Influence of Block-Copolymers’ Composition as Compatibilizers for Epoxy/Silicone Blends
Abstract
:1. Introduction
2. Results and Discussion
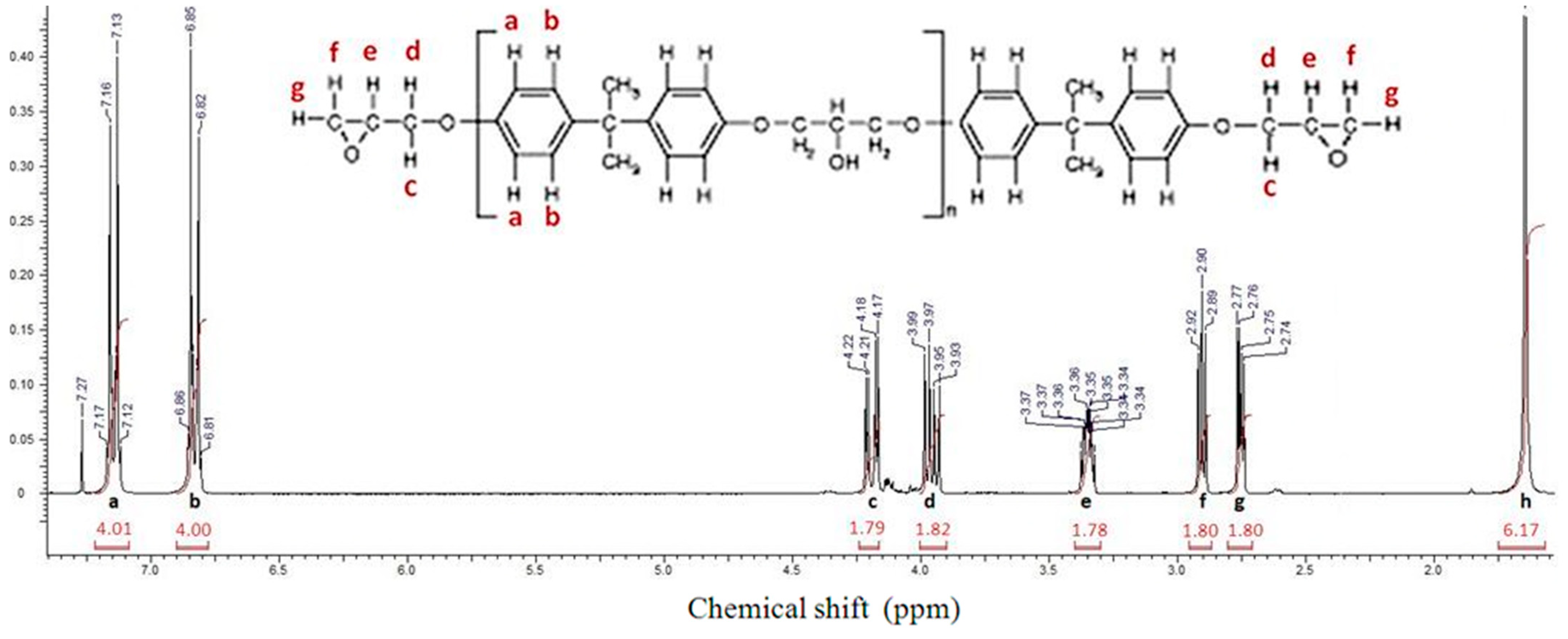
2.1. Determination of the EEW of DGEBA by 1H NMR
2.2. Miscibility Tests of Compatibilizers
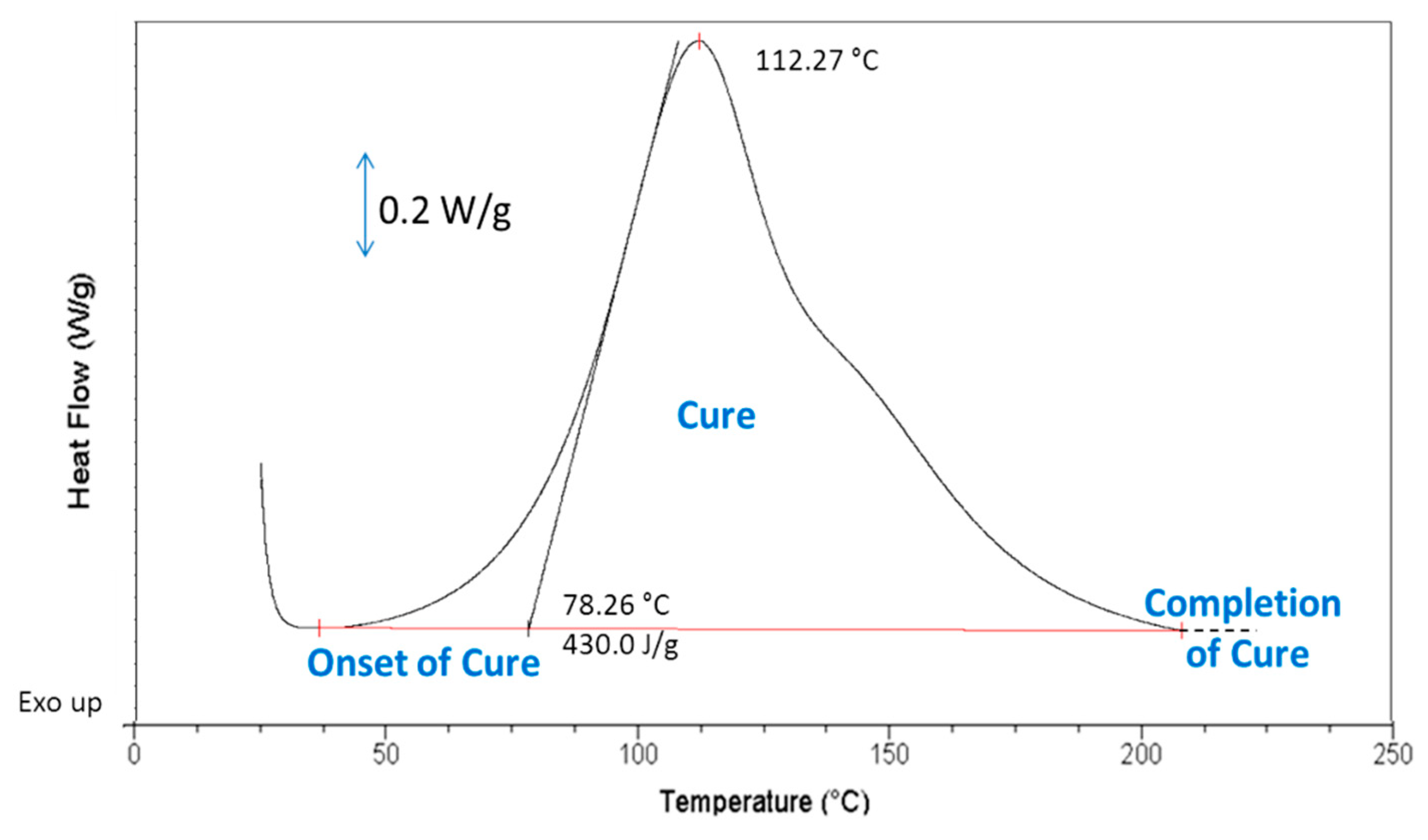
2.3. Curing of Epoxy/Silicone Resins
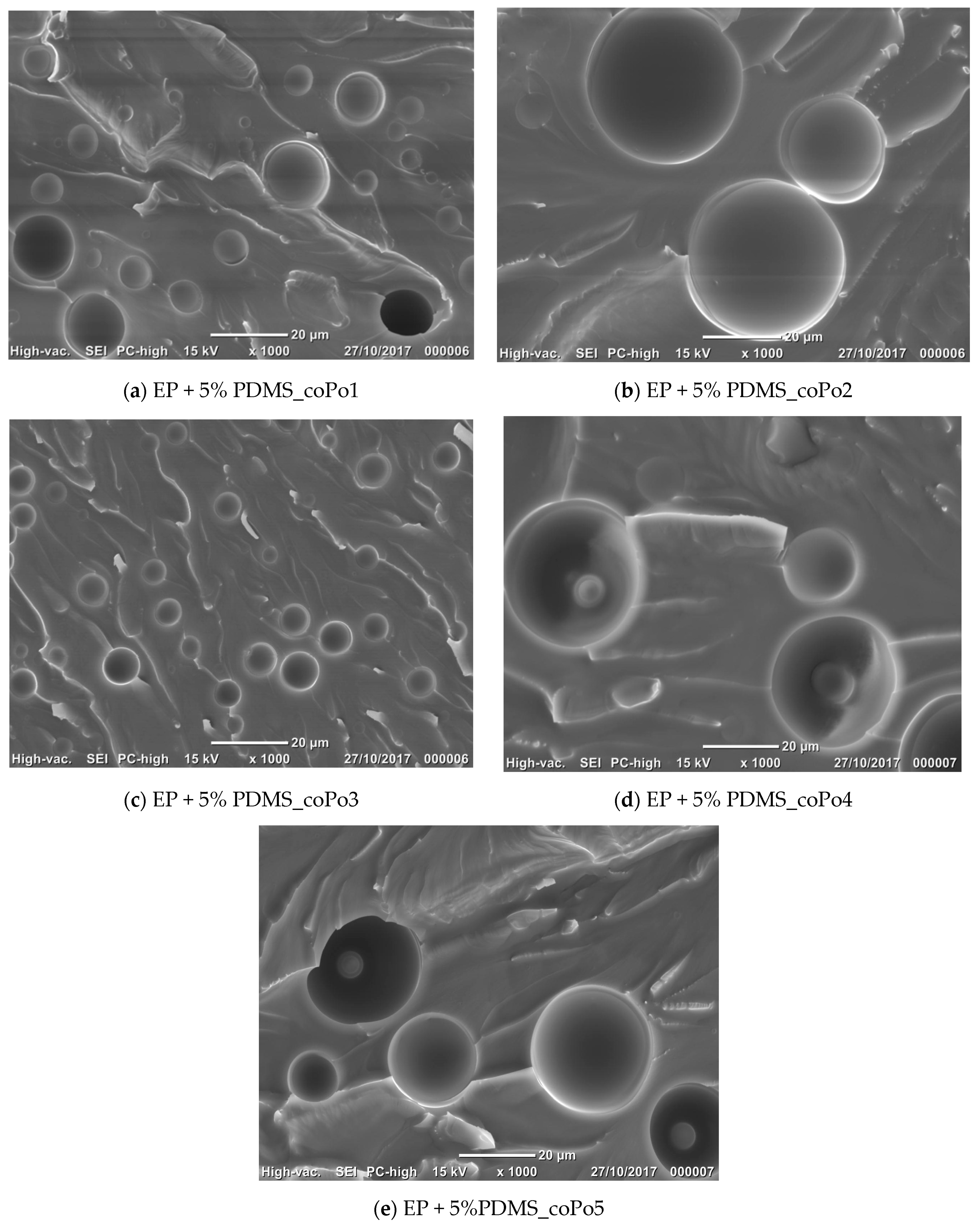
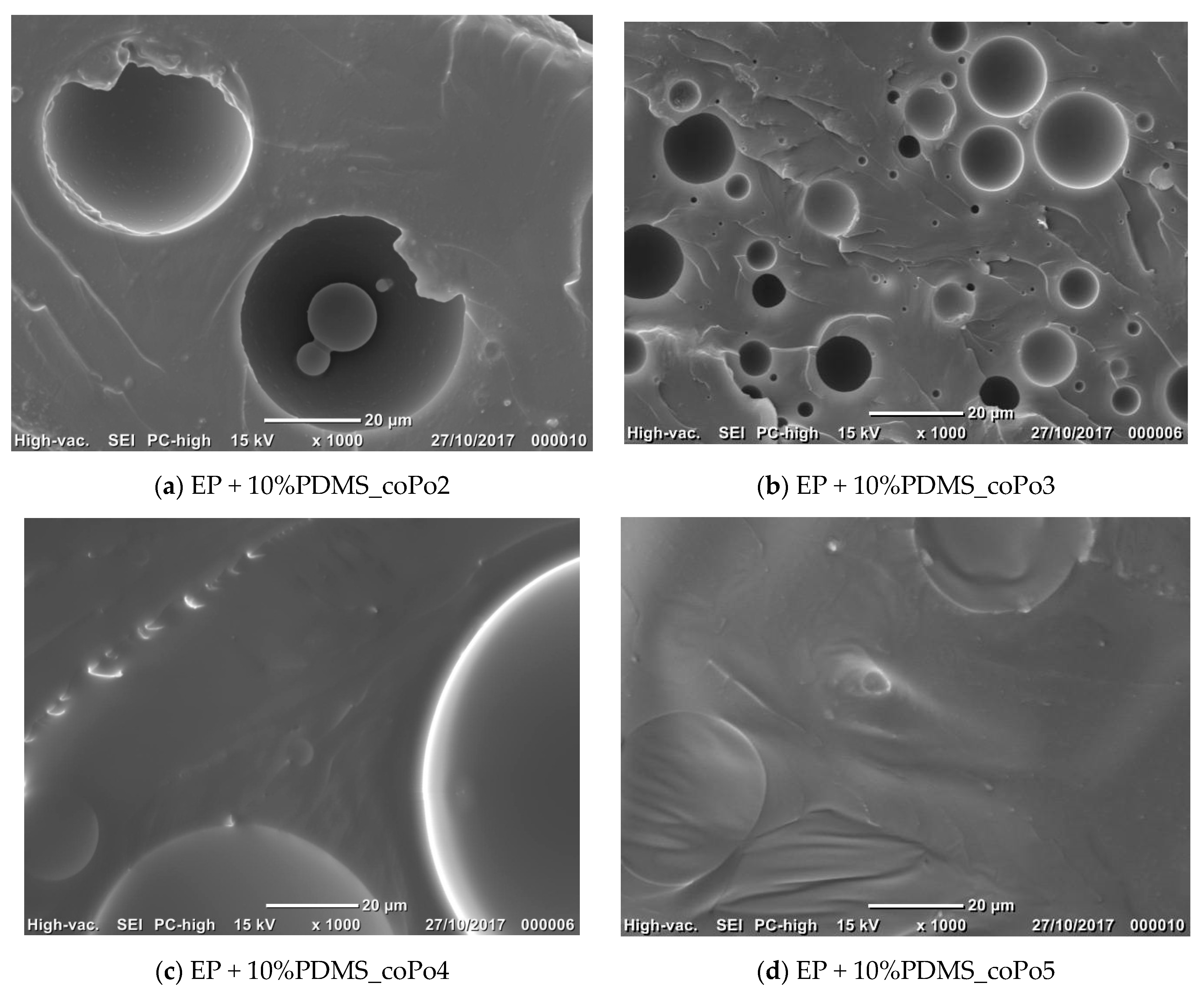
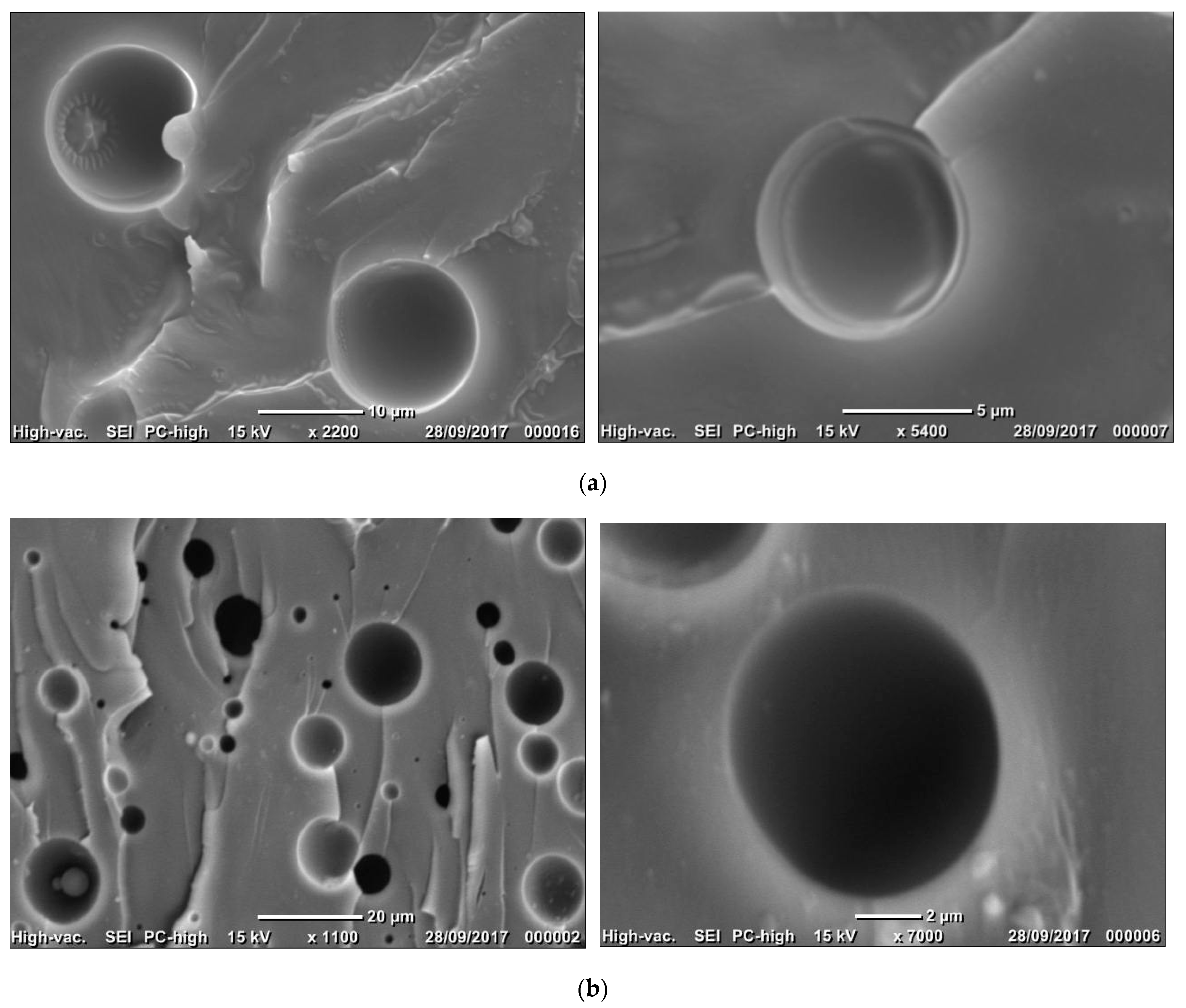
2.4. Morphological Analysis
2.5. Thermal and Dynamic Mechanical Characteristics of Cured Epoxy Formulations
2.6. Contact Angle Measurements
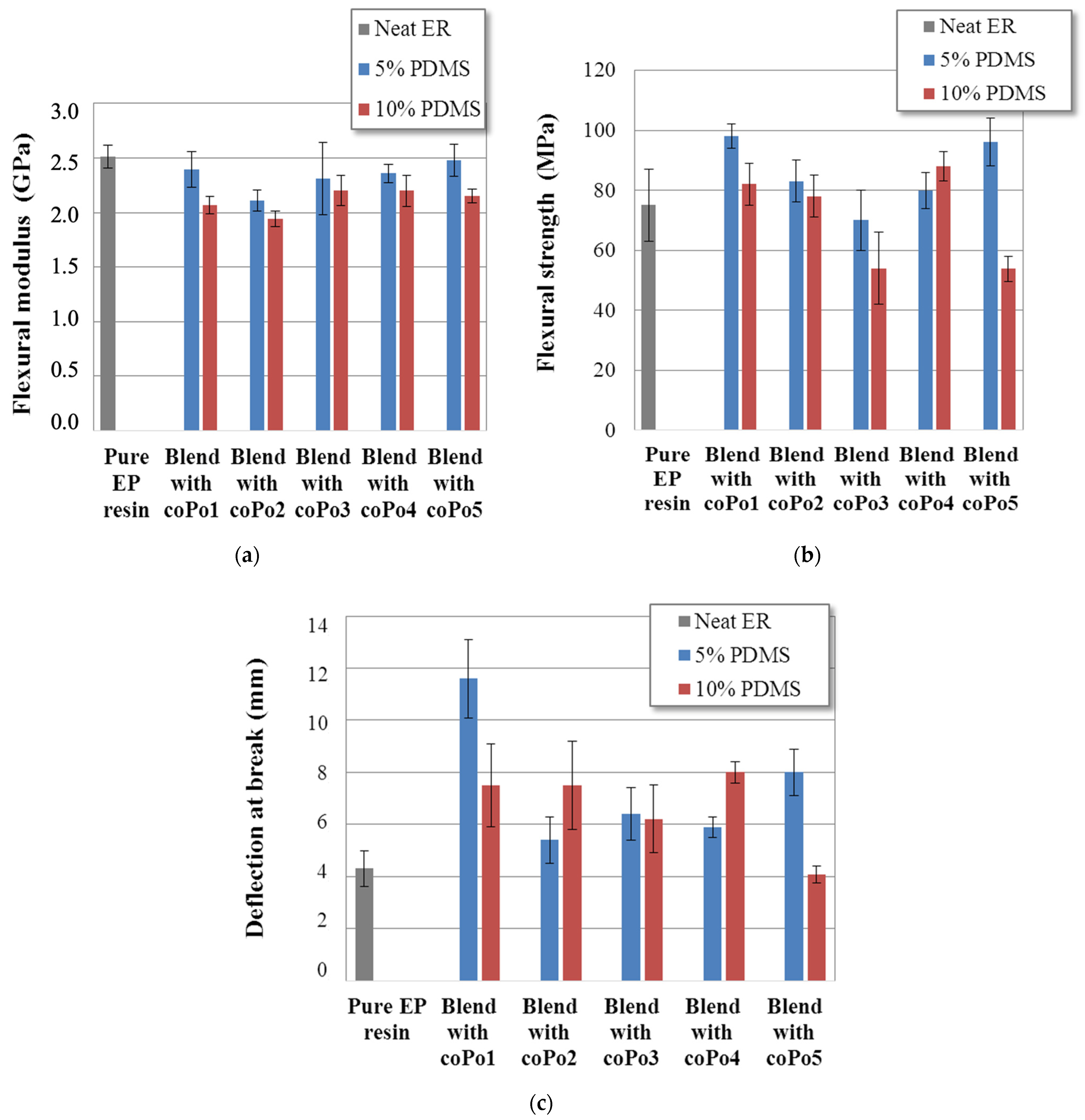
2.7. Flexural and Fracture Behavior
3. Materials and Methods
3.1. Materials
3.2. Preparation and Curing of Epoxy Resin Formulations
3.3. Characterization Methods
3.3.1. 1H NMR Analysis for Determining the Epoxy Equivalent Weight of DGEBA
3.3.2. Miscibility Tests
3.3.3. Morphology Analysis
3.3.4. Differential Scanning Calorimetry (DSC)
3.3.5. Dynamic–Mechanical Analysis (DMA)
3.3.6. Flexural Properties
3.3.7. Contact Angle
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pascault, J.-P.; Williams, R.J.J. Epoxy Polymers: New Materials and Innovations; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010. [Google Scholar]
- Flick, E.W. Epoxy Resins, Curing Agents, Compounds, and Modifiers: An Industrial Guide, 2nd ed.; Noyes Publications: Park Ridge, NJ, USA, 1993. [Google Scholar]
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and Application of Epoxy Resins: A Review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Vidil, T.; Tournilhac, F.; Musso, S.; Robinson, A.; Leibler, L. Control of reactions and network structures of epoxy thermosets. Prog. Polym. Sci. 2016, 62, 126–179. [Google Scholar] [CrossRef]
- Sukanto, H.; Raharjo, W.W.; Ariawan, D.; Triyono, J.; Kaavesina, M. Epoxy resins thermosetting for mechanical engineering. Open Eng. 2021, 11, 797–814. [Google Scholar] [CrossRef]
