Multicomponent X-ray Shielding Using Sulfated Cerium Oxide and Bismuth Halide Composites
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural and Functional Group Analysis of S-CeO2
2.2. XPS Analysis of S-CeO2
2.3. HR-TEM and Element Mapping Analysis of S-CeO2
2.4. FE-SEM and Element Mapping
2.5. X-ray-Shielding Analysis of Multicomponent Halide Composites
3. Materials and Methods
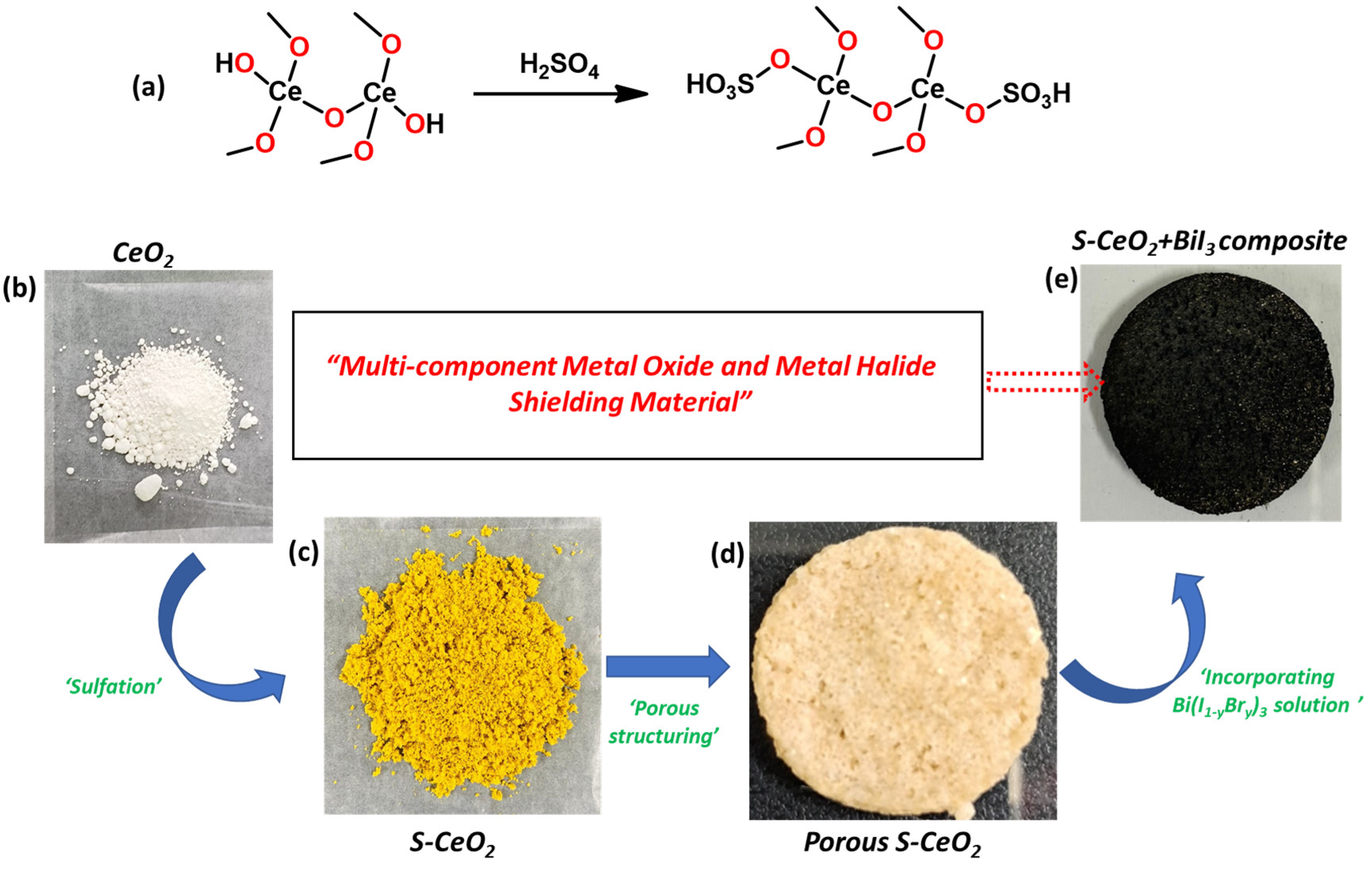
3.1. Synthesis of Sulfated CeO2
3.2. Fabrication of Porous PDMS and PDMS/S-CeO2
3.3. Porous PDMS/BiI3/BiBr3 Salt Solutions with Different Weight Ratios
3.4. Shielding Ability
3.5. Instrumentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zeng, C.; Kang, Q.; Duan, Z.; Qin, B.; Feng, X.; Lu, H.; Lin, Y. Development of Polymer Composites in Radiation Shielding Applications: A Review. J. Inorg. Organomet. Polym. Mater. 2023, 33, 2191–2239. [Google Scholar] [CrossRef]
- Wei, H.; DeSantis, D.; Wei, W.; Deng, Y.; Guo, D.; Savenije, T.J.; Cao, L.; Huang, J. Dopant Compensation in Alloyed CH3NH3PbBr3−xClxPerovskite Single Crystals for Gamma-Ray Spectroscopy. Nat. Mater. 2017, 16, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. Using DNA, Radiation Therapy Gets Personal. Science 2016, 353, 1348–1349. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, T.; Yang, C.; Li, X.; Liu, G.; Yang, Z.; Singh, P.K.; Krishnan, S.; Ding, D. Supramolecular Nanofibers of Curcumin for Highly Amplified Radiosensitization of Colorectal Cancers to Ionizing Radiation. Adv. Funct. Mater. 2018, 28, 1707140. [Google Scholar] [CrossRef]
- Mara, M.W.; Hadt, R.G.; Reinhard, M.E.; Kroll, T.; Lim, H.; Hartsock, R.W.; Alonso-Mori, R.; Chollet, M.; Glownia, J.M.; Nelson, S.; et al. Metalloprotein Entatic Control of Ligand-Metal Bonds Quantified by Ultrafast X-ray Spectroscopy. Science 2017, 356, 1276–1280. [Google Scholar] [CrossRef] [PubMed]
- IARC. Trichloroethylene. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 1995; Volume 63, pp. 75–158. [Google Scholar]
- Fazel, R.; Krumholz, H.M.; Wang, Y.; Ross, J.S.; Chen, J.; Ting, H.H.; Shah, N.D.; Nasir, K.; Einstein, A.J.; Nallamothu, B.K. Exposure to Low-Dose Ionizing Radiation from Medical Imaging Procedures. N. Engl. J. Med. 2009, 361, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Bale, H.A.; Haboub, A.; Macdowell, A.A.; Nasiatka, J.R.; Parkinson, D.Y.; Cox, B.N.; Marshall, D.B.; Ritchie, R.O. Real-Time Quantitative Imaging of Failure Events in Materials under Load at Temperatures above 1600 °C. Nat. Mater. 