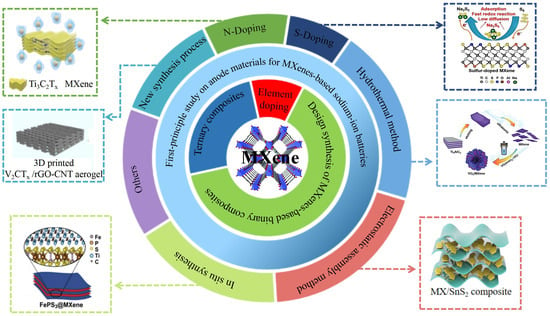
Design and Synthesis Strategy of MXenes-Based Anode Materials for Sodium-Ion Batteries and Progress of First-Principles Research
Abstract
:1. Introduction
2. Design and Synthesis Strategy of Anode Materials for MXenes-Based Sodium-Ion Batteries
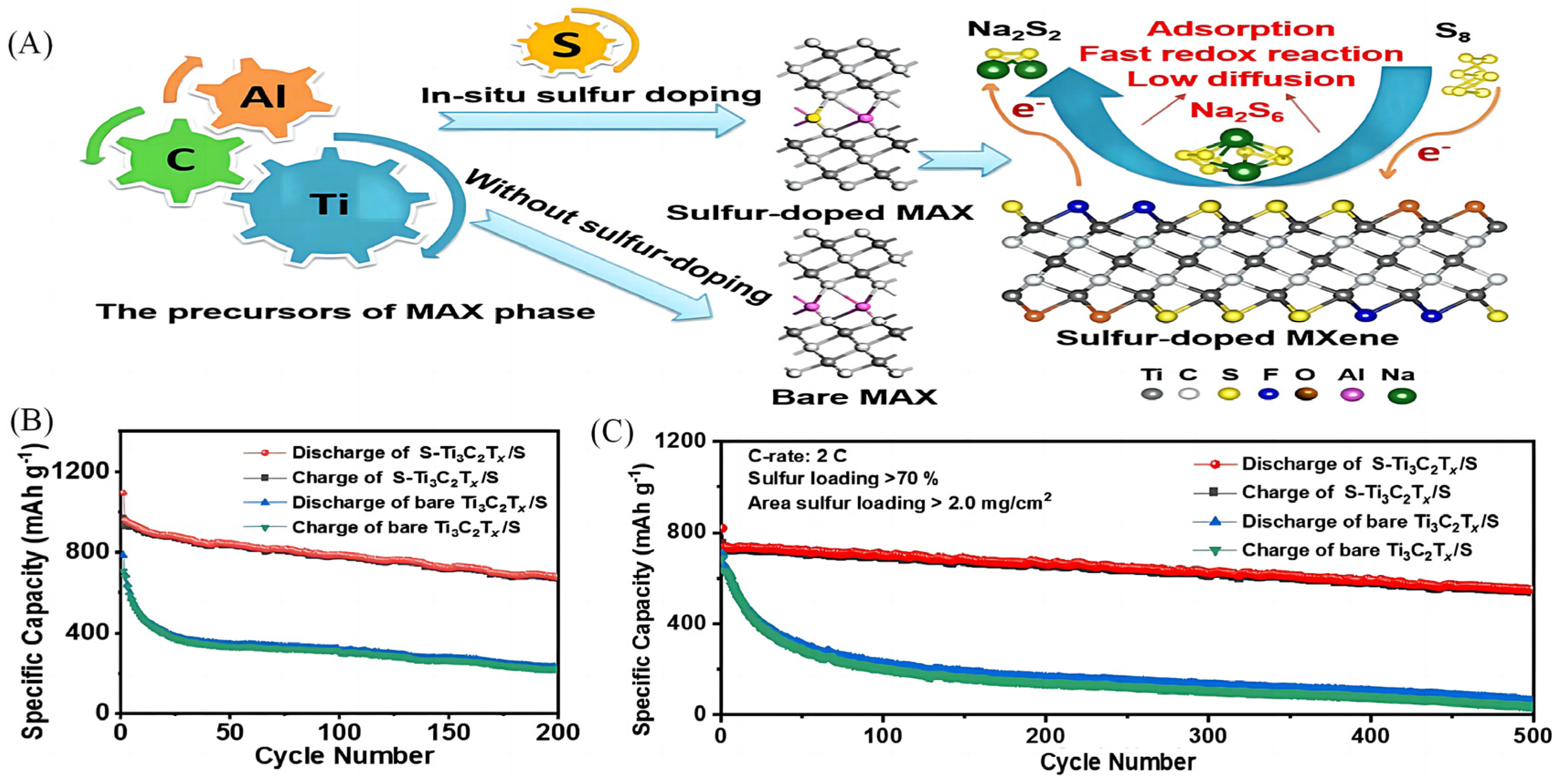
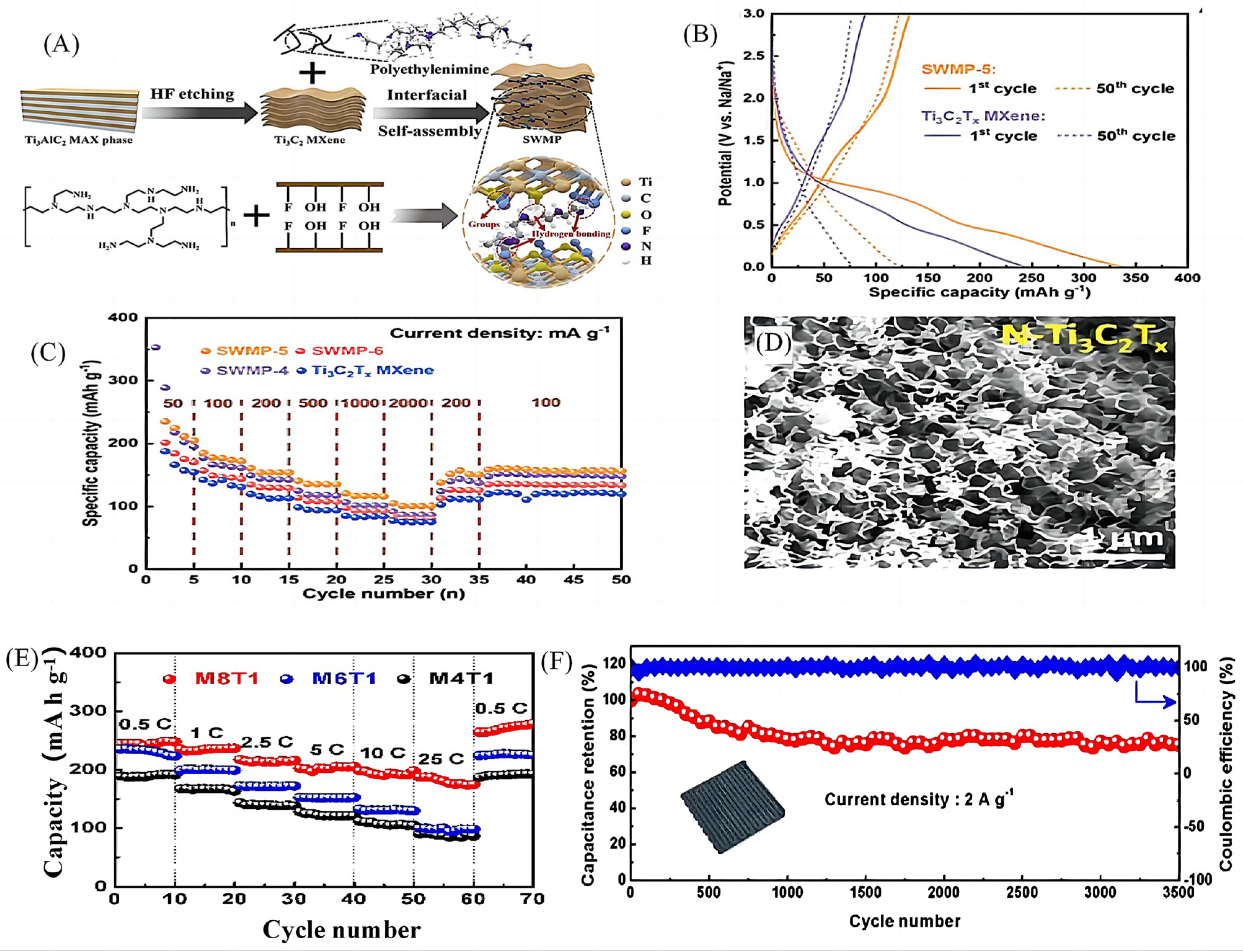
2.1. Design and Synthesis of Elementally-Doped MXenes-Based Materials
2.2. Design of MXenes-Based Binary Composite Synthesis
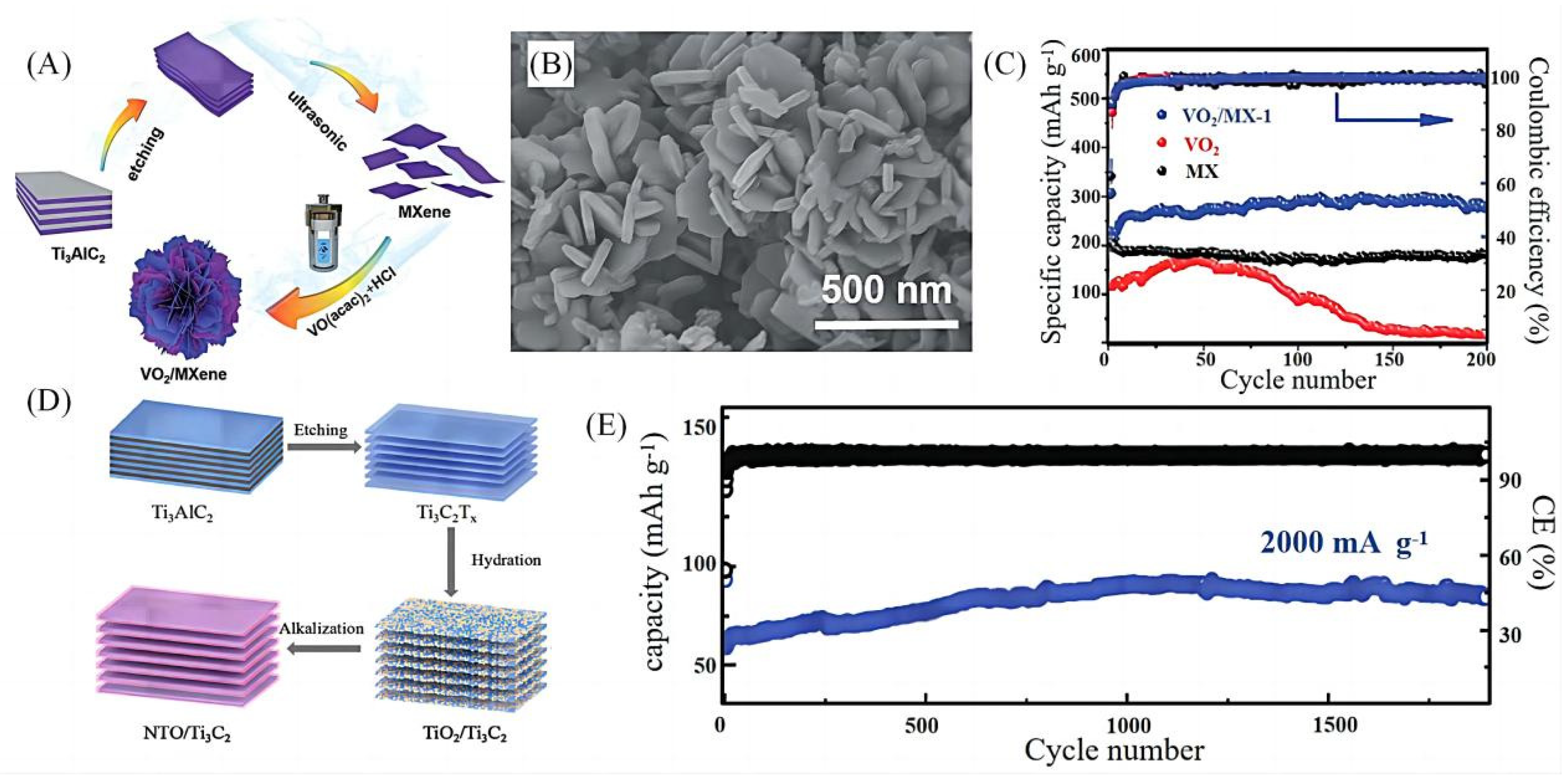
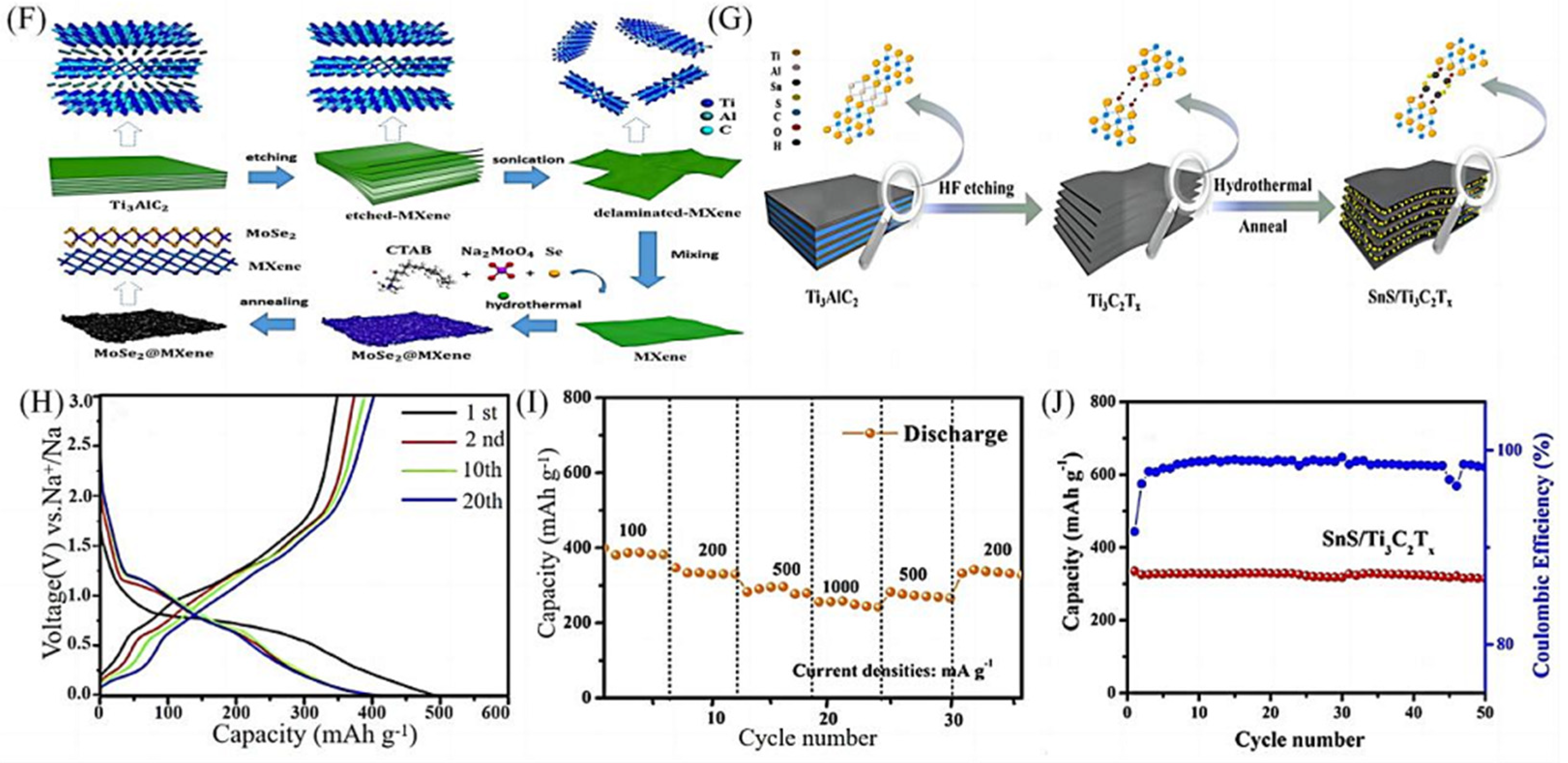
2.2.1. Hydrothermal Methods
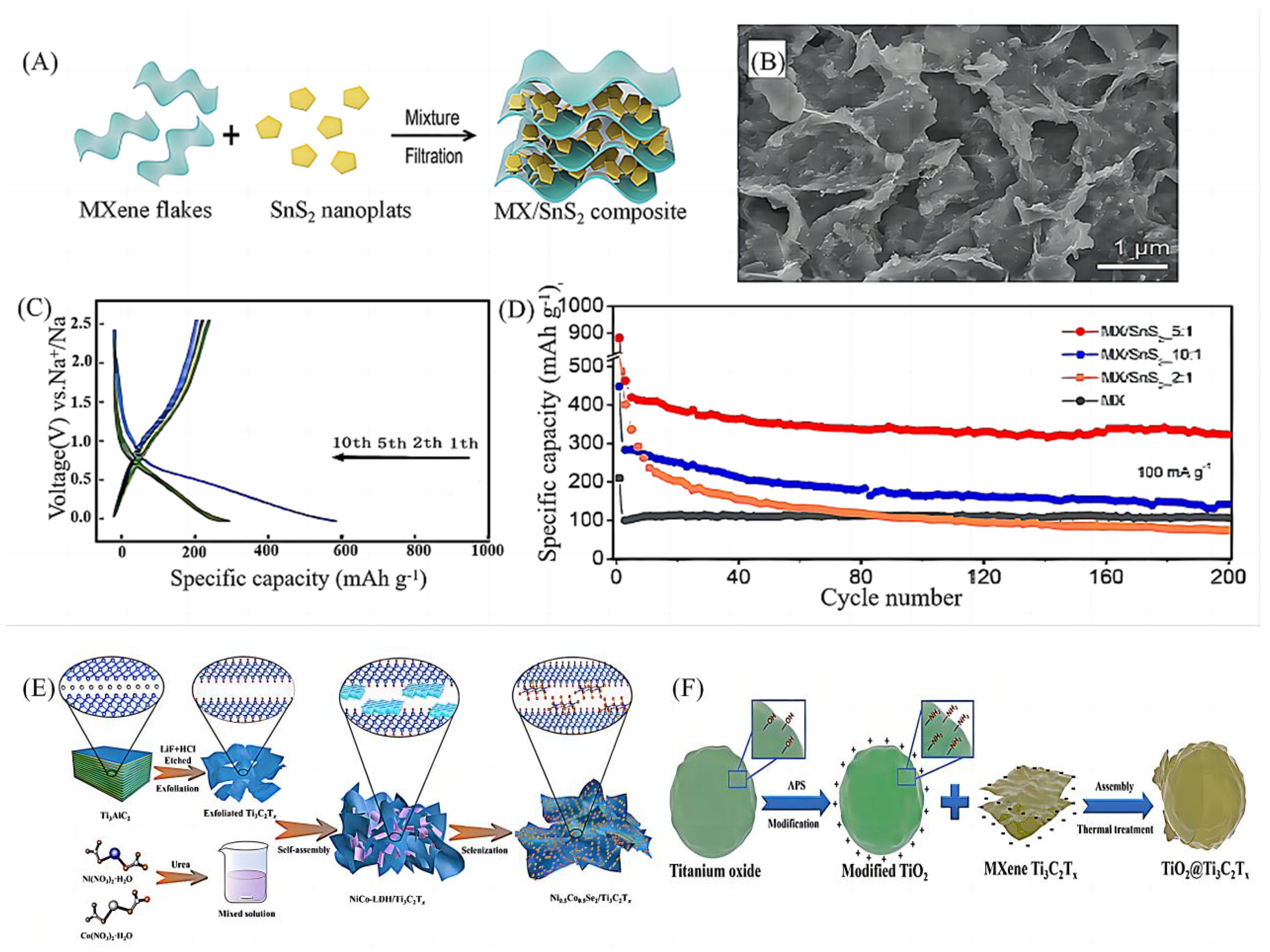
2.2.2. Electrostatic Assembly Method
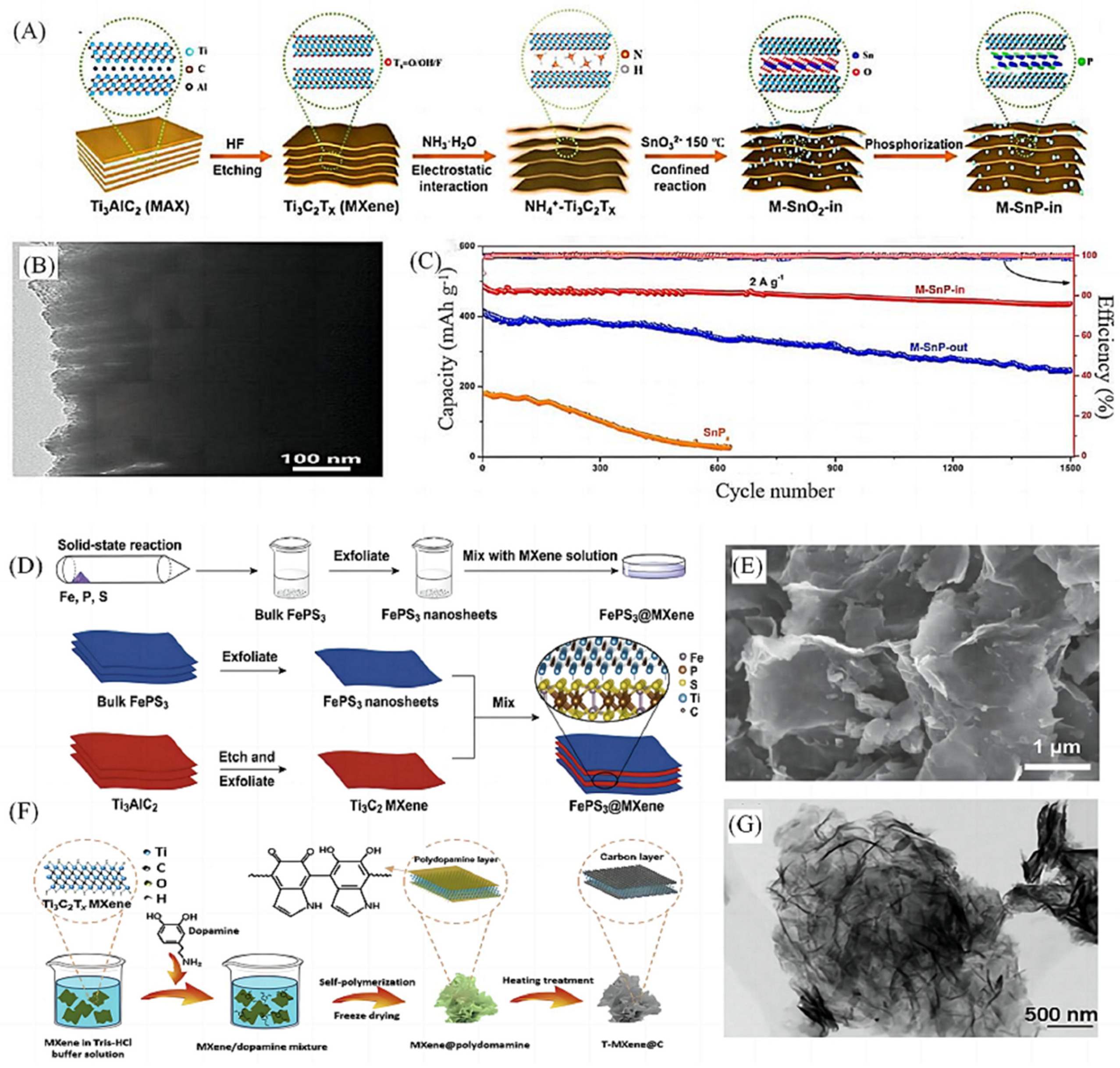
2.2.3. In Situ Synthesis Method
2.2.4. Other Synthesis Strategies
2.3. Design and Synthesis of MXene-Based Ternary Composites
| Serial Number | Material Composition | Preparation Method | First Charge (mAh g−1) | First Discharge (mAh g−1) | Cycling Performance | Rate Capability | References |
|---|---|---|---|---|---|---|---|
| 1 | S-doped Ti3C2Tx | Vacuum freeze drying | 821.7 | 970 | 577 mAh g−1 after 500 cycles | [17] | |
| 2 | S-doped Ti3C2Tx | Electrostatic self-assembly | 325 | 135 mAh g−1 after 1000 cycles at 2.0 A g−1 | 136.6 mAh g−1 at 5 A g−1 | [18] | |
| 3 | S-doped mesoporous Ti3C2Tx film | Electrostatic self-assembly | 354.6 | 345.6 mAh cm−3 after 5000 cycles at 1 A g−1 | 129.2 m Ah g−1 at 1 A g−1 | [20] | |
| 4 | N-doped Ti3C2Tx | Electrostatic self-assembly | 132.6 | 338.5 | 123.4 mAh g−1 after 5000 cycles at 1 A g−1 | 120 mAh g−1 at 1 A g−1 | [24] |
| 5 | N-doped Ti3C2Tx | Electrostatic self-assembly | 1844.7 | 284.2 mAh g−1 after 1000 cycles at 5.0 C | 180.5 mAh g−1at 25 C | [25] | |
| 6 | VO2/MXenes | Hydrothermal method | 229.2 | 280.9 mAh g−1 after 200 cycles at 0.1 A g−1 | 206 mAh g−1 at 1.6 A g−1 | [29] | |
| 7 | NaTi8O13/NaTiO2 | Two-step hydrothermal method | 125 | 162 | 82 mAh g−1 after 1900 cycles at 2.0 A g−1 | 143 mAh g−1 at 0.1 A g−1 | [30] |
| 8 | MoSe2/MXene | Hydrothermal method combined with thermal annealing process | 578 | 826 | 384 mAh g−1 after 400 cycles at 2.0A g−1 | 490 mAh g−1 at 1.0A g−1 | [31] |
| 9 | SnS/Ti3C2Tx | Hydrothermal method combined with thermal annealing process | 348.4 | 495.0 | 320 mAg−1 after 50 cycles at 500 mAg−1 | 255.9 mAh g−1 at 1000 mA g−1 | [32] |
| 10 | CoNiO2/MXene | Hydrothermal method combined with thermal annealing process | 463 | 223 mAh g−1 after 140 cycles at 0.1 A g−1 | 188 mAh g−1 at 0.3 A g−1 | [33] | |
