Nanoscale Ion-Exchange Materials: From Analytical Chemistry to Industrial and Biomedical Applications
Abstract
1. Introduction
2. Rational Design and Fabrication Strategies
2.1. Direct Polymerization
2.2. Post-Polymerization
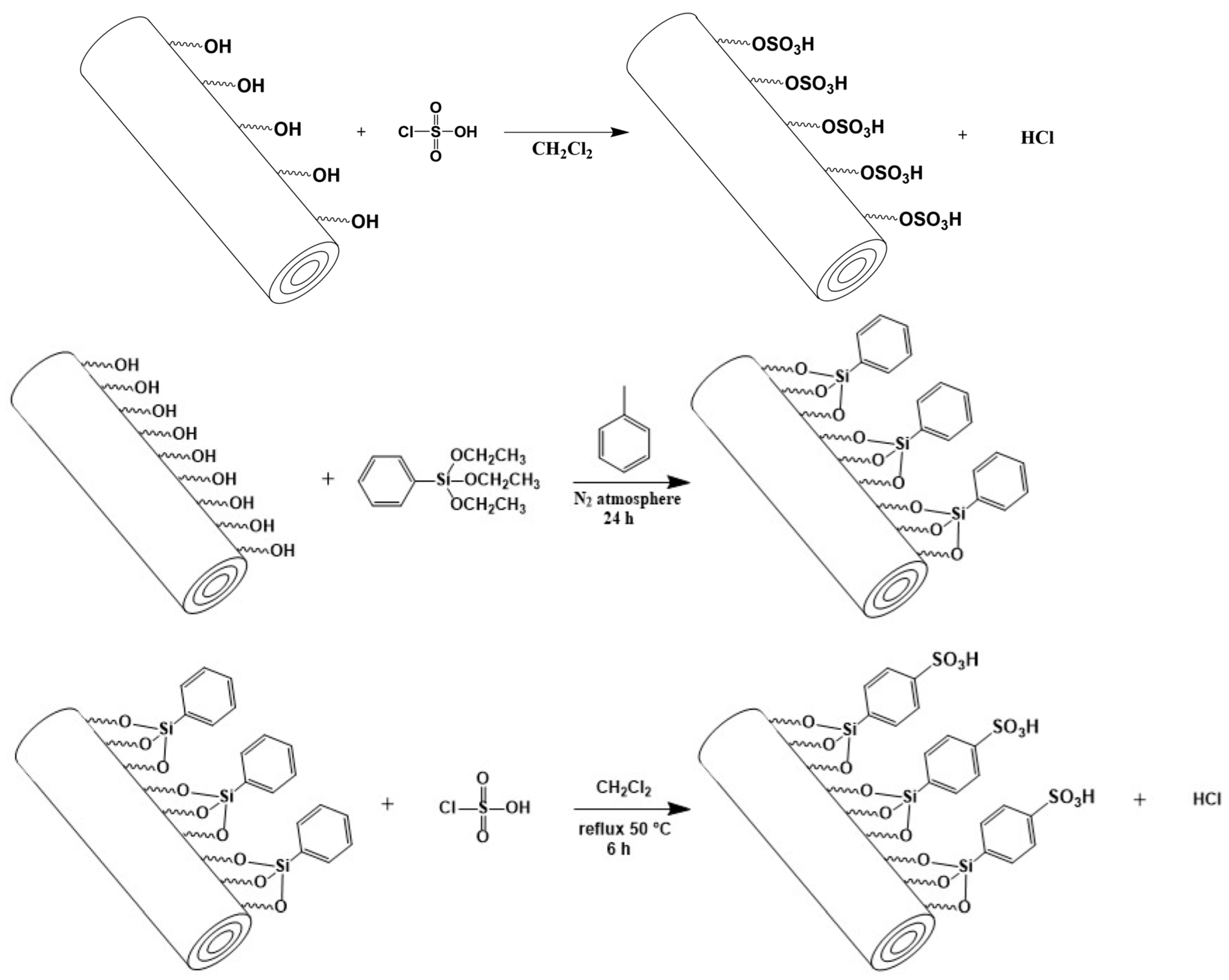
2.3. Non-Synthetic Methodology
3. Characterization
4. Applications
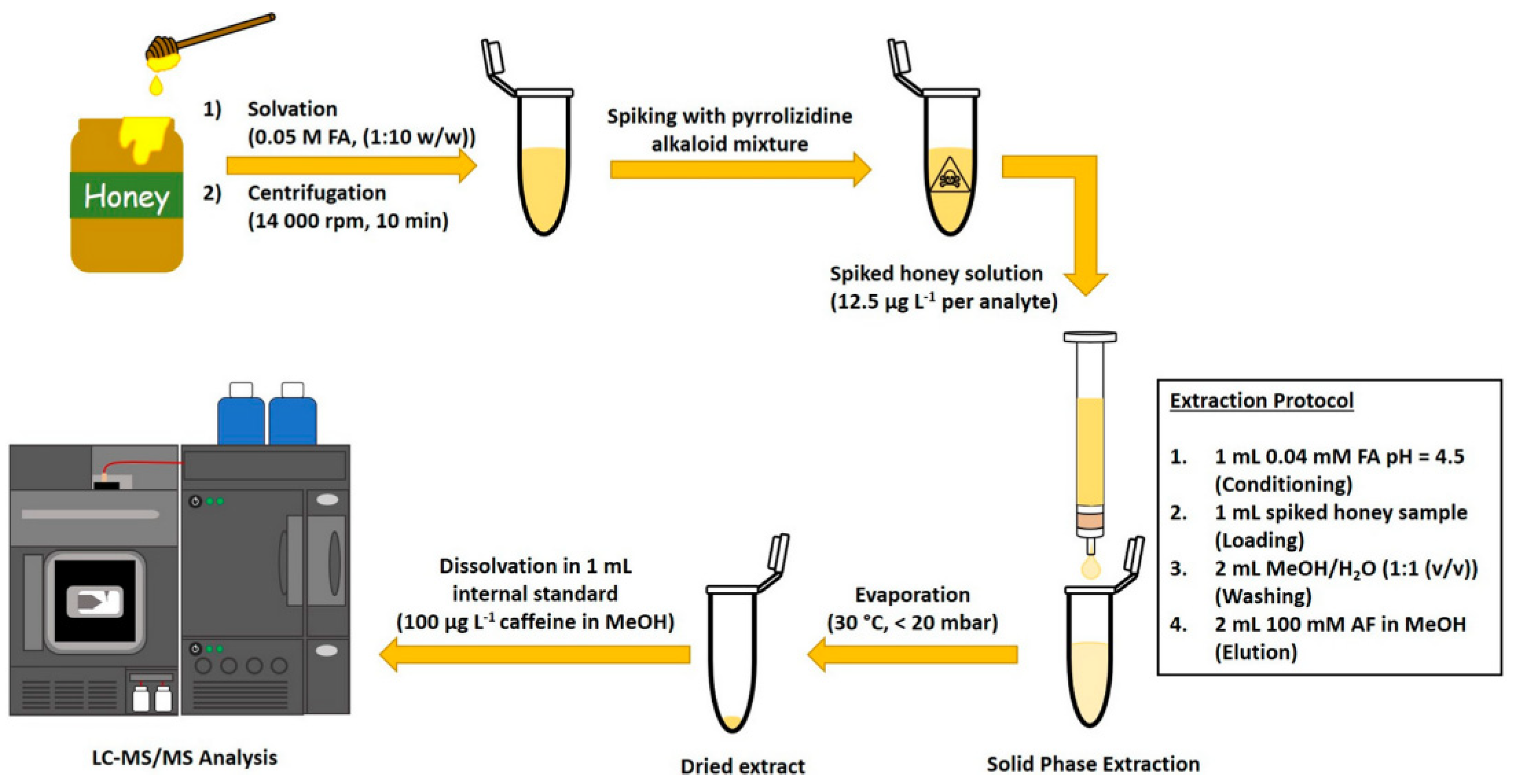
4.1. Solid-Phase Extraction
4.2. Separation Methods
4.3. Miscellaneous Analytical Applications
4.4. Toward Industrial Use
4.5. Drug Delivery
4.6. Delivery of Contrast Agents
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Dardel, F.; Arden, T.V. Ion exchangers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley Online Library: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Van Deventer, J. Selected ion exchange applications in the hydrometallurgical industry. Solv. Extract. Ion Exch. 2011, 29, 695–718. [Google Scholar] [CrossRef]
- Kilislioglu, A. Ion Exchange Technologies; IntechOpen: Rijeka, Croatia, 2012; p. 366. [Google Scholar]
- Sarkar, S.; Sen Gupta, A.K.; Greenleaf, J.E. Ion-exchange technology. In Encyclopedia of Polymer Science and Technology, 4th ed.; Mark, H.F., Ed.; Wiley Online Library: Hoboken, NJ, USA, 2014; Volume 7, pp. 150–194. [Google Scholar]
- Meetei, T.T.; Devi, Y.B.; Chanu, T.T. Ion exchange: The most important chemical reaction on earth after photosynthesis. Int. Res. J. Pure Appl. Chem. 2020, 21, 56798. [Google Scholar] [CrossRef]
- Sharma, V.; Singh, L. Ion exchange resins: A boon for pharmaceutical industry—An overview. Int. J. Pharm. Sci. Rev. Res. 2011, 6, 10–13. [Google Scholar]
- Ramirez, E.; Bringue, R.; Fite, C.; Iborra, M.; Tejero, J.; Cunill, F. Role of ion-exchange resins as catalyst in the reaction-network of transformation of biomass into biofuels. J. Chem. Technol. Biotechnol. 2017, 92, 2775–2786. [Google Scholar] [CrossRef]
- Lee, C.G.; Alvarez, P.J.J.; Nam, A.; Park, S.J.; Do, T.; Choi, U.S.; Lee, S.H. Arsenic(V) removal using an amine-doped acrylic ion exchange fiber: Kinetic, equilibrium, and regeneration studies. J. Hazard. Mater. 2017, 325, 223–229. [Google Scholar] [CrossRef]
- Chu, J.H.; Kang, J.K.; Park, S.J.; Lee, C.G. Application of the anion-exchange resin as a complementary technique to remove residual cyanide complexes in industrial plating wastewater after conventional treatment. Environ. Sci. Pollut. Res. 2020, 27, 41688–41701. [Google Scholar] [CrossRef] [PubMed]
- Dixit, F.; Dutta, R.; Barbeau, B.; Berube, P.; Mohseni, M. PFAS removal by ion exchange resins: A review. Chemosphere 2021, 272, 129777. [Google Scholar] [CrossRef] [PubMed]
- Banert, T.; Peuker, U.A. Synthesis of magnetic beads for bio-separation using the solution method. Chem. Engineer. Commun. 2007, 194, 707–719. [Google Scholar] [CrossRef]
- Mondal, P.; Roy, K.; Bayen, S.P.; Chowdhury, P. Synthesis of polypyrrole nanoparticles and its grafting with silica gel for selective binding of chromium(VI). Talanta 2011, 83, 1482–1486. [Google Scholar] [CrossRef]
- Siva, S.; Sudharsan, S.; Sayee Kannan, R. Synthesis, characterization and ion-exchange properties of novel hybrid polymer nanocomposites for selective and effective mercury(II) removal. RSC Adv. 2015, 5, 79665–79678. [Google Scholar] [CrossRef]
- Dzyazko, Y.S.; Fedina, L.V.; Zakharov, V.V.; Kolomiets, Y.O.; Kudelko, K.O. Composite ion-exchanges for the recycling of liquid waste of dairy industry. Ukr. Chem. J. 2020, 86, 38–52. (In Ukrainian) [Google Scholar] [CrossRef]
