Rafting on the Evidence for Lipid Raft-like Domains as Hubs Triggering Environmental Toxicants’ Cellular Effects
Abstract
1. Introduction
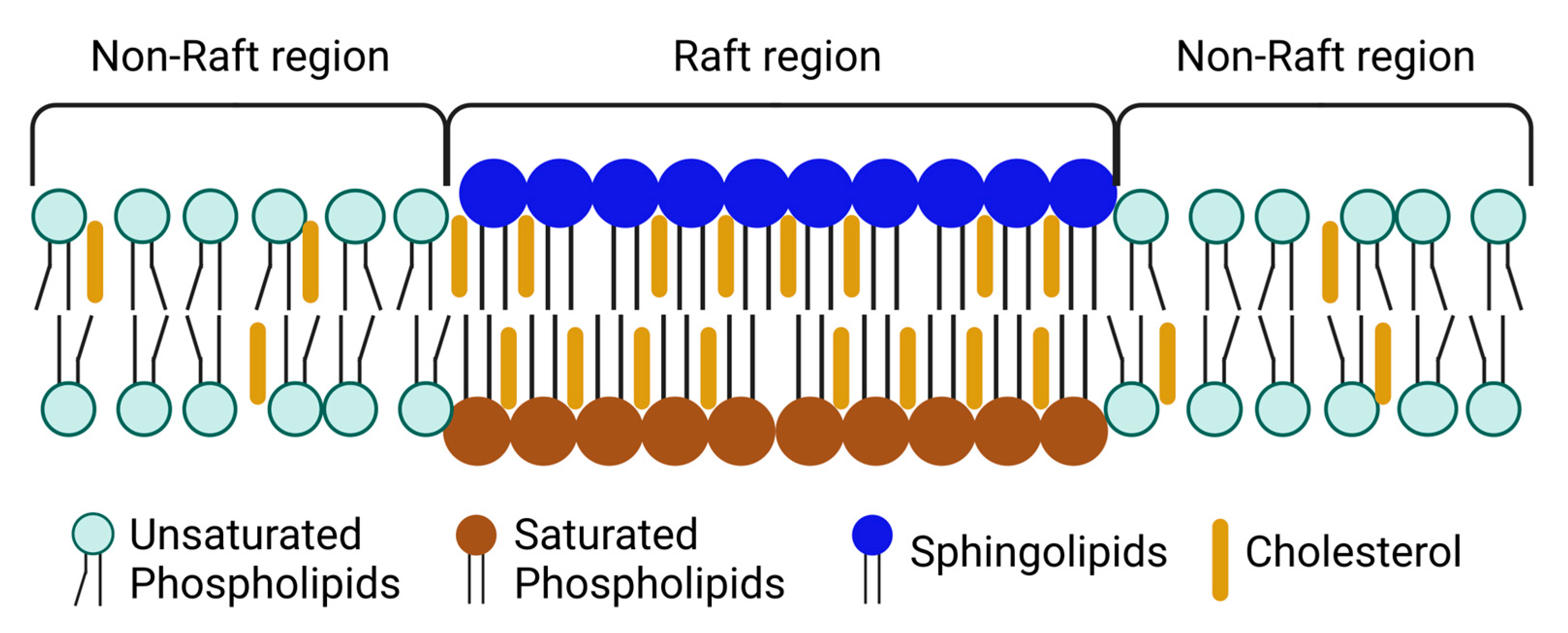
2. Lipid Rafts—Structure and Composition
2.1. Plasma Membrane Domains
2.2. Properties of Lipid Rafts and Composition
2.3. Lipid Rafts as Platforms for Signal Transduction in Cells
3. Lipid Rafts in Cell Signaling and Disease
3.1. Calcium and Redox Signaling in Neurodegeneration
3.2. Inflammation and Atherosclerosis
3.3. Immune Regulation
3.4. Hormone Signaling
3.5. Cell Communication
3.6. Cell Death and Cancer
4. Effects of Environmental Toxicants in Lipid Rafts Organization and Signaling
4.1. Accumulation of Environmental Toxicants in Lipid Rafts and Associated Cellular Effects
4.2. Alterations in Rafts’ Lipid Composition and Associated Cellular Effects
4.2.1. Disruption of Lipid Rafts
4.2.2. Alterations in Membrane Lipids
4.2.3. Alterations in the Levels of Raft-Related Lipids
4.3. Alterations in Rafts’ Proteome and Associated Cellular Effects
4.3.1. Alterations in Lipid Raft-Associated Proteins
4.3.2. Recruitment and Aggregation of Proteins in Lipid Rafts
4.3.3. Activation of Other Signaling Pathways

5. Conclusions and Future Perspectives
- When studying the effect of a toxicant in lipid rafts, several effects can be considered for research since they have already been reported to be common to different toxicant exposures. They include alteration of lipid raft composition (lipids and proteins), alteration of cholesterol content and membrane fluidity, recruitment or displacement of proteins from these membrane domains, and oxidative stress.
- The accumulation of toxicants in lipid rafts is not a clear point, with only PCB77 being reported to accumulate in these membrane domains [102]. Nevertheless, if similar behavior could be demonstrated for additional toxicants, it would strengthen the relevance of these lipid structures in environmental toxicology.
- In the case of exposure to B[a]P, alteration of GM1 and raft protein localization is reported. With this in mind, investigating the effect of other environmental toxicants on the distribution of GM1 can be highly interesting if we consider that the location of GM1 near membrane channels affects their activity [67,68]. Moreover, the effect of B[a]P on the relocation of NHE-1 outside lipid rafts, with consequences for its apoptotic function [151], may be translated to other toxicants.
- The activation of a specific GPCR type via membrane clustering after exposure to vanadium [119], together with the wide range of ligands binding to these receptors and the vast signaling associated, makes these receptors a potential trigger for environmental toxicants, but whether this depends on lipid raft structure is a hypothesis that deserves to be studied.
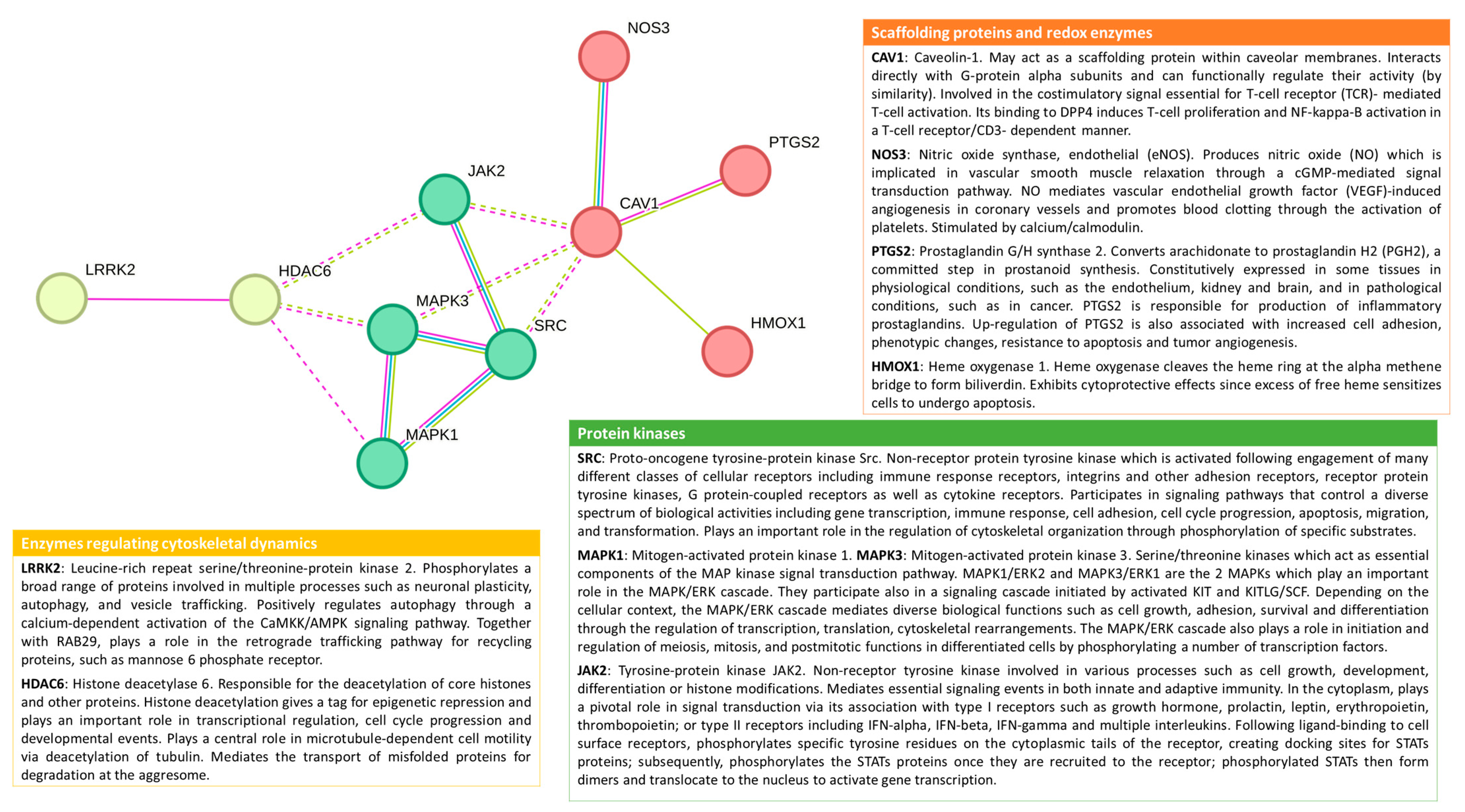
- Another research dimension to be explored is how environmental toxicants can interfere with the interplay of different signals coming from proteins associated with lipid rafts. For example, the lipid rafts are near enzymatic systems producing ROS, like NADPH oxidases and nitric oxide synthases [16,66,78]. This gains even more relevance if we consider that different environmental toxicants are ligands of AhR, leading to activation of membrane NADPH oxidases [4,141].
