Hybrids of 1,4-Naphthoquinone with Thymidine Derivatives: Synthesis, Anticancer Activity, and Molecular Docking Study
Abstract
:1. Introduction
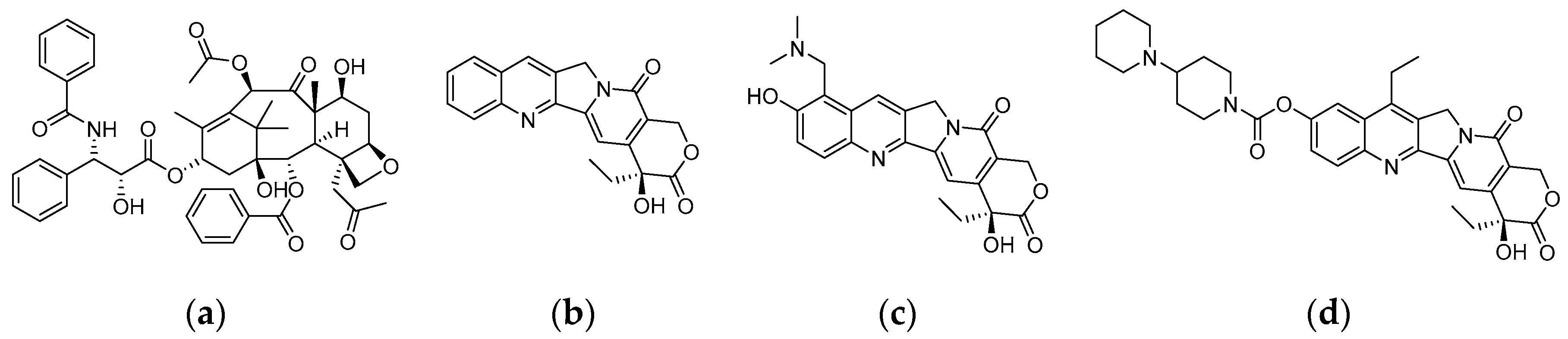
2. Results and Discussion
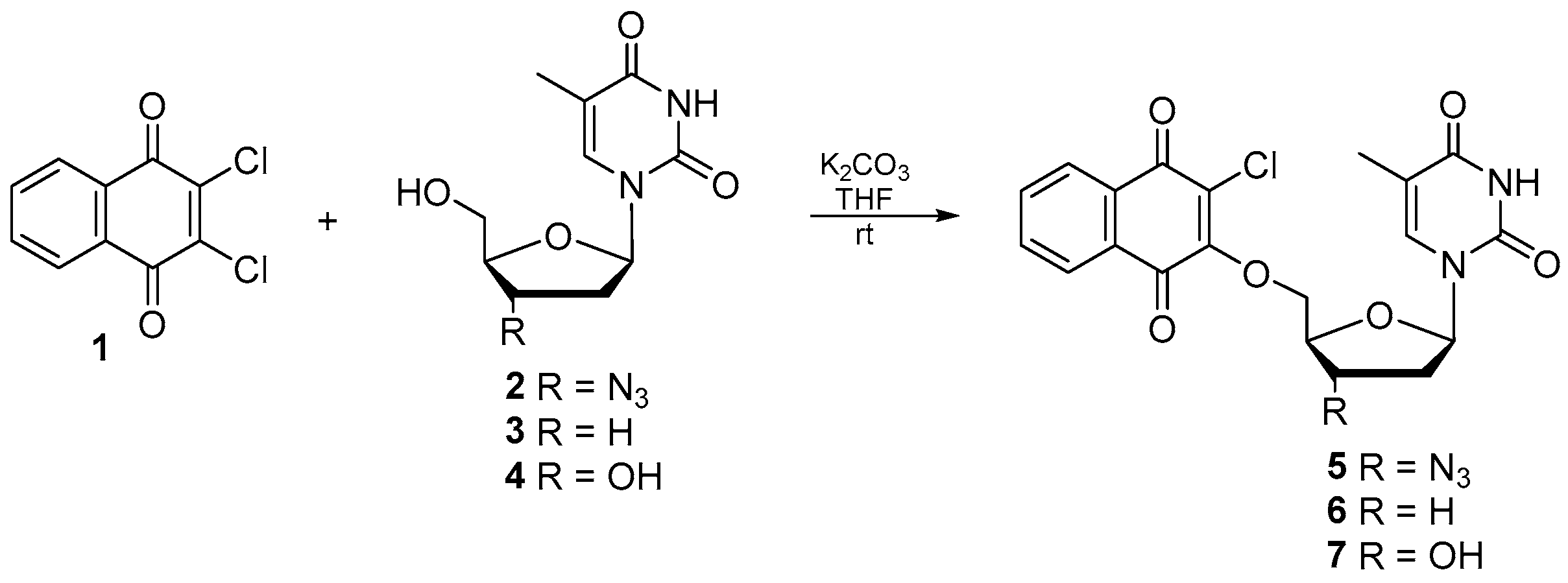
2.1. Chemistry
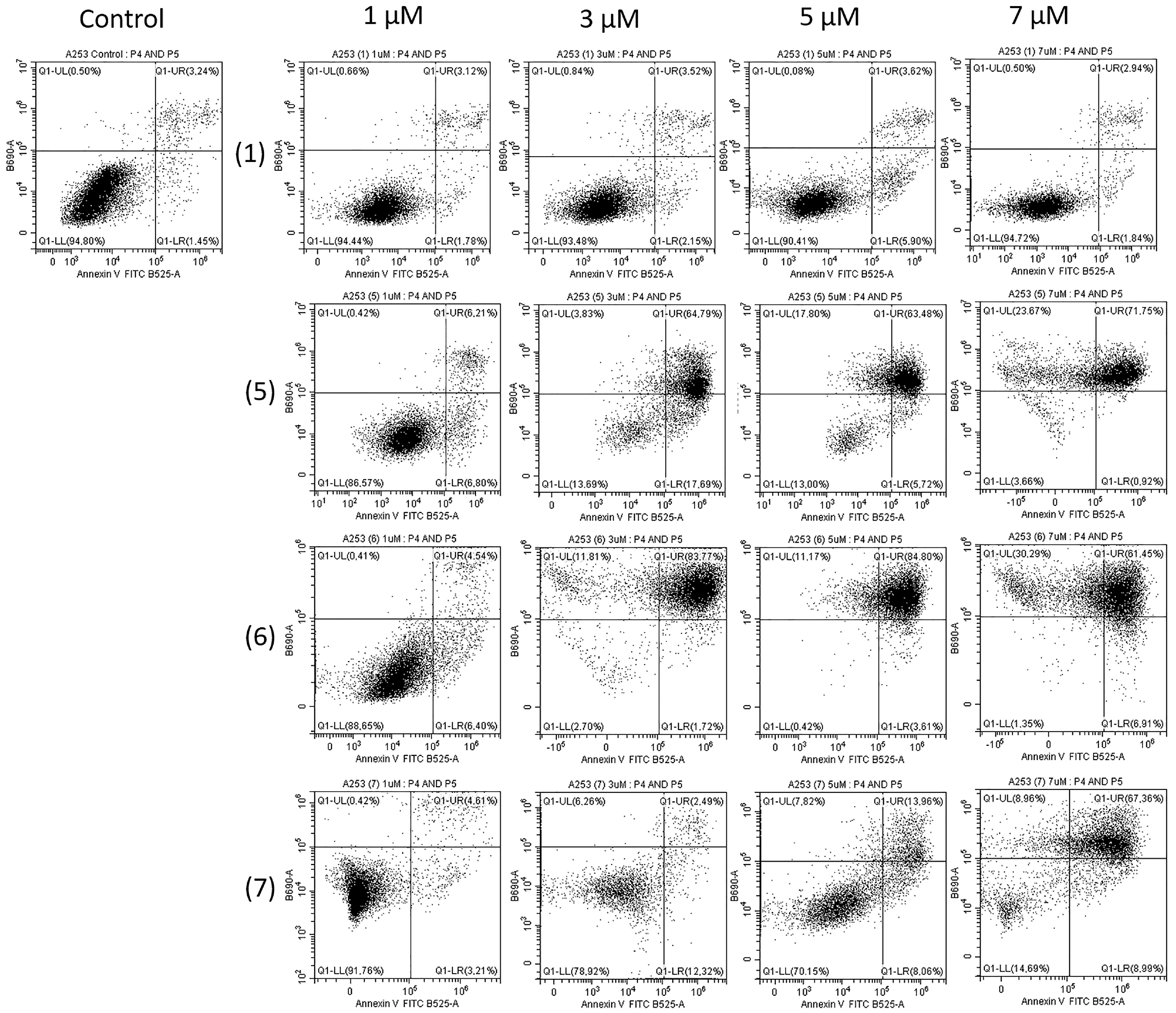
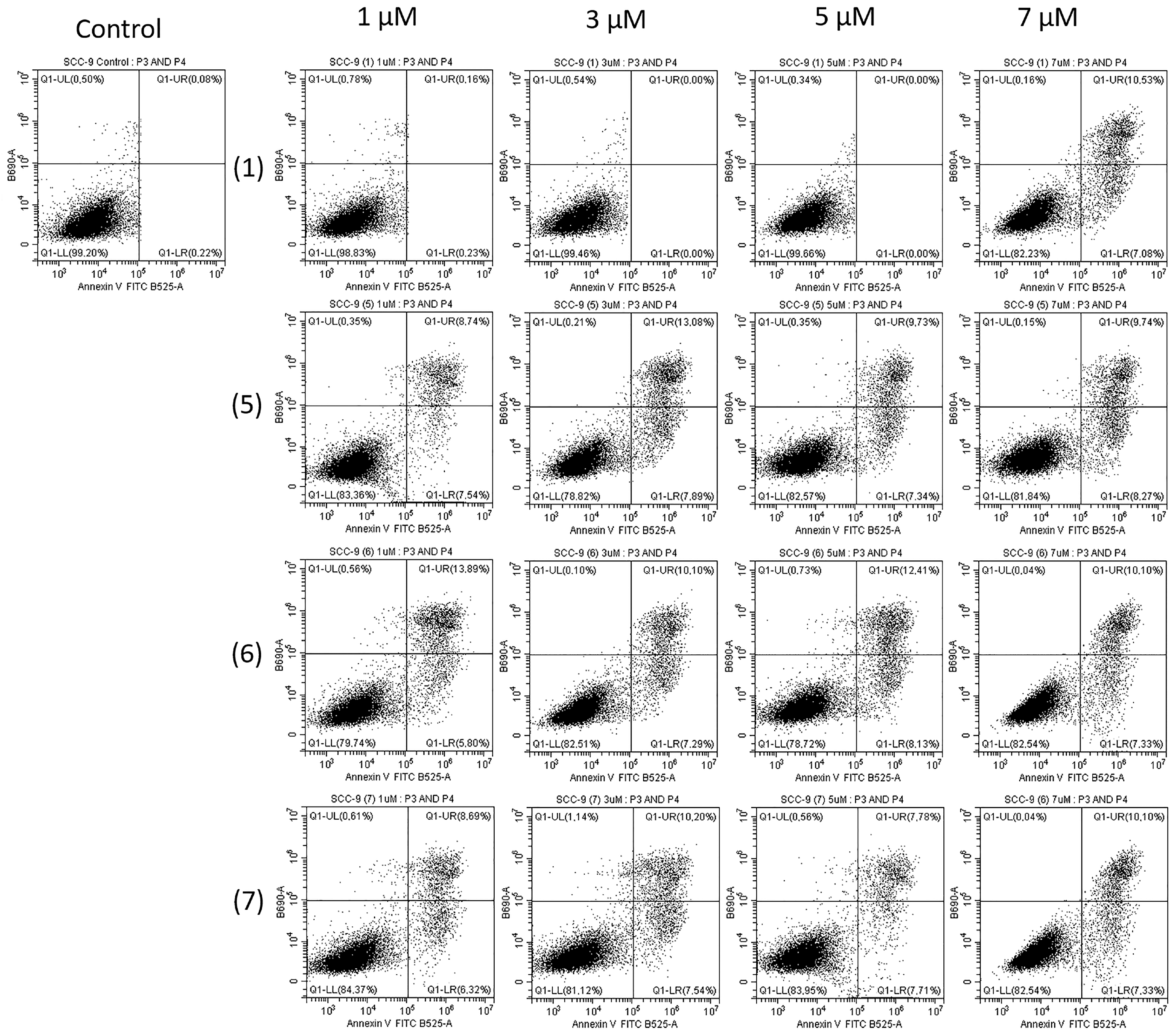
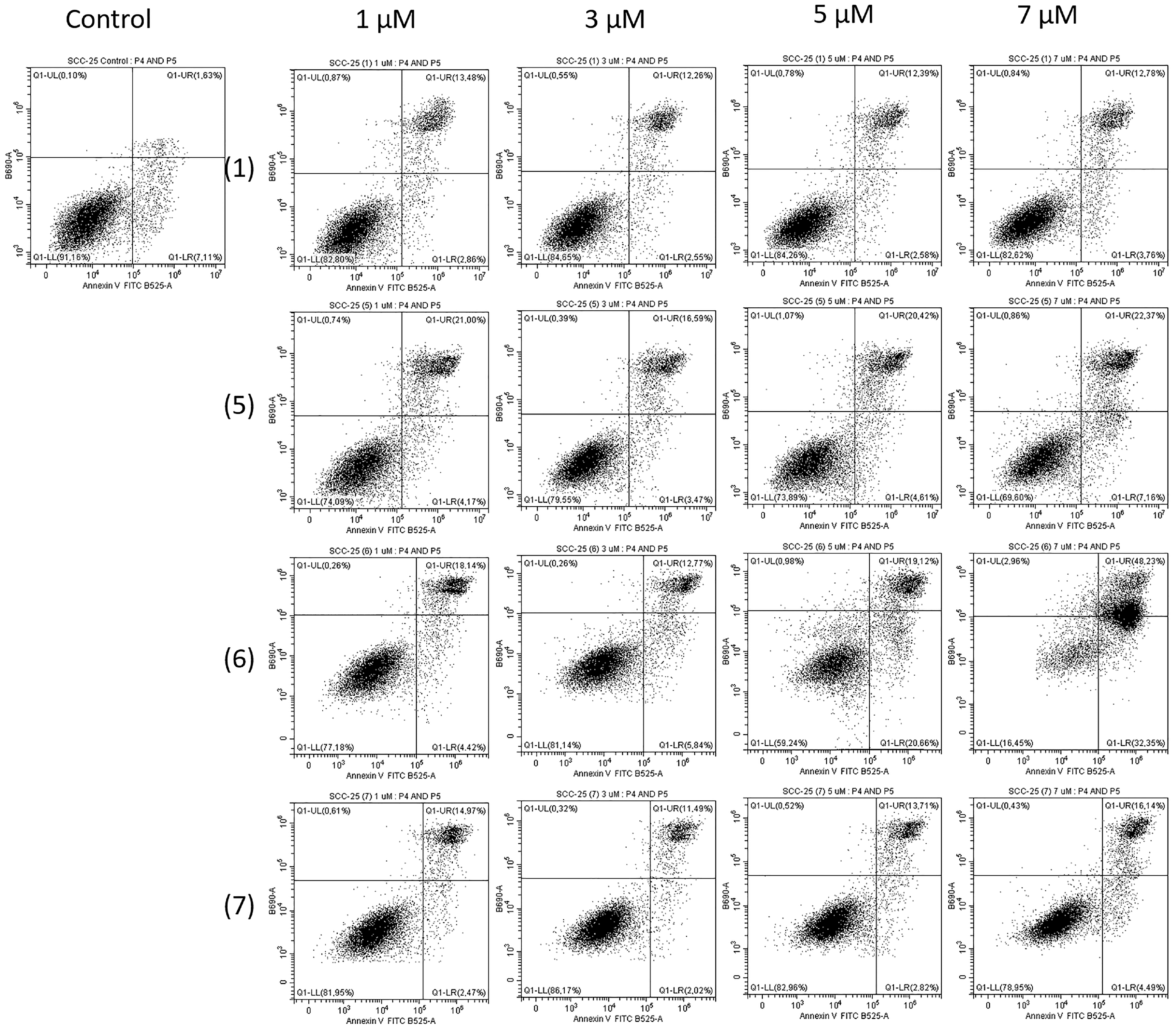
2.2. Biological Study
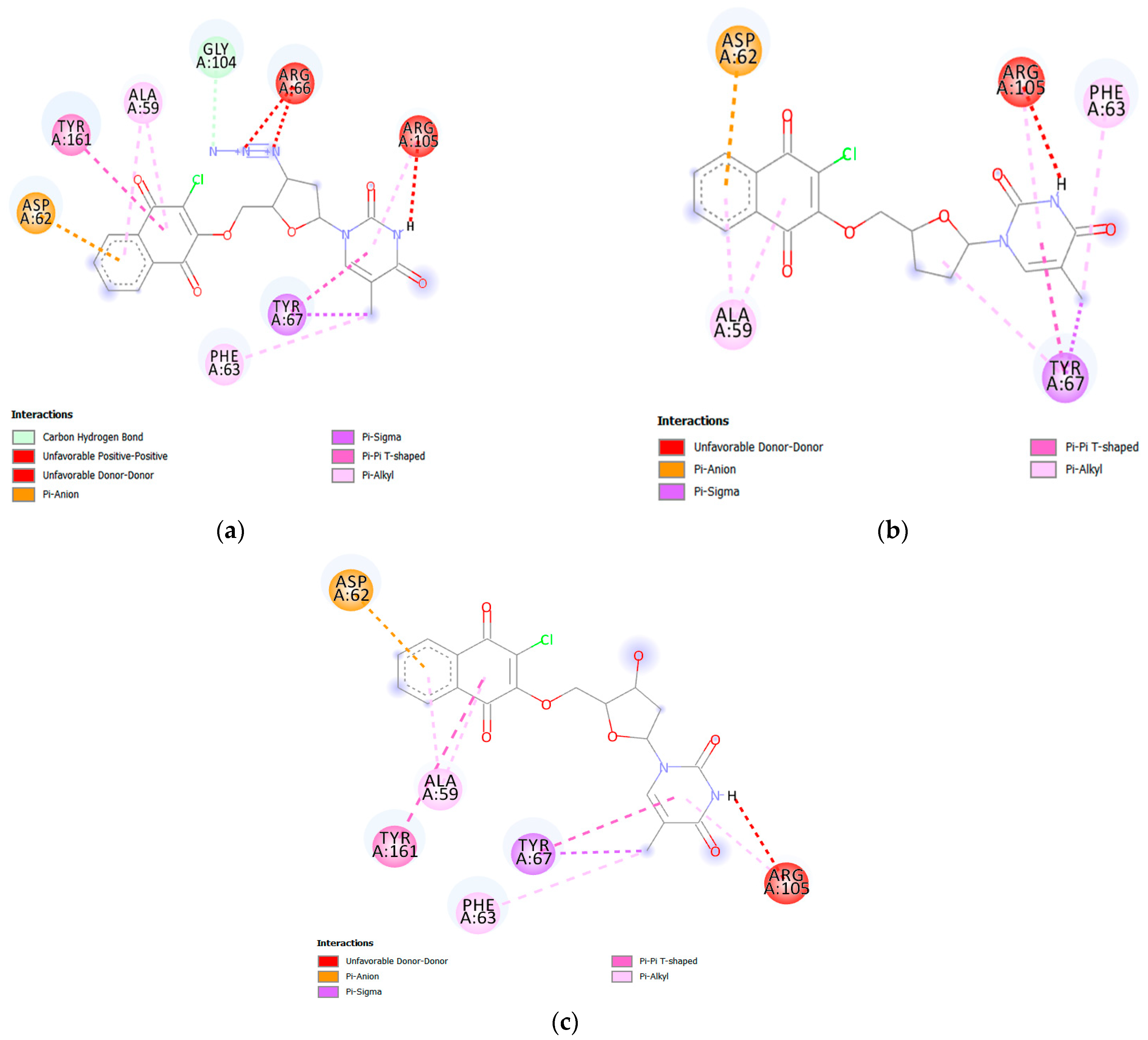
2.3. Molecular Docking
3. Materials and Methods
3.1. Chemistry
General Synthesis of Compounds
