A Microporous Zn(bdc)(ted)0.5 with Super High Ethane Uptake for Efficient Selective Adsorption and Separation of Light Hydrocarbons
Abstract
:1. Introduction
2. Results and Discussion
2.1. XRD Analysis
2.2. Structure Analysis
2.3. SEM Analysis
2.4. Thermogravimetric Analysis
2.5. Porosity Analysis
2.6. Adsorption Isotherms of Hydrocarbons on the Zn(bdc)(ted)0.5
2.7. Isosteric Adsorption Heats (Qst) of Hydrocarbons on the Zn(bdc)(ted)0.5
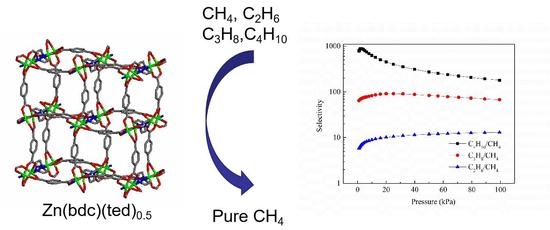
2.8. Ideal Adsorbed Solution Theory Selectivity
2.9. Simulation Results
3. Materials and Methods
3.1. Synthesis
3.2. Characterization
3.3. Adsorption of Light Hydrocarbons
3.4. Simulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cui, W.G.; Hu, T.L.; Bu, X.H. Metal–organic framework materials for the separation and purification of light hydrocarbons. Adv. Mater. 2020, 32, 1806445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, L.; Wang, L.; Cui, X.; Xing, H. Pillar iodination in functional boron cage hybrid supramolecular frameworks for high performance separation of light hydrocarbons. J. Mater. Chem. A 2019, 7, 27560–27566. [Google Scholar]
- Maqsood, K.; Pal, J.; Turunawarasu, D.; Pal, A.J.; Ganguly, S. Performance enhancement and energy reduction using hybrid cryogenic distillation networks for purification of natural gas with high CO2 content. Korean J. Chem. Eng. 2014, 31, 1120–1135. [Google Scholar] [CrossRef]
- Dybtsev, D.N.; Chun, H.; Kim, K. Rigid and Flexible: A highly porous metal–organic framework with unusual guest-dependent dynamic behavior. Angew. Chem. Int. Ed. 2004, 43, 5033–5036. [Google Scholar]
- Weyrich, J.N.; Mson, J.R.; Bazilevskaya, E.A.; Yang, H. Understanding the mechanism for adsorption of Pb(II) ions by Cu-BTC metal–organic frameworks. Molecules 2023, 28, 5443. [Google Scholar]
- Xu, F.; Xian, S.; Xia, Q.; Li, Y.; Li, Z. Effect of textural properties on the adsorption and desorption of toluene on the metal-organic frameworks HKUST-1 and MIL-101. Adsorpt. Sci. Technol. 2013, 31, 325–339. [Google Scholar]
- Wang, B.; Zhang, J.; Xue, Y.; Chong, Y.; Zhao, D.; Cheng, H.; Tian, L.; Zhang, J. Enhanced catalytic activity of TEMPO-mediated aerobic oxidation of alcohols via redox-active metal-organic framework nodes. Molecules 2023, 28, 593. [Google Scholar] [PubMed]
- Jing, H.; Zhao, L.; Song, G.; Li, J.; Wang, Z.; Han, Y.; Wang, Z. Application of a mixed-ligand metal-organic framework in photocatalytic CO2 reduction, antibacterial activity and dye adsorption. Molecules 2023, 28, 5204. [Google Scholar] [CrossRef]
- Wang, J.; Yu, M.; Chen, L.; Li, Z.; Li, S.; Jiang, F.; Hong, M. Construction of a stable lanthanide metal-organic framework as a luminescent probe for rapid naked-eye recognition of Fe3+ and acetone. Molecules 2021, 26, 1695. [Google Scholar]
- Xu, F.; Wang, H.; Teat, S.J.; Liu, W.; Xia, Q.; Li, Z.; Li, J. Synthesis, structure and enhanced photoluminescence properties of two robust, water stable calcium and magnesium coordination networks. Dalton Trans. 2015, 44, 20459–20463. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; He, H.; Yang, Y.; Cui, Y.; Qian, G. Structural variation and switchable nonlinear optical behavior of metal–organic frameworks. Small 2021, 17, 2006649. [Google Scholar]
- Wu, N.; Xu, D.; Wang, Z.; Wang, F.; Liu, J.; Liu, W.; Shao, Q.; Liu, H.; Gao, Q.; Guo, Z. Achieving superior electromagnetic wave absorbers through the novel metal-organic frameworks derived magnetic porous carbon nanorods. Carbon 2019, 145, 433–444. [Google Scholar]
- Qi, X.; Wang, Y.; Li, K.; Wang, J.; Zhang, H.L.; Yu, C.; Wang, H. Enhanced electrical properties and restrained thermal transport in p- and n-type thermoelectric metal–organic framework hybrids. J. Mater. Chem. A 2021, 9, 310–319. [Google Scholar]
- Dong, X.; Zhang, X.; Li, Y.; Xiong, D.; Fu, P.; Afzal, M.; Alarifi, A.; Sakiyama, H. Impact of N-donor auxiliary ligands on two new Co(II)-based MOFs with N-heterocyclic ligands and a magnetism study. New J. Chem. 2022, 46, 11623–11631. [Google Scholar]
- Rajamohan, R.; Raorane, C.J.; Kim, S.C.; Krishnan, M.M.; Lee, Y.R. Supramolecular β-cyclodextrin-quercetin based metal-organic frameworks as an efficient antibiofilm and antifungal agent. Molecules 2023, 28, 3667. [Google Scholar] [PubMed]