- Ellis, B. (Ed.) Chemistry and Technology of Epoxy Resins; Springer: Dordrecht, The Netherlands, 1993. [Google Scholar]
- Thomas, S.; Sinturel, C.; Thomas, R. (Eds.) Micro- and Nanostructured Epoxy/Rubber Blends; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Pearson, R.A.; Yee, A.F. Toughening Mechanisms in Elastomer-Modified Epoxies. J. Mater. Sci. 1989, 24, 2571–2580. [Google Scholar] [CrossRef]
- Thomas, R.; Durix, S.; Sinturel, C.; Omonov, T.; Goossens, S.; Groeninckx, G.; Moldenaers, P.; Thomas, S. Cure Kinetics, Morphology and Miscibility of Modified DGEBA-Based Epoxy Resin—Effects of a Liquid Rubber Inclusion. Polymer 2007, 48, 1695–1710. [Google Scholar] [CrossRef]
- Bagheri, R.; Marouf, B.T.; Pearson, R.A. Rubber-Toughened Epoxies: A Critical Review. Polym. Rev. 2009, 49, 201–225. [Google Scholar] [CrossRef]
- Géhant, S.; Fond, C.; Schirrer, R. Criteria for Cavitation of Rubber Particles: Influence of Plastic Yielding in the Matrix. Int. J. Fract. 2003, 122, 161–175. [Google Scholar] [CrossRef]
- Pearson, R.A.; Yee, A.F. Influence of Particle Size and Particle Size Distribution on Toughening Mechanisms in Rubber-Modified Epoxies. J. Mater. Sci. 1991, 26, 3828–3844. [Google Scholar] [CrossRef]
- Bagheri, R.; Pearson, R.A. Role of Particle Cavitation in Rubber-Toughened Epoxies: II. Inter-Particle Distance. Polymer 2000, 41, 269–276. [Google Scholar] [CrossRef]
- Dittanet, P.; Pearson, R.A. Effect of Bimodal Particle Size Distributions on the Toughening Mechanisms in Silica Nanoparticle Filled Epoxy Resin. Polymer 2013, 54, 1832–1845. [Google Scholar] [CrossRef]
- Lin, K.-F.; Shieh, Y.-D. Core-Shell Particles Designed for Toughening the Epoxy Resins. II. Core-Shell-Particle-Toughened Epoxy Resins. J. Appl. Polym. Sci. 1998, 70, 2313–2322. [Google Scholar] [CrossRef]
- Ramli, R.A.; Laftah, W.A.; Hashim, S. Core–shell Polymers: A Review. RSC Adv. 2013, 3, 15543. [Google Scholar] [CrossRef]