2013, 12, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Chen, L.; Chen, J.; Maiti, D.; Chai, Z.; Liu, Z.; Yang, K. Biomimetic Copper Sulfide for Chemo-Radiotherapy: Enhanced Uptake and Reduced Efflux of Nanoparticles for Tumor Cells under Ionizing Radiation. Adv. Funct. Mater. 2018, 28, 1705161. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, R.; Li, Q.; Liao, J.; Liu, N.; Joshi, N.S.; Shi, B.; Liao, X.; Guo, J. Lightweight and Wearable X-Ray Shielding Material with Biological Structure for Low Secondary Radiation and Metabolic Saving Performance. Adv. Mater. Technol. 2020, 5, 2000240. [Google Scholar] [CrossRef]
- Akman, F.; Ogul, H.; Kaçal, M.R.; Polat, H.; Dilsiz, K.; Turhan, M.F. Impact of Lead (II) Iodide on Radiation Shielding Properties of Polyester Composites. Appl. Phys. A Mater. Sci. Process. 2020, 126, 301–310. [Google Scholar] [CrossRef]
- Chen, J.; Wang, S.; Lin, J.; Chen, D. CsRe2F7@glass Nanocomposites with Efficient Up-/down-Conversion Luminescence: From In Situ Nanocrystallization Synthesis to MultiFunctional Applications. Nanoscale 2019, 11, 22359–22368. [Google Scholar] [CrossRef] [PubMed]
- Christidis, G.; Koch, U.; Poloni, E.; De Leo, E.; Cheng, B.; Koepfli, S.M.; Dorodnyy, A.; Bouville, F.; Fedoryshyn, Y.; Shklover, V.; et al. Broadband, High-Temperature Stable Reflector for Aerospace Thermal Radiation Protection. ACS Appl. Mater. Interfaces 2020, 12, 9925–9934. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Huang, J. Halid lead perovskites for ionizing radiation detection. Nat. Commun. 2019, 10, 1066. [Google Scholar] [CrossRef] [PubMed]
- Azman, N.Z.N.; Siddiqui, S.A.; Hart, R.; Low, I.M. Microstructural Design of Lead Oxide-Epoxy Composites for Radiation Shielding Purposes. J. Appl. Polym. Sci. 2013, 128, 3213–3219. [Google Scholar] [CrossRef]
- More, C.V.; Alsayed, Z.; Badawi, M.S.; Thabet, A.A.; Pawar, P.P. Polymeric Composite Materials for Radiation Shielding: A Review. Environ. Chem. Lett. 2021, 19, 2057–2090. [Google Scholar] [CrossRef] [PubMed]
- Tishkevich, D.I.; Grabchikov, S.S.; Lastovskii, S.B.; Trukhanov, S.V.; Zubar, T.I.; Vasin, D.S.; Trukhanov, A.V.; Kozlovskiy, A.L.; Zdorovets, M.M. Effect of the Synthesis Conditions and Microstructure for Highly Effective Electron Shields Production Based on Bi Coatings. ACS Appl. Energy Mater. 2018, 1, 1695–1702. [Google Scholar] [CrossRef]