- Saha, A.; Deb, M.K.; Mahilang, M.; Kurrey, R.; Sinha, S. Polymeric resins as nano-catalysts: A brief review. J. Ind. Chem. Soc. 2020, 97, 1442–1454. [Google Scholar]
- Zhang, W.; Kochovski, Z.; Lu, Y.; Schmidt, B.V.K.J.; Antonietti, M.; Yuan, J. Internal morphology-controllable self-assembly in poly(ionic liquid) nanoparticles. ACS Nano 2016, 10, 7731–7737. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Kong, L.; Ge, Q.; Zhang, W.; Zhou, X.; Zhang, L.; Wang, X. Antibacterial activities of N-alkyl imidazolium-based poly(ionic liquid) nanoparticles. Polymer Chem. 2019, 10, 209–218. [Google Scholar] [CrossRef]
- Lu, B.; Zhou, G.; Xiao, F.; He, Q.; Zhang, J. Stimuli-responsive poly(ionic liquid) nanoparticles for controlled drug delivery. J. Mater. Chem. B 2020, 8, 7994–8001. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Lu, X.; Cui, K.; Niu, L.; Wei, Y.; Lu, Q. Dual-responsive controlled drug delivery based on ionically assembled nanoparticles. Langmuir 2012, 28, 9413–9420. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Zhang, Y.; Tan, Y.; Luo, X.; Tu, X. Reusable and efficient polymer nanoparticles grafted with hydroxyl-functionalized phosphonium-based ionic liquid catalyst for cycloaddition of CO2 with epoxides. Appl. Catal. A 2016, 514, 43–50. [Google Scholar] [CrossRef]
- Brijmohan, S.B.; Swier, S.; Weiss, R.A.; Shaw, M.T. Synthesis and characterization of cross-linked sulfonated polystyrene nanoparticles. Ind. Eng. Chem. Res. 2005, 44, 8039–8045. [Google Scholar] [CrossRef]
- Wutzel, H.; Samhaber, W.M. Synthesis and characterization of sulphonated core-shell lattices in the size range between 30 and 80 nm. Colloids Surf. A 2008, 319, 84–89. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, Y.; Li, S.; Yang, Z. Interaction of bacteria and ion-exchange particles and its potential in separation for matrix-assisted laser desorption/ionization mass spectrometric identification of bacteria in water. Rapid Commun. Mass Spectrom. 2009, 23, 3983–3993. [Google Scholar] [CrossRef]
- Pinheiro, J.P.; Moura, L.; Fokkink, R.; Farinha, J.P.S. Preparation and characterization of low dispersity anionic multiresponsive core-shell polymer nanoparticles. Langmuir 2012, 28, 5802–5809. [Google Scholar] [CrossRef]
- Fàbregas, A.; Minarro, M.; García-Montoya, E.; Pérez-Lozano, P.; Carrillo, C.; Sarrate, R.; Sánchez, N.; Ticó, J.R.; Suné-Negre, J.M. Impact of physical parameters on particle size and reaction yield when using the ionic gelation method to obtain cationic polymeric chitosan–tripolyphosphate nanoparticles. Int. J. Pharm. 2013, 446, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Reisch, A.; Runser, A.; Arntz, Y.; Mely, Y.; Klymchenko, A.S. Charge-controlled nanoprecipitation as a modular approach to ultrasmall polymer nanocarriers: Making bright and stable nanoparticles. ACS Nano 2015, 9, 5104–5116. [Google Scholar] [CrossRef] [PubMed]
- Dolgonosov, A.M.; Khamizov, R.K.; Kolotilina, N.K.; Shaikhina, S.U.; Evstigneeva, P.V. Preparation, properties and application of colloid solutions of nano-sized ion-exchangers. Sorpt. Chromatogr. Process. 2016, 16, 400–414. (In Russian) [Google Scholar]