- Quan, D.; Ivankovic, A. Effect of Core–shell Rubber (CSR) Nano-Particles on Mechanical Properties and Fracture Toughness of an Epoxy Polymer. Polymer 2015, 66, 16–28. [Google Scholar] [CrossRef]
- Heng, Z.; Zeng, Z.; Zhang, B.; Luo, Y.; Luo, J.; Chen, Y.; Zou, H.; Liang, M. Enhancing Mechanical Performance of Epoxy Thermosets via Designing a Block Copolymer to Self-Organize into “Core-Shell” Nanostructure. RSC Adv. 2016, 6, 77030–77036. [Google Scholar] [CrossRef]
- Brown, H.R.; Schneider, J.A.; Murphy, T.L. Experimental Studies of the Deformation Mechanisms of Core-Shell Rubber-Modified Diglycidyl Ether of Bisphenol-A Epoxy at Cryogenic Temperatures. J. Compos. Mater. 2014, 48, 1279–1296. [Google Scholar] [CrossRef]
- Sprenger, S. The Effects of Silica Nanoparticles in Toughened Epoxy Resins and Fiber-Reinforced Composites; Carl Hanser Verlag GmbH Co., KG: Munich, Germany, 2015. [Google Scholar]
- Cano, L.; Builes, D.H.; Tercjak, A. Morphological and Mechanical Study of Nanostructured Epoxy Systems Modified with Amphiphilic Poly(Ethylene Oxide-b-Propylene Oxide-b-Ethylene Oxide)Triblock Copolymer. Polymer 2014, 55, 738–745. [Google Scholar] [CrossRef]
- Cong, H.; Li, L.; Zheng, S. Formation of Nanostructures in Thermosets Containing Block Copolymers: From Self-Assembly to Reaction-Induced Microphase Separation Mechanism. Polymer 2014, 55, 1190–1201. [Google Scholar] [CrossRef]
- Chen, J.; Kinloch, A.J.; Sprenger, S.; Taylor, A.C. The Mechanical Properties and Toughening Mechanisms of an Epoxy Polymer Modified with Polysiloxane-Based Core-Shell Particles. Polymer 2013, 54, 4276–4289. [Google Scholar] [CrossRef]
- Chruściel, J.J.; Leśniak, E. Modification of Epoxy Resins with Functional Silanes, Polysiloxanes, Silsesquioxanes, Silica and Silicates. Prog. Polym. Sci. 2015, 41, 67–121. [Google Scholar] [CrossRef]
- Kwon, S.-C.; Adachi, T. Strength and Fracture Toughness of Nano and Micron-Silica Particles Bidispersed Epoxy Composites: Evaluated by Fragility Parameter. J. Mater. Sci. 2007, 42, 5516–5523. [Google Scholar] [CrossRef]