- Li, Z.; Zeng, W.; Li, Y. Recent progress in MOF-based electrochemical sensors for non-enzymatic glucose detection. Molecules 2023, 286, 4891. [Google Scholar]
- Yuan, B.; Wang, X.; Zhou, X.; Xiao, J.; Li, Z. Novel room-temperature synthesis of MIL-100(Fe) and its excellent adsorption performances for separation of light hydrocarbons. Chem. Eng. J. 2019, 355, 679–686. [Google Scholar]
- Lv, D.; Liu, Z.; Xu, F.; Wu, H.; Yuan, W.; Yan, J.; Xi, H.; Chen, X.; Xia, Q. A Ni-based metal-organic framework with super-high C3H8 uptake for adsorptive separation of light alkanes. Sep. Purif. Technol. 2021, 266, 118198. [Google Scholar]
- Chen, Y.; Qiao, Z.; Lv, D.; Wu, H.; Shi, R.; Xia, Q.; Wang, H.; Zhou, J.; Li, Z. Selective adsorption of light alkanes on a highly robust indium based metal-organic framework. Ind. Eng. Chem. Res. 2017, 56, 4488–4495. [Google Scholar]
- Shi, R.; Lv, D.; Chen, Y.; Wu, H.; Liu, B.; Xia, Q.; Li, Z. Highly selective adsorption separation of light hydrocarbons with a porphyrinic zirconium metal-organic framework PCN-224. Sep. Purif. Technol. 2018, 207, 262–268. [Google Scholar]
- Allen, F.H.; Bellard, S.; Brice, M.D.; Cartwright, B.A.; Doubleday, A.; Higgs, H.; Hummelink, T.; Hummelink-Peters, B.G.; Kennard, O.; Motherwell, W.D.S.; et al. The cambridge crystallographic data centre: Computer-based search, retrieval, analysis and display of information. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1979, B35, 2331–2339. [Google Scholar]
- Xiang, H.; Ameen, A.; Gorgojo, P.; Siperstein, F.R.; Holmes, S.M.; Fan, X. Selective adsorption of ethane over ethylene on M(bdc)(ted)0.5 (M = Co, Cu, Ni, Zn) metal-organic frameworks (MOFs). Microporous Mesoporous Mater. 2020, 292, 109724. [Google Scholar]
- Lee, J.Y.; Olson, D.H.; Pan, L.; Emge, T.J.; Li, J. Microporous metal–organic frameworks with high gas sorption and separation capacity. Adv. Funct. Mater. 2007, 17, 1255–1262. [Google Scholar]
- Wang, H.; Yao, K.X.; Zhang, Z.J.; Jagiello, J.; Gong, Q.H.; Han, Y.; Li, J. The first example of commensurate adsorption of atomic gas in a MOF and effective separation of xenon from other noble gases. Chem. Sci. 2014, 5, 620–624. [Google Scholar]
- Myers, A.L.; Prausnitz, J.M. Thermodynamics of mixed-gas adsorption. AIChE J. 1965, 11, 121–127. [Google Scholar]
- Yuan, Y.; Wu, H.; Xu, Y.; Lv, D.; Tu, S.; Wu, Y.; Li, Z.; Xia, Q. Selective extraction of methane from C1/C2/C3 on moisture-resistant MIL-142A with interpenetrated networks. Chem. Eng. J. 2020, 395, 125057. [Google Scholar]
- Zhang, Y.; Xiao, H.; Zhou, X.; Wang, X.; Li, Z. Selective adsorption performances of UiO-67 for separation of light hydrocarbons C1, C2, and C3. Ind. Eng. Chem. Res. 2017, 56, 8689–8696. [Google Scholar]
- Fan, W.; Wang, X.; Zhang, X.; Liu, X.; Wang, Y.; Kang, Z.; Dai, F.; Xu, B.; Wang, R.; Sun, D. Fine-tuning the pore environment of the microporous Cu-MOF for high propylene storage and efficient separation of light hydrocarbons. ACS Cent. Sci. 2019, 5, 1261–1268. [Google Scholar]
- Wang, D.; Liu, B.; Yao, S.; Wang, T.; Li, G.; Huo, Q.; Liu, Y. A polyhedral metal-organic framework based on the supermolecular building block strategy exhibiting high performance for carbon dioxide capture and separation of light hydrocarbons. Chem. Commun. 2015, 51, 15287–15289. [Google Scholar]
- Li, J.; Luo, X.; Zhao, N.; Zhang, L.; Huo, Y.; Liu, Y. Two finite binuclear [M2(μ2-OH)(COO)2] (M = Co, Ni) based highly porous Metal–Organic Frameworks with high performance for gas sorption and separation. Inorg. Chem. 2017, 56, 4141–4147. [Google Scholar]
- Huang, Y.; Lin, Z.; Fu, H.; Wang, F.; Shen, M.; Wang, X.; Cao, R. Porous anionic indium–organic framework with enhanced gas and vapor adsorption and separation ability. ChemSusChem 2014, 7, 2647–2653. [Google Scholar] [PubMed]
- Huang, P.; Chen, C.; Hong, Z.; Pang, J.; Wu, M.; Jiang, F.; Hong, M. Azobenzene decorated NbO-type metal–organic framework for high-capacity storage of energy gases. Inorg. Chem. 2019, 58, 11983–11987. [Google Scholar] [PubMed]
- Materials Studio; v7.0; Biovia Software Inc.: San Diego, CA, USA, 2014.