- AhR is a reported target of PCBs, PAHs, PM, and persistent organic pollutants [140,141,146] and the non-canonical AhR signaling pathway [14,141,142] deserves to be explored for the effects of additional environmental toxicants. More specific data is needed to understand how membrane rafts/caveola modulate the activity of transcription factors like AhR and Nrf2, which are highly implicated in the cellular effects of environmental toxicants.
- Finally, to address the role of lipid rafts in triggering a specific cellular mechanism after any toxic exposure, it is important to employ different cell models expressing different membrane receptors and signaling components at lipid rafts since signaling differences were identified in this work. For example, in endothelial cells, the toxicant effects described to be associated with lipid rafts are changes in Nrf2 signaling, an increase in MCP-1 associated with AhR, p38, and JNK signaling pathways, and the displacement of occludin from lipid rafts is also described [140,145,164]. Nevertheless, in neurons, the involvement of lipid rafts in oxidative stress and the increase of cell calcium are potential cellular mechanisms affected by exposure to harmful compounds [13,17,77].
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Simons, K.; Toomre, D. Lipid Rafts and Signal Transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Placidi, G.; Campa, C.C. Deliver on Time or Pay the Fine: Scheduling in Membrane Trafficking. Int. J. Mol. Sci. 2021, 22, 11773. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, J.D.; Subramanian, T.; Liu, K.; King, M.R. Rafting down the Metastatic Cascade: The Role of Lipid Rafts in Cancer Metastasis, Cell Death, and Clinical Outcomes. Cancer Res. 2021, 81, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Lagoa, R.; Marques-da-Silva, D.; Diniz, M.; Daglia, M.; Bishayee, A. Molecular Mechanisms Linking Environmental Toxicants to Cancer Development: Significance for Protective Interventions with Polyphenols. Semin. Cancer Biol. 2022, 80, 118–144. [Google Scholar] [CrossRef]
- Marques-da-Silva, D.; Videira, P.A.; Lagoa, R. Registered Human Trials Addressing Environmental and Occupational Toxicant Exposures: Scoping Review of Immunological Markers and Protective Strategies. Environ. Toxicol. Pharmacol. 2022, 93, 103886. [Google Scholar] [CrossRef]
- Aminov, Z.; Haase, R.F.; Pavuk, M.; Carpenter, D.O. Analysis of the Effects of Exposure to Polychlorinated Biphenyls and Chlorinated Pesticides on Serum Lipid Levels in Residents of Anniston, Alabama. Environ. Health 2013, 12, 108. [Google Scholar] [CrossRef]
- Adetona, A.M.; Adetona, O.; Gogal, R.M.; Diaz-Sanchez, D.; Rathbun, S.L.; Naeher, L.P. Impact of Work Task-Related Acute Occupational Smoke Exposures on Select Proinflammatory Immune Parameters in Wildland Firefighters. J. Occup. Environ. Med. 2017, 59, 679–690. [Google Scholar] [CrossRef]
- Goyal, T.; Mitra, P.; Singh, P.; Ghosh, R.; Sharma, S.; Sharma, P. Association of MicroRNA Expression with Changes in Immune Markers in Workers with Cadmium Exposure. Chemosphere 2021, 274, 129615. [Google Scholar] [CrossRef]
- Parks, C.G.; Santos, A.d.S.E.; Lerro, C.C.; DellaValle, C.T.; Ward, M.H.; Alavanja, M.C.; Berndt, S.I.; Beane Freeman, L.E.; Sandler, D.P.; Hofmann, J.N. Lifetime Pesticide Use and Antinuclear Antibodies in Male Farmers from the Agricultural Health Study. Front. Immunol. 2019, 10, 1476. [Google Scholar] [CrossRef]
- Rider, C.F.; Carlsten, C. Air Pollution and DNA Methylation: Effects of Exposure in Humans. Clin. Epigenetics 2019, 11, 131. [Google Scholar] [CrossRef]
- Rubini, E.; Minacori, M.; Paglia, G.; Macone, A.; Chichiarelli, S.; Altieri, F.; Eufemi, M. Tomato and Olive Bioactive Compounds: A Natural Shield against the Cellular Effects Induced by β-Hexachlorocyclohexane-Activated Signaling Pathways. Molecules 2021, 26, 7135. [Google Scholar] [CrossRef] [PubMed]
- Renu, K.; Mukherjee, A.G.; Wanjari, U.R.; Vinayagam, S.; Veeraraghavan, V.P.; Vellingiri, B.; George, A.; Lagoa, R.; Sattu, K.; Dey, A.; et al. Misuse of Cardiac Lipid upon Exposure to Toxic Trace Elements—A Focused Review. Molecules 2022, 27, 5657. [Google Scholar] [CrossRef] [PubMed]
- Fortalezas, S.; Marques-da-Silva, D.; Gutierrez-Merino, C. Creatine Protects Against Cytosolic Calcium Dysregulation, Mitochondrial Depolarization and Increase of Reactive Oxygen Species Production in Rotenone-Induced Cell Death of Cerebellar Granule Neurons. Neurotox. Res. 2018, 34, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Brinchmann, B.C.; Le Ferrec, E.; Podechard, N.; Lagadic-Gossmann, D.; Shoji, K.F.; Penna, A.; Kukowski, K. Lipophilic Chemicals from Diesel Exhaust Particles Trigger Calcium Response in Human Endothelial Cells via Aryl Hydrocarbon Receptor Non-Genomic Signalling. Int. J. Mol. Sci. 2018, 19, 1429. [Google Scholar] [CrossRef]
- Clark, R.S.; Pellom, S.T.; Booker, B.; Ramesh, A.; Zhang, T.; Shanker, A.; Maguire, M.; Juarez, P.D.; Patricia, M.J.; Langston, M.A.; et al. Validation of Research Trajectory 1 of an Exposome Framework: Exposure to Benzo(a)Pyrene Confers Enhanced Susceptibility to Bacterial Infection. Environ. Res. 2016, 146, 173–184. [Google Scholar] [CrossRef]
- Eum, S.Y.; Andras, I.; Hennig, B.; Toborek, M. NADPH Oxidase and Lipid Raft-Associated Redox Signaling Are Required for PCB153-Induced Upregulation of Cell Adhesion Molecules in Human Brain Endothelial Cells. Toxicol. Appl. Pharmacol. 2009, 240, 299–305. [Google Scholar] [CrossRef]
- Cacciottolo, M.; Morgan, T.E.; Saffari, A.A.; Shirmohammadi, F.; Forman, H.J.; Sioutas, C.; Finch, C.E. Traffic-Related Air Pollutants (TRAP-PM) Promote Neuronal Amyloidogenesis through Oxidative Damage to Lipid Rafts. Free Radic. Biol. Med. 2020, 147, 242–251. [Google Scholar] [CrossRef]
- Tekpli, X.; Rissel, M.; Huc, L.; Catheline, D.; Sergent, O.; Rioux, V.; Legrand, P.; Holme, J.A.; Dimanche-Boitrel, M.T.; Lagadic-Gossmann, D. Membrane Remodeling, an Early Event in Benzo[α]Pyrene-Induced Apoptosis. Toxicol. Appl. Pharmacol. 2010, 243, 68–76. [Google Scholar] [CrossRef]