- 2-Chloro-3-(3′-azido-3′-deoxythymidine)-1,4-naphtoquinone 5: Yield 77%; mp 88-90 °C; 1H NMR (600 MHz, CD3OD) δ, ppm: 1.82 (d, J = 1.2 Hz, 3H, H7t), 2.45 (m, 1H, H2’t), 2.61 (m, 1H, H2’t), 4.16 (m, 1H, H3’t), 4.63 (m, 1H, H4’t), 4.76 (dd, J = 3.6 Hz, J = 12.0 Hz, 1H, H5’t), 5.06 (dd, J = 3.6 Hz, J = 12.0 Hz, 1H, H5’t), 6.18 (t, J = 6.6 Hz, 1H, H1’t), 7.56 (d, J = 1.2 Hz, 1H, H6t), 7.84 (m, 2H, H6q, H7q), 8.07 (m, 1H, H5q), 8.10 (m, 1H, H8q) (Figure S3); 13C NMR (150 MHz, CD3OD) δ, ppm: 11.1 (C7t), 36.1 (C2’t), 60.2 (C4’t), 71.8 (C5’t), 82.8 (C3’t), 84.7 (C1’t), 110.5 (C5t), 126.3 (C8q), 126.4 (C5q), 127.5 (C2q), 130.9 (C9q), 131.0 (C10q), 133.8 (C7q), 134.8 (C6q), 136.3 (C6t), 150.7 (C2t), 156.6 (C3q), 164.7 (C4t), 178.3 (C1q), 179.4 (C4q) (Figure S4); IR (νmax cm−1, ATR): 3177–2926, 2100, 1667, 1595, 1248, 1011; ESI-HRMS m/z [M + Na]+ calcd for C20H16ClN5O6Na+ 480.0687, found 480.0688.
- 2-Chloro-3-(3′-deoxythymidine)-1,4-naphtoquinone 6: Yield 59%; mp 115–117 °C; 1H NMR (600 MHz, CD3OD) δ, ppm: 1.84 (d, J = 1.2 Hz, 3H, H7t), 2.18 (m, 3H, H2’t, 2xH3’t), 2.23 (m, 1H, H2’t), 4.39 (m, 1H, H4’t), 4.70 (dd, J = 3.6 Hz, J = 12.0 Hz, 1H, H5’t), 5.14 (dd, J = 3.6 Hz, J = 12.0 Hz, 1H, H5’t), 6.05 (t, J = 6.6 Hz, 1H, H1’t), 7.55 (d, J = 1.2 Hz, 1H, H6t), 7.82 (m, 2H, H6q, H7q), 8.06 (m, 1H, H5q), 8.09 (m, 1H, H8q) (Figure S5); 13C NMR (150 MHz, CD3OD) δ, ppm: 11.2 (C7t), 25.0 (C3’t), 30.6 (C2’t), 56.1 (C4’t), 73.2 (C5’t), 85.6 (C1’t), 110.0 (C5t), 126.2 (C8q), 126.3 (C5q), 127.1 (C2q), 130.9 (C9q), 131.1 (C10q), 133.8 (C7q), 134.0 (C6q), 136.2 (C6t), 150.7 (C2t), 157.1 (C3q), 164.9 (C4t), 178.5 (C1q), 179.5 (C4q) (Figure S6); IR (νmax cm−1, ATR): 3169–2828, 1697, 1661, 1593, 1246, 1009; ESI-HRMS m/z [M + Na]+ calcd for C20H17ClN2O6Na+ 439.0673, found 439.0667.
- 2-Chloro-3-thymidine-1,4-naphtoquinone 7: Yield 63%; mp 128–130 °C; 1H NMR (600 MHz, acetone-d6) δ, ppm: 1.78 (d, J = 1.2 Hz, 3H, H7t), 2.77 (m, 1H, 2xH2’t), 4.31 (m, 1H, H3’t), 4.73 (d, J = 2.4 Hz, 1H, H5’t), 5.06 (dd, J = 3.6 Hz, J = 12.0 Hz, 1H, H5’t), 5.96 (m, 1H, H4’t), 6.60 (m, 1H, H1’t), 7.56 (d, J = 1.2 Hz, 1H, H6t), 7.84 (m, 2H, H6q, H7q), 8.07 (m, 1H, H5q), 8.10 (m, 1H, H8q), 10.04 (s, 1H, NH) (Figure S7); 13C NMR (150 MHz, acetone-d6) δ, ppm: 11.4 (C7t), 38.3 (C2’t), 68.0 (C4’t), 73.6 (C5’t), 84.2 (C3’t), 84.8 (C1’t), 110.3 (C5t), 126.5 (C8q), 126.6 (C5q), 127.8 (C2q), 130.3 (C9q), 131.1 (C10q), 134.2 (C7q), 134.5 (C6q), 135.5 (C6t), 150.4 (C2t), 156.6 (C3q), 163.2 (C4t), 178.2 (C1q), 179.6 (C4q) (Figure S8); IR (νmax cm−1, ATR): 3071–2928, 1664, 1593, 1246, 1008; ESI-HRMS m/z [M + Na]+ calcd for C20H17ClN2O7Na+ 455.0622, found 455.0608.