- Kim, H.; Lim, J.; Kim, J.; Lee, J.; Seo, Y. Multilayer Structuring of Nonleaded Metal (BiSn)/Polymer/Tungsten Composites for Enhanced γ-Ray Shielding. Adv. Eng. Mater. 2020, 22, 1901448. [Google Scholar] [CrossRef]
- Liu, J.H.; Zhang, Q.P.; Sun, N.; Zhao, Y.; Shi, R.; Zhou, Y.L.; Zheng, J. Elevated Gamma-Rays Shielding Property in Lead-Free Bismuth Tungstate by Nanofabricating Structures. J. Phys. Chem. Solids 2018, 112, 185–189. [Google Scholar] [CrossRef]
- Tekin, H.O.; Sayyed, M.I.; Issa, S.A.M. Gamma Radiation Shielding Properties of the Hematite-Serpentine Concrete Blended with WO3 and Bi2O3 Micro and Nano Particles Using MCNPX Code. Radiat. Phys. Chem. 2018, 150, 95–100. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; El-Khatib, A.M.; Badawi, M.S.; Rashad, A.R.; El-Sharkawy, R.M.; Thabet, A.A. Fabrication, Characterization and Gamma Rays Shielding Properties of Nano and Micro Lead Oxide-Dispersed-High Density Polyethylene Composites. Radiat. Phys. Chem. 2018, 145, 160–173. [Google Scholar] [CrossRef]
- Yılmaz, S.N.; Güngör, A.; Özdemir, T. The Investigations of Mechanical, Thermal and Rheological Properties of Polydimethylsiloxane/Bismuth (III) Oxide Composite for X/Gamma Ray Shielding. Radiat. Phys. Chem. 2020, 170, 108649. [Google Scholar] [CrossRef]
- Nambiar, S.; Osei, E.K.; Yeow, J.T.W. Polymer Nanocomposite-Based Shielding against Diagnostic X-rays. J. Appl. Polym. Sci. 2013, 127, 4939–4946. [Google Scholar] [CrossRef]
- Singh, V.K.; Mukherjee, B.; Aravindh, S.A.; Das, S. Sulfonic acid (SO3H) functionalized two-dimensional MoS2 nanosheets for electrocatalytic hydrogen generation. Appl. Surf. Sci. 2023, 609, 155354. [Google Scholar] [CrossRef]
- Lermontov, S.A.; Malkova, A.N.; Yurkova, L.L.; Baranchikov, Y.A.; Ivanov, V.K. Sulfated Nano-Ceria as a Catalyst of Hex-1-Ene Oligomerization. Nanosyst. Phys. Chem. Math. 2013, 4, 690–695. [Google Scholar]
- Li, C.C.; Zheng, Y.P.; Wang, T.H. Sulfated Mesoporous Au/TiO2 Spheres as a Highly Active and Stable Solid Acid Catalyst. J. Mater. Chem. 2012, 22, 13216–13222. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, Q.; Chen, Y.; Liu, M.; Jin, P.; Yan, Y.; Pan, J. Synthesis of Ceria and Sulfated Zirconia Catalysts Supported on Mesoporous SBA-15 toward Glucose Conversion to 5-Hydroxymethylfurfural in a Green Isopropanol-Mediated System. Ind. Eng. Chem. Res. 2018, 57, 1968–1979. [Google Scholar] [CrossRef]
- Zhao, D.; Yi, B.L.; Zhang, H.M.; Yu, H.M. MnO2/SiO2–SO3H nanocomposite as hydrogen peroxide scavenger for durability improvement in proton exchange membranes. J. Membr. Sci. 2010, 346, 143–151. [Google Scholar] [CrossRef]