- De Gregorio, F.; Pellegrino, M.; Picchietti, S.; Belardinelli, M.C.; Taddei, A.R.; Fausto, A.M.; Rossi, M.; Maggio, R.; Giorgi, F. The Insecticide 1,1,1-Trichloro-2,2-Bis(p-Chlorophenyl) Ethane (DDT) Alters the Membrane Raft Location of the TSH Receptor Stably Expressed in Chinese Hamster Ovary Cells. Toxicol. Appl. Pharmacol. 2011, 253, 121–129. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, W.; Zhou, J.; Chen, M.; Huang, X.; Zhu, Y.; Xie, X.; Li, W.; Zhang, Y.; Kan, H.; et al. Metabolomics Analysis of a Mouse Model for Chronic Exposure to Ambient PM2.5. Environ. Pollut. 2019, 247, 953–963. [Google Scholar] [CrossRef]
- Singh, D.P.; Begum, R.; Kaur, G.; Bagam, P.; Kambiranda, D.; Singh, R.; Batra, S. E-Cig Vapor Condensate Alters Proteome and Lipid Profiles of Membrane Rafts: Impact on Inflammatory Responses in A549 Cells. Cell Biol. Toxicol. 2021, 37, 773–793. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Kumar, M.; Dhatwalia, S.K.; Garg, M.L.; Dhawan, D.K. Acetyl-11-Keto-β-Boswellic Acid Modulates Membrane Dynamics in Benzo(a)Pyrene-Induced Lung Carcinogenesis. Mol. Cell. Biochem. 2019, 460, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Notariale, R.; Längst, E.; Perrone, P.; Crettaz, D.; Prudent, M.; Manna, C. Effect of Mercury on Membrane Proteins Anionic Transport and Cell Morphology in Human Erythrocytes. Cell. Physiol. Biochem. 2022, 56, 500–513. [Google Scholar] [CrossRef]
- Brown, D.A.; London, E. Structure and Origin of Ordered Lipid Domains in Biological Membranes. J. Membr. Biol. 1998, 164, 103–114. [Google Scholar] [CrossRef]
- Hjort Ipsen, J.; Karlström, G.; Mourtisen, O.G.; Wennerström, H.; Zuckermann, M.J. Phase Equilibria in the Phosphatidylcholine-Cholesterol System. BBA—Biomembr. 1987, 905, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Pike, L.J. Lipid Rafts: Bringing Order to Chaos. J. Lipid Res. 2003, 44, 655–667. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional Rafts in Cell Membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Waheed, A.A.; Freed, E.O. The Role of Lipids in Retroviral Replication. In Retrovirus-Cell Interactions; Parent, L.J., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 353–399. [Google Scholar] [CrossRef]
- Shankar, J.; Boscher, C.; Nabi, I.R. Caveolin-1, Galectin-3 and Lipid Raft Domains in Cancer Cell Signalling. Essays Biochem. 2015, 57, 189–201. [Google Scholar] [CrossRef]
- Bartlett, K.; Kim, K. Insight into Tor2, a Budding Yeast Microdomain Protein. Eur. J. Cell Biol. 2014, 93, 87–97. [Google Scholar] [CrossRef]
- Krapf, D. Compartmentalization of the Plasma Membrane. Curr. Opin. Cell Biol. 2018, 53, 15–21. [Google Scholar] [CrossRef]
- Godoy, V.; Riquelme, G. Distinct Lipid Rafts in Subdomains from Human Placental Apical Syncytiotrophoblast Membranes. J. Membr. Biol. 2008, 224, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Toledo, A.; Huang, Z.; Coleman, J.L.; London, E.; Benach, J.L. Lipid Rafts Can Form in the Inner and Outer Membranes of Borrelia Burgdorferi and Have Different Properties and Associated Proteins. Mol. Microbiol. 2018, 108, 63. [Google Scholar] [CrossRef] [PubMed]
- Blouin, C.M.; Prado, C.; Takane, K.K.; Lasnier, F.; Garcia-Ocana, A.; Ferré, P.; Dugail, I.; Hajduch, E. Plasma Membrane Subdomain Compartmentalization Contributes to Distinct Mechanisms of Ceramide Action on Insulin Signaling. Diabetes 2010, 59, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G. Caveolae: Structure, Function, and Relationship to Disease. Annu. Rev. Cell Dev. Biol. 2018, 34, 111–136. [Google Scholar] [CrossRef]
- Yamada, E. The Fine Structure of the Fall Bladder Epithelium of the Mouse. J. Biophys. Biochem. Cytol. 1955, 1, 445. [Google Scholar] [CrossRef]
- Razani, B.; Woodman, S.E.; Lisanti, M.P. Caveolae: From Cell Biology to Animal Physiology. Pharmacol. Rev. 2002, 54, 431–467. [Google Scholar] [CrossRef]
- Martinez-Outschoorn, U.E.; Sotgia, F.; Lisanti, M.P. Caveolae and Signalling in Cancer. Nat. Rev. Cancer 2015, 15, 225–237. [Google Scholar] [CrossRef]
- Hansen, C.G.; Nichols, B.J. Exploring the Caves: Cavins, Caveolins and Caveolae. Trends Cell Biol. 2010, 20, 177–186. [Google Scholar] [CrossRef]
- Filippini, A.; D’alessio, A. Caveolae and Lipid Rafts in Endothelium: Valuable Organelles for Multiple Functions. Biomolecules 2020, 10, 1218. [Google Scholar] [CrossRef]
- Parton, R.G.; Tillu, V.A.; Collins, B.M. Caveolae. Curr. Biol. 2018, 28, R402–R405. [Google Scholar] [CrossRef]
- Sevcsik, E.; Schütz, G.J. With or without Rafts? Alternative Views on Cell Membranes. BioEssays 2016, 38, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Levental, I.; Levental, K.R.; Heberle, F.A. Lipid Rafts: Controversies Resolved, Mysteries Remain. Trends Cell Biol. 2020, 30, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Klotzsch, E.; Schütz, G.J. A Critical Survey of Methods to Detect Plasma Membrane Rafts. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120033. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.G.N.; Kusumi, A. Refinement of Singer-Nicolson Fluid-Mosaic Model by Microscopy Imaging: Lipid Rafts and Actin-Induced Membrane Compartmentalization. Biochim. Biophys. Acta—Biomembr. 2023, 1865, 184093. [Google Scholar] [CrossRef]
- Kusumi, A.; Fujiwara, T.K.; Tsunoyama, T.A.; Kasai, R.S.; Liu, A.A.; Hirosawa, K.M.; Kinoshita, M.; Matsumori, N.; Komura, N.; Ando, H.; et al. Defining Raft Domains in the Plasma Membrane. Traffic 2020, 21, 106–137. [Google Scholar] [CrossRef] [PubMed]
- Regen, S.L. The Origin of Lipid Rafts. Biochemistry 2020, 59, 4617–4621. [Google Scholar] [CrossRef]
- Simons, K.; Ehehalt, R. Cholesterol, Lipid Rafts, and Disease. J. Clin. Investig. 2002, 110, 597–603. [Google Scholar] [CrossRef]
- Kraft, M.L. Sphingolipid Organization in the Plasma Membrane and the Mechanisms That Influence It. Front. Cell Dev. Biol. 2017, 4, 154. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Sampaio, J.L. Membrane Organization and Lipid Rafts. Cold Spring Harb. Perspect. Biol. 2011, 3, a004697. [Google Scholar] [CrossRef]
- Chang, W.J.; Rothberg, K.G.; Kamen, B.A.; Anderson, R.G.W. Lowering the Cholesterol Content of MA104 Cells Inhibits Receptor-Mediated Transport of Folate. J. Cell Biol. 1992, 118, 63–69. [Google Scholar] [CrossRef]
- Ouweneel, A.B.; Thomas, M.J.; Sorci-Thomas, M.G. The Ins and Outs of Lipid Rafts: Functions in Intracellular Cholesterol Homeostasis, Microparticles, and Cell Membranes. J. Lipid Res. 2020, 61, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Fessler, M.B.; Parks, J.S. Intracellular Lipid Flux and Membrane Microdomains as Organizing Principles in Inflammatory Cell Signaling. J. Immunol. 2011, 187, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Fadeyibi, O.; Rybalchenko, N.; Mabry, S.; Nguyen, D.H.; Cunningham, R.L. The Role of Lipid Rafts and Membrane Androgen Receptors in Androgen’s Neurotoxic Effects. J. Endocr. Soc. 2022, 6, bvac030. [Google Scholar] [CrossRef]
