Copper (II) Ions Induced Self-Disproportionation of Enantiomers in Capillary Electrophoresis for the Quantification of Atenolol Enantiomers
Abstract
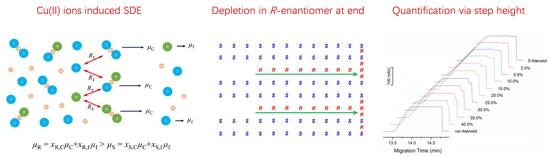
:1. Introduction
2. Principle and Protocol of Quantification
3. Results and Discussion
3.1. Optimization of Conditions
3.2. Analytical Characteristics
3.3. Confirmation of Mechanism
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of BGE and Sample Solution
4.3. Capillary Electrophoresis
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chankvetadze, B. Contemporary theory of enantioseparations in capillary electrophoresis. J. Chromatogr. A 2018, 1567, 2–25. [Google Scholar] [CrossRef]
- Fanali, S.; Chankvetadze, B. Some thoughts about enantioseparations in capillary electrophoresis. Electrophoresis 2019, 40, 2420–2437. [Google Scholar] [CrossRef] [PubMed]
- Chankvetadze, B. Application of enantioselective separation techniques to bioanalysis of chiral drugs and their metabolites. TrAC Trends Anal. Chem. 2021, 143, 116332. [Google Scholar] [CrossRef]
- Qian, H.-L.; Xu, S.-T.; Yan, X.-P. Recent Advances in Separation and Analysis of Chiral Compounds. Anal. Chem. 2023, 95, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Hancu, G.; Lupu, D.; Milan, A.; Budău, M.; Barabás-Hajdu, E. Enantioselective analysis of venlafaxine and its active metabolites: A review on the separation methodologies. Biomed. Chromatogr. 2021, 35, e4874. [Google Scholar] [CrossRef]
- Gogolashvili, A.; Lomsadze, K.; Chankvetadze, L.; Takaishvili, N.; Peluso, P.; Dallocchio, R.; Salgado, A.; Chankvetadze, B. Separation of tetrahydrozoline enantiomers in capillary electrophoresis with cyclodextrin-type chiral selectors and investigation of chiral recognition mechanisms. J. Chromatogr. A 2021, 1643, 462084. [Google Scholar] [CrossRef]
- Grodner, B.; Napiórkowska, M. Dual 2-Hydroxypropyl-β-Cyclodextrin and 5,10,15,20- Tetrakis (4-Hydroxyphenyl) Porphyrin System as a Novel Chiral-Achiral Selector Complex for Enantioseparation of Aminoalkanol Derivatives with Anticancer Activity in Capillary Electrophoresis. Molecules 2021, 26, 993. [Google Scholar] [CrossRef]
- Suliman, F.O.; Al Burtomani, S.K.; Elbashir, A.A.; Schmitz, O.J. Capillary electrophoresis and molecular modeling of the chiral separation of aromatic amino acids using α/β-cyclodextrin and 18-crown-6. Electrophoresis 2021, 42, 1800–1809. [Google Scholar] [CrossRef]
- Nie, L.; Yohannes, A.; Yao, S. Recent advances in the enantioseparation promoted by ionic liquids and their resolution mechanisms. J. Chromatogr. A 2020, 1626, 461384. [Google Scholar] [CrossRef]
- Hu, S.; Sun, T.; Li, R.; Zhang, D.; Zhang, Y.; Yang, Z.; Feng, G.; Guo, X. Comparison of the Performance of Different Bile Salts in Enantioselective Separation of Palonosetron Stereoisomers by Micellar Electrokinetic Chromatography. Molecules 2022, 27, 5233. [Google Scholar] [CrossRef]
- Wang, T.; Cheng, Y.; Zhang, Y.; Zha, J.; Ye, J.; Chu, Q.; Cheng, G. β-cyclodextrin modified quantum dots as pseudo-stationary phase for direct enantioseparation based on capillary electrophoresis with laser-induced fluorescence detection. Talanta 2020, 210, 120629. [Google Scholar] [CrossRef]
