Drug Delivery and Therapy Strategies for Osteoporosis Intervention
Abstract
1. Introduction
2. The Pathophysiology of OP and Clinical Medicaments
3. Engineering of Anti-OP Drugs Delivery
3.1. Targeted Delivery of Anti-OP Drugs
3.2. Developing Supersaturated Drug Delivery Systems
3.3. The Use of Non-Invasive Drug Delivery Systems
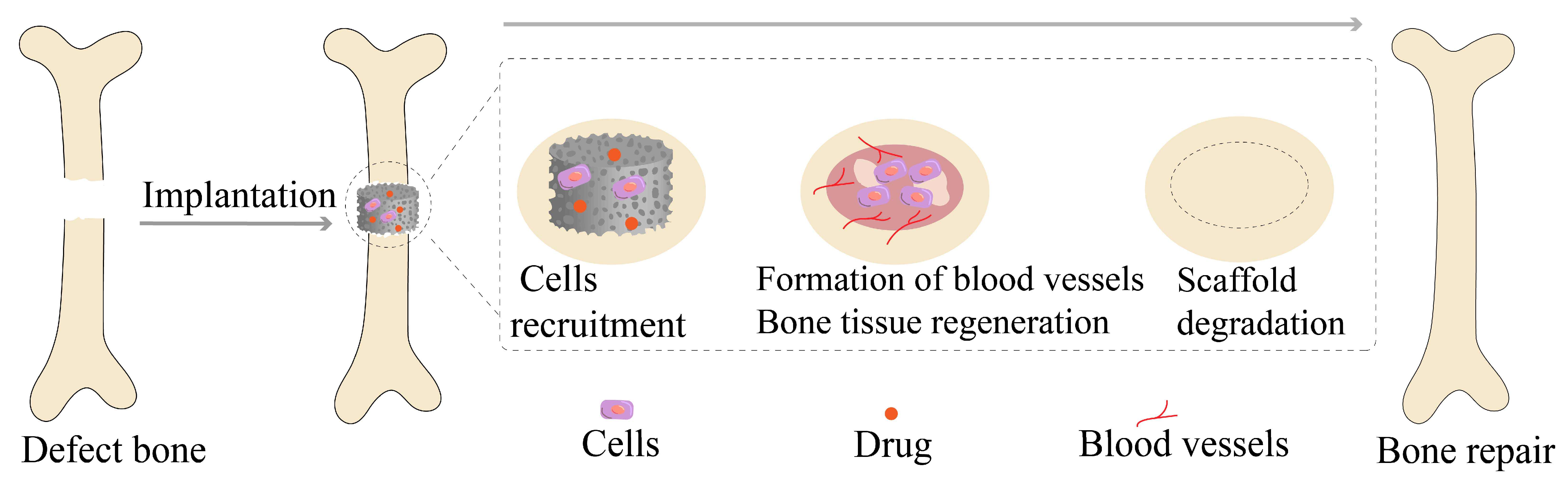
3.4. Scaffolds Implantation and Local Drug Delivery
4. Delivery Vehicles and Strategies for Anti-OP Drugs
4.1. Hydroxyapatite Nanoparticles
4.2. Liposomes
4.3. Emulsions
4.4. Dendrimers
4.5. Micelles
4.6. Other Polymeric Nanoparticles
4.7. Bone Tissue Engineering Scaffolds
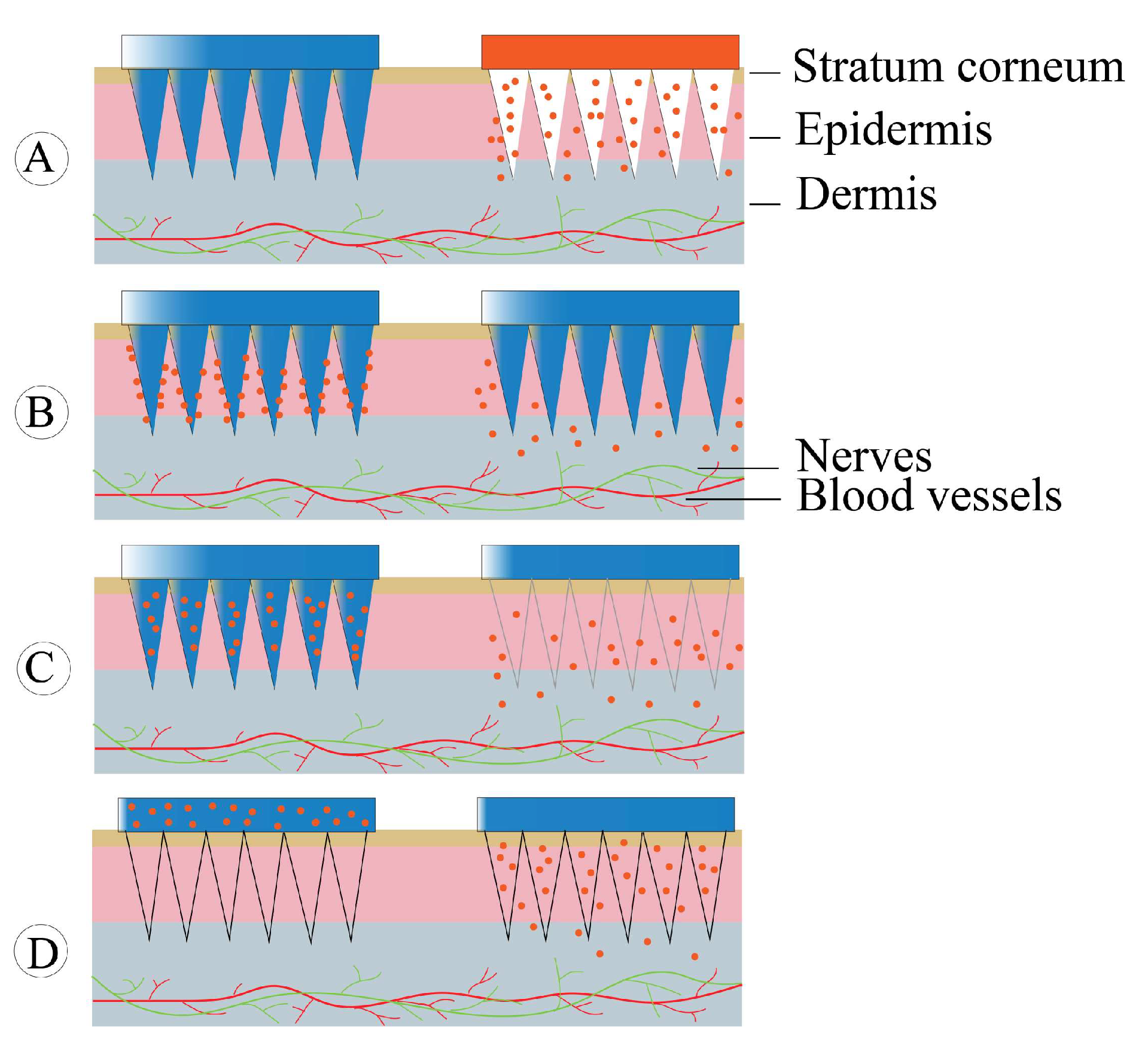
4.8. Microneedles
4.8.1. Solid Microneedles
4.8.2. Coated Microneedles
4.8.3. Dissolving Microneedles
4.8.4. Hollow Microneedles
5. Anti-OP Preparations in Clinical Trials
6. Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cortet, B.; Schott, A.-M.; Désaméricq, G.; Chauny, J.-V.; Samama, P.; Emery, C.; Fagnani, F. Trends in postmenopausal osteoporosis treatment in France during the period 2007–2016: A nationwide claims database analysis. Bone 2022, 154, 116255. [Google Scholar] [CrossRef] [PubMed]
- Poorirani, S.; Taheri, S.L.; Mostafavi, S.A. Scaffolds: A biomaterial engineering in targeted drug delivery for osteoporosis. Osteoporos. Int. 2022, 34, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Sobh, M.M.; Abdalbary, M.; Elnagar, S.; Nagy, E.; Elshabrawy, N.; Abdelsalam, M.; Asadipooya, K.; El-Husseini, A. Secondary Osteoporosis and Metabolic Bone Diseases. J. Clin. Med. 2022, 11, 2382. [Google Scholar] [CrossRef] [PubMed]
- Roux, C.; Briot, K. The crisis of inadequate treatment in osteoporosis. Lancet Rheumatol. 2020, 2, e110–e119. [Google Scholar] [CrossRef]
- Sözen, T.; Özışık, L.; Başaran, N. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Adami, G.; Fassio, A.; Gatti, D.; Viapiana, O.; Benini, C.; Danila, M.I.; Saag, K.G.; Rossini, M. Osteoporosis in 10 years time: A glimpse into the future of osteoporosis. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221083541. [Google Scholar] [CrossRef]
- Cotts, K.G.; Cifu, A.S. Treatment of osteoporosis. JAMA 2018, 319, 1040–1041. [Google Scholar] [CrossRef]
- Berardi, S.; Corrado, A.; Maruotti, N.; Cici, D.; Cantatore, F.P. Osteoblast role in the pathogenesis of rheumatoid arthritis. Mol. Biol. Rep. 2021, 48, 2843–2852. [Google Scholar] [CrossRef]
- Kenkre, J.S.; Bassett, J. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef]
- Liu, D.D.; Zhang, C.Y.; Liu, Y.; Li, J.; Wang, Y.X.; Zheng, S.G. RUNX2 Regulates Osteoblast Differentiation via the BMP4 Signaling Pathway. J. Dent. Res. 2022, 101, 1227–1237. [Google Scholar] [CrossRef]
- He, J.; Zhang, N.; Zhang, J.; Jiang, B.; Wu, F. Migration critically meditates osteoblastic differentiation of bone mesenchymal stem cells through activating canonical Wnt signal pathway. Colloids Surf. B Biointerfaces 2018, 171, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Hemmatian, H.; Bakker, A.D.; Klein-Nulend, J.; van Lenthe, G.H. Aging, Osteocytes, and Mechanotransduction. Curr. Osteoporos. Rep. 2017, 15, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Abarrategi, A.; A Mian, S.; Passaro, D.; Rouault-Pierre, K.; Grey, W.; Bonnet, D. Modeling the human bone marrow niche in mice: From host bone marrow engraftment to bioengineering approaches. J. Exp. Med. 2018, 215, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.E. Epidemiology, etiology, and diagnosis of osteoporosis. Am. J. Obstet. Gynecol. 2006, 194 (Suppl. S2), S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Hardcastle, S.A. Pregnancy and Lactation Associated Osteoporosis. Calcif. Tissue Int. 2022, 110, 531–545. [Google Scholar] [CrossRef]
- Khosla, S.; Amin, S.; Orwoll, E. Osteoporosis in Men. Endocr. Rev. 2008, 29, 441–464. [Google Scholar] [CrossRef]
- Gennari, L.; Becherini, L.; Falchetti, A.; Masi, L.; Massart, F.; Brandi, M. Genetics of osteoporosis: Role of steroid hormone receptor gene polymorphisms. J. Steroid Biochem. Mol. Biol. 2002, 81, 1–24. [Google Scholar] [CrossRef]
- Cannarella, R.; Barbagallo, F.; Condorelli, R.A.; Aversa, A.; La Vignera, S.; Calogero, A.E. Osteoporosis from an Endocrine Perspective: The Role of Hormonal Changes in the Elderly. J. Clin. Med. 2019, 8, 1564. [Google Scholar] [CrossRef]