- Hsieh, T.H.; Kinloch, A.J.; Masania, K.; Lee, J.S.; Taylor, A.C.; Sprenger, S. The Toughness of Epoxy Polymers and Fibre Composites Modified with Rubber Microparticles and Silica Nanoparticles. J. Mater. Sci. 2010, 45, 1193–1210. [Google Scholar] [CrossRef]
- Kumar, A.; Anant, R.; Kumar, K.; Chauhan, S.S.; Kumar, S.; Kumar, R. Anticorrosive and Electromagnetic Shielding Response of a Graphene/TiO 2− Epoxy Nanocomposite with Enhanced Mechanical Properties. RSC Adv. 2016, 6, 113405–113414. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, L.; Sun, W.; Li, S.; Zhu, T.; Liu, W.; Liu, G. Superhydrophobic Epoxy Coating Modified by Fluorographene Used for Anti-Corrosion and Self-Cleaning. Appl. Surf. Sci. 2017, 401, 146–155. [Google Scholar] [CrossRef]
- Atta, A.M.; Al-Lohedan, H.A.; El-saeed, A.M.; Al-Shafey, H.I.; Wahby, M.H. Epoxy Embedded with TiO2 Nanogel Composites as Promising Self-Healing Organic Coatings of Steel. Prog. Org. Coat. 2017, 105, 291–302. [Google Scholar] [CrossRef]
- Cheng, Q.F.; Wang, J.P.; Wen, J.J.; Liu, C.H.; Jiang, K.L.; Li, Q.Q.; Fan, S.S. Carbon Nanotube/Epoxy Composites Fabricated by Resin Transfer Molding. Carbon 2010, 48, 260–266. [Google Scholar] [CrossRef]
- Guo, P.; Chen, X.; Gao, X.; Song, H.; Shen, H. Fabrication and Mechanical Properties of Well-Dispersed Multiwalled Carbon Nanotubes/Epoxy Composites. Compos. Sci. Technol. 2007, 67, 3331–3337. [Google Scholar] [CrossRef]
- Saeb, M.R.; Bakhshandeh, E.; Khonakdar, H.A.; Mäder, E.; Scheffler, C.; Heinrich, G. Cure Kinetics of Epoxy Nanocomposites Affected by MWCNTs Functionalization: A Review. Sci. World J. 2013, 2013, 703708. [Google Scholar] [CrossRef]
- Srivastava, V.K. Modeling and Mechanical Performance of Carbon Nanotube/Epoxy Resin Composites. Mater. Des. 2012, 39, 432–436. [Google Scholar] [CrossRef]
- Adachi, T.; Osaki, M.; Araki, W.; Kwon, S.-C. Fracture Toughness of Nano- and Micro-Spherical Silica-Particle-Filled Epoxy Composites. Acta Mater. 2008, 56, 2101–2109. [Google Scholar] [CrossRef]