- Dzema, D.V.; Kartsova, L.A.; Polikarpova, D.A. Application of the highly basic nano-sized anionite for the capillary electrophoresis separation and on-line concentration of inorganic anions. Anal. Control 2017, 21, 41–48. (In Russian) [Google Scholar] [CrossRef][Green Version]
- Polikarpova, D.; Makeeva, D.; Kartsova, L.; Dolgonosov, A.; Kolotilina, N. Nano-sized anion-exchangers as a stationary phase in capillary electrochromatography for separation and on-line concentration of carboxylic acids. Talanta 2018, 188, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Dolgonosov, A.M.; Kolotilina, N.K.; Yadykov, M.S. Method of Preparation of High-Performance Columns for Ion Chromatography. Russian Patent No. 2499628, 27 November 2013. [Google Scholar]
- Dolgonosov, A.M.; Khamizov, R.K.; Kolotilina, N.K. Nano ion exchangers as modifiers of chromatographic phases and sources of analytical signal. J. Anal. Chem. 2019, 74, 382–392. [Google Scholar] [CrossRef]
- Dolgonosov, A.M.; Khamizov, R.K.; Kolotilina, N.K.; Fokina, O.V. The Method of Optical Emission Analysis of Solutions. Russian Patent No. 2706720, 20 November 2019. [Google Scholar]
- Polikarpova, D.; Makeeva, D.; Kolotilina, N.; Dolgonosov, A.; Peshkova, M.; Kartsova, L. Nanosized cation exchanger for the electrophoretic separation and preconcentration of catecholamines and amino acids. Electrophoresis 2020, 41, 1031–1038. [Google Scholar] [CrossRef]
- Makeeva, D.V.; Polikarpova, D.A.; Kartsova, L.A. Bilayer capillary coatings based on nanosized ion-exchangers for the capillary electrochromatography analyses of biogenic amines and amino acids. Anal. Control 2021, 25, 230–240. (In Russian) [Google Scholar] [CrossRef]
- Shkinev, V.M.; Martynov, L.Y.; Trofimov, D.A.; Dolgonosov, A.M. Ion exchanger-filled track membranes with asymmetric pores for the electrochemical determination of acetylcholine chloride. J. Anal. Chem. 2021, 76, 390–398. [Google Scholar] [CrossRef]
- Dolgonosov, A.M.; Khamizov, R.K.; Kolotilina, N.K. Nano-ion-exchangers—A new class of reactive materials. Sorpt. Chromatogr. Process. 2018, 18, 794–808. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, O.V.; Kolotilina, N.K.; Dolgonosov, A.M.; Khamizov, R.K.; Timerbaev, A.R. A de novo nanoplatform for the delivery of metal-based drugs studied with high-resolution ICP-MS. Talanta 2023, 253, 124035. [Google Scholar] [CrossRef]
- Rank, C.; Yan, L.; Mecking, S.; Winey, K.I. Periodic polyethylene sulfonates from polyesterification: Bulk and nanoparticle morphologies and ionic conductivities. Macromolecules 2019, 52, 8466–8475. [Google Scholar] [CrossRef]
- Smith, G.N.; Canning, S.L.; Derry, M.J.; Jones, E.R.; Neal, T.J.; Smith, A.J. Ionic and nonspherical polymer nanoparticles in nonpolar solvents. Macromolecules 2020, 53, 3148–3156. [Google Scholar] [CrossRef]
- Nasirinezhad, M.; Ghaffarian, S.R.; Tohidian, M. Eco-friendly polyelectrolyte nanocomposite membranes based on chitosan and sulfonated chitin nanowhiskers for fuel cell applications. Iran. Polym. J. 2021, 30, 355–367. [Google Scholar] [CrossRef]
- Zhang, C.; Kong, X.; Yin, J.; Fu, X. Rheology of fresh cement pastes containing polymer nanoparticles. Cem. Concr. Res. 2021, 144, 106419. [Google Scholar] [CrossRef]
- Kim, B.; Zhang, D.; Armstrong, M.S.; Pelczer, I.; Prud’homme, R.K. Formulation of pH-responsive methacrylate-based polyelectrolyte-stabilized nanoparticles for applications in drug delivery. ACS Appl. Nano Mater. 2022, 5, 18770–18778. [Google Scholar] [CrossRef]
