Heterometallic ZnHoMOF as a Dual-Responsive Luminescence Sensor for Efficient Detection of Hippuric Acid Biomarker and Nitrofuran Antibiotics
Abstract
:1. Introduction
2. Result and Discussion
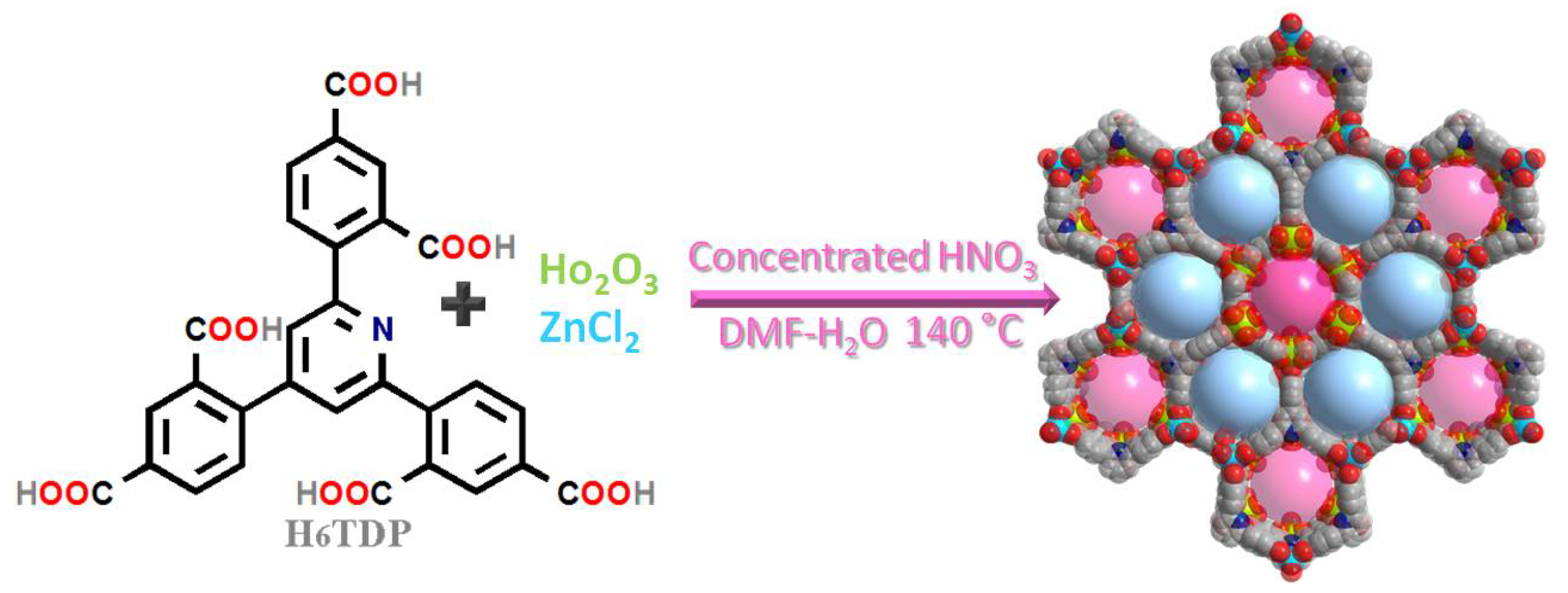
2.1. Simple Structural Description of ZnHoMOF
2.2. Chemical and Thermal Stability of ZnHoMOF
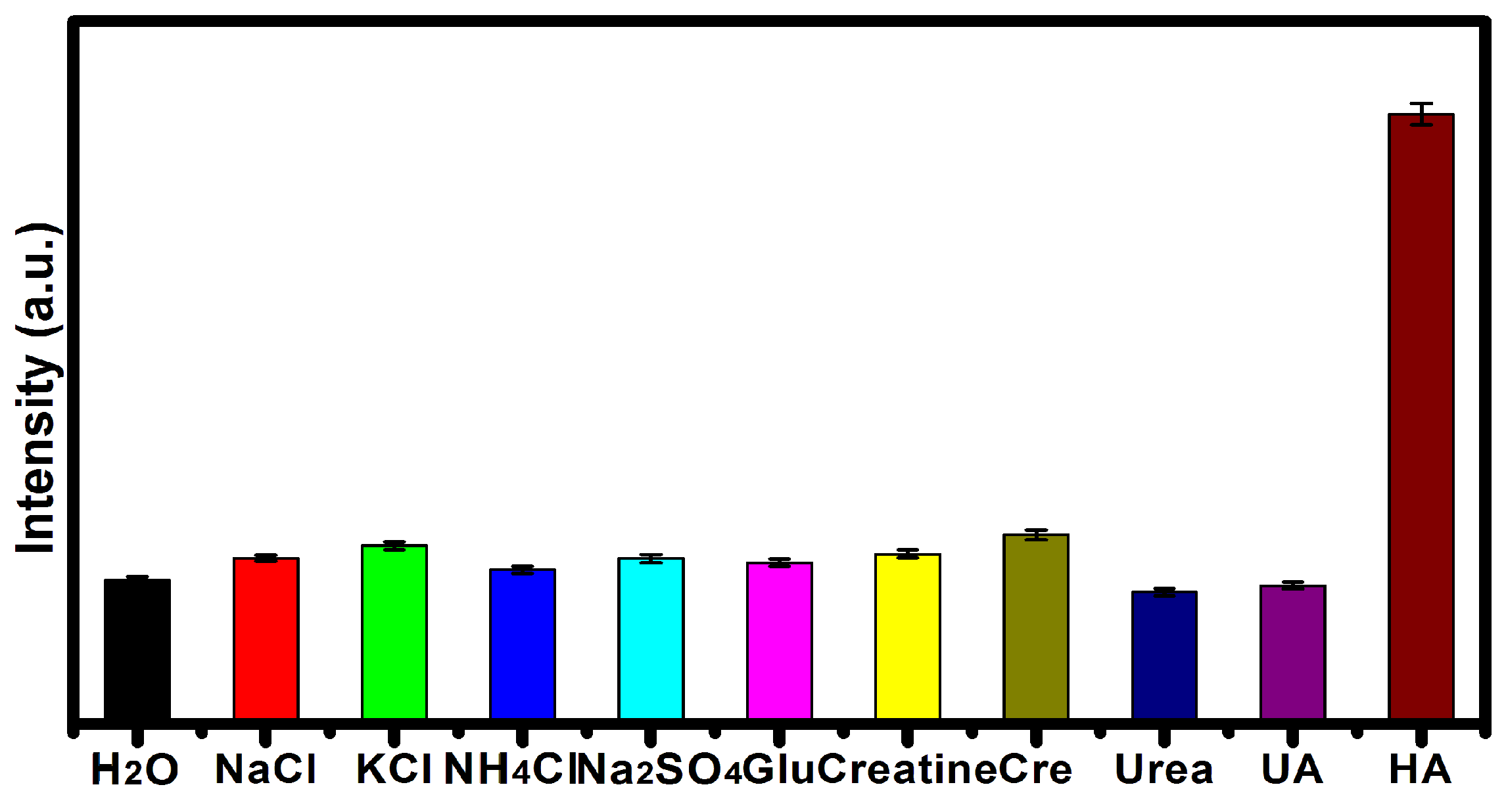
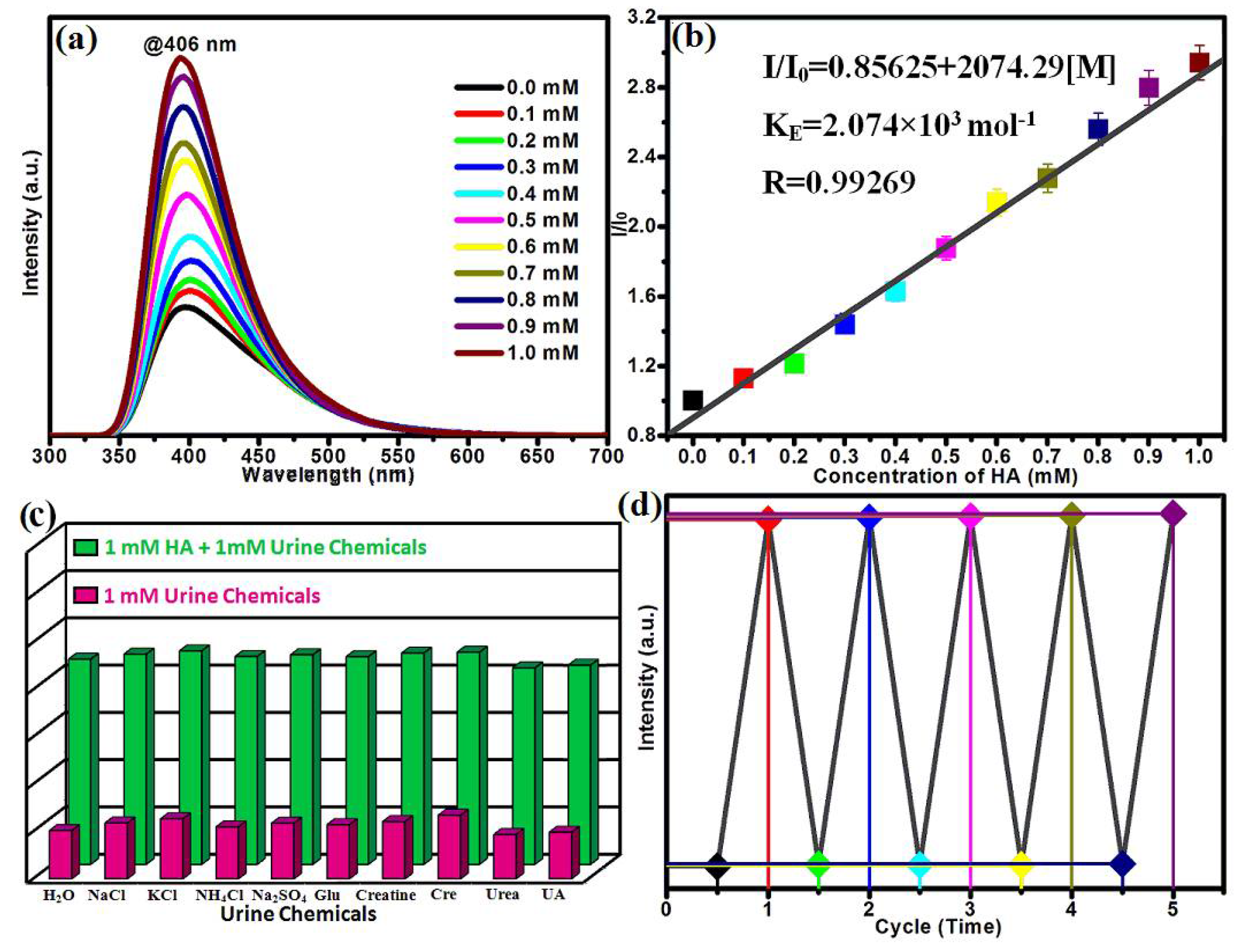
2.3. Detection of the HA
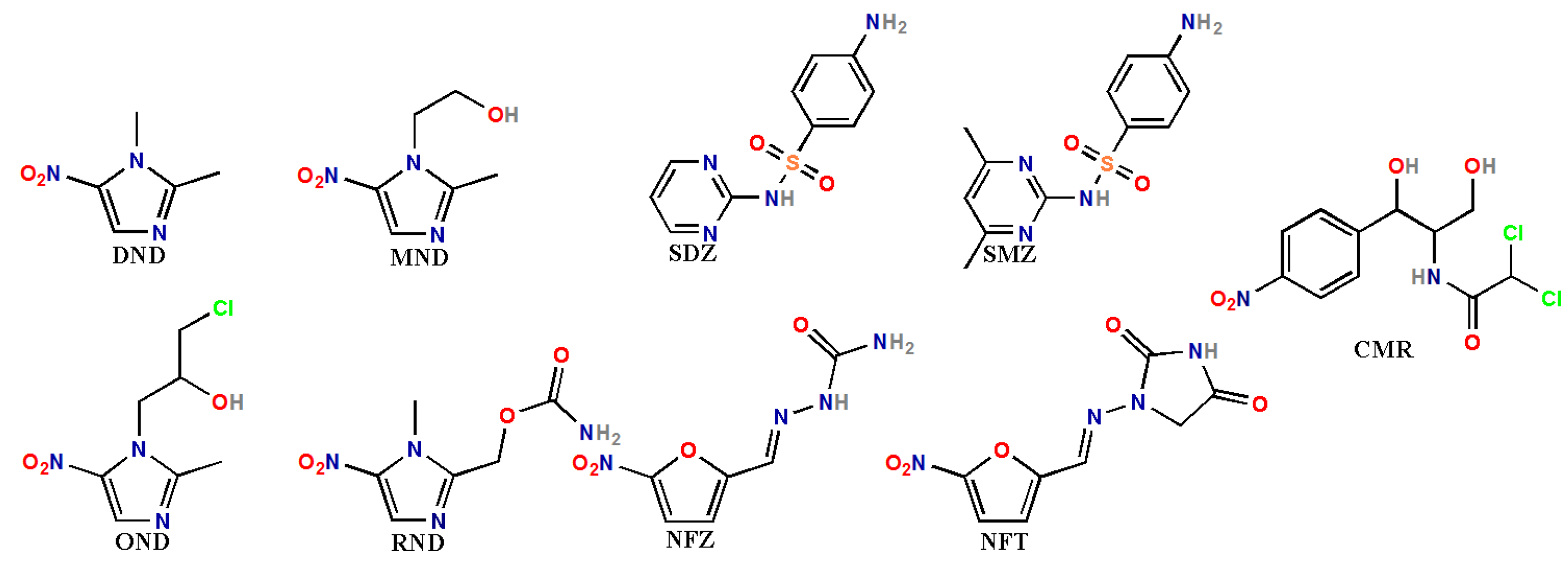
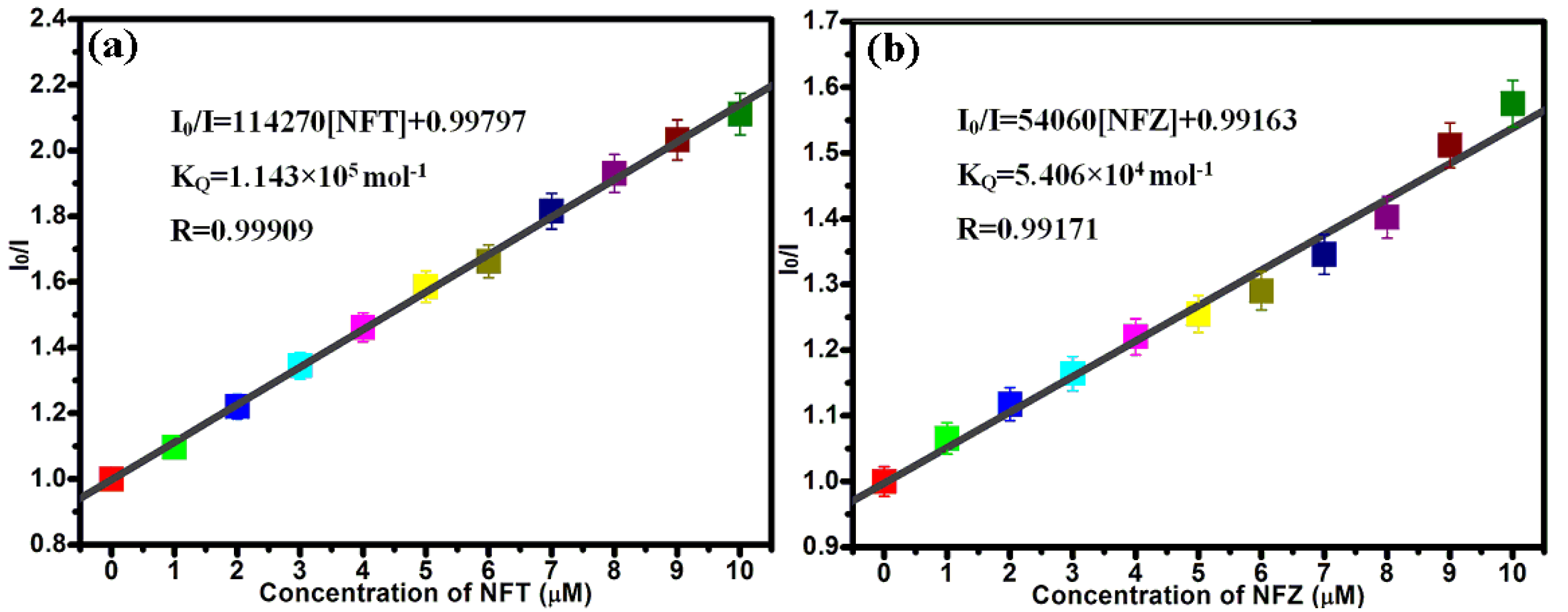
2.4. Detection of Nitrofuran Antibiotics
2.5. Possible Mechanism for Luminescence Sensing
3. Materials and Methods
3.1. Reagents and Apparatus
3.2. Preparation of ZnHoMOF
3.3. Luminescent Sensing Experiments
3.4. Luminescent Titration and Recyclable Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhao, P.; Feng, X.; Huang, D.; Yang, G.; Astruc, D. Basic concepts and recent advances in nitrophenol reduction by gold- and other transition metal nanoparticles. Coord. Chem. Rev. 2015, 2287, 114–136. [Google Scholar] [CrossRef]
- Qin, S.J.; Yan, B. The point-of-care colorimetric detection of the biomarker of phenylamine in the human urine based on Tb3+ functionalized metal-organic framework. Anal. Chim. Acta 2018, 1012, 82–89. [Google Scholar] [CrossRef]
- Desai, A.V.; Manna, B.; Karmakar, A.; Sahu, A.; Ghosh, S.K. A Water-Stable Cationic Metal–Organic Framework as a Dual Adsorbent of Oxoanion Pollutants. Angew. Chem. Int. Ed. 2016, 55, 7811–7815. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.J.; Liu, W.S. Effective luminescence sensing of Fe3+, Cr2O72−, MnO4− and 4-nitrophenol by lanthanide metal–organic frameworks with a new topology type. Dalton Trans. 2019, 48, 12287–12295. [Google Scholar] [CrossRef] [PubMed]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal–organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef]
- Fu, H.R.; Yan, L.B.; Wu, N.T.; Ma, L.F.; Zang, S.Q. Dual-emission MOF⊃dye sensor for ratiometric fluorescence recognition of RDX and detection of a broad class of nitro-compounds. J. Mater. Chem. A 2018, 6, 9183–9191. [Google Scholar] [CrossRef]
- Wang, B.; Lv, X.-L.; Feng, D.; Xie, L.-H.; Zhang, J.; Li, M.; Xie, Y.; Li, J.-R.; Zhou, H.-C. Highly Stable Zr(IV)-Based Metal–Organic Frameworks for the Detection and Removal of Antibiotics and Organic Explosives in Water. J. Am. Chem. Soc. 2016, 138, 6204–6216. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.H.; Yi, J.W.; Han, M.L.; Li, B.; Liu, S.; Wu, Y.P.; Ma, L.F.; Li, D.S. A water-stable terbium(III)–organic framework as a chemosensor for inorganic ions, nitro-containing compounds and antibiotics in aqueous solutions. Chem.-Asian J. 2019, 75, 3694–3701. [Google Scholar] [CrossRef]