- Moloney, A.C.; Kausch, H.H.; Kaiser, T.; Beer, H.R. Parameters Determining the Strength and Toughness of Particulate Filled Epoxide Resins. J. Mater. Sci. 1987, 22, 381–393. [Google Scholar] [CrossRef]
- Marouf, B.T.; Mai, Y.-W.; Bagheri, R.; Pearson, R.A. Toughening of Epoxy Nanocomposites: Nano and Hybrid Effects. Polym. Rev. 2016, 56, 70–112. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Friedrich, K. Fatigue Crack Propagation and Related Failure in Modified, Anhydride-Cured Epoxy Resins. Colloid Polym. Sci. 1992, 270, 549–562. [Google Scholar] [CrossRef]
- Tang, L.-C.; Wang, X.; Wan, Y.-J.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. Mechanical Properties and Fracture Behaviors of Epoxy Composites with Multi-Scale Rubber Particles. Mater. Chem. Phys. 2013, 141, 333–342. [Google Scholar] [CrossRef]
- Ruiz-Pérez, L.; Royston, G.J.; Fairclough, J.P.A.; Ryan, A.J. Toughening by Nanostructure. Polymer 2008, 49, 4475–4488. [Google Scholar] [CrossRef]
- Chrysanthos, M.; Galy, J.; Pascault, J.-P. Preparation and Properties of Bio-Based Epoxy Networks Derived from Isosorbide Diglycidyl Ether. Polymer 2011, 52, 3611–3620. [Google Scholar] [CrossRef]
- Vijayan, P.; AlMaadeed, M.A. “Containers” for Self-Healing Epoxy Composites and Coating: Trends and Advances. Express Polym. Lett. 2016, 10, 506–524. [Google Scholar] [CrossRef]
- Yi, H.; Deng, Y.; Wang, C. Pickering Emulsion-Based Fabrication of Epoxy and Amine Microcapsules for Dual Core Self-Healing Coating. Compos. Sci. Technol. 2016, 133, 51–59. [Google Scholar] [CrossRef]
- Neisiany, R.E.; Lee, J.K.Y.; Khorasani, S.N.; Ramakrishna, S. Self-Healing and Interfacially Toughened Carbon Fibre-Epoxy Composites Based on Electrospun Core–shell Nanofibres. J. Appl. Polym. Sci. 2017, 134, 44956. [Google Scholar] [CrossRef]
- Wu, S.; Yuan, L.; Gu, A.; Zhang, Y.; Liang, G. Synthesis and Characterization of Novel Epoxy Resins-Filled Microcapsules with Organic/Inorganic Hybrid Shell for the Self-Healing of High Performance Resins. Polym. Adv. Technol. 2016, 27, 1544–1556. [Google Scholar] [CrossRef]