- Schlappack, T.; Rainer, M.; Weinberger, N.; Bonn, G.K. Sulfonated halloysite nanotubes as a novel cation exchange material for solid phase extraction of toxic pyrrolizidine alkaloids. Anal. Methods 2022, 14, 2689–2697. [Google Scholar] [CrossRef]
- Schlappack, T.; Weidacher, N.; Huck, C.W.; Bonn, G.K.; Rainer, M. Effective solid phase extraction of toxic pyrrolizidine alkaloids from honey with reusable organosilyl-sulfonated halloysite nanotubes. Separations 2022, 9, 270. [Google Scholar] [CrossRef]
- Sayed Ibrahim, G.P.M.; Isloor, A.M.; Farnood, R. Catalyst- and stabilizer-free rational synthesis of ionic polymer nanoparticles in one step for oil/water separation membranes. ACS Appl. Mater. Interfaces 2022, 14, 45800–45809. [Google Scholar]
- Mikušová, V.; Mikuš, P. Advances in chitosan-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef]
- Dodero, A.; Alberti, S.; Gaggero, G.; Ferretti, M.; Botter, R.; Vicini, S.; Castellano, M. An up-to-date review on alginate nanoparticles and nanofibers for biomedical and pharmaceutical applications. Adv. Mater. Interfaces 2021, 8, 2100809. [Google Scholar] [CrossRef]
- Yasmin, R.; Shah, M.; Khan, S.A.; Ali, R. Gelatin nanoparticles: A potential candidate for medical applications. Nanotechnol. Rev. 2017, 6, 191–207. [Google Scholar] [CrossRef]
- Sayed, S.; Jardine, A. Chitosan derivatives as important biorefinery intermediates. Quaternary tetraalkylammonium chitosan derivatives utilized in anion exchange chromatography for perchlorate removal. Int. J. Mol. Sci. 2015, 16, 9064–9077. [Google Scholar] [CrossRef] [PubMed]
- Hoang, N.H.; Thanh, T.L.; Sangpueak, R.; Treekoon, J.; Saengchan, C.; Thepbandit, W.; Papathoti, N.K.; Kamkaew, A.; Buensanteai, N. Chitosan nanoparticles-based ionic gelation method: A promising candidate for plant disease management. Polymers 2022, 14, 662. [Google Scholar] [CrossRef] [PubMed]
- Benettayeb, A.; Seihoub, F.Z.; Pal, P.; Ghosh, S.; Usman, M.; Chia, C.H.; Usman, M.; Sillanpää, M. Chitosan nanoparticles as potential nano-sorbent for removal of toxic environmental pollutants. Nanomaterials 2023, 13, 447. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Gupta, P. Halloysite-based magnetic manostructures for diverse applications: A review. ACS Appl. Nano Mater. 2023, 6, 13824–13868. [Google Scholar] [CrossRef]
- Silva, S.M.; Peixoto, A.F.; Freire, C. HSO3-functionalized halloysite nanotubes: New acid catalysts for esterification of free fatty acid mixture as hybrid feedstock model for biodiesel production. Appl. Catal. A 2018, 568, 221–230. [Google Scholar] [CrossRef]
- Kuehne, A.J.C. Conjugated polymer nanoparticles toward in vivo theranostics—Focus on targeting, imaging, therapy, and the importance of clearance. Adv. Biosyst. 2017, 1, 1700100. [Google Scholar] [CrossRef]
- Ansari, R.; Samadi, N.; Khodavirdilo, B. Synthesized nano particle derivation of poly(styrene -alternative maleic anhydride) for the removal of the silver(I) ions from aqueous solutions. Iran. J. Anal. Chem. 2017, 4, 50–60. [Google Scholar]
- Samadi, N.; Ansari, R.; Khodavirdilo, B. Synthesized nanoparticles of poly(styrene–alternative-maleic anhydride) and prunus cerasus rock used for removing cadmium(II) ions from aqueous solutions. Asian J. Green Chem. 2018, 2, 338–363. [Google Scholar]