- Wiren, K.M.; Zhang, X.-W.; Olson, D.A.; Turner, R.T.; Iwaniec, U.T. Androgen prevents hypogonadal bone loss via inhibition of resorption mediated by mature osteoblasts/osteocytes. Bone 2012, 51, 835–846. [Google Scholar] [CrossRef]
- Chen, L.-R.; Ko, N.-Y.; Chen, K.-H. Medical Treatment for Osteoporosis: From Molecular to Clinical Opinions. Int. J. Mol. Sci. 2019, 20, 2213. [Google Scholar] [CrossRef] [PubMed]
- Aghebati-Maleki, L.; Dolati, S.; Zandi, R.; Fotouhi, A.; Ahmadi, M.; Aghebati, A.; Nouri, M.; Shakouri, S.K.; Yousefi, M. Prospect of mesenchymal stem cells in therapy of osteoporosis: A review. J. Cell. Physiol. 2019, 234, 8570–8578. [Google Scholar] [CrossRef] [PubMed]
- Ishizu, H.; Arita, K.; Terkawi, M.A.; Shimizu, T.; Iwasaki, N. Risks vs. benefits of switching therapy in patients with postmeno-pausal osteoporosis. Expert Rev. Endocrinol. Metab. 2021, 16, 217–228. [Google Scholar] [CrossRef]
- Snyder, P.J.; Kopperdahl, D.L.; Stephens-Shields, A.J.; Ellenberg, S.S.; Cauley, J.A.; Ensrud, K.E.; Lewis, C.E.; Barrett-Connor, E.; Schwartz, A.V.; Lee, D.C. Effect of testosterone treatment on volumetric bone density and strength in older men with low tes-tosterone: A controlled clinical trial. JAMA Intern. Med. 2017, 177, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Ji, Y.; Cui, Y.; Xu, L.; Liu, H.; Wang, J. Simvastatin-Incorporated Drug Delivery Systems for Bone Regeneration. ACS Bio-mater. Sci. Eng. 2021, 7, 2177–2191. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yujiao, W.; Fang, W.; Linhui, Y.; Ziqi, G.; Zhichen, W.; Zirui, W.; Shengwang, W. The roles of miRNA, lncRNA and circRNA in the development of osteoporosis. Biol. Res. 2020, 53, 1–16. [Google Scholar] [CrossRef]
- Sun, Y.; Kuek, V.; Liu, Y.; Tickner, J.; Yuan, Y.; Chen, L.; Zeng, Z.; Shao, M.; He, W.; Xu, J. MiR-214 is an important regulator of the musculoskeletal metabolism and disease. J. Cell. Physiol. 2019, 234, 231–245. [Google Scholar] [CrossRef]
- Zhong, D.; Xu, G.-Z.; Wu, J.-Z.; Liu, H.; Tang, J.-Y.; Wang, C.-G. Circ-ITCH sponges miR-214 to promote the osteogenic differen-tiation in osteoporosis via upregulating YAP1. Cell Death Dis. 2021, 12, 340. [Google Scholar] [CrossRef]
- Long, L.; Wang, X.; Lei, Y.; Guo, S.; Wang, C.; Dai, W.; Lin, B.; Xie, M.; Xu, H.; Li, S. Icariin: A Potential Alternative Against Osteo-porosis. Nat. Prod. Commun. 2022, 17, 1934578X221134881. [Google Scholar]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Diab, D.L.; Eldeiry, L.S.; Farooki, A.; Harris, S.T.; Hurley, D.L.; Kelly, J.; Lewiecki, E.M. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the di-agnosis and treatment of postmenopausal osteoporosis—2020 update. Endocr. Pract. 2020, 26, 1–46. [Google Scholar] [CrossRef]
- Kishimoto, H.; Noguchi, K.; Takaoka, K. Novel insight into the management of bisphosphonate-related osteonecrosis of the jaw (BRONJ). Jpn. Dent. Sci. Rev. 2019, 55, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Thurner, G.C.; Haybaeck, J.; Debbage, P. Targeting Drug Delivery in the Elderly: Are Nanoparticles an Option for Treating Os-teoporosis? Int. J. Mol. Sci. 2021, 22, 8932. [Google Scholar] [CrossRef] [PubMed]
- Vogel, V.G. Update on raloxifene: Role in reducing the risk of invasive breast cancer in postmenopausal women. Breast Cancer Targets Ther. 2011, 3, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Deal, C.L.; Draper, M.W. Raloxifene: A Selective Estrogen-Receptor Modulator for Postmenopausal Osteoporosis—A Clinical Update on Efficacy and Safety. Womens Health 2006, 2, 199–210. [Google Scholar] [CrossRef]
- Barone, B.; Napolitano, L.; Abate, M.; Cirillo, L.; Reccia, P.; Passaro, F.; Turco, C.; Morra, S.; Mastrangelo, F.; Scarpato, A. The role of testosterone in the elderly: What do we know? Int. J. Mol. Sci. 2022, 23, 3535. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Wong, F.K.; Karponis, D. Calcitonin: A useful old friend. J. Musculoskelet. Neuronal Interact. 2020, 20, 600. [Google Scholar]
- Sun, Y.; Liu, X.; Tan, J.; Lv, D.; Song, W.; Su, R.; Li, L.; Liu, X.; Ouyang, L.; Liao, Y. Strontium ranelate incorporated 3D porous sul-fonated PEEK simulating MC3T3-E1 cell differentiation. Regen. Biomater. 2020, 8, rbaa043. [Google Scholar] [CrossRef]