- Meng, F.; Wang, L.; Cui, J. Controllable Synthesis and Optical Properties of Nano-CeO2 via a Facile Hydrothermal Route. J. Alloys Compd. 2013, 556, 102–108. [Google Scholar] [CrossRef]
- Kuntaiah, K.; Sudarsanam, P.; Reddy, B.M.; Vinu, A. Nanocrystalline Ce1−xSmxO2−δ (x = 0.4) Solid Solutions: Structural Characterization versus CO Oxidation. RSC Adv. 2013, 3, 7953–7962. [Google Scholar] [CrossRef]
- Jaffari, G.H.; Imran, A.; Bah, M.; Ali, A.; Bhatti, A.S.; Qurashi, U.S.; Ismat Shah, S. Identification and Quantification of Oxygen Vacancies in CeO2 Nanocrystals and Their Role in Formation of F-Centers. Appl. Surf. Sci. 2017, 396, 547–553. [Google Scholar] [CrossRef]
- Wang, L.; Meng, F.; Li, K.; Lu, F. Characterization and Optical Properties of Pole-like Nano-CeO2 Synthesized by a Facile Hydrothermal Method. Appl. Surf. Sci. 2013, 286, 269–274. [Google Scholar] [CrossRef]
- Li, H.; Meng, F.; Gong, J.; Fan, Z.; Qin, R. Structural, Morphological and Optical Properties of Shuttle-like CeO2 Synthesized by a Facile Hydrothermal Method. J. Alloys Compd. 2017, 722, 489–498. [Google Scholar] [CrossRef]
- Salusso, D.; Grillo, G.; Manzoli, M.; Signorile, M.; Zafeiratos, S.; Barreau, M.; Damin, A.; Crocellà, V.; Cravotto, G.; Bordiga, S. CeO2 Frustrated Lewis Pairs Improving CO2 and CH3OH Conversion to Monomethylcarbonate. ACS Appl. Mater. Interfaces 2023, 15, 15396–15408. [Google Scholar] [CrossRef] [PubMed]
- Zahran, H.Y.; Mohammed, M.I.; Sayed Yousef, E.; Alqahtani, M.S.; Reben, M.; Algarni, H.; Umar, A.; Albargi, H.B.; Yahia, I.S.; Abdel-wahab, M.S.; et al. Radiation Attenuation Properties of the Quaternary Semiconducting Compounds Cu2CoGe [S, Se, Te]4. Results Phys. 2022, 37, 105488. [Google Scholar] [CrossRef]
- Sakher, E.; Smili, B.; Bououdina, M.; Bellucci, S. Structural Study of Nano-Clay and Its Effectiveness in Radiation Protection against X-rays. Nanomaterials 2022, 12, 2332. [Google Scholar] [CrossRef]
- Jiang, X.; Zhu, X.; Chang, C.; Liu, S.; Luo, X. X-ray shielding structural and properties design for the porous transparent BaSO4/cellulose nanocomposite membranes. Int. J. Biol. Macromol. 2019, 13, 9793–9800. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhong, R.; Xiao, X.; Liao, J.; Liao, X.; Shi, B. Lightweight and Flexible Bi@Bi-La Natural Leather Composites with Superb X-ray Radiation Shielding Performance and Low Secondary Radiation. ACS Appl. Mater. Interfaces 2020, 12, 54117–54126. [Google Scholar] [CrossRef] [PubMed]