| 11 | VO2-NTs/Ti3C2 | Electrostatic self-assembly | 1164 | 2132 | 516 mAh g−1 after 2000 cycles at 5.0 A g−1 | 703 mAh g−1 at 10.0 A g−1 | [35] |
| 12 | Ti3C2Tx/CNT | Electrostatic self-assembly | 179 | 501 | 120 mAh g−1 after 500 cycles at 0.1 A g−1 | [36] | |
| 13 | PDDA-BP/Ti3C2 | Electrostatic self-assembly | 1780 | 2588 | 1112 mAh g−1 after 500 cycles at 0.1 A g−1 | 560 mAh g−1 at 1 A g−1 | [37] |
| 14 | TiO2@Ti3C2Tx | Electrostatic self-assembly | 233.9 | 497.1 | 116 mAh g−1 after 5000 cycles at 0.96 A g−1 | 177 mAh g−1 at 0.12 A g−1 | [40] |
| 15 | M-SnP-In | In situ synthesis | 438.2 | 436.6 mAhg−1 after 1500 cycles at 2 Ag−1 | 438.2 mAhg−1 at 15 A g−1 | [41] | |
| 16 | T-MXene@C | In situ synthesis | 580.6 | 499.4 mAh g−1 after 200 cycles at 0.2 C | 478 mAh g−1 at 0.2 C (1 C = 320 mA g−1) | [44] | |
| 17 | a-VOx/V2C | In situ synthesis | 161 | 54 mAh g−1 after 1800 cycles at 2000 mA g−1 | 96 mAh g−1 at 2 Ah g−1 | [48] | |
| 18 | Cu1.75Se—MXene—CNRib | Microbial electrostatic assembly | 744.2 | 1353.1 | 305.6 mAh g−1 after 400 cycles at 1.0 A g−1 | 435.3, 356.2, 315.7, 274.3, 232.6, and 161.3 mAh g−1 at 0.1 to 5.0 A g−1 | [49] |
| 19 | Nb2CTx/MoS2/CS | Electrostatic self-assembly method + template method | 1270 | 526 mAh g−1 after 100 cycles at 0.1 A g−1 394 mAh g−1 after 900 cycles at 1 A g−1 | 196 mAh g−1 at 20 A g−1 | [50] | |
| 20 | MXene@CoS2/NC | In situ growth method + template method | 660 | 885 | 620 mAhg−1 after 200 cycles at 0.2 A g−1 | 708, 614, 551, 438, and 394 mAh g−1 at 0.2, 0.5, 1, 2, and 5 A g−1 | [51] |
3. First-Principles Study of Anode Materials for MXenes-Based Sodium-Ion Batteries
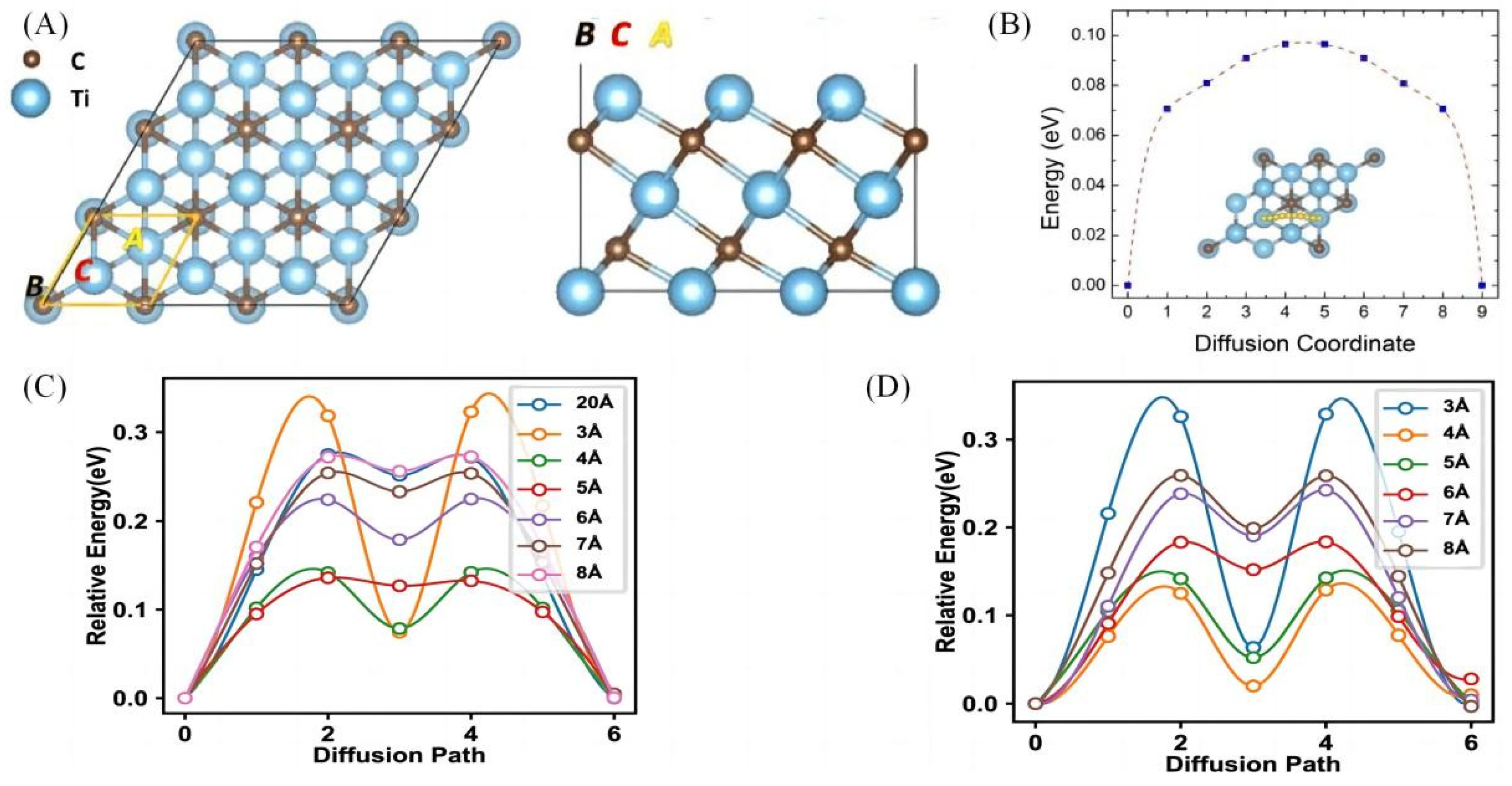
3.1. MXenes Theoretical Computational Study of the Electronic Structure of the Material
3.2. Theoretical Computational Study of Elementally Doped MXenes Materials
3.3. Theoretical Computational Study of MXenes Matrix Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Li, Y.; Guo, J.; Pedersen, K.; Gurevich, L.; Stroe, D.-I. Investigation of multi-step fast charging protocol and aging mechanism for commercial NMC/graphite lithium-ion batteries. J. Energy Chem. 2023, 80, 237–246. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zhang, L.; Tao, H.; Li, Q.; Zhang, J.; Yang, X. Lithiophilicity: The key to efficient lithium metal anodes for lithium batteries. J. Energy Chem. 2023, 77, 123–136. [Google Scholar] [CrossRef]
- Wang, P.; Guo, Y.-J.; Chen, W.-P.; Duan, H.; Ye, H.; Yao, H.-R.; Yin, Y.-X.; Cao, F.-F. Self-supported hard carbon anode from fungus-treated basswood towards sodium-ion batteries. Nano Res. 2022, 16, 3832–3838. [Google Scholar] [CrossRef]