- Chen, S.; Huang, R.; Zou, J.; Liao, D.; Yu, J.; Jiang, X. A sensitive sensor based on MOFs derived nanoporous carbons for electrochemical detection of 4-aminophenol. Ecotoxicol. Environ. Saf. 2020, 191, 110194. [Google Scholar] [CrossRef]
- Guo, L.; Hao, L.; Zhang, L.; Yang, X.; Wang, Q.; Wang, Z.; Wang, C. Metal-organic framework precursors derived Ni-doping porous carbon spheres for sensitive electrochemical detection of acetaminophen. Talanta 2021, 228, 122228. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-D.; Krishna, R.; Li, Y.-Z.; Shi, W.-J.; Hou, L.; Wang, Y.-Y.; Zhu, Z. Boosting Ethane/Ethylene Separation by MOFs through the Amino-Functionalization of Pores. Angew. Chem. Int. Ed. 2022, 61, e202213015. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Sun, Q.; Gao, W.; Perman, J.A.; Sun, F.; Zhu, G.; Aguila, B.; Forrest, K.; Space, B.; Ma, S. A stable metal–organic framework featuring local buffer environment for carbon dioxide fixation. Angew. Chem. Int. Ed. 2018, 57, 4657–4662. [Google Scholar] [CrossRef] [PubMed]
- Han, S.D.; Liu, A.J.; Wei, Q.; Hu, J.X.; Pan, J.; Wang, G.M. Quadruple Photoresponsive Functionality in a Crystalline Hybrid Material: Photochromism, Photomodulated Fluorescence, Magnetism and Nonlinear Optical Properties. Chem. Eur. J. 2021, 27, 7842–7846. [Google Scholar] [CrossRef]
- Chen, X.; Li, M.; Lin, M.; Lu, C.; Kumar, A.; Pan, Y.; Liu, J.; Peng, Y. Current and promising applications of Hf(IV)-based MOFs in clinical cancer therapy. J. Mater. Chem. B 2023, 11, 5693–5714. [Google Scholar] [CrossRef]
- Wang, G.D.; Wang, H.H.; Shi, W.-J.; Hou, L.; Wang, Y.Y.; Zhu, Z. A highly stable MOF with F and N accessible sites for efficient capture and separation of acetylene from ternary mixtures. J. Mater. Chem. A 2021, 9, 24495–24502. [Google Scholar] [CrossRef]
- Shi, W.J.; Li, Y.Z.; Chen, J.; Su, R.H.; Hou, L.; Wang, Y.Y.; Zhu, Z. A new metal-organic framework based on rare [Zn4F4] cores for efficient separation of C2H2. Chem. Commun. 2021, 57, 12788–12791. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, Z.G.; Zhong, M.; Fu, Z.X.; Liu, M.; Yin, J.C.; Shen, Z.; Li, W.; Zhang, J.; Chang, Z.; et al. Strategic Defect Engineering of Metal–Organic Frameworks for Optimizing the Fabrication of Single-Atom Catalysts. Adv. Funct. Mater. 2021, 31, 2103597. [Google Scholar] [CrossRef]