- Rusu, D.; Tsakalos, V.; Navard, P.; Peuvrel-Disdier, E. Le Rôle de l’Interface dans la Mise en Forme des Mélanges de Polymères Immiscibles. In Surfaces, Tribologie et Formage des Matériaux; Les Presses de l’École des Mines: Paris, France, 2001; pp. 207–232. [Google Scholar]
- Gordin, C.; Delaite, C.; Bistac, S.; Schuller, A.-S.; Rusu, D.; Rusu, M. PDMS Migration at Poly(Vinyl Chloride)/Poly(ε-Caprolactone)/Poly(ɛ-Caprolactone)-b-Poly(Dimethylsiloxane) Blends Surfaces. Polym. Test. 2009, 28, 446–451. [Google Scholar] [CrossRef]
- Garcia, F.G.; Soares, B.G. Determination of the Epoxide Equivalent Weight of Epoxy Resins Based on Diglycidyl Ether of Bisphenol A (DGEBA) by Proton Nuclear Magnetic Resonance. Polym. Test. 2003, 22, 51–56. [Google Scholar] [CrossRef]
- Pethrick, R.A.; Miller, C.; Rhoney, I. Influence of Nanosilica Particles on the Cure and Physical Properties of an Epoxy Thermoset Resin. Polym. Int. 2009, 59, 236–241. [Google Scholar] [CrossRef]
- Brostow, W.; Goodman, S.H.; Wahrmund, J. Epoxies. In Handbook of Thermoset Plastics; Elsevier: Amsterdam, The Netherlands, 2014; pp. 191–252. [Google Scholar]
- Levita, G.; De Petris, S.; Marchetti, A.; Lazzeri, A. Crosslink Density and Fracture Toughness of Epoxy Resins. J. Mater. Sci. 1991, 26, 2348–2352. [Google Scholar] [CrossRef]
- Iijima, T.; Yoshioka, N.; Tomoi, M. Effect of Cross−link Density on Modification of Epoxy Resins with Reactive Acrylic Elastomers. Eur. Polym. J. 1992, 28, 573–581. [Google Scholar] [CrossRef]
- Hill, L.W. Calculation of Crosslink Density in Short Chain Networks. Prog. Org. Coat. 1997, 31, 235–243. [Google Scholar] [CrossRef]
- Nakka, J.S.; Jansen, K.M.B.; Ernst, L.J. Effect of Chain Flexibility in the Network Structure on the Viscoelasticity of Epoxy Thermosets. J. Polym. Res. 2011, 18, 1879–1888. [Google Scholar] [CrossRef]