- Zhang, C.; Woolfork, A.G.; Suh, K.; Ovbude, S.; Bi, C.; Elzoeiry, M.; Hage, D.S. Clinical and pharmaceutical applications of affinity ligands in capillary electrophoresis: A review. J. Pharm. Biomed. Anal. 2020, 177, 112882. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xiong, Y.; Qing, G. Comment on Preparation of Vortex Porous Graphene Chiral Membrane for Enantioselective Separation. Anal. Chem. 2021, 93, 4682–4684. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yang, K.; Zhao, X.; Zhang, W.; Liu, Y.; Jiang, J.; Cui, Y. Highly Stable Zr(IV)-Based Metal-Organic Frameworks for Chiral Separation in Reversed-Phase Liquid Chromatography. J. Am. Chem. Soc. 2021, 143, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Lv, H.; Zhao, L. Applications of carbon nanomaterials in chiral separation. TrAC Trends Anal. Chem. 2020, 129, 115941. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, C.; Chen, J.; Xu, G.; Du, Y.; Ma, X.; Sun, X.; Feng, Z.; Huang, Z. Synthesis and application of tetramethylammonium-carboxymethylated-β-cyclodextrin: A novel ionic liquid in capillary electrophoresis enantioseparation. J. Pharmaceut. Biomed. Anal. 2020, 180, 113030. [Google Scholar] [CrossRef]
- Soloshonok, V.A. Remarkable Amplification of the Self-Disproportionation of Enantiomers on Achiral-Phase Chromatography Columns. Angew. Chem. Int. Ed. 2006, 45, 766–769. [Google Scholar] [CrossRef]
- Soloshonok, V.A.; Klika, K.D. Terminology Related to the Phenomenon ‘Self-Disproportionation of Enantiomers’ (SDE). Helv. Chim. Acta 2014, 97, 1583–1589. [Google Scholar] [CrossRef]
- Han, J.; Wzorek, A.; Soloshonok, V.A.; Klika, K.D. The self-disproportionation of enantiomers (SDE): The effect of scaling down, potential problems versus prospective applications, possible new occurrences, and unrealized opportunities? Electrophoresis 2019, 40, 1869–1880. [Google Scholar] [CrossRef]
- Bellec, A.; Guillemin, J.-C. Attempts to explain the self-disproportionation observed in the partial sublimation of enantiomerically enriched carboxylic acids. J. Fluorine Chem. 2010, 131, 545–548. [Google Scholar] [CrossRef]
- Jianlin, H.; Donna, J.N.; Alexander, E.S.; Vadim, A.S. Self-Disproportionation of Enantiomers via Sublimation; New and Truly Green Dimension in Optical Purification. Curr. Org. Synth. 2011, 8, 310–317. [Google Scholar] [CrossRef]
- Peng, C.-H.; Hong, B.-C.; Raja, A.; Chang, C.-W.; Lee, G.-H. Constructing densely functionalized Hajos-Parrish-type ketones with six contiguous stereogenic centers and two quaternary carbons in a formal [2+2+2] cycloaddition cascade. RSC Adv. 2016, 6, 95314–95319. [Google Scholar] [CrossRef]
- Abás, S.; Arróniz, C.; Molins, E.; Escolano, C. Access to the enantiopure pyrrolobenzodiazepine (PBD) dilactam nucleus via self-disproportionation of enantiomers. Tetrahedron 2018, 74, 867–871. [Google Scholar] [CrossRef]
- Cundy, K.C.; Crooks, P.A. Unexpected pehnomenon in the high-performance liquid chromatographic anlaysis of racemic 14C-labelled nicotine: Separation of enantiomers in a totally achiral system. J. Chromatogr. A 1983, 281, 17–33. [Google Scholar] [CrossRef]
- Charles, R.; Gil-Av, E. Self-amplification of optical activity by chromatography on an achiral adsorbent. J. Chromatogr. A 1984, 298, 516–520. [Google Scholar] [CrossRef]
- Mayani, V.J.; Abdi, S.H.R.; Kureshy, R.I.; Khan, N.H.; Agrawal, S.; Jasra, R.V. Enantiomer self-disproportionation of chiral compounds on achiral ordered mesoporous silica M41S and regular silica gel as a stationary phase. Chirality 2009, 21, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-W.; Xu, Z.; Gu, Q.; Shi, X.-X.; Leng, X.-B.; You, S.-L. Chiral counteranion-directed silver-catalyzed asymmetric synthesis of 1,2-dihydroisoquinolines by Friedel-Crafts alkylation reactions. Tetrahedron 2012, 68, 5263–5268. [Google Scholar] [CrossRef]
- Song, W.; Zhou, Y.; Fu, Y.; Xu, W. Self-disproportionation of enantiomers of prazoles via achiral, gravity-driven silica gel column chromatography. Tetrahedron Asymmetry 2013, 24, 909–912. [Google Scholar] [CrossRef]
- Suzuki, Y.; Han, J.; Kitagawa, O.; Aceña, J.L.; Klika, K.D.; Soloshonok, V.A. A comprehensive examination of the self-disproportionation of enantiomers (SDE) of chiral amides via achiral, laboratory-routine, gravity-driven column chromatography. RSC Adv. 2015, 5, 2988–2993. [Google Scholar] [CrossRef]