- Premasekharan, G.; Nguyen, K.; Contreras, J.; Ramon, V.; Leppert, V.J.; Forman, H.J. Iron-Mediated Lipid Peroxidation and Lipid Raft Disruption in Low-Dose Silica-Induced Macrophage Cytokine Production. Free Radic. Biol. Med. 2011, 51, 1184–1194. [Google Scholar] [CrossRef]
- Duan, F.; Zeng, C.; Liu, S.; Gong, J.; Hu, J.; Li, H.; Tan, H. A1-NAchR-Mediated Signaling Through Lipid Raft Is Required for Nicotine-Induced NLRP3 Inflammasome Activation and Nicotine-Accelerated Atherosclerosis. Front. Cell Dev. Biol. 2021, 9, 724699. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tao, J.; Duan, F.; Li, H.; Tan, H. HHcy Induces Pyroptosis and Atherosclerosis via the Lipid Raft-Mediated NOX-ROS-NLRP3 Inflammasome Pathway in ApoE−/− Mice. Cells 2022, 11, 2438. [Google Scholar] [CrossRef]
- Fortalezas, S.; Marques-da-Silva, D.; Gutierrez-Merino, C. Methyl-β-Cyclodextrin Impairs the Phosphorylation of the Β2 Subunit of L-Type Calcium Channels and Cytosolic Calcium Homeostasis in Mature Cerebellar Granule Neurons. Int. J. Mol. Sci. 2018, 19, 3667. [Google Scholar] [CrossRef]
- Dietrich, C.; Bagatolli, L.A.; Volovyk, Z.N.; Thompson, N.L.; Levi, M.; Jacobson, K.; Gratton, E. Lipid Rafts Reconstituted in Model Membranes. Biophys. J. 2001, 80, 1417–1428. [Google Scholar] [CrossRef]
- Palestini, P.; Calvi, C.; Conforti, E.; Daffara, R.; Botto, L.; Miserocchi, G. Compositional Changes in Lipid Microdomains of Air-Blood Barrier Plasma Membranes in Pulmonary Interstitial Edema. J. Appl. Physiol. 2003, 95, 1446–1452. [Google Scholar] [CrossRef][Green Version]
- Ledeen, R.W.; Wu, G. The Multi-Tasked Life of GM1 Ganglioside, a True Factotum of Nature. Trends Biochem. Sci. 2015, 40, 407–418. [Google Scholar] [CrossRef]
- Van Heyningen, S. Cholera Toxin: Interaction of Subunits with Ganglioside GM1. Science 1974, 183, 656–657. [Google Scholar] [CrossRef] [PubMed]
- Day, C.A.; Kenworthy, A.K. Functions of Cholera Toxin B-Subunit as a Raft Cross-Linker. Essays Biochem. 2015, 57, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Kenworthy, A.K.; Schmieder, S.S.; Raghunathan, K.; Tiwari, A.; Wang, T.; Kelly, C.V.; Lencer, W.I. Cholera Toxin as a Probe for Membrane Biology. Toxins 2021, 13, 543. [Google Scholar] [CrossRef] [PubMed]
- Samhan-Arias, A.K.; López-Sánchez, C.; Marques-da-Silva, D.; Lagoa, R.; Garcia-Lopez, V.; García-Martínez, V.; Gutierrez-Merino, C. High Expression of Cytochrome b5 Reductase Isoform 3/Cytochrome b5 System in the Cerebellum and Pyramidal Neurons of Adult Rat Brain. Brain Struct. Funct. 2016, 221, 2147–2162. [Google Scholar] [CrossRef]
- Samhan-Arias, A.K.; Garcia-Bereguiain, M.A.; Martin-Romero, F.J.; Gutierrez-Merino, C. Clustering of Plasma Membrane-Bound Cytochrome B5 Reductase within “lipid Raft” Microdomains of the Neuronal Plasma Membrane. Mol. Cell. Neurosci. 2009, 40, 14–26. [Google Scholar] [CrossRef]
- Puljko, B.; Stojanović, M.; Ilic, K.; Kalanj-Bognar, S.; Mlinac-Jerkovic, K. Start Me Up: How Can Surrounding Gangliosides Affect Sodium-Potassium ATPase Activity and Steer towards Pathological Ion Imbalance in Neurons? Biomedicines 2022, 10, 1518. [Google Scholar] [CrossRef]
- Ilic, K.; Lin, X.; Malci, A.; Stojanović, M.; Puljko, B.; Rožman, M.; Vukelić, Ž.; Heffer, M.; Montag, D.; Schnaar, R.L.; et al. Plasma Membrane Calcium ATPase-Neuroplastin Complexes Are Selectively Stabilized in GM1-Containing Lipid Rafts. Int. J. Mol. Sci. 2021, 22, 13590. [Google Scholar] [CrossRef]
- Lucero, H.A.; Robbins, P.W. Lipid Rafts-Protein Association and the Regulation of Protein Activity. Arch. Biochem. Biophys. 2004, 426, 208–224. [Google Scholar] [CrossRef]
- Kurzchalia, T.V.; Parton, R.G. Membrane Microdomains and Caveolae. Curr. Opin. Cell Biol. 1999, 11, 424–431. [Google Scholar] [CrossRef]
- Galbiati, F.; Razani, B.; Lisanti, M.P. Emerging Themes in Lipid Rafts and Caveolae. Cell 2001, 106, 403–411. [Google Scholar] [CrossRef]
- Vassilieva, E.V.; Ivanov, A.I.; Nusrat, A. Flotillin-1 Stabilizes Caveolin-1 in Intestinal Epithelial Cells. Biochem. Biophys. Res. Commun. 2009, 379, 460–465. [Google Scholar] [CrossRef]
- Foster, L.J.; De Hoog, C.L.; Mann, M. Unbiased Quantitative Proteomics of Lipid Rafts Reveals High Specificity for Signaling Factors. Proc. Natl. Acad. Sci. USA 2003, 100, 5813–5818. [Google Scholar] [CrossRef]
- Magee, A.I.; Parmryd, I. Detergent-Resistant Membranes and the Protein Composition of Lipid Rafts. Genome Biol. 2003, 4, 234. [Google Scholar] [CrossRef][Green Version]
- Marques-da-Silva, D.; Samhan-Arias, A.K.; Tiago, T.; Gutierrez-Merino, C. L-Type Calcium Channels and Cytochrome B5 Reductase Are Components of Protein Complexes Tightly Associated with Lipid Rafts Microdomains of the Neuronal Plasma Membrane. J. Proteom. 2010, 73, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Marques-da-Silva, D.; Gutierrez-Merino, C. L-Type Voltage-Operated Calcium Channels, N-Methyl-d-Aspartate Receptors and Neuronal Nitric-Oxide Synthase Form a Calcium/Redox Nano-Transducer within Lipid Rafts. Biochem. Biophys. Res. Commun. 2012, 420, 257–262. [Google Scholar] [CrossRef]
- Marques-da-Silva, D.; Gutierrez-Merino, C. Caveolin-Rich Lipid Rafts of the Plasma Membrane of Mature Cerebellar Granule Neurons Are Microcompartments for Calcium/Reactive Oxygen and Nitrogen Species Cross-Talk Signaling. Cell Calcium 2014, 56, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Samhan-Arias, A.K.; Marques-da-Silva, D.; Yanamala, N.; Gutierrez-Merino, C. Stimulation and Clustering of Cytochrome b 5 Reductase in Caveolin-Rich Lipid Microdomains Is an Early Event in Oxidative Stress-Mediated Apoptosis of Cerebellar Granule Neurons. J. Proteom. 2012, 75, 2934–2949. [Google Scholar] [CrossRef] [PubMed]
- Fortalezas, S.; Poejo, J.; Samhan-Arias, A.K.; Gutierrez-Merino, C. Cholesterol-Rich Plasma Membrane Submicrodomains Can Be a Major Extramitochondrial Source of Reactive Oxygen Species in Partially Depolarized Mature Cerebellar Granule Neurons in Culture. J. Neurophysiol. Neurol. Disord. 2019, 5, 1–22. [Google Scholar]
- Tiago, T.; Palma, P.S.; Gutierrez-Merino, C.; Aureliano, M. Peroxynitrite-Mediated Oxidative Modifications of Myosin and Implications on Structure and Function. Free Radic. Res. 2010, 44, 1317–1327. [Google Scholar] [CrossRef]