- Samadi, N.; Ansari, R.; Khodavirdilo, B. Synthesized nano particle derivation of poly(styrene-co-maleic anhydride) and sour cherry rock for removing nickel(II) ion from aqueous solutions. Toxicol. Rep. 2019, 5, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Dolgonosov, A.M.; Khamizov, R.K. Properties and application of nano-ion-exchangers. In Ion Exchange—A Continuing Success Story (IEx 2016); Cox, M., Ed.; Robinson College Publ.: Cambridge, UK, 2016; p. 135. [Google Scholar]
- Öztürk Er, E.; Dalgıç Bozyiğit, G.; Büyükpınar, Ç.; Bakırdere, S. Magnetic nanoparticles based solid phase extraction methods for the determination of trace elements. Crit. Rev. Anal. Chem. 2022, 52, 231–249. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.I.; El-Nouby, M.A.M.; Kimani, P.K.; Lim, L.W.; Rabea, E.I. A review of the modern principles and applications of solid-phase extraction techniques in chromatographic analysis. Anal. Sci. 2022, 38, 1457–1487. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, A.F.; Fernandes, A.C.; Pereira, C.; Pires, J.; Freire, C. Physicochemical characterization of organosilylated halloysite clay nanotubes. Microporous Mesoporous Mater. 2016, 219, 145–154. [Google Scholar] [CrossRef]
- Ansari, R.; Khoshbakht Fahim, N. Application of polypyrrole coated on wood sawdust for removal of Cr(VI) ion from aqueous solutions. React. Funct. Polym. 2007, 67, 367–374. [Google Scholar] [CrossRef]
- Li, S.; Guo, Z.; Liu, Y.; Yang, Z.; Hui, H.K. Integration of microfiltration and anion-exchange nanoparticles-based magnetic separation with MALDI mass spectrometry for bacterial analysis. Talanta 2009, 80, 313–320. [Google Scholar] [CrossRef]
- Çimen, D.; Bereli, N.; Günaydın, S.; Denizli, A. Preparation of magnetic poly(ethylene glycol dimethacrylate-N-methacryloyl-(L)-glutamic acid) particles for thrombin purification. J. Chromatogr. B 2023, 1219, 123653. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J. Handbook of Ion Chromatography; Wiley-VCH: Weinheim, Germany, 2016; p. 1522. [Google Scholar]
- Paull, B.; Michalski, R. Ion exchange/ion chromatography—Principles and Applications. In Encyclopedia of Analytical Science, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 178–189. [Google Scholar]
- Liu, X.; Pohl, C.; Woodruff, A.; Chen, J. Chromatographic evaluation of reversed-phase/anion-exchange/cation exchange trimodal stationary phases prepared by electrostatically driven self-assembly process. J. Chromatogr. A 2011, 1218, 3407–3412. [Google Scholar] [CrossRef] [PubMed]
- Pobozẏ, E.; Trojanowicz, M. Application of capillary electrophoresis for determination of inorganic analytes in waters. Molecules 2021, 26, 6972. [Google Scholar] [CrossRef]
- Gao, Z.; Zhong, W. Recent (2018–2020) development in capillary electrophoresis. Anal. Bioanal. Chem. 2022, 414, 115–130. [Google Scholar] [CrossRef]
- Yan, C.; Xue, Y.; Wang, Y. Chapter 9—Capillary electrochromatography. In Separation Methods; Handbooks in Separation Science; Elsevier: Amsterdam, The Netherlands, 2018; pp. 209–233. [Google Scholar]
- Palmer, C.P.; McCarney, J.P. Recent progress in the use of soluble ionic polymers as pseudostationary phases for electrokinetic chromatography. Electrophoresis 2004, 25, 4086–4094. [Google Scholar] [CrossRef] [PubMed]
- The Global Market for Nanomaterials 2010–2030. Available online: https://www.futuremarketsinc.com/the-global-market-for-nanomaterials-2010-2022 (accessed on 9 April 2023).