- Guo, B.B.; Yin, J.C.; Li, N.; Fu, Z.X.; Han, X.; Xu, J.; Bu, X.H. Recent Progress in Luminous Particle-Encapsulated Host–Guest Metal-Organic Frameworks for Optical Applications. Adv. Opt. Mater. 2021, 9, 2100283. [Google Scholar] [CrossRef]
- Ma, D.Y.; Li, Z.; Zhu, J.X.; Zhou, Y.P.; Chen, L.L.; Mai, X.F.; Liufu, M.L.; Wu, Y.B.; Li, Y.W. In-verse and highly selective separation of CO2 /C2H2 on a thulium–organic framework. J. Mater. Chem. A 2020, 8, 11933–11937. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Lu, J.J.; Lin, H.Y.; Wang, X.L.; Chang, Z.H.; Chen, Y.Z.; Zhang, Y.C. Polyoxometalate-based metal-organic complexes constructed from a new bis-pyrimidine-amide ligand with high capacitance performance and selectivity for the detection of Cr(VI). Chin. Chem. Lett. 2022, 33, 4389–4394. [Google Scholar] [CrossRef]
- Geng, J.; Chen, Y.; Xie, S.; Lin, H.; Wang, X. Ultraversatile Fluorescent Sensors Based on Two CoII/NiII Coordination Polymers for Identifying Various Antibiotics via the Turn-On/Off Effect and Detecting pH. Inorg. Chem. 2023, 62, 5158–5167. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Q.; Fan, M.; Sun, J.; Su, Z.M.; Li, X.; Wang, X. Novel Eu-MOF-based mixed matrix membranes and 1D Eu-MOF-based ratiometric fluorescent sensor for the detection of metronidazole and PA in water. Dye. Pigment. 2022, 197, 109812. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Q.; Li, J.; Su, Z.M.; Li, X.; Wang, X.; Sun, J.; Zhou, C.; Hu, X. A dual-emitting mixed-lanthanide MOF with high water-stability for ratiometric fluorescence sensing of Fe3+ and ascorbic acid. J. Mater. Chem. C 2021, 9, 562–568. [Google Scholar] [CrossRef]
- Li, Z.Y.; Yao, Z.Q.; Feng, R.; Sun, M.H.; Shan, X.T.; Su, Z.H.; Li, W.; Bu, X.H. A highly stable terbium metal-organic framework for efficient detection of picric acid in water. Chin. Chem. Lett. 2021, 32, 3095–3098. [Google Scholar] [CrossRef]
- Zhong, Y.; Peng, Z.; Peng, Y.; Li, B.; Pan, Y.; Ouyang, Q.; Sakiyama, H.; Muddassir, M.; Liu, J. Construc-tion of Fe-doped ZIF-8/DOX nanocomposites for ferroptosis strategy on the treatment of breast cancer. J. Mater. Chem. B 2023, 11, 6335–6345. [Google Scholar] [CrossRef]
