEP) compared to an aqueous solution of propolis [12] and wax from Tetragonula spp. bees [14]. These species include T. biroi, T. fusco balteata, T. laeviceps and T. sapiens [34], which produce more propolis than other bee species [35]. On the other hand, 60Co γ-irradiation was mainly used in five out of six articles [7,8,9,11,12], with only one unique application of X-rays in ionizing radiation [10], with the radiation range from 1 to 6 Gy.
For non-human animals, male rats were used more frequently, primarily Wistar rats [21,22,23,24,25,27,28], which were followed by Sprague–Dawley rats [26]. In addition, CBA mice [15,16,17,18,19,20] were also utilized. Various types of propolis were employed, including EEP [19,20,21], water-soluble derivative of propolis (WSDP) [15,16,17,18,28], and hydroalcoholic extract of red propolis (HERP) [27]. In terms of radiation, X-rays were utilized in one study at a dose rate of 15 Gy [21], while γ-irradiation was mainly obtained using 60Co [15,16,17,18,19,20,23,24,25,26], which was followed by 137Cs [22,28]. The radiation range for both types of radiation was from 1 to 15 Gy, with the latter applied to the entire cranium. Additionally, non-ionizing radiation in the form of UV-B was only detected in one article [27], where it was applied at a dosage of 1.6 J/cm2 for 60 min per day over a period of 6 days. For humans, several studies were conducted involving male participants [29,30], female participants [32], or both genders [31,33], all aged 18 years or older. The dosage forms of propolis used in these studies were EEP [29], WSDP [30,31], or capsules [32]. In three out of five articles [29,30,31], the use of 60Co γ-irradiation was predominant, with a radiation range of 2 Gy [31] to 4 Gy [29,30]. The human subjects in these studies were individuals with head and neck malignancies [31], patients diagnosed with oral cavity or oropharynx cancer [33], and breast cancer patients [32]. The studies observed various improvements in several parameters, such as the prevention and healing of radiotherapy-induced mucositis [31], protection against DNA damage induced by ionizing radiation in leukocytes of breast cancer patients [32], and propolis treatment resulting in a reduction in TNF-α and IL-1β levels as well as a decrease in the incidence of oral candidiasis episodes [33]. These findings suggest that propolis could serve as a beneficial complementary option for the prevention and treatment of the primary acute oral toxicities associated with radiotherapy [33]. In the included studies for this manuscript, it is clear that the studies prior to 2011 did not have the complete characterization of the propolis extracts. This could be attributed to two reasons. Firstly, it was probably due to the variability of bioactive compounds among different batches of propolis used, and secondly, it could be due to the limited development of analytical techniques, particularly chromatography coupled with mass spectrometry, in analytical laboratories at that time. The only study [16] in 2005 provided a partial characterization of the propolis used. It mainly focused on caffeic acid, naringenin, chrysin, pinocembrin, galangin, and total polyphenols. In 2011, our group [9] emphasized the importance of comprehensively characterizing the extracts used in studies investigating the biological activities of propolis. This advancement allowed subsequent articles to include detailed analyses of the propolis composition used in radioprotection studies within a few months [10] and in the following years [13,14,25,27,33]. Table 4 presents the broad composition of these preparations or extracts, emphasizing the significance of their composition. Furthermore, some compounds of this table are reflected in Figure 1, Figure 2 and Figure 3.

Table 4.
Type of propolis and main compounds of selected propolis.
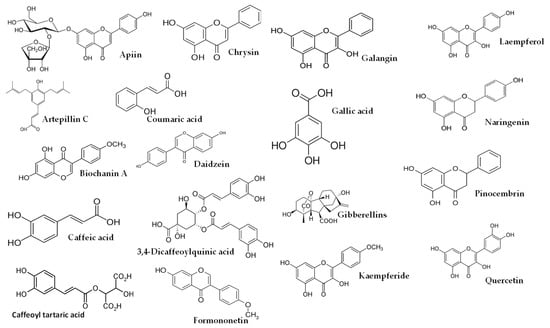
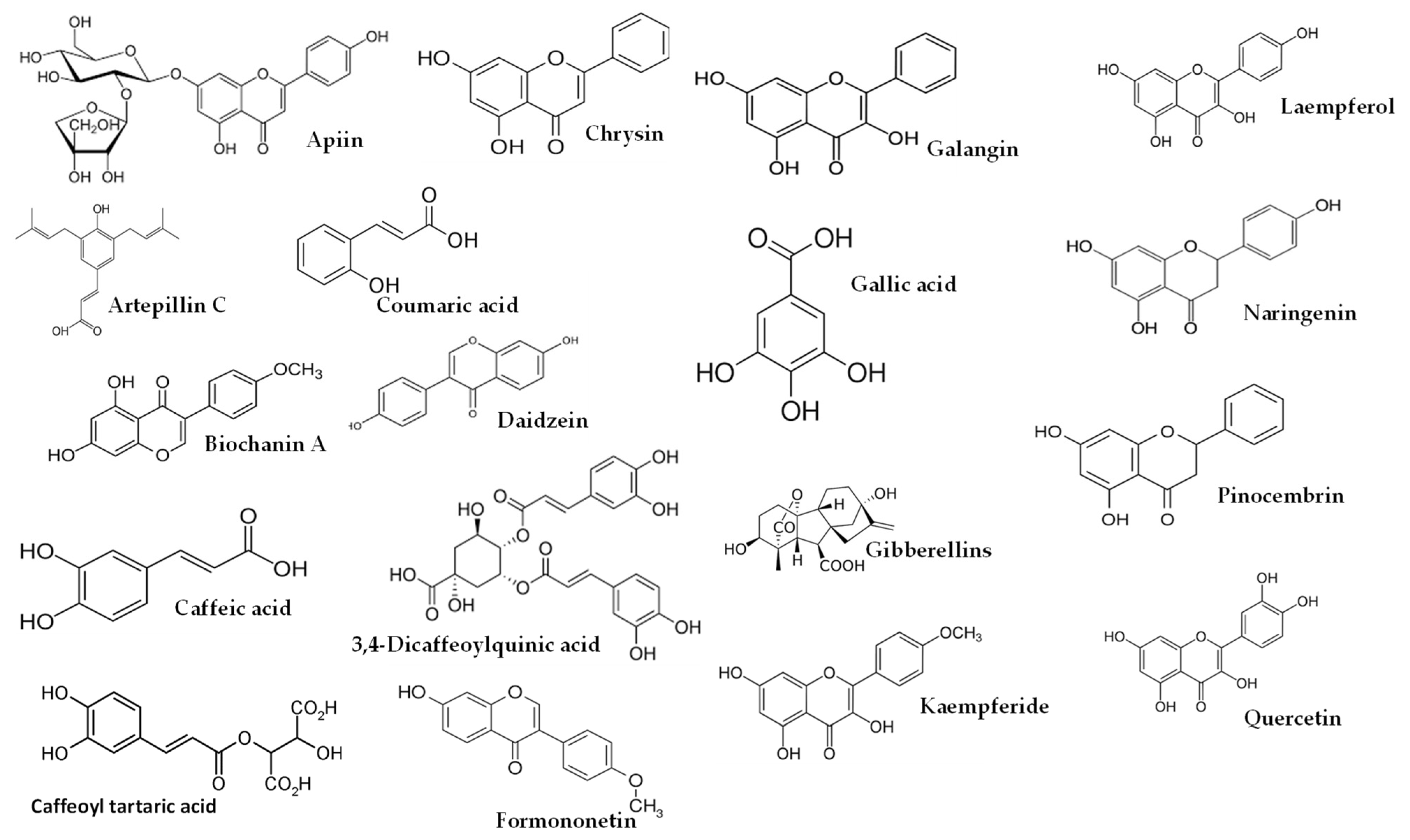
Figure 1.
Some of compounds detected from Brazilian, Croatian and Egyptian propolis.
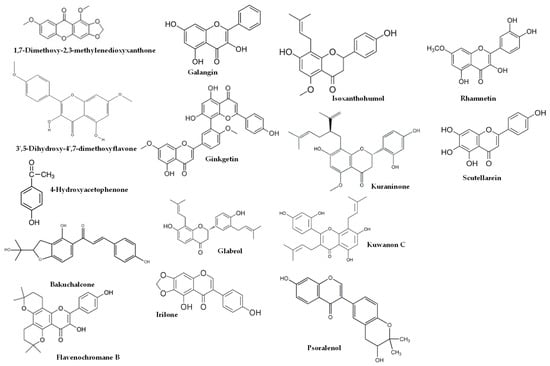
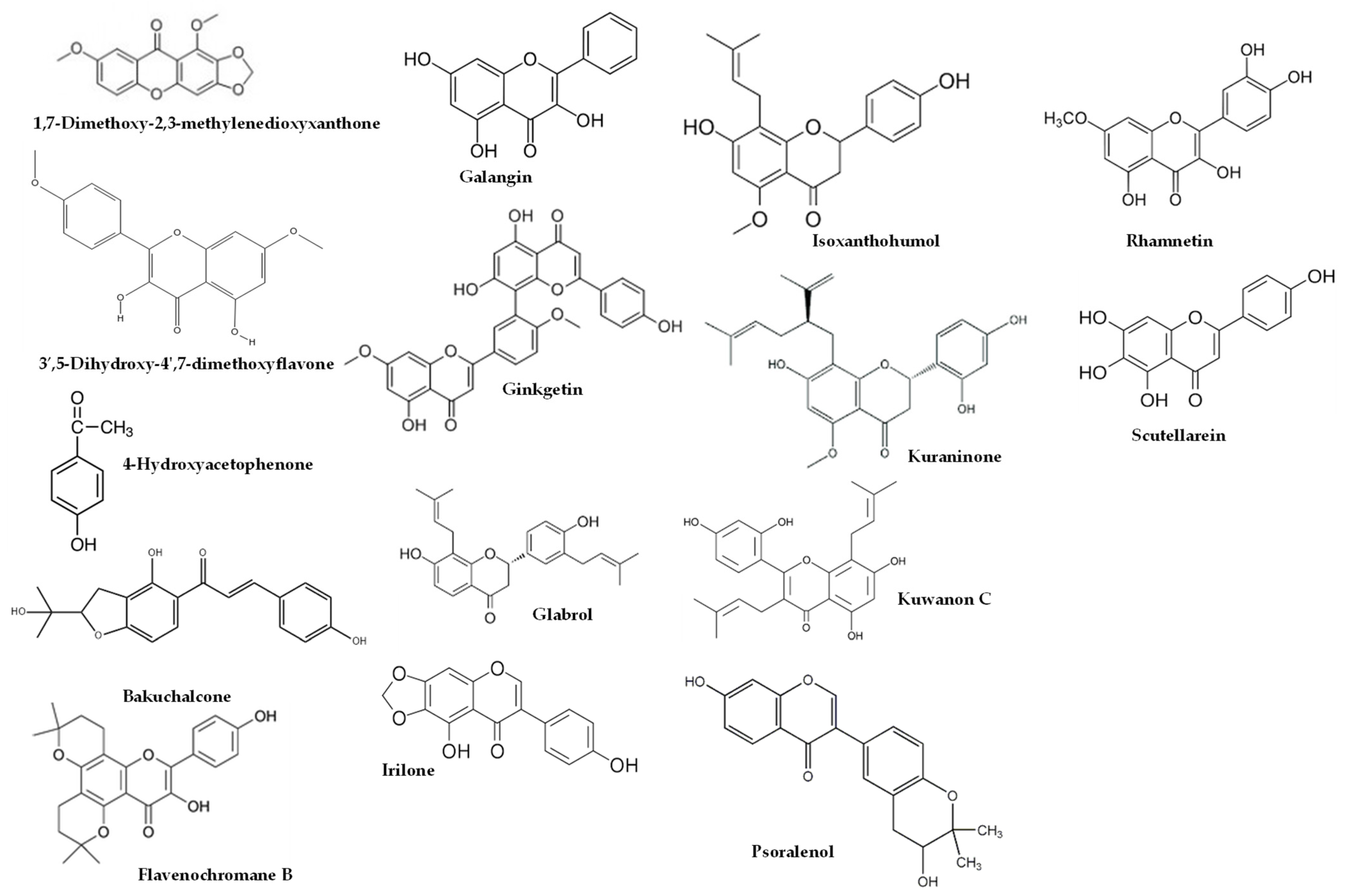
Figure 2.
Some of compounds detected from propolis commercialized in Spain and European propolis.
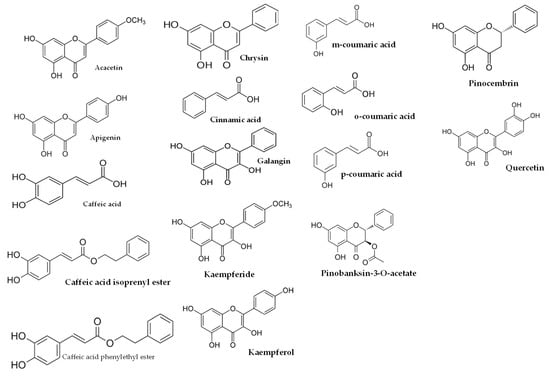
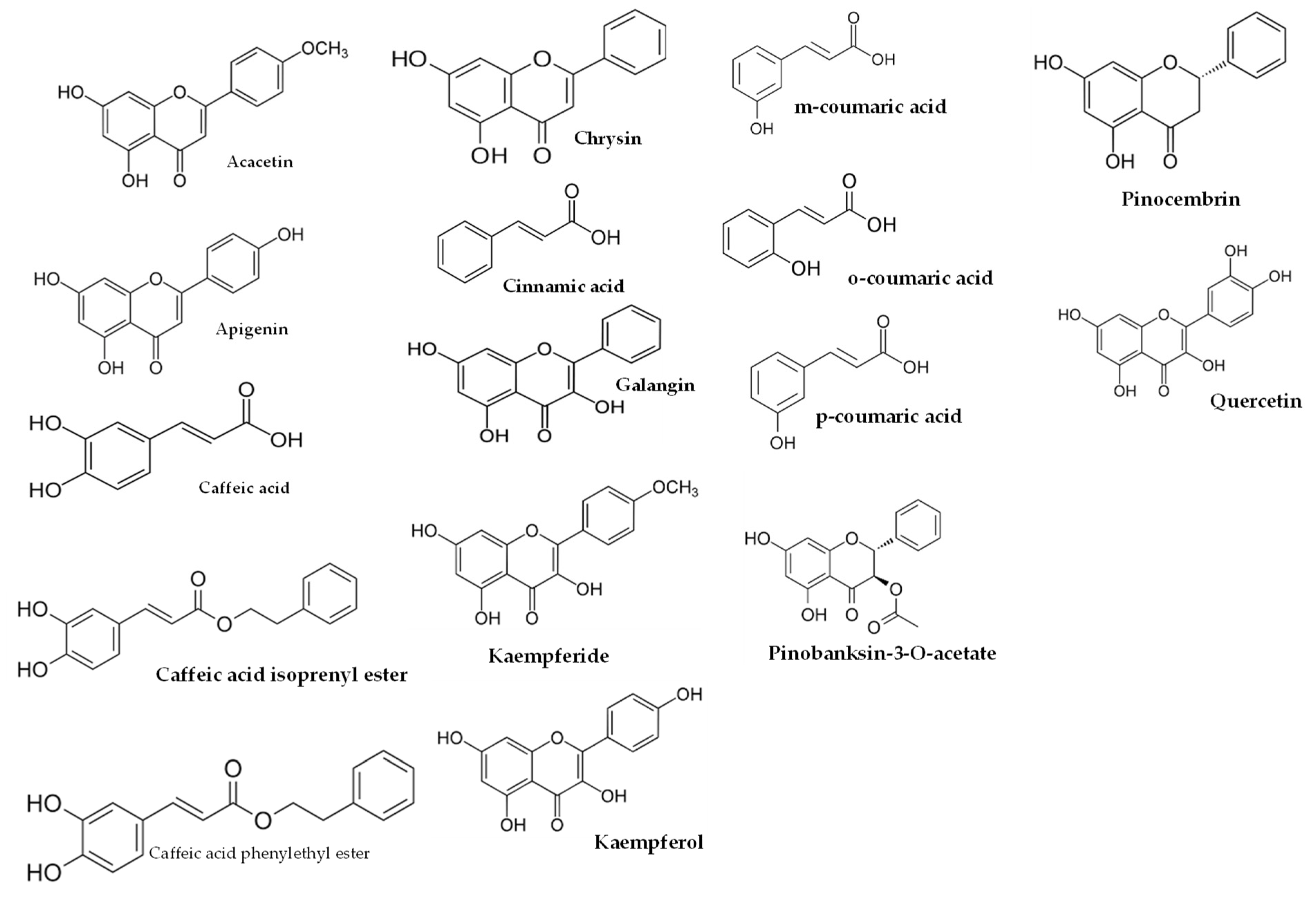
Figure 3.