- Jones, F.N.; Nichols, M.E.; Pappas, S.P. Organic Coatings: Science and Technology, 4th ed.; Wiley-Interscience: Hoboken, NJ, USA, 2017. [Google Scholar]
- Romo-Uribe, A.; Arcos-Casarrubias, J.A.; Flores, A.; Valerio-Cárdenas, C.; González, A.E. Influence of Rubber on the Curing Kinetics of DGEBA Epoxy and the Effect on the Morphology and Hardness of the Composites. Polym. Bull. 2014, 71, 1241–1262. [Google Scholar] [CrossRef]
- Romo-Uribe, A.; Arcos-Casarrubias, J.A.; Reyes-Mayer, A.; Guardian-Tapia, R. PDMS Nanodomains in DGEBA Epoxy Induce High Flexibility and Toughness. Polym. Plast. Technol. Eng. 2017, 56, 96–107. [Google Scholar] [CrossRef]



| Copolymer Code | Miscibility at 25 °C and 33 °C | Order of Addition in the Thermosetting Formulation | |
|---|---|---|---|
| DGEBA | PDMS | ||
| coPo1 | PM | PM/IMM | Pre-mixed with DGEBA |
| coPo2 | PM/IMM | M | Pre-mixed with PDMS |
| coPo3 | M | PM/IMM | Pre-mixed with DGEBA |
| coPo4 | M | PM/IMM | Pre-mixed with DGEBA |
| coPo5 | PM/IMM | M | Pre-mixed with PDMS |
| Formulation Code | ΔHcure (J·g−1) | Tonset (°C) | Tpeak (°C) |
|---|---|---|---|
| Pure epoxy resin (EP) | 430 | 78.0 | 112.3 |
| EP + 5% PDMS and coPo3 | 405 | 76.5 | 111.7 |
| EP + 10% PDMS and coPo3 | 383 | 75.7 | 112.1 |
| Formulations | Morphological Characteristics | ||
|---|---|---|---|
| Mean Diameter (µm) | Size Distribution | ||
| Uncompatibilized epoxy/silicone blends | Broad | ||
| coPo1 | 5% PDMS | 8.6 | Narrow |
| 10% PDMS | 11.7 | Narrow | |
| coPo2 | 5% PDMS | 9.0 | Narrow |
| 10% PDMS | 29.4 | Broad | |
| coPo3 | 5% PDMS | 5.2 | Narrow |
| 10% PDMS | 7.8 | Narrow | |
| coPo4 | 5% PDMS | 28.3 | Broad |
| 10% PDMS | 48.1 | Broad | |
| coPo5 | 5% PDMS | 8.4 | Narrow |
| 10% PDMS | 26.5 | Broad | |
| Formulations | Tg (°C) by DSC | Tg (°C) by DMA | G’rubbery @ (Tg + 30 °C) (MPa) | |
|---|---|---|---|---|
| Pure epoxy resin | 155 | 155 | 9.8 | |
| coPo1 | 5% PDMS | 152 | 154 | 7.7 |
| 10% PDMS | 148 | 153 | 6.5 | |
| coPo2 | 5% PDMS | 153 | 154 | 7.9 |
| 10% PDMS | 151 | 155 | 7.1 | |