- Shi, C.; Wu, T.; He, Y.; Zhang, Y.; Fu, D. Recent advances in bone-targeted therapy. Pharmacol. Ther. 2020, 207, 107473. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Y.; Shi, C.; Wu, T.; Cui, Y.; Wang, S.; Liu, P.; Feng, X.; He, Y.; Fu, D. Remote-controllable bone-targeted delivery of es-tradiol for the treatment of ovariectomy-induced osteoporosis in rats. J. Nanobiotechnol. 2021, 19, 248. [Google Scholar] [CrossRef]
- Senra, M.R.; Lima, R.B.d.; Souza, D.d.H.S.; Marques, M.d.F.V.; Monteiro, S.N. Thermal characterization of hydroxyapatite or carbonated hydroxyapatite hybrid composites with distinguished collagens for bone graft. J. Mater. Res. Technol. 2020, 9, 7190–7200. [Google Scholar] [CrossRef]
- Sawamoto, K.; Álvarez, J.V.; Herreño, A.M.; Otero-Espinar, F.J.; Couce, M.L.; Alméciga-Díaz, C.J.; Tomatsu, S. Bone-Specific Drug Delivery for Osteoporosis and Rare Skeletal Disorders. Curr. Osteoporos. Rep. 2020, 18, 515–525. [Google Scholar] [CrossRef]
- Farrell, K.B.; Karpeisky, A.; Thamm, D.H.; Zinnen, S. Bisphosphonate conjugation for bone specific drug targeting. Bone Rep. 2018, 9, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Ordikhani, F.; Zandi, N.; Mazaheri, M.; Luther, G.A.; Ghovvati, M.; Akbarzadeh, A.; Annabi, N. Targeted nanomedicines for the treatment of bone disease and regeneration. Med. Res. Rev. 2021, 41, 1221–1254. [Google Scholar] [CrossRef] [PubMed]
- Cremers, S.; Drake, M.T.; Ebetino, F.H.; Bilezikian, J.P.; Russell, R.G.G. Pharmacology of bisphosphonates. Br. J. Pharmacol. 2019, 85, 1052–1062. [Google Scholar] [CrossRef]
- Yang, X.; Chen, S.; Liu, X.; Yu, M.; Liu, X. Drug delivery based on nanotechnology for target bone disease. Curr. Drug. Deliv. 2019, 16, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Neale, J.R.; Richter, N.B.; Merten, K.E.; Taylor, K.G.; Singh, S.; Waite, L.C.; Emery, N.K.; Smith, N.B.; Cai, J.; Pierce, W.M., Jr. Bone selective effect of an estradiol conjugate with a novel tetracycline-derived bone-targeting agent. Bioorg. Med. Chem. Lett. 2009, 19, 680–683. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Katsumi, H.; Hibino, N.; Isobe, Y.; Yagi, Y.; Kusamori, K.; Sakane, T.; Yamamoto, A. Development of PEGylated carboxylic acid-modified polyamidoamine dendrimers as bone-targeting carriers for the treatment of bone diseases. J. Control. Release 2017, 262, 10–17. [Google Scholar] [CrossRef]
- Sun, Y.; Ye, X.; Cai, M.; Liu, X.; Xiao, J.; Zhang, C.; Wang, Y.; Yang, L.; Liu, J.; Li, S. Osteoblast-targeting-peptide modified nanoparticle for siRNA/microRNA delivery. ACS Nano 2016, 10, 5759–5768. [Google Scholar] [CrossRef]
- Gencturk, A.; Kahraman, E.; Güngör, S.; Özhan, G.; Özsoy, Y.; Sarac, A.S. Polyurethane/hydroxypropyl cellulose electrospun nanofiber mats as potential transdermal drug delivery system: Characterization studies and in vitro assays. Artif. Cells Nanomed. Biotechnol. 2017, 45, 655–664. [Google Scholar] [CrossRef]
- Chen, H.; Deng, J.; Yao, X.; He, Y.; Li, H.; Jian, Z.; Tang, Y.; Zhang, X.; Zhang, J.; Dai, H. Bone-targeted erythrocyte-cancer hybrid membrane-camouflaged nanoparticles for enhancing photothermal and hypoxia-activated chemotherapy of bone invasion by OSCC. J. Nanobiotechnol. 2021, 19, 342. [Google Scholar] [CrossRef]
- Nielsen, J.J.; Low, S.A.; Ramseier, N.T.; Hadap, R.V.; Young, N.A.; Wang, M.; Low, P.S. Analysis of the bone fracture targeting properties of osteotropic ligands. J. Control. Release 2021, 329, 570–584. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, P.; Kim, S.; Kang, H.C.; Shim, M.S. Targeted siRNA delivery using aptamer-siRNA chimeras and aptamer-conjugated nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1543. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.-W.; Li, F.-X.-Z.; Liu, Y.-W.; Rao, S.-S.; Yin, H.; Huang, J.; Chen, C.-Y.; Hu, Y.; Zhang, Y.; Tan, Y.-J.; et al. Aptamer-functionalized exosomes from bone marrow stromal cells target bone to promote bone regeneration. Nanoscale 2019, 11, 20884–20892. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jia, H.; Hu, A.; Liu, R.; Zeng, X.; Wang, H. Nanoparticles Targeting Delivery Antagomir-483-5p to Bone Marrow Mes-enchymal Stem Cells Treat Osteoporosis by Increasing Bone Formation. Curr. Stem Cell Res. Ther. 2023, 18, 115–126. [Google Scholar] [CrossRef]