- Tong, Y.; Wu, Y.; Liu, Z.; Yin, Y.; Sun, Y.; Li, H. Fabricating multi-porous carbon anode with remarkable initial coulombic efficiency and enhanced rate capability for sodium-ion batteries. Chin. Chem. Lett. 2023, 34, 107443. [Google Scholar] [CrossRef]
- Zhou, D.; Ning, D.; Wang, J.; Liu, J.; Zhang, G.; Xiao, Y.; Zheng, J.; Li, Y.; Li, J.; Liu, X. Clarification of underneath capacity loss for O3-type Ni, co free layered cathodes at high voltage for sodium ion batteries. J. Energy Chem. 2023, 77, 479–486. [Google Scholar] [CrossRef]
- Xu, Y.; Xiong, W.; Huang, J.; Tang, X.; Wang, H.; Liu, W.; Xiao, D.; Guo, Y.; Zhang, Y. Pressure-induced growth of coralloid-like FeF2 nanocrystals to enable high-performance conversion cathode. J. Energy Chem. 2023, 79, 291–300. [Google Scholar] [CrossRef]
- Liu, C.; Lu, Q.; Gorbunov, M.V.; Omar, A.; Gonzalez Martinez, I.G.; Zhao, P.; Hantusch, M.; Permana, A.D.C.; He, H.; Gaponik, N.; et al. Ultrasmall CoS nanoparticles embedded in heteroatom-doped carbon for sodium-ion batteries and mechanism explorations via synchrotron X-ray techniques. J. Energy Chem. 2023, 79, 373–381. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366, 969–981. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Gogotsi, Y. Two-dimensional heterostructures for energy storage. Nat. Energy 2017, 2, 17089. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Mathis, T.S.; Sarycheva, A.; Hatter, C.B.; Uzun, S.; Levitt, A.; Gogotsi, Y. Selective Etching of Silicon from Ti(3)SiC(2) (MAX) To Obtain 2D Titanium Carbide (MXene). Angew Chem. Int. Ed. Engl. 2018, 57, 5444–5448. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Q.; Zhu, K.; Ye, K.; Wang, G.; Cao, D.; Yan, J. 3D Porous Oxidation-Resistant MXene/Graphene Architectures Induced by In Situ Zinc Template toward High-Performance Supercapacitors. Adv. Funct. Mater. 2021, 31, 2101087. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotis, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Zhan, C.; Naguib, M.; Lukatskaya, M.; Kent, P.R.C.; Gogotsi, Y.; Jiang, D.E. Understanding the MXene Pseudocapacitance. Phys. Chem. Lett. 2018, 9, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Dall’Agnese, Y.; Naguib, M.; Gogotis, Y.; Barsoum, M.W.; Zhuang, H.L.; Kent, P.R.C. Prediction and Characterization of MXene Nanosheet Anodes for Non-Lithium-Ion Batteries. ACS Nano 2014, 8, 9606–9615. [Google Scholar] [CrossRef]