- Nanomaterials Market—Growth, Tends, COVID-19 Impact, and Forecasts (2023–2028). Available online: https://www.mordorintelligence.com/industry-reports/nanomaterials-market (accessed on 9 April 2023).
- Tenson, T.J.; Baby, R. Recent advances in proton exchange membrane fuel cells: A view. Int. Adv. Res. J. Sci. Eng. Technol. 2017, 4, 34–40. [Google Scholar]
- Wang, T.; Bao, Y.; Zhuang, M.; Li, J.; Chen, J.; Xu, H. Nanoscale engineering of conducting polymers for emerging applications in soft electronics. Nano Res. 2021, 14, 3112–3125. [Google Scholar] [CrossRef]
- Juttner, K.; Mangold, K.-M.; Weidlich, C.; Leuze, M.; Bouzek, K. Nano-scale conducting polymers as ion exchangers and electrocatalyst support. Proc. Electrochem. Soc. 2001, 2001–2023, 428–437. [Google Scholar]
- Mecking, S.; Schwab, E.; Thomann, R. Microheterogenization of catalytically active rhodium complexes by ionic binding to charged polymer nanoparticles. In Proceedings of the Abstract Papers, American Chemical Society—221st National Meeting, San Diego, CA, USA, 1–5 April 2001. INOR-285. [Google Scholar]
- Sun, X.; Wang, Q.; Zhan, J.; Yang, T.; Zhao, Y.; Sun, C.K.; Aisha, M.; Guo, M.; Tang, S.; Zhao, H.; et al. Superhydrophobic conductive suede fabrics based on carboxylated multiwalled carbon nanotubes and polydopamine for wearable pressure sensors. ACS Appl. Nano Mater. 2023, 6, 10746–10757. [Google Scholar] [CrossRef]
- Grumezescu, A.M. (Ed.) Design of Nanostructures for Theranostics Applications; Elsevier: Amsterdam, Netherlands, 2018; p. 666. [Google Scholar]
- Tekade, R.K. (Ed.) Basic Fundamentals of Drug Delivery; Academic Press: Cambridge, USA, 2019; p. 792. [Google Scholar]
- Mohajer, F.; Mirhosseini-Eshkevari, B.; Ahmadi, S.; Ghasemzadeh, M.A.; Mohammadi Ziarani, G.; Badiei, A.; Farshidfar, N.; Varma, R.S.; Rabiee, N.; Iravani, S. Advanced nanosystems for cancer therapeutics: A review. ACS Appl. Nano Mater. 2023, 6, 7123–7149. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.; Wang, T.; Xiong, F.; Sun, C.R.; Yao, X.K.; Huang, W. Platinum drug-incorporating polymeric nanosystems for precise cancer therapy. Small 2023, 19, 2208241. [Google Scholar] [CrossRef]
- Naskar, S.; Koutsu, K.; Sharma, S. Chitosan-based nanoparticles as drug delivery systems: A review on two decades of research. J. Drug Target. 2018, 27, 379–393. [Google Scholar] [CrossRef]
- Shanmuganathan, R.; Edison, N.T.J.I.; Lewis Oscar, F.; Kumar, P.; Shanmugan, S.; Pugazhendhi, A. Chitosan nanopolymers: An overview of drug delivery against cancer. Int. J. Biol. Macromol. 2019, 130, 727–736. [Google Scholar] [CrossRef]
- Wang, S.; Xu, J.; Li, W.; Sun, S.; Gao, S.; Hou, Y. Magnetic nanostructures: Rational design and fabrication strategies toward diverse applications. Chem. Rev. 2022, 122, 5411–5475. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-C.; Huang, S.-Y.; Fan, W.-L.; Lee, A.-W.; Chiu, C.-W.; Lee, D.-J.; Lai, J.-Y. Water-soluble single-chain polymeric nanoparticles for highly selective cancer chemotherapy. ACS Appl. Polym. Mater. 2021, 3, 474–484. [Google Scholar] [CrossRef]
- Sogomonyan, A.S.; Deyev, S.M.; Shipunova, V.O. Internalization-responsive poly(lactic-co-glycolic acid) nanoparticles for image-guided photodynamic therapy against HER2-positive breast cancer. ACS Appl. Nano Mater. 2023, 6, 11402–11415. [Google Scholar] [CrossRef]