- Wzorek, A.; Sato, A.; Drabowicz, J.; Soloshonok, V.A.; Klika, K.D. Remarkable magnitude of the self-disproportionation of enantiomers (SDE) via achiral chromatography: Application to the practical-scale enantiopurification of β-amino acid esters. Amino Acids 2016, 48, 605–613. [Google Scholar] [CrossRef]
- Han, J.; Wzorek, A.; Kwiatkowska, M.; Soloshonok, V.A.; Klika, K.D. The self-disproportionation of enantiomers (SDE) of amino acids and their derivatives. Amino Acids 2019, 51, 865–889. [Google Scholar] [CrossRef] [PubMed]
- Bulko, F.; Májek, M.; Putala, M. Deracemization of Binaphthyl by Suzuki Diarylation: The Role of Electronic and Steric Effects. J. Org. Chem. 2022, 87, 9316–9329. [Google Scholar] [CrossRef] [PubMed]
- Soloshonok, V.A.; Ueki, H.; Yasumoto, M.; Mekala, S.; Hirschi, J.S.; Singleton, D.A. Phenomenon of Optical Self-Purification of Chiral Non-Racemic Compounds. J. Am. Chem. Soc. 2007, 129, 12112–12113. [Google Scholar] [CrossRef] [PubMed]
- Tonner, R.; Soloshonok, V.A.; Schwerdtfeger, P. Theoretical investigations into the enantiomeric and racemic forms of α-(trifluoromethyl)lactic acid. Phys. Chem. Chem. Phys. 2011, 13, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, V.; Murzin, D.Y.; Klika, K.D. NMR and molecular modeling of the dimeric self-association of the enantiomers of 1,1’-bi-2-naphthol and 1-phenyl-2,2,2- trifluoroethanol in the solution state and their relevance to enantiomer self-disproportionation on achiral-phase chromatography (ESDAC). Org. Biomol. Chem. 2009, 7, 537–542. [Google Scholar] [CrossRef]
- Terada, S.; Hirai, M.; Honzawa, A.; Kitagawa, O.; Kamizela, A.; Wzorek, A.; Soloshonok, V.A. Possible Case of Halogen Bond-Driven Self-Disproportionation of Enantiomers (SDE) via Achiral Chromatography. Chem. Eur. J. 2017, 23, 14631–14638. [Google Scholar] [CrossRef]
- Pietrusiewicz, K.M.; Borkowski, M.; Strzelecka, D.; Kielar, K.; Kicińska, W.; Karevych, S.; Jasiński, R.; Demchuk, O.M. A General Phenomenon of Spontaneous Amplification of Optical Purity under Achiral Chromatographic Conditions. Symmetry 2019, 11, 680. [Google Scholar] [CrossRef] [Green Version]
- Hosaka, T.; Imai, T.; Wzorek, A.; Marcinkowska, M.; Kolbus, A.; Kitagawa, O.; Soloshonok, V.A.; Klika, K.D. The self-disproportionation of enantiomers (SDE) of α-amino acid derivatives: Facets of steric and electronic properties. Amino Acids 2019, 51, 283–294. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Wzorek, A.; Kolbus, A.; Urbaniak, M.; Han, J.; Soloshonok, V.A.; Klika, K.D. Flurbiprofen: A Study of the Behavior of the Scalemate by Chromatography, Sublimation, and NMR. Symmetry 2021, 13, 543. [Google Scholar] [CrossRef]
- Wzorek, A.; Sato, A.; Drabowicz, J.; Soloshonok, V.A. Self-disproportionation of Enantiomers (SDE) of Chiral Nonracemic Amides via Achiral Chromatography. Isr. J. Chem. 2016, 56, 977–989. [Google Scholar] [CrossRef]
- Schmid, M.G.; Grobuschek, N.; Lecnik, O.; Gübitz, G. Chiral ligand-exchange capillary electrophoresis. J. Biochem. Bioph. Meth. 2001, 48, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.G.; Gübitz, G. Chiral separation by ligand-exchange. Maced. J. Chem. Chem. Eng. 2011, 30, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Schmid, M.G. Chiral metal-ion complexes for enantioseparation by capillary electrophoresis and capillary electrochromatography: A selective review. J. Chromatogr. A 2012, 1267, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qi, L.; Mao, L.; Chen, Y. Chiral separation using capillary electromigration techniques based on ligand exchange principle. J. Sep. Sci. 2012, 35, 1236–1248. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; He, M.; Hu, B. Chiral speciation and determination of selenomethionine enantiomers in selenized yeast by ligand-exchange micellar electrokinetic capillary chromatography after solid phase extraction. J. Chromatogr. A 2012, 1268, 173–179. [Google Scholar] [CrossRef]