Some of compounds of propolis wax from Tetragonula sp. bees.
As shown in Table 4, higher dosages had an impact on the composition of the studies using EEP, resulting in a higher proportion of bioactive lipophilic compounds such as flavones, flavonols, and flavanones. Furthermore, this manuscript covered several propolis origins, including Brazilian [13,16,27,33], Croatian [13,16], Egyptian [25], European [10], and Iranian [24] propolis, as well as propolis commercialized in Spain [9]. It is worth noting that green or red propolis originated from Brazil. In the studies conducted by Park et al. [39,40] and Shimizu et al. [41], various constituents of propolis were identified, including quercetin, apigenin, kaempferol, acacetin, melliferone, moronic acid, anwuweizonic acid, betulonic acid, 4-hydroxy-3-methoxy-propiophenone, 4-hydroxy-3-methoxybenzaldehyde, 3-(3,4-dimethoxyphenyl)-2-propenal, and acetoxitremetone. However, major compounds such as artepillin C and chrysin were not found in their analyses. In addition, Santos et al. [11] suggested that propolis of Brazilian origin was reported to lack caffeic acid phenethyl ester (CAPE) in its chemical composition. Until 2016, the commercially available propolis was primarily extracted Brazilian green propolis. However, a new type of propolis emerged and was widely known as Brazilian Organic Propolis (BOP) [33,34]. BOP is characterized by its mild flavor and is produced under organic conditions in conservation areas. It is noteworthy that BOP is free from heavy metals and pesticides, making it a desirable option. BOP has demonstrated interesting bioactive properties. For instance, BOP type 4 has significant antioxidant activity, which is mainly due to its high content of Artepillin C. On the other hand, BOP type 6 has shown remarkable anti-inflammatory activity by reducing NF-kB activation and TNF-α release [33]. These properties are particularly relevant, since oral mucositis pathogenesis is closely related to inflammatory pathways and the production of reactive oxygen species. As a result, both types of BOP have become subjects of investigation for their potential in addressing oral mucositis [42]. Another type of propolis is Brazilian red propolis, which contains significant amounts of flavonoids, such as pinocembrin, formononetin, and isoliquiritigenin [43]. Additionally, HERP (hydroalcoholic extract of red propolis) exhibits antioxidant properties [44,45] and demonstrates anti-inflammatory effects [46]. Moreover, HERP is hypothesized to have the potential to protect the skin from damage caused by UV-B radiation, which is attributed to the presence of benzophenone in HERP, a compound commonly used in the formulation of organic sunscreens. These unique properties make HERP a compelling subject of interest in research and potential applications.
On the other hand, the mechanisms of action of propolis are grouped in four properties [9,47]. (i) The first is antioxidant activity, as propolis is rich in various bioactive compounds, including flavonoids, phenolic acids, and polyphenols, which have strong antioxidant properties. When exposed to ionizing radiation, the body generates free radicals that can cause cellular damage. Propolis’ antioxidants can neutralize these free radicals, reducing oxidative stress and preventing further damage to cells. (ii) The second mechanism is DNA repair stimulation. In fact, radiation can cause breaks and lesions in DNA strands, leading to mutations and cell death. Propolis may enhance the body’s natural DNA repair mechanisms, helping to fix some of the radiation-induced DNA damage and increasing the chances of cell survival. (iii) The third mechanism is anti-inflammatory effects. Propolis exhibits anti-inflammatory properties, which can help mitigate inflammation caused by radiation exposure. Inflammation contributes to tissue damage and can exacerbate the harmful effects of radiation and (iv) immune system modulation. Propolis has been shown to modulate the immune system, potentially enhancing the body’s defense against radiation-induced damage. By supporting the immune system, propolis may aid in the recovery of damaged tissues.