- Kaewpirom, S.; Chousangsuntorn, K.; Boonsang, S. Evaluation of Micro- and Nano-Bismuth (III) Oxide Coated Fabric for Environmentally Friendly X-ray Shielding Materials. ACS Omega 2022, 7, 28248–28257. [Google Scholar] [CrossRef]
- Aral, N.; Banu Nergis, F.; Candan, C. An alternative X-ray shielding material based on coated textiles. Text. Res. J. 2016, 86, 803–811. [Google Scholar] [CrossRef]
- Maghrabi, H.A.; Vijayan, A.; Deb, P.; Wang, L. Bismuth oxide-coated fabrics for X-ray shielding. Text. Res. J. 2016, 86, 649–658. [Google Scholar] [CrossRef]
- Muhammad, N.A.; Armynah, B.; Tahir, D. High transparent wood composite for effective X-ray shielding applications. Mat. Resea. Bull. 2022, 154, 111930. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Xiao, X.; Zhong, R.; Liao, J.; Guo, J.; Liao, X.; Shi, B. Research on X-ray shielding performance of wearable Bi/Ce-natural leather composite materials. J. Hazar. Mat. 2020, 398, 122943. [Google Scholar] [CrossRef]
- Zarei, M.; Sina, S.; Hashemi, S.A. Superior X-ray radiation shielding of biocompatible platform based on reinforced polyaniline by decorated graphene oxide, with interconnected tungsten–bismuth–tin complex. Rad. Phys. Chem. 2021, 188, 109588. [Google Scholar] [CrossRef]





| Sample Name | A0 | A2 | A4 | A6 | A8 | A10 |
|---|---|---|---|---|---|---|
| Powder A (g) BiBr3 | 0 | 0.2 | 0.4 | 0.6 | 0.8 | 1 |
| Powder B (g) BiI3 | 1 | 0.8 | 0.6 | 0.4 | 0.2 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahalingam, S.; Kwon, D.-S.; Kang, S.-G.; Kim, J. Multicomponent X-ray Shielding Using Sulfated Cerium Oxide and Bismuth Halide Composites. Molecules 2023, 28, 6045. https://doi.org/10.3390/molecules28166045
Mahalingam S, Kwon D-S, Kang S-G, Kim J. Multicomponent X-ray Shielding Using Sulfated Cerium Oxide and Bismuth Halide Composites. Molecules. 2023; 28(16):6045. https://doi.org/10.3390/molecules28166045
Chicago/Turabian StyleMahalingam, Shanmugam, Dae-Seong Kwon, Seok-Gyu Kang, and Junghwan Kim. 2023. "Multicomponent X-ray Shielding Using Sulfated Cerium Oxide and Bismuth Halide Composites" Molecules 28, no. 16: 6045. https://doi.org/10.3390/molecules28166045
APA StyleMahalingam, S., Kwon, D.-S., Kang, S.-G., & Kim, J. (2023). Multicomponent X-ray Shielding Using Sulfated Cerium Oxide and Bismuth Halide Composites. Molecules, 28(16), 6045. https://doi.org/10.3390/molecules28166045