- Jin, J.; Xue, J.; Liu, Y.; Yang, G.; Wang, Y.Y. Recent progresses in luminescent metal-organic frameworks (LMOFs) as sensors for the detection of anions and cations in aqueous solution. Dalton Trans. 2021, 50, 1950–1972. [Google Scholar] [CrossRef]
- Wang, G.D.; Li, Y.Z.; Shi, W.J.; Zhang, B.; Hou, L.; Wang, Y.Y. A robust cluster-based Eu-MOF as multi-functional fluorescence sensor for detection of antibiotics and pesticides in water. Sens. Actuators B Chem. 2021, 331, 129377. [Google Scholar] [CrossRef]
- Ma, L.N.; Zhang, B.; Wang, Z.H.; Hou, L.; Zhu, Z.; Wang, Y.Y. Efficient Gas and VOC Separation and Pesticide Detection in a Highly Stable Interpenetrated Indium-Organic Framework. Inorg. Chem. 2021, 60, 10698–10706. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Li, D.; Li, Y.; Sakiyama, H.; Muddassir, M.; Pan, Y.; Srivastava, D.; Kumar, A. A 3,8-connected Cd(II)-based metal–organic framework as an appropriate luminescent sensor for the antibiotic sulfasalazine. Crystengcomm 2022, 24, 7157–7165. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, N.; Wang, L.; Wang, H.; Lu, J.; Li, Y.; Dou, J.; Wang, S. Detection Enhancement of One Multifunctional Cd-Metal–Organic Framework toward Tetracycline Antibiotics by Simply Mixing Eu3+ in Suspension. Inorg. Chem. 2023, 62, 3573–3584. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.-J.; Hao, J.-N.; Xu, X.-Y.; Lian, X.; Yan, B. Highly sensing probe for biological metabolite of benzene series pollutants based on recyclable Eu3+ functionalized metal-organic frameworks hybrids. Sens. Actuators B Chem. 2017, 253, 852–859. [Google Scholar] [CrossRef]
- Hao, J.-N.; Yan, B. Recyclable lanthanide-functionalized MOF hybrids to determine hippuric acid in urine as a biological index of toluene exposure. Chem. Commun. 2015, 51, 14509–14512. [Google Scholar] [CrossRef]
- Li, W.; Zhao, D.; Li, W.; Wen, R.; Liu, X.; Liu, L.; Li, T.; Fan, L. Chemorobust dye-encapsulated framework as dual-emission self-calibrating ratiometric sensor for intelligent detection of toluene exposure biomarker in urine. Spectrochim. Acta A 2023, 296, 122637. [Google Scholar] [CrossRef]