3. Materials and Methods
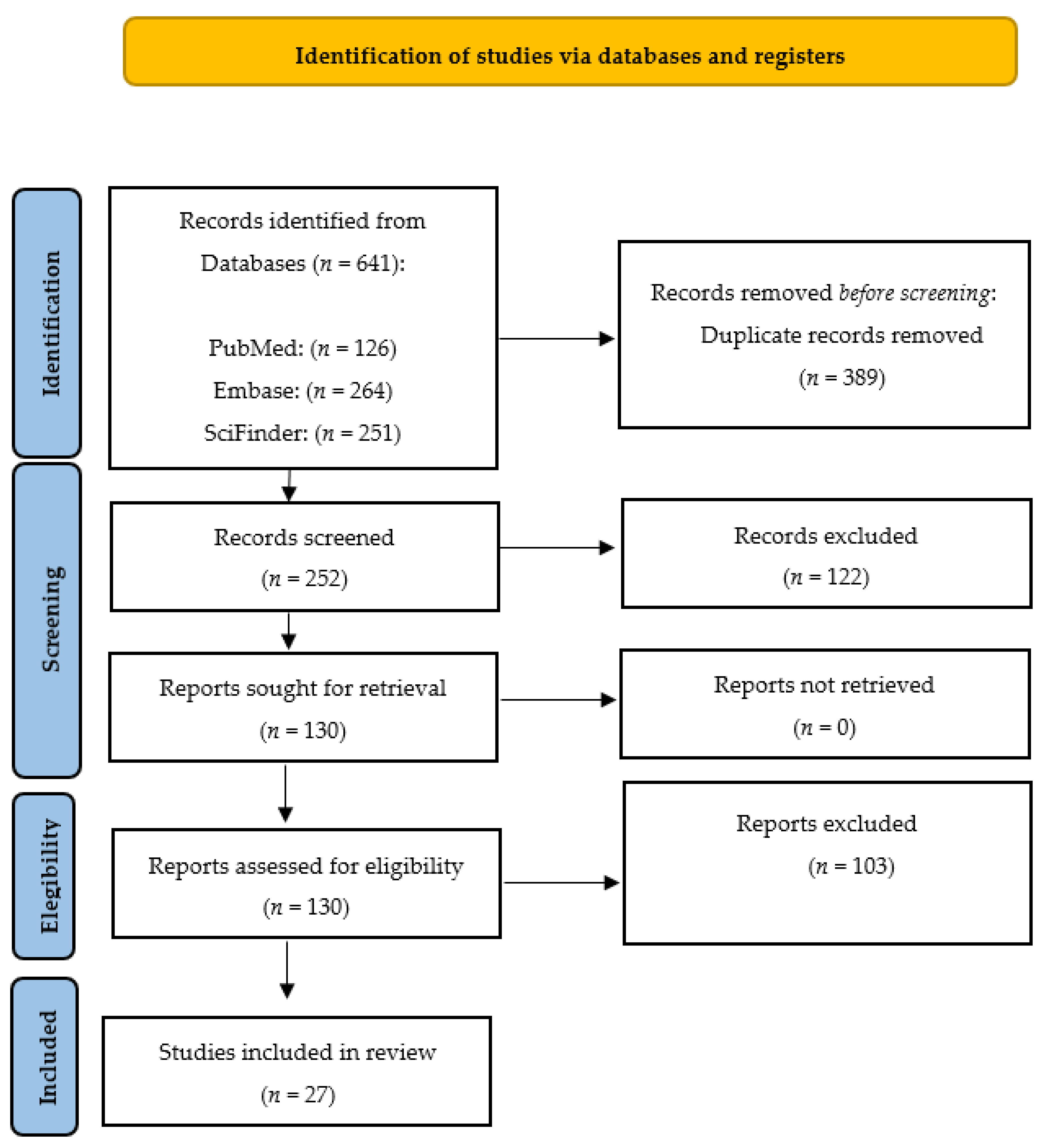
We conducted a systematic review following the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [48] (Figure 4). Our search was performed using the PubMed, SciFinder and Embase databases. In PubMed, we used the medical subject headings (MeSH) terms ‘propolis’, ‘radioprotection’, and ‘radiation’. In Embase, we searched for the terms ‘vitamin’, ‘radioprotection’, and ‘radiation’. We employed Boolean operators (AND, OR) to combine the keywords in our search. The search was limited to literature published in the English language over the last 50 years and updated to 1 June 2023. The inclusion criteria were articles written in English from original papers, review papers, theses and experimental procedures. The exclusion criteria for this study were manuscripts with unrelated abstracts, books, letters, conference literature, case reports, editorials, and pilot studies. The full-text articles of all the shortlisted studies were then thoroughly examined to determine their eligibility. Two teams of paired reviewers, each with expertise in medical and health assessment and training in research methodology, conducted the screening process independently. The three sections of the articles, namely titles, abstracts, and full texts (if eligible), were meticulously reviewed. Additionally, the reviewers assessed the applicability of the studies and collected relevant data for the reviews. The teams worked separately to ensure a comprehensive and unbiased assessment of the articles under consideration.
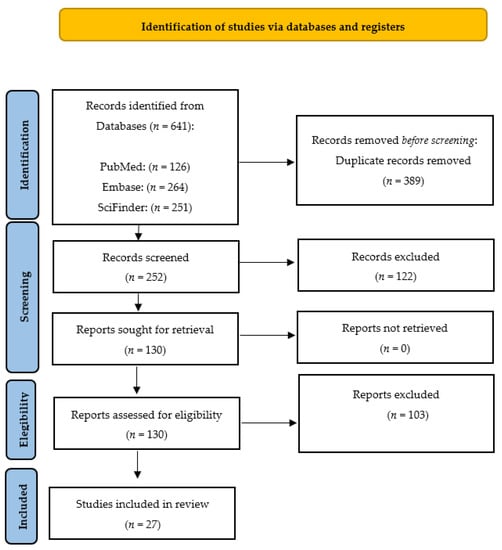
Figure 4.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 flow diagram representing the searching and selection process for this review [48].