- Ma, P.; Fang, D.; Liu, Y.; Shang, Y.; Shi, Y.; Yang, H.Y. MXene-Based Materials for Electrochemical Sodium-Ion Storage. Adv. Sci. 2021, 8, 2003185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sun, S.; Lin, J.; Zhong, L.; Chen, L.; Guo, J.; Yin, J.; Alshareef, H.N.; Qiu, X.; Zhang, W. Defect Engineering of Disordered Carbon Anodes with Ultra-High Heteroatom Doping Through a Supermolecule-Mediated Strategy for Potassium-Ion Hybrid Capacitors. Nano-Micro Lett. 2023, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Shuck, C.E.; Zhang, W.; Guo, X.; Gogotsi, Y.; Wang, G. Boosting Performance of Na-S Batteries Using Sulfur-Doped Ti(3)C(2)T(x) MXene Nanosheets with a Strong Affinity to Sodium Polysulfides. ACS Nano 2019, 13, 11500–11509. [Google Scholar] [CrossRef]
- Sun, S.; Xie, Z.; Yan, Y.; Wu, S. Hybrid energy storage mechanisms for sulfur-decorated Ti3C2 MXene anode material for high-rate and long-life sodium-ion batteries. Chem. Eng. J. 2019, 366, 460–467. [Google Scholar] [CrossRef]
- Li, J.; Yan, D.; Hou, S.; Li, Y.; Lu, T.; Yao, Y.; Pan, L. Improved sodium-ion storage performance of Ti3C2Tx MXenes by sulfur doping. J. Mater. Chem. A 2018, 6, 1234–1243. [Google Scholar] [CrossRef]
- Li, G.; Lian, S.; Song, F.; Chen, S.; Wu, Z.; Xie, X.; Zhang, N. Surface Chemistry and Mesopore Dual Regulation by Sulfur-Promised High Volumetric Capacity of Ti(3)C(2)T(x) Films for Sodium-Ion Storage. Small 2021, 17, 2103626. [Google Scholar] [CrossRef]
- Lu, C.; Yang, L.; Yan, B.; Sun, L.; Zhang, P.; Zhang, W.; Sun, Z. Nitrogen-Doped Ti3C2 MXene: Mechanism Investigation and Electrochemical Analysis. Adv. Funct. Mater. 2020, 30, 2000852. [Google Scholar] [CrossRef]
- Ji, X.; Chen, X.; Zhang, L.; Meng, C.; He, Y.; Zhang, X.; Wang, Z.; Yu, R. Anchoring nitrogen-doped Co2P nanoflakes on NiCo2O4 nanorod arrays over nickel foam as high-performance 3D electrode for alkaline hydrogen evolution. Green Energy Environ. 2023, 8, 470–477. [Google Scholar] [CrossRef]
- Gao, M.; Li, J.; Wang, Y.; Liang, W.; Yang, Z.; Chen, Y.; Deng, W.; Wang, Z.; Ao, T.; Chen, W. Flexible nitrogen-doped carbon nanofiber-reinforced hierarchical hollow iron oxide nanorods as a binder-free electrode for efficient capacitive deionization. Desalination 2023, 549, 116360. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, W.; Liu, S.; Zhang, T.; Song, Z.; Shao, W.; Mao, R.; Yao, M.; Jian, X.; Hu, F. Highly stable Ti3C2Tx MXene-based sandwich-like structure via interfacial self-assembly of nitrogen-rich polymer network for superior sodium-ion storage performance. Chem. Eng. J. 2023, 451, 138763. [Google Scholar] [CrossRef]