- Zhao, D.; Li, W.; Wen, R.; Lei, N.; Li, W.; Liu, X.; Zhang, X.; Fan, L. Eu(III)-Functionalized MOF-Based Dual-Emission Ratiometric Sensor Integrated with Logic Gate Operation for Efficient Detection of Hippuric Acid in Urine and Serum. Inorg. Chem. 2023, 62, 2715–2725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lei, M.; Yan, H.; Wang, J.; Shi, Y. A Water-Stable 3D Luminescent Metal-Organic Framework Based on Heterometallic [EuIII6ZnII] Clusters Showing Highly Sensitive, Selective, and Reversible Detection of Ronidazole. Inorg. Chem. 2017, 56, 7610–7614. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Wang, Y.L.; Xue, Z.; Han, S.D.; Xue, Z.Z.; Pan, J. Heterometallic-Organic Framework from [Cu2I2] and [PbO]n Chains: Photoluminescence, Sensing, and Photocatalytic Performance. Cryst. Growth Des. 2021, 21, 5261–5267. [Google Scholar] [CrossRef]
- Zhao, D.; Li, W.; Wen, R.; Li, W.; Liu, X.; Zhang, X.; Fan, L. Tb(III) functionalized MOF based self-calibrating sensor integrated with logic gate operation for efficient epinephrine detection in serum. J. Rare Earths 2023, in press. [CrossRef]
- Wang, F.; Zhao, D.; Li, W.; Zhang, H.; Li, B.; Hu, T.; Fan, L. Rod-shaped units based cobalt(II) organic framework as an efficient electrochemical sensor for uric acid detection in serum. Microchem. J. 2023, 185, 108154. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, X.; Yan, B. A visual logic alarm sensor for diabetic patients towards diabetic polyneuropathy based on a metal–organic framework functionalized by dual-cation exchange. J. Mater. Chem. C 2021, 9, 3440–3446. [Google Scholar] [CrossRef]
- Liu, T.Y.; Qu, X.L.; Yan, B. A sensitive metal–organic framework nanosensor with cation-introduced chirality for enantioselective recognition and determination of quinine and quinidine in human urine. J. Mater. Chem. C 2020, 8, 14579–14586. [Google Scholar] [CrossRef]
- Wang, N.; Li, S.; Li, Z.; Gong, Y.; Li, X. A Zn(II)–Metal–Organic Framework Based on 4-(4-Carboxy phenoxy) Phthalate Acid as Luminescent Sensor for Detection of Acetone and Tetracycline. Molecules 2023, 28, 999. [Google Scholar] [CrossRef]
- Li, L.; Zou, J.; Han, Y.; Liao, Z.; Lu, P.; Nezamzadeh-Fjhieh, A.; Liu, J.; Peng, Y. Recent advances in Al(III)/In(III)-based MOFs for the detection of pollutants. New J. Chem. 2022, 46, 19577–19592. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, S.; Shao, L.; Ren, Y.; Jiang, J.; Wang, S.; Tang, H.; Deng, H.; Xia, T. Highly Sensitive Adsorption and Detection of Iodide in Aqueous Solution by a Post-Synthesized Zirconium-Organic Framework. Molecules 2022, 27, 8547. [Google Scholar] [CrossRef]