3.2. Biological Study
3.2.1. Materials
3.2.2. Compounds Treatment
3.2.3. Cell Culture
3.2.4. MTT Test
3.2.5. FITC Annexin V Apoptosis Detection Kit II
3.2.6. Statistical Analysis
3.3. Molecular Docking Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of iIncidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Cancer Today. Available online: https://gco.iarc.fr/today/home (accessed on 26 June 2023).
- Starzyńska, A.; Sobocki, B.K.; Alterio, D. Current challenges in head and neck cancer management. Cancers 2022, 14, 358. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Karamouzis, M.V.; Raben, D.R.; Ferris, L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Hoskins, C. Drug development: Lessons from nature. Biomed. Rep. 2017, 6, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martínez, R.A.; Cánovas-Díaz, M.; de Diego Puente, T. A compressive review about Taxol®: History and future challenges. Molecules 2020, 25, 5986. [Google Scholar] [CrossRef]
- Marupudi, N.I.; Han, J.E.; Li, K.W.; Renard, W.M.; Tyler, B.M.; Brem, H. Paclitaxel: A review of adverse toxicities and novel delivery strategies. Expert. Opin. Drug Saf. 2007, 6, 609–621. [Google Scholar] [CrossRef]
- Venditto, V.J.; Simanek, E.E. Cancer therapies utilizing the camptothecins: A review of in vivo literature. Mol. Pharm. 2010, 7, 307–349. [Google Scholar] [CrossRef]
- Jayaram, K.; Prasad, M.N.V. Rapid in vitro multiplication of Drosera indica L.: A vulnerable, medicinally important insectivorous plant. Plant Biotechnol. Rep. 2007, 1, 79–84. [Google Scholar] [CrossRef]
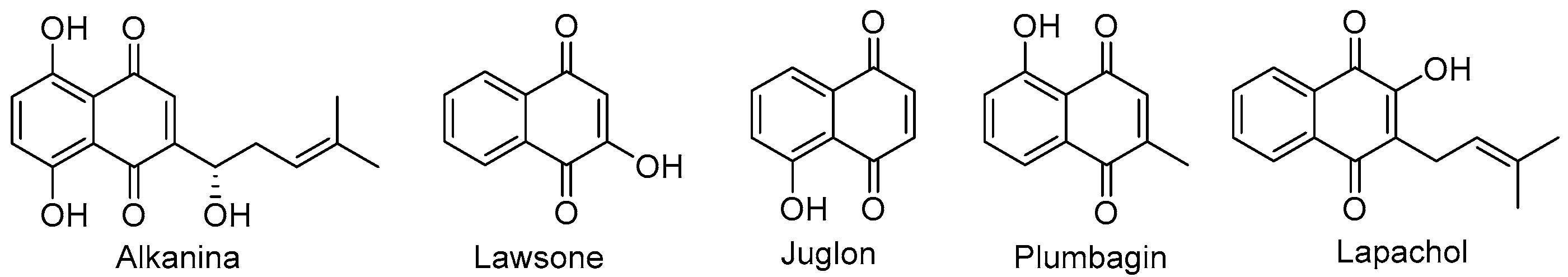
- Hook, I.L.I. Naphthoquinone contents of in vitro cultured plants and cell suspensions of Dionaea muscipula and Drosera species. Plant Cell Tissue Organ Cult. 2001, 67, 281–285. [Google Scholar] [CrossRef]
- Widhalm, J.R.; Rhodes, D. Biosynthesis and molecular actions of specialized 1,4-naphthoquinone natural products produced by horticultural plants. Hortic. Res. 2016, 3, 16046. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Tovar, G.; Vega-Rodríguez, S.; Leyva, E.; Loredo-Carrillo, S.; de Loera, D.; López-López, L.I. The relevance and insights on 1,4-naphthoquinones as antimicrobial and antitumoral molecules: A systematic review. Pharmaceuticals 2023, 16, 496. [Google Scholar] [CrossRef] [PubMed]
- Karcz, W.; Burdach, Z.; Rudnicka, M. The effects of 1,4-naphthoquinone (NQ) and naphthazarin (5,8-dihydroxy-1,4-naphthoquinone, DHNQ) individually and in combination on growth and oxidative stress in maize (Zea mays L.) seedlings. Plants 2023, 12, 900. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, B.; Walczak, J.; Kapsiak, M.; Barnaś, K.; Dziuba, J.; Suchoń, A. Impact of cytotoxic plant naphthoquinones, juglone, plumbagin, lawsone and 2-methoxy-1,4-naphthoquinone, on Chlamydomonas reinhardtii reveals the biochemical mechanism of juglone toxicity by rapid depletion of plastoquinol. Plant Physiol. Biochem. 2023, 197, 107660. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Liu, L.; He, B.; Zhao, W.; Zhang, W.; Xiao, L.; Deng, M.; Zhong, X.; Zeng, S.; Qi, X.L.M. Activation of xanthine oxidase by 1,4-naphthoquinones: A novel potential research topic for diet management and risk assessment. Food Chem. 2023, 424, 36264. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Islam, M.R.; Akash, S.; Shohag, S.; Ahmed, L.; Supti, F.A.; Rauf, A.; Aljohani, A.S.M.; Al Abdulmonem, W.; Khalil, A.; et al. Naphthoquinones and derivatives as potential anticancer agents: An updated review. Chem. Biol. Interact. 2022, 368, 110198. [Google Scholar] [CrossRef]