- Gupta, N.; DeFranco, A.L. Lipid Rafts and B Cell Signaling. Semin. Cell Dev. Biol. 2007, 18, 616–626. [Google Scholar] [CrossRef]
- Delos Santos, R.C.; Garay, C.; Antonescu, C.N. Charming Neighborhoods on the Cell Surface: Plasma Membrane Microdomains Regulate Receptor Tyrosine Kinase Signaling. Cell. Signal. 2015, 27, 1963–1976. [Google Scholar] [CrossRef]
- Janes, P.W.; Ley, S.C.; Magee, A.I.; Kabouridis, P.S. The Role of Lipid Rafts in T Cell Antigen Receptor (TCR) Signalling. Semin. Immunol. 2000, 12, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Maselli, A.; Pierdominici, M.; Vitale, C.; Ortona, E. Membrane Lipid Rafts and Estrogenic Signalling: A Functional Role in the Modulation of Cell Homeostasis. Apoptosis 2015, 20, 671–678. [Google Scholar] [CrossRef]
- Li, B.; Qin, Y.; Yu, X.; Xu, X.; Yu, W. Lipid Raft Involvement in Signal Transduction in Cancer Cell Survival, Cell Death and Metastasis. Cell Prolif. 2022, 55, e13167. [Google Scholar] [CrossRef] [PubMed]
- Mollinedo, F.; Gajate, C. Lipid Rafts as Signaling Hubs in Cancer Cell Survival/Death and Invasion: Implications in Tumor Progression and Therapy. J. Lipid Res. 2020, 61, 611–635. [Google Scholar] [CrossRef] [PubMed]
- Marin, R. Lipid Rafts as Molecular Platforms of Neuronal Toxicity and Survival: Two Sides of the Same Coin. In Lipid Rafts: Properties and Role in Signaling; Nils, T., Sten, J., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2018; ISBN 978-1-53613-624-1. [Google Scholar]
- Moll, T.; Marshall, J.N.G.; Soni, N.; Zhang, S.; Cooper-Knock, J.; Shaw, P.J. Membrane Lipid Raft Homeostasis Is Directly Linked to Neurodegeneration. Essays Biochem. 2021, 65, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.H.; Robb, E.L.; Voltakis, I.; Ho, M.; Evin, G.; Li, Q.X.; Culvenor, J.G.; Masters, C.L.; Cherny, R.A.; Bush, A.I. Paradoxical Condensation of Copper with Elevated β-Amyloid in Lipid Rafts under Cellular Copper Deficiency Conditions. Implications for Alzheimer Disease. J. Biol. Chem. 2009, 284, 21899–21907. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Zhang, J.; Miyazawa, S.; Liu, Q.; Farzan, M.R.; Yao, W.D. Association of Membrane Rafts and Postsynaptic Density: Proteomics, Biochemical, and Ultrastructural Analyses. J. Neurochem. 2011, 119, 64. [Google Scholar] [CrossRef]
- Eid, A.; Mhatre-Winters, I.; Sammoura, F.M.; Edler, M.K.; von Stein, R.; Hossain, M.M.; Han, Y.; Lisci, M.; Carney, K.; Konsolaki, M.; et al. Effects of DDT on Amyloid Precursor Protein Levels and Amyloid Beta Pathology: Mechanistic Links to Alzheimer’s Disease Risk. Environ. Health Perspect. 2022, 130, 87005. [Google Scholar] [CrossRef]
- Morris, G.; Walder, K.; Puri, B.K.; Berk, M.; Maes, M. The Deleterious Effects of Oxidative and Nitrosative Stress on Palmitoylation, Membrane Lipid Rafts and Lipid-Based Cellular Signalling: New Drug Targets in Neuroimmune Disorders. Mol. Neurobiol. 2016, 53, 4638–4658. [Google Scholar] [CrossRef]
- Evangelisti, E.; Wright, D.; Zampagni, M.; Cascella, R.; Fiorillo, C.; Bagnoli, S.; Relini, A.; Nichino, D.; Scartabelli, T.; Nacmias, B.; et al. Lipid Rafts Mediate Amyloid-Induced Calcium Dyshomeostasis and Oxidative Stress in Alzheimer’s Disease. Curr. Alzheimer Res. 2013, 10, 143–153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Poejo, J.; Salazar, J.; Mata, A.M.; Gutierrez-merino, C. Binding of Amyloid β(1–42)-calmodulin Complexes to Plasma Membrane Lipid Rafts in Cerebellar Granule Neurons Alters Resting Cytosolic Calcium Homeostasis. Int. J. Mol. Sci. 2021, 22, 1984. [Google Scholar] [CrossRef]
- Mattson, M.P.; Chan, S.L. Dysregulation of Cellular Calcium Homeostasis in Alzheimer’s Disease: Bad Genes and Bad Habits. J. Mol. Neurosci. 2001, 17, 205–224. [Google Scholar] [CrossRef] [PubMed]
- Sattler, R.; Tymianski, M. Molecular Mechanisms of Calcium-Dependent Excitotoxicity. J. Mol. Med. 2000, 78, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.W. Calcium: Still Center-Stage in Hypoxic-Ischemic Neuronal Death. Trends Neurosci. 1995, 18, 58–60. [Google Scholar] [CrossRef]
- Jiang, L.; Fernandes, D.; Mehta, N.; Bean, J.L.; Michaelis, M.L.; Zaidi, A. Partitioning of the Plasma Membrane Ca2+-ATPase into Lipid Rafts in Primary Neurons: Effects of Cholesterol Depletion. J. Neurochem. 2007, 102, 378–388. [Google Scholar] [CrossRef]
- Duan, W.; Zhou, J.; Li, W.; Zhou, T.; Chen, Q.; Yang, F.; Wei, T. Plasma Membrane Calcium ATPase 4b Inhibits Nitric Oxide Generation through Calcium-Induced Dynamic Interaction with Neuronal Nitric Oxide Synthase. Protein Cell 2013, 4, 286–298. [Google Scholar] [CrossRef]
- Legler, D.F.; Micheau, O.; Doucey, M.A.; Tschopp, J.; Bron, C. Recruitment of TNF Receptor 1 to Lipid Rafts Is Essential for TNFα-Mediated NF-ΚB Activation. Immunity 2003, 18, 655–664. [Google Scholar] [CrossRef]
- Miller, Y.I.; Navia-Pelaez, J.M.; Corr, M.; Yaksh, T.L. Lipid Rafts in Glial Cells: Role in Neuroinflammation and Pain Processing. J. Lipid Res. 2020, 61, 655–666. [Google Scholar] [CrossRef]
- Lim, E.J.; Májková, Z.; Xu, S.; Bachas, L.; Arzuaga, X.; Smart, E.; Tseng, M.T.; Toborek, M.; Hennig, B. Coplanar Polychlorinated Biphenyl-Induced CYP1A1 Is Regulated through Caveolae Signaling in Vascular Endothelial Cells. Chem. Biol. Interact. 2008, 176, 71–78. [Google Scholar] [CrossRef]
- Shihata, W.A.; Michell, D.L.; Andrews, K.L.; Chin-Dusting, J.P.F. Caveolae: A Role in Endothelial Inflammation and Mechanotransduction? Front. Physiol. 2016, 7, 628. [Google Scholar] [CrossRef]
- Sorci-Thomas, M.G.; Thomas, M.J. Microdomains, Inflammation, and Atherosclerosis. Circ. Res. 2016, 118, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Navia-Pelaez, J.M.; Agatisa-Boyle, C.; Choi, S.-H.; Sak Kim, Y.; Li, S.; Alekseeva, E.; Weldy, K.; Miller, Y.I. Differential Expression of Inflammarafts in Macrophage Foam Cells and in Nonfoamy Macrophages in Atherosclerotic Lesions. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Vieth, J.A.; Kim, M.K.; Glaser, D.; Stiles, K.; Schreiber, A.D.; Worth, R.G. FcγRIIa Requires Lipid Rafts, but Not Co-Localization into Rafts, for Effector Function. Inflamm. Res. 2013, 62, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Scheel-Toellner, D.; Wang, K.; Singh, R.; Majeed, S.; Raza, K.; Curnow, S.J.; Salmon, M.; Lord, J.M. The Death-Inducing Signalling Complex Is Recruited to Lipid Rafts in Fas-Induced Apoptosis. Biochem. Biophys. Res. Commun. 2002, 297, 876–879. [Google Scholar] [CrossRef]