- Barker, J.A.; Henderson, D. What is “liquid”? Understanding the states of matter. Rev. Mod. Phys. 1976, 48, 587–671. [Google Scholar]
- Rappé, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard III, W.A.; Skif, W.M. UFF, A full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar]












| Adsorbates | (mmol/g) | b1 (kPa−1) | n1 | (mmol/g) | b2 (kPa−1) | n2 | R2 |
|---|---|---|---|---|---|---|---|
| CH4 | 16.5729 | 1.359 × 10−4 | 0.9918 | 16.573 | 1.0359 × 10−4 | 0.9918 | 0.9999 |
| C2H6 | 7.07662 | 0.0022 | 0.8282 | 7.0766 | 0.0022 | 0.8282 | 0.9999 |
| C3H8 | 1.50532 | 0.0016 | 0.2973 | 5.5847 | 0.0588 | 0.8818 | 0.9999 |
| C4H10 | 3.50191 | 3.031 | 0.3748 | 3.8683 | 0.3413 | 1.4425 | 0.9999 |
| Adsorbents | BET | C2H6 | CH4 | C2H6/CH4 | References |
|---|---|---|---|---|---|
| (m2 g−1) | (mmol/g) | (mmol/g) | Selectivity | ||
| CTGU-15 | 3163.7 | 2.13 | 0.4 | 5.2 | [18] |
| PCN-224 | 2704 | 2.93 | 0.48 | 12 | [20] |
| MIL-100(Fe) | 2482 | 2.22 | 0.36 | 6 | [17] |
| MIL-142A | 1424.7 | 3.82 | 0.54 | 14.5 | [26] |
| UIO-67 | 2590.6 | 4.26 | 0.56 | 8.1 | [27] |
| Iso-MOF-1 | 3211 | 3.19 | 0.38 | - | [28] |
| InOF-1 | 982 | 4.14 | 0.64 | 17 | [19] |
| JLU-Liu22 | 1487 | 3.3 | 0.71 | 14.4 | [29] |
| JLU-Liu37 | 1795 | 4.42 | 0.45 | 11 | [30] |
| JLU-Liu38 | 1784 | 4.96 | 0.48 | 15 | [30] |
| FJI-C1 | 1726.3 | 3.93 | 0.45 | 24 | [31] |
| FJI-H23 | 3740.4 | 6.28 | 0.67 | 14.7 | [32] |
| Zn(bdc)(ted)0.5 | 1904 | 4.9 | 0.4 | 13 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.; Wu, Y.; Wu, J.; Lv, D.; Yan, J.; Wang, X.; Chen, X.; Liu, Z.; Peng, J. A Microporous Zn(bdc)(ted)0.5 with Super High Ethane Uptake for Efficient Selective Adsorption and Separation of Light Hydrocarbons. Molecules 2023, 28, 6000. https://doi.org/10.3390/molecules28166000
Xu F, Wu Y, Wu J, Lv D, Yan J, Wang X, Chen X, Liu Z, Peng J. A Microporous Zn(bdc)(ted)0.5 with Super High Ethane Uptake for Efficient Selective Adsorption and Separation of Light Hydrocarbons. Molecules. 2023; 28(16):6000. https://doi.org/10.3390/molecules28166000
Chicago/Turabian StyleXu, Feng, Yilu Wu, Juan Wu, Daofei Lv, Jian Yan, Xun Wang, Xin Chen, Zewei Liu, and Junjie Peng. 2023. "A Microporous Zn(bdc)(ted)0.5 with Super High Ethane Uptake for Efficient Selective Adsorption and Separation of Light Hydrocarbons" Molecules 28, no. 16: 6000. https://doi.org/10.3390/molecules28166000
APA StyleXu, F., Wu, Y., Wu, J., Lv, D., Yan, J., Wang, X., Chen, X., Liu, Z., & Peng, J. (2023). A Microporous Zn(bdc)(ted)0.5 with Super High Ethane Uptake for Efficient Selective Adsorption and Separation of Light Hydrocarbons. Molecules, 28(16), 6000. https://doi.org/10.3390/molecules28166000