- Fan, Z.; Wei, C.; Yu, L.; Xia, Z.; Cai, J.; Tian, Z.; Zou, G.; Dou, S.; Sun, J. 3D Printing of Porous Nitrogen-Doped Ti3C2 MXene Scaffolds for High-Performance Sodium-Ion Hybrid Capacitors. ACS Nano 2020, 14, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Yu, S.; Choi, W.; Kim, S.-O. Hierarchically Designed Nitrogen-Doped MoS2/Silicon Oxycarbide Nanoscale Heterostructure as High-Performance Sodium-Ion Battery Anode. Acs Nano 2021, 15, 7409–7420. [Google Scholar] [CrossRef]
- Xia, Q.; Liang, Y.; Lin, Z.; Wang, S.; Lai, W.; Yuan, D.; Dou, Y.; Gu, Q.; Wang, J.; Liu, H.; et al. Confining Ultrathin 2D Superlattices in Mesoporous Hollow Spheres Renders Ultrafast and High-Capacity Na-Ion Storage. Adv. Energy Mater. 2020, 10, 2001033. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, Y.; Zhao, Z.; Yuan, C.; Lou, X. A Ternary Fe1-xS@Porous Carbon Nanowires/Reduced Graphene Oxide Hybrid Film Electrode with Superior Volumetric and Gravimetric Capacities for Flexible Sodium Ion Batteries. Adv. Energy Mater. 2019, 9, 1803052. [Google Scholar] [CrossRef]
- Wu, F.; Jiang, Y.; Ye, Z.; Huang, Y.; Wang, Z.; Li, S.; Mei, Y.; Xie, M.; Li, L.; Chen, R. A 3D flower-like VO2/MXene hybrid architecture with superior anode performance for sodium ion batteries. J. Mater. Chem. A 2019, 7, 1315–1322. [Google Scholar] [CrossRef]
- Sun, X.; Tan, K.; Liu, Y.; Zhang, J.; Hou, L.; Yuan, C. In-situ growth of hybrid NaTi8O13/NaTiO2 nanoribbons on layered MXene Ti3C2 as a competitive anode for high-performance sodium-ion batteries. Chin. Chem. Lett. 2020, 31, 2254–2258. [Google Scholar] [CrossRef]
- Xu, E.; Zhang, Y.; Wang, H.; Zhu, Z.; Quan, J.; Chang, Y.; Li, P.; Yu, D.; Jiang, Y. Ultrafast kinetics net electrode assembled via MoSe2/MXene heterojunction for high-performance sodium-ion batteries. Chem. Eng. J. 2020, 385, 123839. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, B.; Hu, L.; Xu, Q.; Li, Y.; Liu, D.; Xu, M. Synthesis of SnS nanoparticle-modified MXene (Ti3C2Tx) composites for enhanced sodium storage. J. Alloys Compd. 2018, 732, 448–453. [Google Scholar] [CrossRef]
- Tao, M.; Zhang, Y.; Zhan, R.; Guo, B.; Xu, Q.; Xu, M. A chemically bonded CoNiO2 nanoparticles/MXene composite as anode for sodium-ion batteries. Mater. Lett. 2018, 230, 173–176. [Google Scholar] [CrossRef]
- Wang, J.; Du, C.F.; Xue, Y.; Tan, X.; Kang, J.; Gao, Y.; Yu, H.; Yan, Q. MXenes as a versatile platform for reactive surface modification and superior sodium-ion storages. Exploration 2021, 1, 20210024. [Google Scholar] [CrossRef]