- Gajate, C.; Gonzalez-Camacho, F.; Mollinedo, F. Involvement of Raft Aggregates Enriched in Fas/CD95 Death-Inducing Signaling Complex in the Antileukemic Action of Edelfosine in Jurkat Cells. PLoS ONE 2009, 4, e5044. [Google Scholar] [CrossRef]
- Molnár, E.; Swamy, M.; Holzer, M.; Beck-García, K.; Worch, R.; Thiele, C.; Guigas, G.; Boye, K.; Luescher, I.F.; Schwille, P.; et al. Cholesterol and Sphingomyelin Drive Ligand-Independent T-Cell Antigen Receptor Nanoclustering. J. Biol. Chem. 2012, 287, 42664–42674. [Google Scholar] [CrossRef]
- Miguel, L.; Owen, D.M.; Lim, C.; Liebig, C.; Evans, J.; Magee, A.I.; Jury, E.C. Primary Human CD4 + T Cells Have Diverse Levels of Membrane Lipid Order That Correlate with Their Function. J. Immunol. 2011, 186, 3505–3516. [Google Scholar] [CrossRef]
- Jury, E.C.; Kabouridis, P.S.; Flores-Borja, F.; Mageed, R.A.; Isenberg, D.A. Altered Lipid Raft–Associated Signaling and Ganglioside Expression in T Lymphocytes from Patients with Systemic Lupus Erythematosus. J. Clin. Investig. 2004, 113, 1176–1187. [Google Scholar] [CrossRef]
- Krishnan, S.; Nambiar, M.P.; Warke, V.G.; Fisher, C.U.; Mitchell, J.; Delaney, N.; Tsokos, G.C. Alterations in Lipid Raft Composition and Dynamics Contribute to Abnormal T Cell Responses in Systemic Lupus Erythematosus. J. Immunol. 2004, 172, 7821–7831. [Google Scholar] [CrossRef]
- Flores-Borja, F.; Kabouridis, P.S.; Jury, E.C.; Isenberg, D.A.; Mageed, R.A. Altered Lipid Raft-Associated Proximal Signaling and Translocation of CD45 Tyrosine Phosphatase in B Lymphocytes from Patients with Systemic Lupus Erythematosus. Arthritis Rheum. 2007, 56, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Karsalia, R.; Morrissey, A.; Bamezai, A.K. Cholesterol-Dependent Plasma Membrane Order (Lo) Is Critical for Antigen-Specific Clonal Expansion of CD4+ T Cells. Sci. Rep. 2021, 11, 13970. [Google Scholar] [CrossRef] [PubMed]
- Saeki, K.; Miura, Y.; Aki, D.; Kurosaki, T.; Yoshimura, A. The B Cell-Specific Major Raft Protein, Raftlin, Is Necessary for the Integrity of Lipid Raft and BCR Signal Transduction. EMBO J. 2003, 22, 3015–3026. [Google Scholar] [CrossRef] [PubMed]
- Kumbul, Y.Ç.; Yasan, H.; Okur, E.; Tüz, M.; Sivrice, M.E.; Akın, V.; Şirin, F.B.; Doğan Kıran, E. The Role of Raftlin in the Pathogenesis of Chronic Rhinosinusitis with Nasal Polyps. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 3519–3523. [Google Scholar] [CrossRef]
- Latif, R.; Ando, T.; Davies, T.F. Lipid Rafts Are Triage Centers for Multimeric and Monomeric Thyrotropin Receptor Regulation. Endocrinology 2007, 148, 3164–3175. [Google Scholar] [CrossRef]
- Smith, S.M.L.; Lei, Y.; Liu, J.; Cahill, M.E.; Hagen, G.M.; Barisas, B.G.; Roess, D.A. Luteinizing Hormone Receptors Translocate to Plasma Membrane Microdomains after Binding of Human Chorionic Gonadotropin. Endocrinology 2006, 147, 1789–1795. [Google Scholar] [CrossRef]
- Althumairy, D.; Murakami, H.A.; Zhang, D.; Barisas, B.G.; Roess, D.A.; Crans, D.C. Effects of Vanadium(IV) Compounds on Plasma Membrane Lipids Lead to G Protein-Coupled Receptor Signal Transduction. J. Inorg. Biochem. 2020, 203, 110873. [Google Scholar] [CrossRef]
- Marin, R.; Diaz, M. Estrogen Interactions with Lipid Rafts Related to Neuroprotection. Impact of Brain Ageing and Menopause. Front. Neurosci. 2018, 12, 128. [Google Scholar] [CrossRef]
- Canerina-Amaro, A.; Hernandez-Abad, L.G.; Ferrer, I.; Quinto-Alemany, D.; Mesa-Herrera, F.; Ferri, C.; Puertas-Avendaño, R.A.; Diaz, M.; Marin, R. Lipid Raft ER Signalosome Malfunctions in Menopause and Alzheimer’s Disease. Front. Biosci.—Sch. 2017, 9, 111–126. [Google Scholar] [CrossRef]
- Mesa-Herrera, F.; Marín, R.; Torrealba, E.; Santos, G.; Díaz, M. Neuronal ER-Signalosome Proteins as Early Biomarkers in Prodromal Alzheimer’s Disease Independent of Amyloid-β Production and Tau Phosphorylation. Front. Mol. Neurosci. 2022, 15, 879146. [Google Scholar] [CrossRef]
- Marques-Da-Silva, D.; Rodrigues, J.R.; Lagoa, R. Anthocyanins, Effects in Mitochondria and Metabolism. In Mitochondrial Physiology and Vegetal Molecules Therapeutic Potential of Natural Compounds on Mitochondrial Health; de Oliveira, M.R., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 267–300. [Google Scholar] [CrossRef]
- Ghanbari-Movahed, M.; Shafiee, S.; Burcher, J.T.; Lagoa, R.; Farzaei, M.H.; Bishayee, A. Anticancer Potential of Apigenin and Isovitexin with Focus on Oncogenic Metabolism in Cancer Stem Cells. Metabolites 2023, 13, 404. [Google Scholar] [CrossRef] [PubMed]
- Svensson, K.J.; Christianson, H.C.; Wittrup, A.; Bourseau-Guilmain, E.; Lindqvist, E.; Svensson, L.M.; Mörgelin, M.; Belting, M. Exosome Uptake Depends on ERK1/2-Heat Shock Protein 27 Signaling and Lipid Raft-Mediated Endocytosis Negatively Regulated by Caveolin-1. J. Biol. Chem. 2013, 288, 17713–17724. [Google Scholar] [CrossRef]
- Skryabin, G.O.; Komelkov, A.V.; Savelyeva, E.E.; Tchevkina, E.M. Lipid Rafts in Exosome Biogenesis. Biochemistry 2020, 85, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Schubert, A.L.; Schubert, W.; Spray, D.C.; Lisanti, M.P. Connexin Family Members Target to Lipid Raft Domains and Interact with Caveolin-1. Biochemistry 2002, 41, 5754–5764. [Google Scholar] [CrossRef] [PubMed]
- Martins-Marques, T.; Ribeiro-Rodrigues, T.; Batista-Almeida, D.; Aasen, T.; Kwak, B.R.; Girao, H. Biological Functions of Connexin43 Beyond Intercellular Communication. Trends Cell Biol. 2019, 29, 835–847. [Google Scholar] [CrossRef]
- Tekpli, X.; Huc, L.; Lacroix, J.; Rissel, M.; Poët, M.; Noël, J.; Dimanche-Boitrel, M.T.; Counillon, L.; Lagadic-Gossmann, D. Regulation of Na+/H+ Exchanger 1 Allosteric Balance by Its Localization in Cholesterol- and Caveolin-Rich Membrane Microdomains. J. Cell. Physiol. 2008, 216, 207–220. [Google Scholar] [CrossRef]
- Casaburi, I.; Chimento, A.; De Luca, A.; Nocito, M.; Sculco, S.; Avena, P.; Trotta, F.; Rago, V.; Sirianni, R.; Pezzi, V. Cholesterol as an Endogenous ERRα Agonist: A New Perspective to Cancer Treatment. Front. Endocrinol. 2018, 9, 525. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Tomiyama, A.; Sasaki, N.; Yamaguchi, H.; Shirakihara, T.; Nakashima, K.; Kumagai, K.; Takeuchi, S.; Toyooka, T.; Otani, N.; et al. Intracellular Cholesterol Level Regulates Sensitivity of Glioblastoma Cells against Temozolomide-Induced Cell Death by Modulation of Caspase-8 Activation via Death Receptor 5-Accumulation and Activation in the Plasma Membrane Lipid Raft. Biochem. Biophys. Res. Commun. 2018, 495, 1292–1299. [Google Scholar] [CrossRef]