- Stapleton, M.; Sawamoto, K.; Alméciga-Díaz, C.J.; Mackenzie, W.G.; Mason, R.W.; Orii, T.; Tomatsu, S. Development of Bone Targeting Drugs. Int. J. Mol. Sci. 2017, 18, 1345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, Y.; He, Y.; Boucetta, H.; Wu, J.; Chen, Z.; He, W. Lipid carriers for mRNA delivery. Acta Pharm. Sin. B, 2022; in press. [Google Scholar]
- Thapa Magar, K.; Boafo, G.F.; Li, X.; Chen, Z.; He, W. Liposome-based delivery of biological drugs. Chin. Chem. Lett. 2022, 33, 587–596. [Google Scholar] [CrossRef]
- Nasra, S.; Bhatia, D.; Kumar, A. Recent advances in nanoparticle-based drug delivery systems for rheumatoid arthritis treatment. Nanoscale Adv. 2022, 4, 3479–3494. [Google Scholar] [CrossRef]
- Magar, K.T.; Boafo, G.F.; Zoulikha, M.; Jiang, X.; Li, X.; Xiao, Q.; Xing, X.; Wang, X.; Fan, L.; Wu, Z.; et al. Metal phenolic net-work-stabilized nanocrystals of andrographolide to alleviate macrophage-mediated inflammation in-vitro. Chin. Chem. Lett. 2023, 34, 107453. [Google Scholar] [CrossRef]
- Sharma, A.; Arora, K.; Mohapatra, H.; Sindhu, R.K.; Bulzan, M.; Cavalu, S.; Paneshar, G.; Elansary, H.O.; El-Sabrout, A.M.; Mahmoud, E.A.; et al. Supersaturation-Based Drug Delivery Systems: Strategy for Bioavailability Enhancement of Poorly Water-Soluble Drugs. Molecules 2022, 27, 2969. [Google Scholar] [CrossRef]
- Fontana, M.C.; Laureano, J.V.; Forgearini, B.; dos Santos, J.; Pohlmann, A.R.; Guterres, S.S.; de Araujo, B.V.; Beck, R.C.R. Spray-dried raloxifene submicron particles for pulmonary delivery: Development and in vivo pharmacokinetic evaluation in rats. Int. J. Pharm. 2020, 585, 119429. [Google Scholar] [CrossRef]
- Zakir, F.; Ahmad, A.; Mirza, M.A.; Kohli, K.; Ahmad, F.J. Exploration of a transdermal nanoemulgel as an alternative therapy for postmenopausal osteoporosis. J. Drug Deliv. Sci. Technol. 2021, 65, 102745. [Google Scholar] [CrossRef]
- Ansari, M.D.; Khan, I.; Solanki, P.; Pandit, J.; Jahan, R.N.; Aqil, M.; Sultana, Y. Fabrication and optimization of raloxifene loaded spanlastics vesicle for transdermal delivery. J. Drug Deliv. Sci. Technol. 2022, 68, 103102. [Google Scholar] [CrossRef]
- Dhaval, M.; Vaghela, P.; Patel, K.; Sojitra, K.; Patel, M.; Patel, S.; Dudhat, K.; Shah, S.; Manek, R.; Parmar, R. Lipid-based emulsion drug delivery systems—A comprehensive review. Drug Deliv. Transl. Res. 2022, 12, 1616–1639. [Google Scholar] [CrossRef] [PubMed]
- Wibel, R.; Jörgensen, A.M.; Laffleur, F.; Spleis, H.; Claus, V.; Bernkop-Schnürch, A. Oral delivery of calcitonin-ion pairs: In vivo proof of concept for a highly lipophilic counterion. Int. J. Pharm. 2023, 631, 122476. [Google Scholar] [CrossRef]
- Kumar, R.; Islam, T. Nurunnabi Mucoadhesive carriers for oral drug delivery. J. Control. Release 2022, 351, 504–559. [Google Scholar] [CrossRef]
- Liu, L.; Yang, H.; Lou, Y.; Wu, J.Y.; Miao, J.; Lu, X.Y.; Gao, J.Q. Enhancement of oral bioavailability of salmon calcitonin through chitosan-modified, dual drug-loaded nanoparticles. Int. J. Pharm. 2019, 557, 170–177. [Google Scholar] [CrossRef]
- Phatale, V.; Vaiphei, K.K.; Jha, S.; Patil, D.; Agrawal, M.; Alexander, A. Overcoming skin barriers through advanced transdermal drug delivery approaches. J. Control. Release 2022, 351, 361–380. [Google Scholar] [CrossRef]
- Villanueva-Martínez, A.; Hernández-Rizo, L.; Ganem-Rondero, A. Evaluating two nanocarrier systems for the transdermal delivery of sodium alendronate. Int. J. Pharm. 2020, 582, 119312. [Google Scholar] [CrossRef]
- Guillot, A.J.; Cordeiro, A.S.; Donnelly, R.F.; Montesinos, M.C.; Garrigues, T.M.; Melero, A. Microneedle-Based Delivery: An Over-view of Current Applications and Trends. Pharmaceutics 2020, 12, 569. [Google Scholar] [CrossRef]
- Kulkarni, D.; Gadade, D.; Chapaitkar, N.; Shelke, S.; Pekamwar, S.; Aher, R.; Ahire, A.; Avhale, M.; Badgule, R.; Bansode, R.; et al. Polymeric Microneedles: An Emerging Paradigm for Advanced Biomedical Applications. Sci. Pharm. 2023, 91, 27. [Google Scholar] [CrossRef]
- Li, Y.; Ju, X.J.; Fu, H.; Zhou, C.H.; Gao, Y.; Wang, J.; Xie, R.; Wang, W.; Liu, Z.; Chu, L.Y. Composite Separable Microneedles for Transdermal Delivery and Controlled Release of Salmon Calcitonin for Osteoporosis Therapy. ACS Appl. Mater. Interfaces 2023, 15, 638–650. [Google Scholar] [CrossRef]