- Ogata, M.; Tomokuni, K.; Takatsuka, Y. Urinary excretion of hippuric acid and m- or p-methylhippuric acid in the urine of persons exposed to vapours of toluene and m- or p-xylene as a test of exposure. Brit. J. Industr. Med. 1970, 27, 43–50. [Google Scholar] [CrossRef]
- Siqueira, M.E.P.B.; Paiva, M.J.N. Hippuric acid in urine: Reference values. Rev. Saúde Pública 2002, 36, 723–727. [Google Scholar] [CrossRef]
- Fan, L.; Wang, F.; Zhao, D.; Sun, X.; Chen, H.; Wang, H.; Zhang, X. Two cadmium(II) coordination polymers as multi-functional luminescent sensors for the detection of Cr(VI) anions, dichloronitroaniline pesticide, and nitrofuran antibiotic in aqueous media. Spectrochim. Acta A 2020, 239, 118467. [Google Scholar] [CrossRef]
- Fan, L.; Zhao, D.; Li, B.; Chen, X.; Wang, F.; Deng, Y.; Niu, Y.; Zhang, X. An exceptionally stable luminescent cadmium(II) metal–organic framework as a dual-functional chemosensor for detecting Cr(VI) anions and nitro-containing antibiotics in aqueous media. Crystengcomm 2021, 23, 1218–1225. [Google Scholar] [CrossRef]
- Fan, L.; Wang, F.; Zhao, D.; Peng, Y.; Deng, Y.; Luo, Y.; Zhang, X. A self-penetrating and chemically stable zinc (II)-organic framework as multi-responsive chemo-sensor to detect pesticide and antibiotics in water. Appl. Organomet. Chem. 2020, 34, e5960. [Google Scholar] [CrossRef]
- Fan, L.; Zhao, D.; Zhang, H.; Wang, F.; Li, B.; Yang, L.; Deng, Y.; Zhang, X. A hydrolytically stable amino-functionalized Zinc(II) metal-organic framework containing nanocages for selective gas adsorption and luminescent sensing. Micropor. Mesopor. Mat. 2021, 326, 111396. [Google Scholar] [CrossRef]
- Zhang, Q.; Lei, M.; Kong, F.; Yang, Y. A water-stable homochiral luminescent MOF constructed from an achiral acylamide-containing dicarboxylate ligand for enantioselective sensing of penicillamine. Chem. Commun. 2018, 54, 10901–10904. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lei, M.; Zhang, T.; Zhang, Q.; Zhang, R.; Yang, M. A water-stable multi-responsive luminescent Zn-MOF sensor for detecting TNP, NZF and Cr2O72− in aqueous media. Dalton Trans. 2021, 50, 3816–3824. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.A.; Mullangi, D.; Deng, Z.; Wang, Y.; Peh, S.B.; Wei, F.; Wang, J.; Brown, C.M.; Zhao, D.; Canepa, P.; et al. Aluminum formate, Al(HCOO)3: An earth-abundant, scalable, and highly selective material for CO2 capture. Sci. Adv. 2022, 8, ade1473. [Google Scholar] [CrossRef] [PubMed]