- Liu, H.; He, P.; Cao, J.; Duan, T.; Kong, Q.; Yao, W. Multidimensional VO2 nanotubes/Ti3C2 MXene composite for efficient electrochemical lithium/sodium-ion storage. J. Power Sources 2022, 521, 230946. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, M.-Q.; Anasori, B.; Maleski, K.; Ren, C.E.; Li, J.; Byles, B.W.; Pomerantseva, E.; Wang, G.; Gogotsi, Y. Porous heterostructured MXene/carbon nanotube composite paper with high volumetric capacity for sodium-based energy storage devices. Nano Energy 2016, 26, 513–523. [Google Scholar] [CrossRef]
- Zhao, R.; Qian, Z.; Liu, Z.; Zhao, D.; Hui, X.; Jiang, G.; Wang, C.; Yin, L. Molecular-level heterostructures assembled from layered black phosphorene and Ti3C2 MXene as superior anodes for high-performance sodium ion batteries. Nano Energy 2019, 65, 104037. [Google Scholar] [CrossRef]
- Wu, Y.; Nie, P.; Wu, L.; Dou, H.; Zhang, X. 2D MXene/SnS2 composites as high-performance anodes for sodium ion batteries. Chem. Eng. J. 2018, 334, 932–938. [Google Scholar] [CrossRef]
- Dong, X.; Zhao, R.; Sun, B.; Zhang, T.; Wang, B.; He, Y.; Gao, T.; Chao, D.; Zhou, G. Confining homogeneous Ni0.5Co0.5Se2 nanoparticles in Ti3C2T MXene architectures for enhanced sodium storage performance. Appl. Surf. Sci. 2022, 605, 154847. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, J.; Song, J.; Wu, W.; Liu, H.; Wang, G. MXene encapsulated titanium oxide nanospheres for ultra-stable and fast sodium storage. Energy Storage Mater. 2018, 14, 306–313. [Google Scholar] [CrossRef]
- Tang, J.; Peng, X.; Lin, T.; Huang, X.; Luo, B.; Wang, L. Confining ultrafine tin monophosphide in Ti3C2Tx interlayers for rapid and stable sodium ion storage. eScience 2021, 1, 203–211. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, Y.; Xu, N.; Lian, X.; Li, L.; Hu, Y.; Peng, S. Facile Synthesis of FePS(3) Nanosheets@MXene Composite as a High-Performance Anode Material for Sodium Storage. Nanomicro Lett. 2020, 12, 54. [Google Scholar]
- Ming, K.; Zhang, Z.; Li, H. In situ growth of NaTiO2 nanotubes on Ti3C2Fx for enhanced sodium ion batteries. Mater. Lett. 2022, 309, 131457. [Google Scholar] [CrossRef]
- Zhang, P.; Soomro, R.A.; Guan, Z.; Sun, N.; Xu, B. 3D carbon-coated MXene architectures with high and ultrafast lithium/sodium-ion storage. Energy Storage Mater. 2020, 29, 163–171. [Google Scholar] [CrossRef]
- Zhao, M.-Q.; Trainor, N.; Ren, C.E.; Torelli, M.; Anasori, B.; Gogotsi, Y. Scalable manufacturing of large and flexible sheets of MXene/graphene hetero-structures. Adv. Mater. Technol. 2019, 4, 1800639. [Google Scholar]