- Abourehab, M.A.; Alsubaiyel, A.M.; Alshehri, S.; Alzhrani, R.M.; Almalki, A.H.; Abduljabbar, M.H.; Venkatesan, K.; Kamal, M. Laboratory determination and thermodynamic analysis of alendronate solubility in supercritical carbon dioxide. J. Mol. Liq. 2022, 367, 120242. [Google Scholar] [CrossRef]
- Villanueva-Martínez, A.; Merino, V.; Ganem-Rondero, A. Transdermal formulations and strategies for the treatment of osteoporosis. J. Drug Deliv Sci. Technol. 2022, 69, 103111. [Google Scholar] [CrossRef]
- Lima, A.C.; Ferreira, H.; Reis, R.L.; Neves, N.M. Biodegradable polymers: An update on drug delivery in bone and cartilage dis-eases. Expert Opin. Drug Deliv. 2019, 16, 795–813. [Google Scholar] [CrossRef]
- Wang, X.; Guo, W.; Li, L.; Yu, F.; Li, J.; Liu, L.; Fang, B.; Xia, L. Photothermally triggered biomimetic drug delivery of Teriparatide via reduced graphene oxide loaded chitosan hydrogel for osteoporotic bone regeneration. Chem. Eng. J. 2021, 413, 127413. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Ding, J.; Yu, L. Blending strategy to modify PEEK-based orthopedic implants. Compos. B Eng. 2022, 250, 110427. [Google Scholar] [CrossRef]
- Chiang, C.W.; Chen, C.H.; Manga, Y.B.; Huang, S.C.; Chao, K.M.; Jheng, P.R.; Wong, P.C.; Nyambat, B.; Satapathy, M.K.; Chuang, E.Y. Facilitated and Controlled Strontium Ranelate Delivery Using GCS-HA Nanocarriers Embedded into PEGDA Coupled with Decortication Driven Spinal Regeneration. Int. J. Nanomed. 2021, 16, 4209–4224. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Bordone, M.; Bettencourt, A. Management of bone diseases: Looking at scaffold-based strategies for drug delivery. Drug Deliv. Transl. Res. 2023, 13, 79–104. [Google Scholar] [CrossRef]
- Boafo, G.F.; Shi, Y.; Xiao, Q.; Magar, K.T.; Zoulikha, M.; Xing, X.; Teng, C.; Brobbey, E.; Li, X.; Jiang, X.; et al. Targeted co-delivery of daunorubicin and cytarabine based on the hyaluronic acid prodrug modified liposomes. Chin. Chem. Lett. 2022, 33, 4600–4604. [Google Scholar] [CrossRef]
- Choi, J.B.; Kim, Y.K.; Byeon, S.M.; Park, J.E.; Bae, T.S.; Jang, Y.S.; Lee, M.H. Fabrication and Characterization of Biodegradable Gelatin Methacrylate/Biphasic Calcium Phosphate Composite Hydrogel for Bone Tissue Engineering. Nanomaterials 2021, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Agrawal, M.K. A historical perspective of liposomes-a bio nanomaterial. Mater. Today Proc. 2021, 45, 2963–2966. [Google Scholar] [CrossRef]
- Neslihan Gursoy, R.; Benita, S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed. Pharmacother. 2004, 58, 173–182. [Google Scholar] [CrossRef]
- de Souza, M.P.C.; de Camargo, B.A.F.; Spósito, L.; Fortunato, G.C.; Carvalho, G.C.; Marena, G.D.; Meneguin, A.B.; Bauab, T.M.; Chorilli, M. Highlighting the use of micro and nanoparticles based-drug delivery systems for the treatment of Helicobacter pylori infections. Crit. Rev. Microbiol. 2021, 47, 435–446. [Google Scholar] [CrossRef]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, E.; Yang, J.; Cao, Z. Strategies to improve micelle stability for drug delivery. Nano Res. 2018, 11, 4985–4998. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef]
- Lara-Ochoa, S.; Ortega-Lara, W.; Guerrero-Beltrán, C.E. Hydroxyapatite Nanoparticles in Drug Delivery: Physicochemistry and Applications. Pharmaceutics 2021, 13, 1642. [Google Scholar] [CrossRef]
- Fouad-Elhady, E.A.; Aglan, H.A.; Hassan, R.E.; Ahmed, H.H.; Sabry, G.M. Modulation of bone turnover aberration: A target for management of primary osteoporosis in experimental rat model. Heliyon 2020, 6, e03341. [Google Scholar] [CrossRef]