- Mullangi, D.; Evans, H.A.; Yildirim, T.; Wang, Y.; Deng, Z.; Zhang, Z.; Mai, T.T.; Wei, F.; Wang, J.; Walker, A.R.H.; et al. Noncryogenic Air Separation Using Aluminum Formate Al(HCOO)3 (ALF). J. Am. Chem. Soc. 2023, 145, 9850–9856. [Google Scholar] [CrossRef] [PubMed]
- Maity, R.; Chakraborty, D.; Nandi, S.; Yadav, A.K.; Mullangi, D.; Vinod, C.P.; Vaidhyanathan, R.V. Aqueous-Phase Differentiation and Speciation of Fe3+ and Fe2+ Using Water-Stable Photoluminescent Lanthanide-Based Metal–Organic Framework. ACS Appl. Nano Mater. 2019, 2, 5169–5178. [Google Scholar] [CrossRef]
- Zhou, Y.-N.; Wang, L.; Yu, J.-H.; Ding, T.-Y.; Zhang, X.; Jiao, C.-Q.; Li, X.; Sun, Z.-G.; Zhu, Y.-Y. Two Stable Cd-MOFs as Dual-Functional Materials with Luminescent Sensing of Antibiotics and Proton Conduction. Inorg. Chem. 2022, 61, 20111–20122. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.R.; Zhao, Y.; Zhou, Z.; Yang, X.G.; Ma, L.F. Neutral ligand TIPA-based two 2D metal–organic frameworks: Ultrahigh selectivity of C2H2/CH4 and efficient sensing and sorption of Cr(VI). Dalton Trans. 2018, 47, 3725–3732. [Google Scholar] [CrossRef]
- Dong, B.X.; Pan, Y.M.; Liu, W.L.; Teng, Y.L. An ultrastable luminescent metal-organic framework for selective sensing of nitroaromatic compounds and nitroimidazole based drug molecules. Cryst. Growth. Des. 2018, 18, 431–440. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, Q.; Zhang, G. A carbazole-functionalized metal–organic framework for efficient detection of antibiotics, pesticides and nitroaromatic compounds. Dalton Trans. 2019, 48, 2683–2691. [Google Scholar] [CrossRef]
- Zhu, Q.Q.; Zhou, Q.S.; Zhang, H.W.; Zhang, W.W.; Lu, D.Q.; Guo, M.T.; Yuan, Y.; Sun, F.; He, H. Design and construction of a metal–organic framework as an efficient luminescent sensor for detecting antibiotics. Inorg. Chem. 2020, 59, 1323–1331. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, H.; Lv, H.; Li, Q.; Zhang, X. Nanochannel-based heterometallic {ZnIIHoIII}–organic framework with high catalytic activity for the chemical fixation of CO2. RSC Adv. 2021, 11, 9731–9739. [Google Scholar] [CrossRef]






| MOFs | Detection Signal | KE (M−1) | LODs | Reference |
|---|---|---|---|---|
| [Co3(ndc)(HCOO)3(μ3-OH)] | 421 nm | 1.219 × 103 | 4.6 ppm | [33] |
| [Ni(H2TPTA)(bimb)] | 441 nm | 8.22 × 103 | 4.1 ppm | [34] |
| ZnHoMOF | 406 nm | 2.074 × 103 | 0.7 ppm | This work |
| Added (μM) | Found (μM) | RSD (%) | Recovery (%) |