- Costa Souza, R.M.; Montenegro Pimentel, L.M.L.; Ferreira, L.K.M.; Pereira, V.R.A.; Santos, A.C.D.S.; Dantas, W.M.; Silva, C.J.O.; De Medeiros Brito, R.M.; Andrade, J.L.; De Andrade-Neto, V.F.; et al. Biological activity of 1,2,3-triazole-2-amino-1,4-naphthoquinone derivatives and their evaluation as therapeutic strategy for malaria control. Eur. J. Med. Chem. 2023, 255, 115400. [Google Scholar] [CrossRef]
- Kadela-Tomanek, M.; Jastrzębska, M.; Chrobak, E.; Bębenek, E.; Latocha, M. Hybrids of 1,4-quinone with quinoline derivatives: Synthesis, biological activity, and molecular docking with DT-Diaphorase (NQO1). Molecules 2022, 27, 6206. [Google Scholar] [CrossRef]
- Kadela-Tomanek, M.; Jastrzębska, M.; Chrobak, E.; Bębenek, E. Lipophilicity and ADMET analysis of quinoline-1,4-quinone hybrids. Pharmaceutics 2023, 15, 34. [Google Scholar] [CrossRef]
- Devi, M.; Kumar, P.; Singh, R.; Narayan, L.; Kumar, A.; Sindhu, J.; Lal, S.; Hussain, K.; Singh, D. A Comprehensive review on synthesis, biological profile and photophysical studies of heterocyclic compounds derived from 2,3-diamino-1,4-naphthoquinone. J. Mol. Struct. 2022, 1269, 133786. [Google Scholar] [CrossRef]
- Ozkan, T.; Hekmatshoar, Y.; Karabay, A.Z.; Koc, A.; Gunes, B.A.; Gurel, A.K.; Sunguroglu, A. Assessment of azithromycin as an anticancer agent for treatment of imatinib sensitive and resistant CML cells. Leuk. Res. 2021, 102, 106523. [Google Scholar] [CrossRef] [PubMed]
- Chirumarry, S.; Soung, N.K.; Han, J.; Kim, E.Y.; Ryu, E.K.; Lee, Y.H.; Shin, S.Y.; Gunasekaran, P.; Bang, J.K. Antibacterial AZT derivative regulates metastasis of breast cancer cells. Eur. J. Med. Chem. 2020, 193, 112233. [Google Scholar] [CrossRef] [PubMed]
- Broder, S. The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antivir. Res. 2010, 85, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mitsuya, H.; Weinhold, K.J.; Furman, P.A.; St Clair, M.H.; Lehrman, S.N.; Gallo, R.C.; Bolognesi, D.; Barry, D.W.; Broder, S. 3′-Azido-3′-deoxythymidine (BW A509U): An antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc. Natl. Acad. Sci. USA 1985, 82, 7096–7100. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, I.; Falcone, A.; Calabresi, P.; Goulette, F.A.; Darnowski, J.W. 5-Fluorouracil enhances azidothymidine cytotoxicity: In vitro, in vivo, and biochemical studies. Cancer Res. 1990, 50, 4026–4031. [Google Scholar] [PubMed]
- Darnowski, J.W.; Goulette, F.A. 3′-Azido-3′-deoxythymidine cytotoxicity and metabolism in the human colon tumor cell line HCT-8. Biochem. Pharmacol. 1994, 48, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Dang Thi, T.A.; Kim Tuyet, N.T.; Pham The, C.; Thanh Nguyen, H.; Ba Thi, C.; Doan Duy, T.; D’hooghe, M.; Van Nguyen, T. Synthesis and cytotoxic evaluation of novel ester-triazole-linked triterpenoid-AZT conjugates. Bioorg. Med. Chem. Lett. 2014, 24, 5190–5194. [Google Scholar] [CrossRef]
- Bębenek, E.; Jastrzębska, M.; Kadela-Tomanek, M.; Chrobak, E.; Orzechowska, B.; Zwolińska, K.; Latocha, M.; Mertas, A.; Czuba, Z.; Boryczka, S. Novel triazole hybrids of betulin: Synthesis and biological activity profile. Molecules 2017, 22, 1876. [Google Scholar] [CrossRef]
- Bębenek, E.; Kadela-Tomanek, M.; Chrobak, E.; Wietrzyk, J.; Sadowska, J.; Boryczka, S. New acetylenic derivatives of betulin and betulone, synthesis and cytotoxic activity. Med. Chem. Res. 2017, 26, 1–8. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, H.; Wei, Q.; Zhou, Q. Structure-activity relationship study of anticancer thymidine-quinoxaline conjugates under the low radiance of long wavelength ultraviolet light for photodynamic therapy. Eur. J. Med. Chem. 2016, 107, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, Z.; Yang, R.; Qian, T.; Zhou, Q. Naphthyl quinoxaline thymidine conjugate is a potent anticancer agent post UVA activation and elicits marked inhibition of tumor growth through vaccination. Eur. J. Med. Chem. 2019, 171, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Celewicz, L.; Jóźwiak, A.; Ruszkowski, P.; Laskowska, H.; Olejnik, A.; Czarnecka, A.; Hoffmann, M.; Hładoń, B. Synthesis and anticancer activity of 5′-chloromethylphosphonates of 3′-azido-3′-deoxythymidine (AZT). Bioorg. Med. Chem. 2011, 19, 6375–6382. [Google Scholar] [CrossRef] [PubMed]