Author Contributions
B.I., A.M. (Ana Melero), A.M. (Alegría Montoro), N.S.O. and J.M.S. were responsible for the conceptualization, design, developed methodology, validation and data curation and writing—review and editing; B.I., A.M. (Ana Melero), A.M. (Alegría Montoro), N.S.O. and J.M.S. were primarily responsible for the investigation and writing—original draft preparation; J.M.S. were responsible for the writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Spanish Nuclear Safety Council (2696/SRO). I.L. was supported by a Fellowship of the ‘University Junior International Entrepreneurs’ program (UJIE 2019) of University of Valencia and Generalitat Valenciana. N.S.O. was supported by the Universidad de Alicante, Ministerio de Universidades and the European Union “NextGeneration EU/PRTR” through 2022–2024 Margarita Salas grant (MARSALAS22-23).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Simone-Finstrom, M.; Spivak, M. Propolis and bee health: The natural history and significance of resin use by honey bees. Apidologie 2010, 41, 295–311. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.P.; Wang, K.; Li, G.Q.; Hu, F.L. Recent advances in the chemical composition of propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef] [PubMed]
- Kurek-Górecka, A.; Keskin, Ş.; Bobis, O.; Felitti, R.; Górecki, M.; Otręba, M.; Rzepecka-Stojko, A. Comparison of the antioxidant activity of propolis samples from different geographical regions. Plants 2022, 11, 1203. [Google Scholar] [CrossRef]
- Przybyłek, I.; Karpiński, T.M. Antibacterial properties of propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [PubMed]
- Sforcin, J.M. Biological properties and therapeutic applications of propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Zulhendri, F.; Chandrasekaran, K.; Kowacz, M.; Ravalia, M.; Kripal, K.; Fearnley, J.; Perera, C.O. Antiviral, antibacterial, antifungal, and antiparasitic properties of propolis: A review. Foods 2021, 10, 1360. [Google Scholar] [CrossRef]
- Spigoti, G.; Bartolini, P.; Okazaki, K.; Tsutsumi, S. Protective effect of propolis on radiation-induced chromosomal damage on Chinese hamster ovary cells (CHO-K1). In Proceedings of the 2009 International Nuclear Atlantic Conference-INAC 2009, Rio de Janeiro, RJ, Brazil, 27 September–2 October 2009. [Google Scholar]
- Yalcin, C.O.; Aliyazicioglu, Y.; Demir, S.; Turan, I.; Bahat, Z.; Misir, S.; Deger, O. Evaluation of the radioprotective effect of Turkish propolis on foreskin fibroblast cells. J. Cancer Res. Ther. 2016, 12, 990–994. [Google Scholar]
- Montoro, A.; Barquinero, J.F.; Almonacid, M.; Montoro, A.; Sebastià, N.; Verdú, G.; Sahuquillo, V.; Serrano, J.; Saiz, M.; Villaescusa, J.I.; et al. Concentration-Dependent Protection by Ethanol Extract of Propolis against γ-Ray-Induced Chromosome Damage in Human Blood Lymphocytes. Evid. Based Complement. Alternat. Med. 2011, 2011, 174853. [Google Scholar] [CrossRef]
- Hehlgans, S.; Lange, I.; Eke, I.; Kammerer, B.; Cordes, N. Human head and neck squamous cell carcinoma cell lines are differentially radiosensitised by the honeybee product Propolis. Int. J. Radiat. Biol. 2011, 87, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.S.; Tsutsumi, S.; Vieira, D.P.; Bartolini, P.; Okazaki, K. Effect of Brazilian propolis (AF-08) on genotoxicity, cytotoxicity and clonogenic death of Chinese hamster ovary (CHO-K1) cells irradiated with 60Co gamma-radiation. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 762, 17–23. [Google Scholar] [CrossRef]
- Nogueira, N.D.S.G.; Campos, T.P.R.; Montaño Valencia, C.J.; Falcão, P.L. In vitro radiation protection of peripheral blood mononuclear cells by Tetragona Clavipes Propolis. In Proceedings of the 2017 International Nuclear Atlantic Conference-INAC 2017, Belo Horizonte, MG, Brazil, 22–27 October 2017. [Google Scholar]
- Yoshino, Y.; Marunaka, K.; Kobayashi, M.; Matsunaga, H.; Shu, S.; Matsunaga, T.; Ikari, A. Protective effects of ethanol extract of Brazilian green propolis and apigenin against weak ultraviolet ray-B-induced barrier dysfunction via suppressing nitric oxide production and mislocalization of claudin-1 in HaCaT cells. Int. J. Mol. Sci. 2021, 22, 10326. [Google Scholar] [CrossRef] [PubMed]