- Zhang, T.; Jiang, X.; Li, G.; Yao, Q.; Lee, J.Y. A Red-Phosphorous-Assisted Ball-Milling Synthesis of Few-Layered Ti3C2Tx(MXene) Nanodot Composite. ChemNanoMat 2018, 4, 56–60. [Google Scholar]
- Shen, Y.; Li, Y.; Deng, S.; Pan, G.; Xiong, Q.; Ding, X.; Lu, Y.; Liu, Q.; Xia, X.; Wang, X.; et al. TiC/C core/shell nanowires arrays as advanced anode of sodium ion batteries. Chin. Chem. Lett. 2020, 31, 846–850. [Google Scholar] [CrossRef]
- Zhang, W.; Peng, J.; Hua, W.; Liu, Y.; Wang, J.; Liang, Y.; Lai, W.; Jiang, Y.; Huang, Y.; Zhang, W.; et al. Architecting Amorphous Vanadium Oxide/MXene Nanohybrid via Tunable Anodic Oxidation for High-Performance Sodium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2100757. [Google Scholar] [CrossRef]
- Cao, J.; Li, J.; Li, D.; Yuan, Z.; Zhang, Y.; Shulga, V.; Sun, Z.; Han, W. Strongly Coupled 2D Transition Metal Chalcogenide-MXene-Carbonaceous Nanoribbon Heterostructures with Ultrafast Ion Transport for Boosting Sodium/Potassium Ions Storage. Nanomicro Lett. 2021, 13, 113. [Google Scholar] [CrossRef]
- Yuan, Z.; Cao, J.; Valerii, S.; Xu, H.; Wang, L.; Han, W. MXene-Bonded hollow MoS2/Carbon sphere strategy for high-performance flexible sodium ion storage. Chem. Eng. J. 2022, 430, 132755. [Google Scholar] [CrossRef]
- Li, Q.; Jiao, Q.; Yan, Y.; Li, H.; Zhou, W.; Gu, T.; Shen, X.; Lu, C.; Zhao, Y.; Zhang, Y.; et al. Optimized Co-S bonds energy and confinement effect of hollow MXene@CoS2/NC for enhanced sodium storage kinetics and stability. Chem. Eng. J. 2022, 450, 137922. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Z.; Wang, H.; Li, W.; Wang, B.; Xu, J.; Xu, T.; Zang, J.; Kong, D.; Li, X.; et al. 3D-Printed Sodiophilic V(2)CT(x)/rGO-CNT MXene Microgrid Aerogel for Stable Na Metal Anode with High Areal Capacity. ACS Nano 2022, 16, 9105–9116. [Google Scholar] [CrossRef]
- Fang, Y.; Lian, R.; Li, H.; Zhang, Y.; Gong, Z.; Zhu, K.; Ye, K.; Yan, J.; Wang, G.; Gao, Y.; et al. Induction of Planar Sodium Growth on MXene (Ti(3)C(2)T(x))-Modified Carbon Cloth Hosts for Flexible Sodium Metal Anodes. ACS Nano 2020, 14, 8744–8753. [Google Scholar] [CrossRef] [PubMed]
- Er, D.; Li, J.; Naguib, M.; Gogotsi, Y.; Shenoy, V.B. Ti(3)C(2) MXene as a high capacity electrode material for metal (Li, Na, K, Ca) ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 11173–11179. [Google Scholar] [CrossRef]
- Dai, L.; Zhao, J.; Li, Q.; Chen, M.; Li, H.; Qu, K.; Li, R. Understanding the tunable sodium storage performance in pillared MXenes: A first-principles study. Phys. Chem. Chem. Phys. 2022, 24, 27184–27194. [Google Scholar] [CrossRef]