- Mao, Z.; Li, Y.; Yang, Y.; Fang, Z.; Chen, X.; Wang, Y.; Kang, J.; Qu, X.; Yuan, W.; Dai, K. Osteoinductivity and antibacterial properties of strontium ranelate-loaded poly (lactic-co-glycolic acid) microspheres with assembled silver and hydroxyapatite nanoparti-cles. Front. Pharmacol. 2018, 9, 368. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Kornicka-Garbowska, K.; Patej, A.; Sobierajska, P.; Kotela, A.; Turlej, E.; Kepska, M.; Bienko, A.; Wiglusz, R.J. Ami-nopropyltriethoxysilane (APTES)-Modified Nanohydroxyapatite (nHAp) Incorporated with Iron Oxide (IO) Nanoparticles Promotes Early Osteogenesis, Reduces Inflammation and Inhibits Osteoclast Activity. Materials 2022, 15, 2095. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Sun, J.; Tan, L.; Yan, Q.; Li, L.; Chen, L.; Liu, X.; Bin, S. Enhanced osteogenesis and therapy of osteoporosis using simvastatin loaded hybrid system. Bioact. Mater. 2020, 5, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wang, L.; Yang, X.; Feng, Y.; Li, Y.; Feng, W. Poly(N-isopropylacrylamide)-based smart hydrogels: Design, properties and applications. Prog. Mater. Sci. 2021, 115, 100702. [Google Scholar] [CrossRef]
- Kotak, D.J.; Devarajan, P.V. Bone targeted delivery of salmon calcitonin hydroxyapatite nanoparticles for sublingual osteoporosis therapy (SLOT). Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102153. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, A.I.; Giofrè, S.; Seneci, P.; Passarella, D.; Pellegrino, S. Stimulus-responsive liposomes for biomedical applications. Drug Discov. Today 2021, 26, 1794–1824. [Google Scholar] [CrossRef]
- Leitgeb, M.; Knez, Ž.; Primožič, M. Sustainable technologies for liposome preparation. J. Supercrit. Fluids 2020, 165, 104984. [Google Scholar] [CrossRef]
- Nirwan, N.; Nikita; Sultana, Y.; Vohora, D. Liposomes as multifaceted delivery system in the treatment of osteoporosis. Expert Opin. Drug Deliv. 2021, 18, 761–775. [Google Scholar] [CrossRef]
- Cai, Y.; Gao, T.; Fu, S.; Sun, P. Development of zoledronic acid functionalized hydroxyapatite loaded polymeric nanoparticles for the treatment of osteoporosis. Exp. Ther. Med. 2018, 16, 704–710. [Google Scholar] [CrossRef]
- Xiao, Q.; Li, X.; Liu, C.; Jiang, Y.; He, Y.; Zhang, W.; Azevedo, H.S.; Wu, W.; Xia, Y.; He, W. Improving cancer immunotherapy via co-delivering checkpoint blockade and thrombospondin-1 downregulator. Acta Pharm. Sin. B 2023, 13, 3503–3517. [Google Scholar] [CrossRef]
- Xiao, Q.; Li, X.; Liu, C.; Yang, Y.; Hou, Y.; Wang, Y.; Su, M.; He, W. Liposome-based anchoring and core-encapsulation for combi-natorial cancer therapy. Chin. Chem. Lett. 2022, 33, 4191–4196. [Google Scholar] [CrossRef]
- Liu, X.-M.; Zhang, Y.; Chen, F.; Khutsishvili, I.; Fehringer, E.V.; Marky, L.A.; Bayles, K.W.; Wang, D. Prevention of Orthopedic Device-Associated Osteomyelitis Using Oxacillin-Containing Biomineral-Binding Liposomes. Pharm. Res. 2012, 29, 3169–3179. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wei, J.; Lyu, J.; Bian, T.; Liu, Z.; Huang, J.; Pi, F.; Li, C.; Zhong, Z. Bone-targeting drug delivery system of biomineral-binding liposomes loaded with icariin enhances the treatment for osteoporosis. J. Nanobiotechnol. 2019, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Lu, Y.; Qi, J.; Zhu, Q.; Chen, Z.; Wu, W. Adapting liposomes for oral drug delivery. Acta Pharm. Sin. B 2019, 9, 36–48. [Google Scholar] [CrossRef]
- Gradauer, K.; Barthelmes, J.; Vonach, C.; Almer, G.; Mangge, H.; Teubl, B.; Roblegg, E.; Dünnhaupt, S.; Fröhlich, E.; Bernkop-Schnürch, A.; et al. Liposomes coated with thiolated chitosan enhance oral peptide delivery to rats. J. Control. Release 2013, 172, 872–878. [Google Scholar] [CrossRef]
- Ewert, K.K.; Scodeller, P.; Simón-Gracia, L.; Steffes, V.M.; Wonder, E.A.; Teesalu, T.; Safinya, C.R. Cationic Liposomes as Vectors for Nucleic Acid and Hydrophobic Drug Therapeutics. Pharmaceutics 2021, 13, 1365. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, L.; Wang, H.; Wang, Y.; Tan, Y.; Dang, L.; Wang, K.; Sun, Z.; Li, G.; Cao, X. Targeted silencing of miRNA-132-3p expression rescues disuse osteopenia by promoting mesenchymal stem cell osteogenic differentiation and osteogenesis in mice. Stem Cell Res. Ther. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.-Y.; Ima-Nirwana, S. The role of tocotrienol in preventing male osteoporosis—A review of current evidence. Int. J. Mol. Sci. 2019, 20, 1355. [Google Scholar] [CrossRef]
- Mohamad, N.-V.; Ima-Nirwana, S.; Chin, K.-Y. Therapeutic potential of annatto tocotrienol with self-emulsifying drug delivery system in a rat model of postmenopausal bone loss. BioMedicine 2021, 137, 111368. [Google Scholar] [CrossRef]
- Liao, Y.; Zhong, L.; Liu, L.; Xie, L.; Tang, H.; Zhang, L.; Li, X. Comparison of surfactants at solubilizing, forming and stabilizing nanoemulsion of hesperidin. J. Food Eng. 2020, 281, 110000. [Google Scholar] [CrossRef]