- George, K.S.; Wu, S. Lipid Raft: A Floating Island of Death or Survival. Toxicol. Appl. Pharmacol. 2012, 259, 311–319. [Google Scholar] [CrossRef]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Mechanisms of Membrane Toxicity of Hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef]
- Buff, K.; Berndt, J. Interaction of DDT (1,1,1-Trichloro-2,2-BIS(p-Chlorophenyl)-Ethane with Liposomal Phospholipids. BBA—Biomembr. 1981, 643, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Antunes-Madeira, M.C.; Madeira, V.M.C. Membrane Fluidity as Affected by the Organochlorine Insecticide DDT. BBA—Biomembr. 1990, 1023, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Escher, B.I.; Goss, K.U. Capacities of Membrane Lipids to Accumulate Neutral Organic Chemicals. Environ. Sci. Technol. 2011, 45, 5912–5921. [Google Scholar] [CrossRef] [PubMed]
- Broniatowski, M.; Binczycka, M.; Wójcik, A.; Flasiński, M.; Wydro, P. Polycyclic Aromatic Hydrocarbons in Model Bacterial Membranes—Langmuir Monolayer Studies. Biochim. Biophys. Acta—Biomembr. 2017, 1859, 2402–2412. [Google Scholar] [CrossRef]
- Yang, H.; Li, H.; Liu, L.; Zhou, Y.; Long, X. Molecular Simulation Studies on the Interactions of 2,4,6-Trinitrotoluene and Its Metabolites with Lipid Membranes. J. Phys. Chem. B 2019, 123, 6481–6491. [Google Scholar] [CrossRef]
- Subuddhi, U.; Mishra, A.K. Prototropism of 1-Hydroxypyrene in Liposome Suspensions: Implications towards Fluorescence Probing of Lipid Bilayers in Alkaline Medium. Photochem. Photobiol. Sci. 2006, 5, 283–290. [Google Scholar] [CrossRef]
- Majkova, Z.; Smart, E.; Toborek, M.; Hennig, B. Up-Regulation of Endothelial Monocyte Chemoattractant Protein-1 by Coplanar PCB77 Is Caveolin-1-Dependent. Toxicol. Appl. Pharmacol. 2009, 237, 1–7. [Google Scholar] [CrossRef]
- Vogel, C.F.A.; Van Winkle, L.S.; Esser, C.; Haarmann-Stemmann, T. The Aryl Hydrocarbon Receptor as a Target of Environmental Stressors—Implications for Pollution Mediated Stress and Inflammatory Responses. Redox Biol. 2020, 34, 101530. [Google Scholar] [CrossRef]
- Rey-Barroso, J.; Alvarez-Barrientos, A.; Rico-Leo, E.; Contador-Troca, M.; Carvajal-Gonzalez, J.M.; Echarri, A.; Del Pozo, M.A.; Fernandez-Salguero, P.M. The Dioxin Receptor Modulates Caveolin-1 Mobilization during Directional Migration: Role of Cholesterol. Cell Commun. Signal. 2014, 12, 57. [Google Scholar] [CrossRef]
- Hennig, B.; Reiterer, G.; Majkova, Z.; Oesterling, E.; Meerarani, P.; Toborek, M. Modification of Environmental Toxicity by Nutrients. Implications in Atherosclerosis. Cardiovasc. Toxicol. 2005, 5, 153–160. [Google Scholar] [CrossRef]
- Ramadass, P.; Meerarani, P.; Toborek, M.; Robertson, L.W.; Hennig, B. Dietary Flavonoids Modulate PCB-Induced Oxidative Stress, CYP1A1 Induction, and AhR-DNA Binding Activity in Vascular Endothelial Cells. Toxicol. Sci. 2003, 76, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Petriello, M.C.; Han, S.G.; Newsome, B.J.; Hennig, B. PCB 126 Toxicity Is Modulated by Cross-Talk between Caveolae and Nrf2 Signaling. Toxicol. Appl. Pharmacol. 2014, 277, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Oesterling, E.; Toborek, M.; Hennig, B. Benzo[a]Pyrene Induces Intercellular Adhesion Molecule-1 through a Caveolae and Aryl Hydrocarbon Receptor Mediated Pathway. Toxicol. Appl. Pharmacol. 2008, 232, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Collin, A.; Hardonnière, K.; Chevanne, M.; Vuillemin, J.; Podechard, N.; Burel, A.; Dimanche-Boitrel, M.T.; Lagadic-Gossmann, D.; Sergent, O. Cooperative Interaction of Benzo[a]Pyrene and Ethanol on Plasma Membrane Remodeling Is Responsible for Enhanced Oxidative Stress and Cell Death in Primary Rat Hepatocytes. Free Radic. Biol. Med. 2014, 72, 11–22. [Google Scholar] [CrossRef]
- Gorria, M.; Tekpli, X.; Sergent, O.; Huc, L.; Gaboriau, F.; Rissel, M.; Chevanne, M.; Dimanche-Boitrel, M.-T.; Lagadic-Gossmann, D. Membrane Fluidity Changes Are Associated with Benzo[a]Pyrene-Induced Apoptosis in F258 Cells: Protection by Exogenous Cholesterol. Ann. N. Y. Acad. Sci. 2006, 1090, 108–112. [Google Scholar] [CrossRef]
- Tekpli, X.; Rivedal, E.; Gorria, M.; Landvik, N.E.; Rissel, M.; Dimanche-Boitrel, M.T.; Baffet, G.; Holme, J.A.; Lagadic-Gossmann, D. The B[a]P-Increased Intercellular Communication via Translocation of Connexin-43 into Gap Junctions Reduces Apoptosis. Toxicol. Appl. Pharmacol. 2010, 242, 231–240. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. Regulation of the Mevalonate Pathway. Nature 1990, 343, 425–430. [Google Scholar] [CrossRef]
- Tekpli, X.; Huc, L.; Sergent, O.; Dendelé, B.; Dimanche-Boitrel, M.T.; Holme, J.A.; Lagadic-Gossmann, D. NHE-1 Relocation Outside Cholesterol-Rich Membrane Microdomains Is Associated with Its Benzo[a]Pyrene-Related Apoptotic Function. Cell. Physiol. Biochem. 2012, 29, 657–666. [Google Scholar] [CrossRef]
- Dendelé, B.; Tekpli, X.; Hardonnière, K.; Holme, J.A.; Debure, L.; Catheline, D.; Arlt, V.M.; Nagy, E.; Phillips, D.H.; Øvrebø, S.; et al. Protective Action of N-3 Fatty Acids on Benzo[a]Pyrene-Induced Apoptosis through the Plasma Membrane Remodeling-Dependent NHE1 Pathway. Chem. Biol. Interact. 2014, 207, 41–51. [Google Scholar] [CrossRef]
- Bazzoni, G.B.; Bollini, A.N.; Hernández, G.N.; Contini, M.D.C.; Chiarotto, M.M.; Rasia, M.L. In Vivo Effect of Aluminium upon the Physical Properties of the Erythrocyte Membrane. J. Inorg. Biochem. 2005, 99, 822–827. [Google Scholar] [CrossRef]
- Yilmaz, B.; Sandal, S.; Chen, C.H.; Carpenter, D.O. Effects of PCB 52 and PCB 77 on Cell Viability, [Ca2+] i Levels and Membrane Fluidity in Mouse Thymocytes. Toxicology 2006, 217, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y. Ortho-Substituted but Not Coplanar PCBs Rapidly Kill Cerebellar Granule Cells. Toxicol. Sci. 2004, 79, 147–156. [Google Scholar] [CrossRef]