- Kodama, S.; Aizawa, S.-i.; Taga, A.; Yamamoto, A.; Honda, Y.; Suzuki, K.; Kemmei, T.; Hayakawa, K. Determination of α-hydroxy acids and their enantiomers in fruit juices by ligand exchange CE with a dual central metal ion system. Electrophoresis 2013, 34, 1327–1333. [Google Scholar] [CrossRef]
- Meng, R.; Kang, J. Determination of the stereoisomeric impurities of sitafloxacin by capillary electrophoresis with dual chiral additives. J. Chromatogr. A 2017, 1506, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bao, P.; Qiao, J.; Zhang, H.; Qi, L. Chiral ligand exchange capillary electrophoresis with L-dipeptides as chiral ligands for separation of Dns-D,L-amino acids. Talanta 2020, 217, 121069. [Google Scholar] [CrossRef]
- Qi, L.; Qiao, J. Progress of chiral ligand-exchange capillary electrophoresis for enantioseparation. J. Chromatogr. A 2022, 1679, 463381. [Google Scholar] [CrossRef]
- Bontchev, P.R.; Pantcheva, I.N.; Gochev, G.P.; Mehandjiev, D.R.; Ivanov, D.S. Complexes of copper(II) with the β-blocker atenolol. Transition Met. Chem. 2000, 25, 196–199. [Google Scholar] [CrossRef]
- Gölcü, A.; Yücesoy, C.; Serin, S. Synthesis and Characterization of Metal Complexes of Acebutolol, Atenolol, and Propranolol Antihypertension Drugs. Syn. React. Inorg. Met. Chem. 2004, 34, 1259–1275. [Google Scholar] [CrossRef]
- Gölcü, A.; Yücesoy, C.; Serin, S. Spectrophotometric determination of some beta-blockers in dosage forms based on complex formation with Cu(II) and Co(II). Il Farmaco 2004, 59, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xu, X.; Huang, L.; Lin, J.; Chen, G. Microemulsion electrokinetic chromatography coupling with field amplified sample injection and electroosmotic flow suppressant for analysis of some quinolizidine alkaloids. J. Chromatogr. A 2008, 1198–1199, 220–225. [Google Scholar] [CrossRef] [PubMed]



| Calibration range | 0~30% (R-atenolol, m/m) |
| Regression equation b | H = 0.9988C + 1.6855 |
| Coefficient of determination, R2 | 0.9989 |
| Recovery (%) | |
| 5% added | 97.6 |
| 10% added | 102.5 |
| 15% added | 98.2 |
| 20% added | 101.2 |
| 25% added | 99.0 |
| 30% added | 97.4 |
| Repeatability (RSD, %) c | |
| Intra-day | 5.1 |
| Inter-day | 7.3 |
| 0 | 3.0 mM | 4.0 mM | 5.0 mM | |
|---|---|---|---|---|
| rac-atenolol | 0.61 ± 0.01 | 2.76 ± 0.01 | 3.06 ± 0.01 | 3.49 ± 0.02 |
| S-atenolol | 0.61 ± 0.02 | 2.74 ± 0.02 | 3.05 ± 0.01 | 3.51 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S. Copper (II) Ions Induced Self-Disproportionation of Enantiomers in Capillary Electrophoresis for the Quantification of Atenolol Enantiomers. Molecules 2023, 28, 5908. https://doi.org/10.3390/molecules28155908
Hu S. Copper (II) Ions Induced Self-Disproportionation of Enantiomers in Capillary Electrophoresis for the Quantification of Atenolol Enantiomers. Molecules. 2023; 28(15):5908. https://doi.org/10.3390/molecules28155908
Chicago/Turabian StyleHu, Shaoqiang. 2023. "Copper (II) Ions Induced Self-Disproportionation of Enantiomers in Capillary Electrophoresis for the Quantification of Atenolol Enantiomers" Molecules 28, no. 15: 5908. https://doi.org/10.3390/molecules28155908
APA StyleHu, S. (2023). Copper (II) Ions Induced Self-Disproportionation of Enantiomers in Capillary Electrophoresis for the Quantification of Atenolol Enantiomers. Molecules, 28(15), 5908. https://doi.org/10.3390/molecules28155908