- Nayanathara, U.; Rossi Herling, B.; Ansari, N.; Zhang, C.; Logan, S.R.; Beach, M.A.; Smith, S.A.; Boase, N.R.B.; Johnston, A.P.R.; Such, G.K. Impact of the core architecture of dual pH-responsive polymer nanoparticles on intracellular delivery of doxorubicin. ACS Appl. Nano Mater. 2023, 6, 10015–10022. [Google Scholar] [CrossRef]
- Zhang, L.; Li, M.; Wang, Y.; Liu, Y.; Zhang, F.; Lin, Z.; Zhang, Y.; Ma, M.; Wang, S. Hollow-polydopamine-nanocarrier-based near-infrared-light/pH-responsive drug delivery system for diffusive alveolar hemorrhage treatment. Front. Chem. 2023, 11, 1222107. [Google Scholar] [CrossRef]
- Ou, H.; Li, J.; Chen, C.; Gao, H.; Ding, D. Organic/polymer photothermal nanoagents for photoacoustic imaging and photothermal therapy in vivo. Sci. China Mater. 2019, 62, 1740–1758. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Q.; Pan, X.; Zhang, J. Insight into fluorescence imaging and bioorthogonal reactions in biological analysis. Top. Curr. Chem. 2021, 379, 10. [Google Scholar] [CrossRef]
- Mieog, J.S.D.; Achterberg, F.B.; Zlitni, A.; Hutteman, M.; Burggraaf, J.; Swijnenburg, R.-J.; Gioux, S.; Vahrmeijer, A.L. Fundamentals and developments in fluorescence-guided cancer surgery. Nat. Rev. Clin. Oncol. 2022, 19, 9–22. [Google Scholar] [CrossRef]
- Poznanski, P.; Hameed, A.; Orczyk, W. Chitosan and chitosan nanoparticles: Parameters enhancing antifungal activity. Molecules 2023, 28, 2996. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F. Properties and applications of halloysite nanotubes: Recent research advances and future prospects. Appl. Clay Sci. 2015, 112–113, 75–93. [Google Scholar] [CrossRef]

| Matrix | Ionizable Groups | Fabrication | Shape/Size (nm) | Properties | Application | Ref. |
|---|---|---|---|---|---|---|
| PS | Sulfonic acid groups (SG) | Emulsion or emulsifier-free polymerization | Spherical/40–90 | IEC, 0.7–2.2 meq g−1 | - | [21] |
| PS | Trimethylamine groups | Emulsion polymerization followed by surface functionalization | Spherical/150 | IEC, 2.35 mmol g−1 | Binding of BSA (after the preparation of magnetic/ion-exchange composite beads) | [11] |
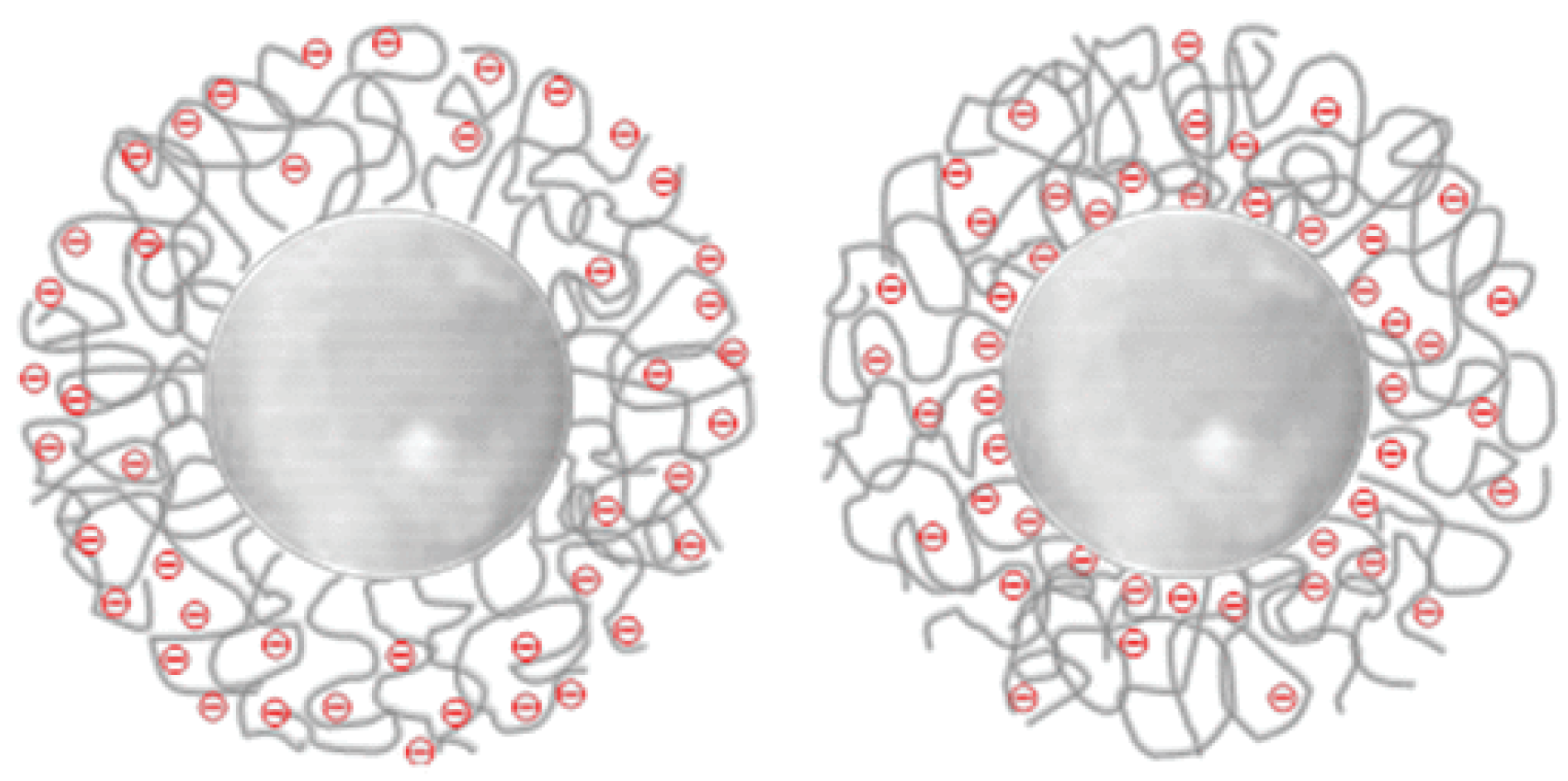
| Polystyrene modified with sodium styrene sulfonate | SG | Emulsion polymerization followed by surface modification by a two-step polymerization reaction | Spherical/30–80 | Surface charge density, 5.9–27.7 µC cm−2 | - | [22] |
| Polypyrrole grafted onto functionalized silica gel | Pyrrolium groups | Micelle technique | Opal-like/100 | IEC, 1.78 meq g−1 | Adsorption of Cr(VI) | [12] |
| Poly-d,l-aspartic acid | Carboxylic groups | Commercial product (Chemicell GmbH, Berlin, Germany) | Spherical/100 | - | Capturing of bacteria (after coating on magnetite nanoparticles) | [23] |
| PMMA covered with poly(N-isopropylacrylamide) | Carboxylic groups | Two-stage emulsion polymerization | Spherical/90–260 | - | - | [24] |
| Chitosan | Tripolyphosphate groups | Ionic gelation | Nonspherical/105–209 | - | - | [25] |
| PMMA, poly(d,l-lactide-co-glycolide) or polylcaprolactone | Carboxylate, sulfonate or trimethylammonium groups | Functionalization and nanoprecipitation | Spherical/15 | - | Loading of a fluorescent contrast agent (after postmodification by surfactants) | [26] |
| PS | SG or trimethylamine groups | Grinding of commercial cation or anion exchanger | Nonspherical/50–300 | IEC, 0.1 and 0.95 meq mL−1 for cation and anion NIE, respectively | Luminescence analysis of metals | [27] |
| CE of inorganic anions (in urine) | [28] | |||||
| CEC of carboxylic acids (in wine) | [29] | |||||