- Bedia, C.; Dalmau, N.; Jaumot, J.; Tauler, R. Phenotypic Malignant Changes and Untargeted Lipidomic Analysis of Long-Term Exposed Prostate Cancer Cells to Endocrine Disruptors. Environ. Res. 2015, 140, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Yun, U.J.; Lee, J.H.; Shim, J.; Yoon, K.; Goh, S.H.; Yi, E.H.; Ye, S.K.; Lee, J.S.; Lee, H.; Park, J.; et al. Anti-Cancer Effect of Doxorubicin Is Mediated by Downregulation of HMG-Co A Reductase via Inhibition of EGFR/Src Pathway. Lab. Investig. 2019, 99, 1157–1172. [Google Scholar] [CrossRef]
- Lagoa, R.; Gañán, C.; López-Sánchez, C.; García-Martínez, V.; Gutierrez-Merino, C. The Decrease of NAD(P)H:Quinone Oxidoreductase 1 Activity and Increase of ROS Production by NADPH Oxidases Are Early Biomarkers in Doxorubicin Cardiotoxicity. Biomarkers 2014, 19, 142–153. [Google Scholar] [CrossRef]
- Busso, I.T.; Silva, G.B.; Carreras, H.A. Organic Compounds Present in Airborne Particles Stimulate Superoxide Production and DNA Fragmentation: Role of NOX and Xanthine Oxidase in Animal Tissues. Environ. Sci. Pollut. Res. 2016, 23, 16653–16660. [Google Scholar] [CrossRef]
- Kim, C.; Ashrap, P.; Watkins, D.J.; Mukherjee, B.; Rosario-Pabón, Z.Y.; Vélez-Vega, C.M.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. Maternal Metals/Metalloid Blood Levels Are Associated with Lipidomic Profiles Among Pregnant Women in Puerto Rico. Front. Public Health 2022, 9, 2248. [Google Scholar] [CrossRef] [PubMed]
- Corsetto, P.A.; Ferrara, G.; Buratta, S.; Urbanelli, L.; Montorfano, G.; Gambelunghe, A.; Chiaradia, E.; Magini, A.; Roderi, P.; Colombo, I.; et al. Changes in Lipid Composition during Manganese-Induced Apoptosis in PC12 Cells. Neurochem. Res. 2016, 41, 258–269. [Google Scholar] [CrossRef]
- Salzer, U.; Prohaska, R. Stomatin, Flotillin-1, and Flotillin-2 Are Major Integral Proteins of Erythrocyte Lipid Rafts. Blood 2001, 97, 1141–1143. [Google Scholar] [CrossRef]
- Kwiatkowska, K.; Matveichuk, O.V.; Fronk, J.; Ciesielska, A. Flotillins: At the Intersection of Protein S-Palmitoylation and Lipid-Mediated Signaling. Int. J. Mol. Sci. 2020, 21, 2283. [Google Scholar] [CrossRef]
- Eum, S.Y.; Jaraki, D.; András, I.E.; Toborek, M. Lipid Rafts Regulate PCB153-Induced Disruption of Occludin and Brain Endothelial Barrier Function through Protein Phosphatase 2A and Matrix Metalloproteinase-2. Toxicol. Appl. Pharmacol. 2015, 287, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Ni, I.; Ji, C.; Vij, N. Second-Hand Cigarette Smoke Impairs Bacterial Phagocytosis in Macrophages by Modulating CFTR Dependent Lipid-Rafts. PLoS ONE 2015, 10, e0121200. [Google Scholar] [CrossRef] [PubMed]
- Winter, P.W.; Al-Qatati, A.; Wolf-Ringwall, A.L.; Schoeberl, S.; Chatterjee, P.B.; Barisas, B.G.; Roess, D.A.; Crans, D.C. The Anti-Diabetic Bis(Maltolato)Oxovanadium(Iv) Decreases Lipid Order While Increasing Insulin Receptor Localization in Membrane Microdomains. Dalt. Trans. 2012, 41, 6419–6430. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Kim, H.P.; Lee, S.-J.; Wang, X.; Wang, Y.; Ifedigbo, E.; Watkins, S.C.; Ohba, M.; Ryter, S.W.; Vyas, Y.M.; et al. Protein Kinase Cα and ζ Differentially Regulate Death-Inducing Signaling Complex Formation in Cigarette Smoke Extract-Induced Apoptosis. J. Immunol. 2008, 180, 4668–4678. [Google Scholar] [CrossRef]
- Tappe, M.; Null, V. Requirements for Tires from the Environmental View Point. In Proceedings of the Tire Technology Expo Conference, Hamburg, Germany, 18–20 May 2002. [Google Scholar]
- Beretta, E.; Gualtieri, M.; Botto, L.; Palestini, P.; Miserocchi, G.; Camatini, M. Organic Extract of Tire Debris Causes Localized Damage in the Plasma Membrane of Human Lung Epithelial Cells. Toxicol. Lett. 2007, 173, 191–200. [Google Scholar] [CrossRef]
- Singh, D.P.; Kaur, G.; Bagam, P.; Pinkston, R.; Batra, S. Membrane Microdomains Regulate NLRP10- and NLRP12-Dependent Signalling in A549 Cells Challenged with Cigarette Smoke Extract. Arch. Toxicol. 2018, 92, 1767–1783. [Google Scholar] [CrossRef]
- Fedida-Metula, S.; Feldman, B.; Koshelev, V.; Levin-Gromiko, U.; Voronov, E.; Fishman, D. Lipid Rafts Couple Store-Operated Ca2+ Entry to Constitutive Activation of PKB/Akt in a Ca2+/Calmodulin-, Src- and PP2A-Mediated Pathway and Promote Melanoma Tumor Growth. Carcinogenesis 2012, 33, 740–750. [Google Scholar] [CrossRef]
- Janes, P.W.; Ley, S.C.; Magee, A.I. Aggregation of Lipid Rafts Accompanies Signaling via the T Cell Antigen Receptor. J. Cell Biol. 1999, 147, 447–461. [Google Scholar] [CrossRef]
- Ghare, S.; Patil, M.; Hote, P.; Suttles, J.; McClain, C.; Barve, S.; Joshi-Barve, S. Ethanol Inhibits Lipid Raft-Mediated TCR Signaling and IL-2 Expression: Potential Mechanism of Alcohol-Induced Immune Suppression. Alcohol. Clin. Exp. Res. 2011, 35, 1435–1444. [Google Scholar] [CrossRef]
- Goddard, A.D.; Watts, A. Regulation of G Protein-Coupled Receptors by Palmitoylation and Cholesterol. BMC Biol. 2012, 10, 27. [Google Scholar] [CrossRef]
- Zheng, H.; Pearsall, E.A.; Hurst, D.P.; Zhang, Y.; Chu, J.; Zhou, Y.; Reggio, P.H.; Loh, H.H.; Law, P.Y. Palmitoylation and Membrane Cholesterol Stabilize μ-Opioid Receptor Homodimerization and G Protein Coupling. BMC Cell Biol. 2012, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Le Ferrec, E.; Øvrevik, J. G-Protein Coupled Receptors (GPCR) and Environmental Exposure. Consequences for Cell Metabolism Using the β-Adrenoceptors as Example. Curr. Opin. Toxicol. 2018, 8, 14–19. [Google Scholar] [CrossRef]
- Shahid, A.; Chen, M.; Lin, C.; Andresen, B.T.; Parsa, C.; Orlando, R.; Huang, Y. The β-Blocker Carvedilol Prevents Benzo(a)Pyrene-Induced Lung Toxicity, Inflammation and Carcinogenesis. Cancers 2023, 15, 583. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques-da-Silva, D.; Lagoa, R. Rafting on the Evidence for Lipid Raft-like Domains as Hubs Triggering Environmental Toxicants’ Cellular Effects. Molecules 2023, 28, 6598. https://doi.org/10.3390/molecules28186598
Marques-da-Silva D, Lagoa R. Rafting on the Evidence for Lipid Raft-like Domains as Hubs Triggering Environmental Toxicants’ Cellular Effects. Molecules. 2023; 28(18):6598. https://doi.org/10.3390/molecules28186598
Chicago/Turabian StyleMarques-da-Silva, Dorinda, and Ricardo Lagoa. 2023. "Rafting on the Evidence for Lipid Raft-like Domains as Hubs Triggering Environmental Toxicants’ Cellular Effects" Molecules 28, no. 18: 6598. https://doi.org/10.3390/molecules28186598
APA StyleMarques-da-Silva, D., & Lagoa, R. (2023). Rafting on the Evidence for Lipid Raft-like Domains as Hubs Triggering Environmental Toxicants’ Cellular Effects. Molecules, 28(18), 6598. https://doi.org/10.3390/molecules28186598