| coPo3 | 5% PDMS | 155 | 158 | 10 |
| 10% PDMS | 153 | 154 | 9.8 | |
| coPo4 | 5% PDMS | 150 | 159 | 9.4 |
| 10% PDMS | 148 | 155 | 7.1 | |
| coPo5 | 5% PDMS | 151 | 156 | 7.6 |
| 10% PDMS | 150 | 157 | 4.7 | |
| Contact Angle (°) | |||
|---|---|---|---|
| Pure DGEBA/IPD epoxy resin | 80 ± 2 | ||
| Epoxy formulations with 5 wt% PDMS | Epoxy formulations with 10 wt% PDMS | ||
| coPo1 | 74 ± 2 | coPo1 | 83 ± 2 |
| coPo2 | 86 ± 5 | coPo2 | 95 ± 2 |
| coPo3 | 77 ± 3 | coPo3 | 85 ± 2 |
| coPo4 | 81 ± 3 | coPo4 | 87 ± 3 |
| coPo5 | 86 ± 3 | coPo5 | 92 ± 3 |
| Components | Molar Mass * (g·mol−1) | Specificities/Equivalent Weight * (g·eq−1) | Functionality Type/Number | Possible Reaction with |
|---|---|---|---|---|
| DGEBA | < 700 | Not indicated | Epoxy/F = 2 | -NH2; -OH |
| IPD | M = 170 | AHEW = 42.58 | -NH2/F = 4 | epoxy groups |
| PDMS | Mw = 28,000 | trimethylsiloxy terminated | -/F = 0 | - |
| coPo1 | Mw = 2400 | PO/silicone molar ratio = 18/15 | -OH/F = 2 | epoxy groups |
| coPo2 | Mw = 4100 | EEW = 2050 long silicone chain | Epoxy/F = 2 | -NH2; -OH |
| coPo3 | Mw = 2900 | EO/silicone molar ratio = 24/20 | -OH/F = 2 | epoxy groups |
| coPo4 | Mw = 900 | EO/PO/silicone molar ratio = 10/4/1 | -OH/F = 1 | epoxy groups |
| coPo5 | Mw = 11,800 | EEW = 3900 long PDMS chain | Epoxy/F = 3 | -NH2; -OH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delaite, C.; Bistac, S.; Rusu, D. Influence of Block-Copolymers’ Composition as Compatibilizers for Epoxy/Silicone Blends. Molecules 2023, 28, 6300. https://doi.org/10.3390/molecules28176300
Delaite C, Bistac S, Rusu D. Influence of Block-Copolymers’ Composition as Compatibilizers for Epoxy/Silicone Blends. Molecules. 2023; 28(17):6300. https://doi.org/10.3390/molecules28176300
Chicago/Turabian StyleDelaite, Christelle, Sophie Bistac, and Daniela Rusu. 2023. "Influence of Block-Copolymers’ Composition as Compatibilizers for Epoxy/Silicone Blends" Molecules 28, no. 17: 6300. https://doi.org/10.3390/molecules28176300