- Kadela-Tomanek, M.; Bębenek, E.; Chrobak, E.; Latocha, M.; Boryczka, S. Alkoxy and enediyne eerivatives containing 1,4-benzoquinone subunits-synthesis and antitumor activity. Molecules 2017, 22, 447. [Google Scholar] [CrossRef] [PubMed]
- Kadela-Tomanek, M.; Bębenek, E.; Chrobak, E.; Marciniec, K.; Latocha, M.; Kuśmierz, D.; Jastrzębska, M.; Boryczka, S. Betulin-1,4-quinone hybrids: Synthesis, anticancer activity and molecular docking study with NQO1 enzyme. Eur. J. Med. Chem. 2019, 177, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Kadela-Tomanek, M.; Jastrzębska, M.; Marciniec, K.; Chrobak, E.; Bębenek, E.; Latocha, M.; Kuśmierz, D.; Boryczka, S. Design, synthesis and biological activity of 1,4-quinone moiety attached to betulin derivatives as potent DT-diaphorase substrate. Bioorg. Chem. 2021, 106, 104478. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Weerapreeyakul, N.; Nonpunya, A.; Barusrux, S.; Thitimetharoch, T.; Sripanidkulchai, B. Evaluation of the anticancer potential of six herbs against a hepatoma cell line. Chin Med. 2012, 7, 15. [Google Scholar] [CrossRef]
- Yip, K.W.; Reed, J.C. Bcl-2 family proteins and cancer. Oncogene 2008, 27, 6398–6406. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Toure, B.B.; Miller-Moslin, K.; Yusuff, N.; Perez, L.; Dore, M.; Joud, C.; Michael, W.; DiPietro, L.; van der Plas, S.; McEwan, M.; et al. Crystal Structure of human Bcl-2 in complex with a small molecule inhibitor targeting Bcl-2 BH3 domain interactions. ACS Med. Chem. Lett. 2013, 4, 186–190. [Google Scholar]
- Dessault Systemes BIOVIA. Available online: https://www.3dsbiovia.com/products/collaborative-science/biovia-discovery-studio/ (accessed on 12 June 2023).

| Physicochemical Properties | Compound 5 | Compound 6 | Compound 7 |
|---|---|---|---|
| MW [g/mol] | 457.82 | 416.81 | 432.81 |
| TPSA [Å] | 157.21 | 107.46 | 127.69 |
| log P | 2.64 | 2.63 | 1.83 |
| S [µM] | 31.4 | 116 | 488 |
| nHA | 9 | 6 | 7 |
| nHD | 1 | 1 | 2 |
| nRT | 5 | 4 | 4 |
| Compound | ΔG [kcal/mol] |
|---|---|
| 1 | −6.70 |
| 5 | −8.60 |
| 6 | −8.70 |
| 7 | −8.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadela-Tomanek, M.; Krzykawski, K.; Halama, A.; Kubina, R. Hybrids of 1,4-Naphthoquinone with Thymidine Derivatives: Synthesis, Anticancer Activity, and Molecular Docking Study. Molecules 2023, 28, 6644. https://doi.org/10.3390/molecules28186644
Kadela-Tomanek M, Krzykawski K, Halama A, Kubina R. Hybrids of 1,4-Naphthoquinone with Thymidine Derivatives: Synthesis, Anticancer Activity, and Molecular Docking Study. Molecules. 2023; 28(18):6644. https://doi.org/10.3390/molecules28186644
Chicago/Turabian StyleKadela-Tomanek, Monika, Kamil Krzykawski, Adrianna Halama, and Robert Kubina. 2023. "Hybrids of 1,4-Naphthoquinone with Thymidine Derivatives: Synthesis, Anticancer Activity, and Molecular Docking Study" Molecules 28, no. 18: 6644. https://doi.org/10.3390/molecules28186644
APA StyleKadela-Tomanek, M., Krzykawski, K., Halama, A., & Kubina, R. (2023). Hybrids of 1,4-Naphthoquinone with Thymidine Derivatives: Synthesis, Anticancer Activity, and Molecular Docking Study. Molecules, 28(18), 6644. https://doi.org/10.3390/molecules28186644