- Bai, L.-N.; Kong, L.-Y.; Wen, J.; Ma, N.; Gao, H.; Zhang, X.-T. First-principles study of high performance lithium/sodium storage of Ti3C2T2 nanosheets as electrode materials. Chin. Phys. B 2020, 29, 016802. [Google Scholar] [CrossRef]
- Lu, C.; Li, A.; Li, G.; Yan, Y.; Zhang, M.; Yang, Q.; Zhou, W.; Guo, L. S-Decorated Porous Ti(3)C(2) MXene Combined with In Situ Forming Cu(2) Se as Effective Shuttling Interrupter in Na-Se Batteries. Adv. Mater. 2021, 33, 2008414. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, M.; Xu, L.-C.; Zhao, W.; Li, R.; Yang, Z.; Liu, R.-P.; Li, X. Achieving superior high-capacity batteries with the lightest Ti2C MXene anode by first-principles calculations: Overarching role of S-functionate (Ti2CS2) and multivalent cations carrier. J. Power Sources 2020, 451, 227791. [Google Scholar] [CrossRef]
- Liao, S.; Qin, W.; Sun, B.; Wu, M.; Xu, B. Electronic and magnetic properties in nitrogen-d oped 2D titanium carbides (MXenes): Insight from first-principles calculations. Solid State Commun. 2021, 340, 114549. [Google Scholar] [CrossRef]
- Xia, Y.; Que, L.; Yu, F.; Deng, L.; Liang, Z.; Jiang, Y.; Sun, M.; Zhao, L.; Wang, Z. Tailoring Nitrogen Terminals on MXene Enables Fast Charging and Stable Cycling Na-Ion Batteries at Low Temperature. Nanomicro Lett. 2022, 14, 143. [Google Scholar] [CrossRef]
- Kajiyama, S.; Szabova, L.; Sodeyama, K.; Iinuma, H.; Morita, R.; Gotoh, K.; Tateyama, Y.; Okubo, M.; Yamada, A. Sodium-Ion Intercalation Mechanism in MXene Nanosheets. ACS Nano 2016, 10, 3334–3341. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, D.; Zhang, H.; Zhang, J.; Zhao, Y. Design and Synthesis Strategy of MXenes-Based Anode Materials for Sodium-Ion Batteries and Progress of First-Principles Research. Molecules 2023, 28, 6292. https://doi.org/10.3390/molecules28176292
Su D, Zhang H, Zhang J, Zhao Y. Design and Synthesis Strategy of MXenes-Based Anode Materials for Sodium-Ion Batteries and Progress of First-Principles Research. Molecules. 2023; 28(17):6292. https://doi.org/10.3390/molecules28176292
Chicago/Turabian StyleSu, Dan, Hao Zhang, Jiawei Zhang, and Yingna Zhao. 2023. "Design and Synthesis Strategy of MXenes-Based Anode Materials for Sodium-Ion Batteries and Progress of First-Principles Research" Molecules 28, no. 17: 6292. https://doi.org/10.3390/molecules28176292
APA StyleSu, D., Zhang, H., Zhang, J., & Zhao, Y. (2023). Design and Synthesis Strategy of MXenes-Based Anode Materials for Sodium-Ion Batteries and Progress of First-Principles Research. Molecules, 28(17), 6292. https://doi.org/10.3390/molecules28176292