- Zakir, F.; Ahmad, A.; Farooq, U.; Mirza, M.A.; Tripathi, A.; Singh, D.; Shakeel, F.; Mohapatra, S.; Ahmad, F.J.; Kohli, K. Design and development of a commercially viable in situ nanoemulgel for the treatment of postmenopausal osteoporosis. Nanomedicine 2020, 15, 1167–1187. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ma, E.; Zhao, H.; Long, Y.; Zheng, C.; Duan, M. Preliminary evaluation of a novel oral delivery system for rhPTH1-34: In vitro and in vivo. Int. J. Pharm. 2011, 420, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Kheraldine, H.; Rachid, O.; Habib, A.M.; Al Moustafa, A.-E.; Benter, I.F.; Akhtar, S. Emerging innate biological properties of nano-drug delivery systems: A focus on PAMAM dendrimers and their clinical potential. Adv. Drug Deliv. Rev. 2021, 178, 113908. [Google Scholar] [CrossRef]
- Ren, M.; Li, Y.; Zhang, H.; Li, L.; He, P.; Ji, P.; Yang, S. An oligopeptide/aptamer-conjugated dendrimer-based nanocarrier for dual-targeting delivery to bone. J. Mater. Chem. B 2021, 9, 2831–2844. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, A.; Marturano, A.; Placella, G.; Staderini, E.; Domingo, L.I.; Cerulli, G.G.; Tiribuzi, R.; Blasi, P. Amelogenin-Derived Peptides in Bone Regeneration: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 9224. [Google Scholar] [CrossRef]
- Ahmed, R.; Aucamp, M.; Ebrahim, N.; Samsodien, H. Supramolecular assembly of rifampicin and PEGylated PAMAM dendrimer as a novel conjugate for tuberculosis. J. Drug Deliv. Sci. Technol. 2021, 66, 102773. [Google Scholar] [CrossRef]
- Zheng, X.; Xie, J.; Zhang, X.; Sun, W.; Zhao, H.; Li, Y.; Wang, C. An overview of polymeric nanomicelles in clinical trials and on the market. Chin. Chem. Lett. 2021, 32, 243–257. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- del Pozo-Acebo, L.; Hazas, M.-C.L.d.L.; Tomé-Carneiro, J.; Gil-Cabrerizo, P.; San-Cristobal, R.; Busto, R.; García-Ruiz, A.; Dávalos, A. Bovine Milk-Derived Exosomes as a Drug Delivery Vehicle for miRNA-Based Therapy. Int. J. Mol. Sci. 2021, 22, 1105. [Google Scholar] [CrossRef]
- Cai, M.; Yang, L.; Zhang, S.; Liu, J.; Sun, Y.; Wang, X. A bone-resorption surface-targeting nanoparticle to deliver anti-miR214 for osteoporosis therapy. Int. J. Nanomed. 2017, 12, 7469–7482. [Google Scholar] [CrossRef]
- Yang, J.; Qin, L.; Huang, J.; Li, Y.; Xu, S.; Wang, H.; Zhu, S.; Wang, J.; Zhu, B.; Li, F. Astragalus polysaccharide attenuates LPS-related inflammatory osteolysis by suppressing osteoclastogenesis by reducing the MAPK signalling pathway. J. Cell. Mol. Med. 2021, 25, 6800–6814. [Google Scholar] [CrossRef]
- Wu, D.; Cline-Smith, A.; Shashkova, E.; Perla, A.; Katyal, A.; Aurora, R. T-Cell Mediated Inflammation in Postmenopausal Oste-oporosis. Front. Immunol. 2021, 12, 687551. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Tan, X.; Huang, J.; Huang, H.; Zou, P.; Hu, J. Atorvastatin-loaded micelles with bone-targeted ligand for the treatment of osteoporosis. Drug Deliv. 2017, 24, 1067–1076. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xie, Y.; Liu, C.; Huang, H.; Huang, J.; Deng, A.; Zou, P.; Tan, X. Bone-targeted delivery of simvastatin-loaded PEG-PLGA micelles conjugated with tetracycline for osteoporosis treatment. Drug Deliv. Transl. Res. 2018, 8, 1090–1102. [Google Scholar] [CrossRef]
- Fazil, M.; Hassan, Q.; Baboota, S.; Ali, J. Biodegradable intranasal nanoparticulate drug delivery system of risedronate sodium for osteoporosis. Drug Deliv. 2016, 23, 2428–2438. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Chen, S.; Bhatia, S.S.; Li, B.; Liang, H.; Liu, C.; Liang, Z.; Liu, J.; Li, H.; Liu, Z.; et al. Bone-targeted polymeric nanoparticles as alendronate carriers for potential osteoporosis treatment. Polym. Test. 2022, 110, 107584. [Google Scholar] [CrossRef]
- Santhosh, S.; Mukherjee, D.; Anbu, J.; Murahari, M.; Teja, B.V. Improved treatment efficacy of risedronate functionalized chitosan nanoparticles in osteoporosis: Formulation development, in vivo, and molecular modelling studies. J. Microencapsul. 2019, 36, 338–355. [Google Scholar] [CrossRef]