|---|---|---|---|
| 50 | 50.63 | 2.23 | 96.7 |
| 100 | 102.19 | 1.68 | 99.8 |
| 150 | 153.87 | 1.53 | 101.0 |
| 200 | 198.73 | 2.56 | 98.2 |
| MOFs | Analyte | KQ (M−1) | LODs | Reference |
|---|---|---|---|---|
| [Cd(tptc)0.5(bpz)] | NFT | 3.87 × 104 | 0.06 ppm | [46] |
| [Cd(tptc)0.5(bpy)] | NFT | 7.63 × 103 | 0.18 ppm | [46] |
| [Cd10(DDB)4(bpz)8] | NFT | 1.88 × 105 | 0.02 ppm | [47] |
| [Zn(tptc)0.5(bimb)] | NFT | 1.273 × 105 | 0.02 ppm | [48] |
| [Zn(NH2−TCB)] | NFT | 6.59 × 104 | 0.07 ppm | [49] |
| ZnHoMOF | NFT | 1.143 × 105 | 0.04 ppm | This work |
| [Cd10(DDB)4(bpz)8] | NFZ | 1.05 × 105 | 0.03 ppm | [47] |
| [Zn (tptc)0.5(bimb)] | NFZ | 9.556 × 104 | 0.03 ppm | [48] |
| [Zn(NH2−TCB)] | NFZ | 4.85 × 104 | 0.06 ppm | [49] |
| ZnHoMOF | NFZ | 5.406 × 104 | 0.05 ppm | This work |
| Analyte | Added (μM) | Found (μM) | RSD (%) | Recovery (%) |
|---|---|---|---|---|
| NFT | 1 | 1.03 | 2.04 | 103.0 |
| 2 | 1.99 | 1.88 | 99.5 | |
| 3 | 2.98 | 2.15 | 99.9 | |
| 4 | 3.97 | 2.16 | 99.3 | |
| 5 | 5.02 | 2.12 | 100.4 | |
| NFZ | 1 | 1.05 | 2.05 | 105.0 |
| 2 | 1.97 | 1.87 | 98.5 | |
| 3 | 2.96 | 2.17 | 98.7 | |
| 4 | 3.95 | 2.13 | 98.8 | |
| 5 | 5.04 | 2.08 | 100.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, J.; Li, W.; Li, W.; Liu, L.; Zhao, D.; Liu, X.; Hu, T.; Fan, L. Heterometallic ZnHoMOF as a Dual-Responsive Luminescence Sensor for Efficient Detection of Hippuric Acid Biomarker and Nitrofuran Antibiotics. Molecules 2023, 28, 6274. https://doi.org/10.3390/molecules28176274
Yin J, Li W, Li W, Liu L, Zhao D, Liu X, Hu T, Fan L. Heterometallic ZnHoMOF as a Dual-Responsive Luminescence Sensor for Efficient Detection of Hippuric Acid Biomarker and Nitrofuran Antibiotics. Molecules. 2023; 28(17):6274. https://doi.org/10.3390/molecules28176274
Chicago/Turabian StyleYin, Jingrui, Wenqian Li, Wencui Li, Liying Liu, Dongsheng Zhao, Xin Liu, Tuoping Hu, and Liming Fan. 2023. "Heterometallic ZnHoMOF as a Dual-Responsive Luminescence Sensor for Efficient Detection of Hippuric Acid Biomarker and Nitrofuran Antibiotics" Molecules 28, no. 17: 6274. https://doi.org/10.3390/molecules28176274
APA StyleYin, J., Li, W., Li, W., Liu, L., Zhao, D., Liu, X., Hu, T., & Fan, L. (2023). Heterometallic ZnHoMOF as a Dual-Responsive Luminescence Sensor for Efficient Detection of Hippuric Acid Biomarker and Nitrofuran Antibiotics. Molecules, 28(17), 6274. https://doi.org/10.3390/molecules28176274