- Asih, S.C.; Pratami, D.K.; Yohda, M.; Fukutami, Y.; Faried, A.; Sahlan, M. The role of propolis Tetragonula sp. in oxidative stress and its protective effect against UV radiation on cells. Int. J. App. Pharm. 2022, 14, 123–128. [Google Scholar] [CrossRef]
- Orsolic, N.; Tadic, Z.; Benkovic, V.; Horvat, A.; Lojkic, M.; Basic, I. Radioprotective effect of a water-soluble derivative of propolis in mice. Mellifera 2004, 4, 45–52. [Google Scholar]
- Oršolić, N.; Bašić, I. Antitumor, hematostimulative and radioprotective action of water-soluble derivative of propolis (WSDP). Biomed. Pharmacother. 2005, 59, 561–570. [Google Scholar] [CrossRef]
- Oršolić, N.; Benković, V.; Horvat-Knežević, A.; Kopjar, N.; Kosalec, I.; Bakmaz, M.; Bašić, I. Assessment by survival analysis of the radioprotective properties of propolis and its polyphenolic compounds. Biol. Pharm. Bull. 2007, 30, 946–951. [Google Scholar] [CrossRef]
- Benković, V.; Orsolić, N.; Knežević, A.H.; Ramić, S.; Ðikić, D.; Bašić, I.; Kopjar, N. Evaluation of the radioprotective effects of propolis and flavonoids in gamma-irradiated mice: The alkaline comet assay study. Biol. Pharm. Bull. 2008, 31, 167–172. [Google Scholar] [CrossRef]
- Benkovic, V.; Knezevic, A.H.; Dikic, D.; Lisicic, D.; Orsolic, N.; Basic, I.; Kopjar, N. Radioprotective effects of propolis and quercetin in γ-irradiated mice evaluated by the alkaline comet assay. Phytomedicine 2008, 15, 851–858. [Google Scholar] [CrossRef]
- Benković, V.; Horvat Knežević, A.; Đikić, D.; Lisičić, D.; Oršolić, N.; Bašić, I.; Kopjar, N. Radioprotective effects of quercetin and ethanolic extract of propolis in gamma-irradiated mice. Arh. za Hig. Rada Toksikol. 2009, 60, 129–138. [Google Scholar] [CrossRef]
- Ghassemi, L.; Zabihi, E.; Mahdavi, R.; Seyedmajidi, M.; Akram, S.; Motallebnejad, M. The effect of ethanolic extract of propolis on radiation-induced mucositis in rats. Saudi Med. J. 2010, 31, 622–626. [Google Scholar]
- El-Ghazaly, M.A.; Rashed, R.R.; Khayyal, M.T. Anti-ulcerogenic effect of aqueous propolis extract and the influence of radiation exposure. Int. J. Radiat. Biol. 2011, 87, 1045–1051. [Google Scholar] [CrossRef]
- Motallebnejad, M.; Abedi, S.M.; Seyedmajidi, M.; Moghadamnia, A.A.; Ashrafpour, M.; Salehi, M.; Ghorbani, A. Evaluation of protective effect of propolis on parotid salivary glands in gamma-irradiated rats. J. Contemp. Dent. Pract. 2014, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Aghel, S.; Pouramir, M.; Moghadamnia, A.A.; Moslemi, D.; Molania, T.; Ghassemi, L.; Motallebnejad, M. Effect of Iranian propolis on salivary total antioxidant capacity in gamma-irradiated rats. J. Dent. Res. Dent. Clin. Dent. Prospects 2014, 8, 235. [Google Scholar] [PubMed]
- Hamieda, S.F.; Hassan, A.I.; Abdou, M.I.; Khalil, W.A.; Abd-el Nour, K.N. Evaluation of radioprotective effects of some bee products and their flavonoid constituents: In vivo study on male rats. Rom. J. Biophys. 2015, 25, 13–34. [Google Scholar]
- Demir, E.; Taysi, S.; Al, B.; Demir, T.; Okumus, S.; Saygili, O.; Bagci, C. The effects of Nigella sativa oil, thymoquinone, propolis, and caffeic acid phenethyl ester on radiation-induced cataract. Wien. Klin. Wochenschr. 2016, 128, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Batista, C.M.; Alves, A.V.F.; Queiroz, L.A.; Lima, B.S.; Araújo, A.A.S.; de Albuquerque Júnior, R.L.C.; Cardoso, J.C. The photoprotective and anti-inflammatory activity of red propolis extract in rats. J. Photochem. Photobiol. B Biol. 2018, 180, 198–207. [Google Scholar] [CrossRef]
- Khayyal, M.T.; Abdel-Naby, D.H.; El-Ghazaly, M.A. Propolis extract protects against radiation-induced intestinal mucositis through anti-apoptotic mechanisms. Environ. Sci. Pollut. Res. 2019, 26, 24672–24682. [Google Scholar] [CrossRef]
- Benković, V.; Kopjar, N.; Kneževic, A.H.; Đikić, D.; Bašić, I.; Ramić, S.; Viculin, T.; Knežević, F.; Orolić, N. Evaluation of Radioprotective Effects of Propolis and Quercetin on Human White Blood Cells in Vitro. Biol. Pharm. Bull. 2008, 31, 1778–1785. [Google Scholar] [CrossRef]
- Benkovic, V.; Knezevic, A.H.; Orsolic, N.; Basic, I.; Ramic, S.; Viculin, T.; Kopjar, N. Evaluation of radioprotective effects of propolis and its flavonoid constituents: In vitro study on human white blood cells. Phytother. Res. 2009, 23, 1159–1168. [Google Scholar] [CrossRef]