| IC of alkali metals and ammonium | [30,31] | |||||
| ICP-OES of metals | [32] | |||||
| CE of catecholamines and amino acids (in urine) | [33] | |||||
| CEC of catecholamines and amino acids | [34] | |||||
| CEC of carboxylic acids (in wine) | [35] | |||||
| Loading of an anticancer drug (doxorubicin) | [36] | |||||
| Loading of an anticancer drug (cisplatin) | [37] | |||||
| Aliphatic polyester | SG | Polyesterification | Platelet/400 | - | - | [38] |
| Poly(stearyl methacrylate)– poly(benzyl methacrylate) with incorporated 2-((methacryloyloxy)ethyl)-trimethylammonium | Trimethylamine groups | Polymerization-induced self-assembly | Spherical and nonspherical (worms, vesicles) | - | - | [39] |
| Chitosan | SG | Extraction by HCl (from shell chitin), dialysis, freeze-drying, modification by propane- 1,3-sultone | Whiskers/diameter, 15–30; length, 150–300 | IEC, 0.60–0.91 mmol g−1 | Preparation of nanocomposite polymer membranes for direct methanol fuel cell | [40] |
| Copolymers of styrene, acrylic acid, N-dimethyl acryl amide, and methyl allyl polyoxyethylene ether | Carboxylic groups | Precipitation polymerization | Nonsperical/7–146 | Charge density, 0.09–1.5 meq g−1 | Modification of the rheological properties of cement pastes | [41] |
| Poly(lactic acid) covered with methyl methacrylate polymer | Carboxylic groups | Flash nanoprecipitation | Spherical/59–454 | Surface charge (no data) | Loading of antimalarial drug (lumefantrine) | [42] |
| Halloysite | SG | Direct sulfonation or organosilylation and sulfonation | Tubes | - | SPE of pyrrolizidine alkaloids | [43,44] |
| Poly(2-(dimethylamino) ethyl methacrylate-co-4-vinylbenzyl chloride) | Quaternary ammonium groups | Quarterization precipitation polymerization | Spherical/50–80 | - | Oil/water emulsion separation (after the incorporation into polysulfone ultrafiltration membranes) | [45] |
| Advantages | Disadvantages |
|---|---|
| Large specific surface area High IEC Surface-to-volume area Reactivity Stability in suspension Rational design strategies Solid preparation technology Reusability Wealth of analytically and technically attractive properties | Outdated methodology for assessing ion-exchange properties Shortage of large-scale manufacturing approaches No real-world applications for environmental remediation Uneasiness of NIE-based devices and unitsMarginal biosafety Lack of targeted delivery function |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matczuk, M.; Ruzik, L.; Keppler, B.K.; Timerbaev, A.R. Nanoscale Ion-Exchange Materials: From Analytical Chemistry to Industrial and Biomedical Applications. Molecules 2023, 28, 6490. https://doi.org/10.3390/molecules28186490
Matczuk M, Ruzik L, Keppler BK, Timerbaev AR. Nanoscale Ion-Exchange Materials: From Analytical Chemistry to Industrial and Biomedical Applications. Molecules. 2023; 28(18):6490. https://doi.org/10.3390/molecules28186490
Chicago/Turabian StyleMatczuk, Magdalena, Lena Ruzik, Bernhard K. Keppler, and Andrei R. Timerbaev. 2023. "Nanoscale Ion-Exchange Materials: From Analytical Chemistry to Industrial and Biomedical Applications" Molecules 28, no. 18: 6490. https://doi.org/10.3390/molecules28186490
APA StyleMatczuk, M., Ruzik, L., Keppler, B. K., & Timerbaev, A. R. (2023). Nanoscale Ion-Exchange Materials: From Analytical Chemistry to Industrial and Biomedical Applications. Molecules, 28(18), 6490. https://doi.org/10.3390/molecules28186490