- Bolouri, A.J.; Pakfetrat, A.; Tonkaboni, A.; Aledavood, S.A.; Najafi, M.F.; Delavarian, Z.; Mohtashami, A. Preventing and therapeutic effect of propolis in radiotherapy induced mucositis of head and neck cancers: A triple-blind, randomized, placebo-controlled trial. Iran. J. Cancer Prev. 2015, 8, e4019. [Google Scholar]
- Ebeid, S.A.; Abd El Moneim, N.A.; El-Benhawy, S.A.; Hussain, N.G.; Hussain, M.I. Assessment of the radioprotective effect of propolis in breast cancer patients undergoing radiotherapy. New perspective for an old honey bee product. J. Radiat. Res. Appl. Sci. 2016, 9, 431–440. [Google Scholar] [CrossRef]
- Fernandes, P.M.; Rosalen, P.L.; Fernandes, D.T.; Dias-Neto, E.; Alencar, S.M.; Bueno-Silva, B.; Lopes, M.A. Brazilian organic propolis for prevention and treatment of radiation-related oral acute toxicities in head and neck cancer patients: A double-blind randomized clinical trial. Front. Pharmacol. 2022, 13, 973255. [Google Scholar] [CrossRef] [PubMed]
- Pratami, D.K.; Mun’im, A.; Sahlan, M.; Sundowo, A. Phytochemical profile and anti-oxidant activity of propolis ethanolic extract from tetragonula bee. Pharmacog. J. 2018, 10, 128–135. [Google Scholar] [CrossRef]
- Suriawanto, N. Nesting sites characteristics of stingless bees (Hymenoptera: Apidae) in Central Sulawesi, Indonesia. J. Insect. Biodivers. 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Athikomkulchai, S.; Awale, S.; Ruangrungsi, N.; Ruchirawat, S.; Kadota, S. Chemical constituents of Thai propolis. Fitoterapia 2013, 88, 96–100. [Google Scholar] [CrossRef]
- Sanpa, S.; Popova, M.; Tunkasiri, T.; Eitssayeam, S.; Bankova, V.; Chantawannakul, P. Chemical profiles and antimicrobial activities of Thai propolis collected from Apis mellifera. Chiang Mai J. Sci. 2017, 44, 438–448. [Google Scholar]
- Miyata, R.; Motoyama, T.; Nakano, S.; Ito, S.; Mukaide, K.; Vongsak, B.; Kumazawa, S. Catechol-O-methyltransferase inhibitors isolated from Thai propolis. Nat. Prod. Commun. 2021, 16, 1934578. [Google Scholar] [CrossRef]
- Park, Y.K.; Alencar, S.M.; Aguiar, C.L. Botanical origin and chemical composition of Brazilian propolis. J. Agric. Food Chem. 2002, 50, 2502–2506. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Paredes-Guzman, J.F.; Aguiar, C.L.; Alencar, S.M.; Fujiwara, F.Y. Chemical constituents in Baccharis dracunculifolia as the main botanical origin of southeastern Brazilian propolis. J. Agric. Food Chem. 2004, 52, 1100–1103. [Google Scholar] [CrossRef]
- Shimizu, T.; Takeshita, Y.; Takamori, Y.; Kai, H.; Sawamura, R.; Yoshida, H.; Kurokawa, M. Efficacy of Brazilian propolis against herpes simplex virus type 1 infection in mice and their modes of antiherpetic efficacies. Evid. Based Complement. Alternat. Med. 2011, 2011, 976196. [Google Scholar] [CrossRef]
- Nani, B.D.; Sardi, J.D.C.O.; Lazarini, J.G.; Silva, D.R.; Massariolli, A.P.; Cunha, T.M.; Rosalen, P.L. Anti-inflammatory and anti-Candida effects of Brazilian organic propolis, a promising source of bioactive molecules and functional food. J. Agric. Food Chem. 2019, 68, 2861–2871. [Google Scholar] [CrossRef]
- Daugsch, A.; Moraes, C.S.; Fort, P.; Park, Y.K. Brazilian red propolis—Chemical composition and botanical origin. Evid. Based Complement. Alternat. Med. 2008, 5, 435–441. [Google Scholar] [CrossRef]
- Frozza, C.O.d.S.; Garcia, C.S.C.; Gambato, G.; de Souza, M.D.O.; Salvador, M.; Moura, S.; Padilha, F.F.; Seixas, F.K.; Collares, T.; Borsuk, S.; et al. Chemical characterization, antioxidant and cytotoxic activities of Brazilian red propolis. Food Chem. Toxicol. 2013, 52, 137–142. [Google Scholar] [CrossRef]
- Mendonça, L.S.D.; Mendonça, F.M.R.D.; Araújo, Y.L.F.M.D.; Araújo, E.D.D.; Ramalho, S.A.; Narain, N.; Cardoso, J.C. Chemical markers and antifungal activity of red propolis from Sergipe, Brazil. Food Sci. Technol. 2015, 35, 291–298. [Google Scholar] [CrossRef]
- Cavendish, R.L.; de Souza Santos, J.; Neto, R.B.; Paixão, A.O.; Oliveira, J.V.; de Araujo, E.D.; Gomes, M.Z. Antinociceptive and anti-inflammatory effects of Brazilian red propolis extract and formononetin in rodents. J. Ethnopharmacol. 2015, 173, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, S.; Piccolella, S.; Manti, L.; Pacifico, S. Could polyphenols really be a good radioprotective strategy? Molecules 2021, 26, 4969. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).