- Rahmani, S.; Hakimi, S.; Esmaeily, A.; Samadi, F.Y.; Mortazavian, E.; Nazari, M.; Mohammadi, Z.; Tehrani, N.R.; Tehrani, M.R. Novel chitosan based nanoparticles as gene delivery systems to cancerous and noncancerous cells. Int. J. Pharm. 2019, 560, 306–314. [Google Scholar] [CrossRef]
- Zeng, K.; Ma, L.; Yang, W.; Lei, S.; Wang, M.; You, Y.; Zhao, Y.; Ge, X. Biodegradable nano-organosilica gene carrier for high-efficiency gene transfection. J. Mater. Chem. B 2020, 8, 2483–2494. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, K.; Bu, W.; Xu, X.; Jin, H.; Chang, B.; Wang, B.; Sun, Y.; Yang, B.; Zheng, C. Effective delivery of bone morphogenetic protein 2 gene using chitosan–polyethylenimine nanoparticle to promote bone formation. RSC Adv. 2016, 6, 34081–34089. [Google Scholar] [CrossRef]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Arumugam, B.; Balagangadharan, K.; Selvamurugan, N. Syringic acid, a phenolic acid, promotes osteoblast differentiation by stimulation of Runx2 expression and targeting of Smad7 by miR-21 in mouse mesenchymal stem cells. J. Cell Commun. Signal. 2018, 12, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Shuai, C.; Feng, P.; Gao, C.; Peng, S.; Yang, Y. Antibacterial capability, physicochemical properties, and biocompatibility of nTiO2 incorporated polymeric scaffolds. Polymers 2018, 10, 328. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, D.; Zhang, T.; Chen, C.; Song, Y.; Liu, S.; Su, Y.; Guo, S. Effect of the vascularized bone components on the survival of vascularized composite allografts. J. Surg. Res. 2018, 224, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Perić Kačarević, Ž.; Rider, P.; Alkildani, S.; Retnasingh, S.; Pejakić, M.; Schnettler, R.; Gosau, M.; Smeets, R.; Jung, O.; Barbeck, M. An introduction to bone tissue engineering. Int. J. Artif. Organs 2019, 43, 69–86. [Google Scholar] [CrossRef]
- Anupama, D.V.K.; Ray, S.; Arora, U.; Mitra, S.; Sionkowska, A.; Jaiswal, A.K. Dual drug delivery platforms for bone tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 969843. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Gao, M.; Yang, K.; Tan, L.; Zhao, W. A Degradable and Osteogenic Mg-Based MAO-MT-PLGA Drug/Ion Delivery System for Treating an Osteoporotic Fracture. Pharmaceutics 2022, 14, 1481. [Google Scholar] [CrossRef]
- Bakhshi, R.; Mohammadi-Zerankeshi, M.; Mehrabi-Dehdezi, M.; Alizadeh, R.; Labbaf, S.; Abachi, P. Additive manufacturing of PLA-Mg composite scaffolds for hard tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2023, 138, 105655. [Google Scholar] [CrossRef]
- Chen, M.; Huang, L.; Shen, X.; Li, M.; Luo, Z.; Cai, K.; Hu, Y. Construction of multilayered molecular reservoirs on a titanium alloy implant for combinational drug delivery to promote osseointegration in osteoporotic conditions. Acta Biomater. 2020, 105, 304–318. [Google Scholar] [CrossRef]
- Che, L.; Wang, Y.; Sha, D.; Li, G.; Wei, Z.; Liu, C.; Yuan, Y.; Song, D. A biomimetic and bioactive scaffold with intelligently pulsatile teriparatide delivery for local and systemic osteoporosis regeneration. Bioact. Mater. 2022, 19, 75–87. [Google Scholar] [CrossRef]
- Kuang, L.; Huang, J.; Liu, Y.; Li, X.; Yuan, Y.; Liu, C. Injectable Hydrogel with NIR Light-Responsive, Dual-Mode PTH Release for Osteoregeneration in Osteoporosis. Adv. Funct. Mater. 2021, 31, 2105383. [Google Scholar] [CrossRef]
- Gan, M.; Zhou, Q.; Ge, J.; Zhao, J.; Wang, Y.; Yan, Q.; Wu, C.; Yu, H.; Xiao, Q.; Wang, W.; et al. Precise in-situ release of microRNA from an injectable hydrogel induces bone regeneration. Acta Biomater. 2021, 135, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cai, Q.; Zhu, Y.; Zhu, W. A simple hydrogel scaffold with injectability, adhesivity and osteogenic activity for bone regeneration. Biomater. Sci. 2021, 9, 960–972. [Google Scholar] [CrossRef]
- He, X.-Y.; Yu, H.-M.; Lin, S.; Li, Y.-Z. Advances in the application of mesenchymal stem cells, exosomes, biomimetic materials, and 3D printing in osteoporosis treatment. Cell. Mol. Biol. Lett. 2021, 26, 47. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Hu, L.; Pan, P.; Tarafder, S.; Du, M.; Geng, Y.; Xu, G.; Chen, L.; Chen, J.; Lee, C.H. Icariin-releasing 3D printed scaffold for bone regeneration. Compos. Part B Eng. 2022, 232, 109625. [Google Scholar] [CrossRef]
- Chen, Q.; Zou, B.; Lai, Q.; Wang, Y.; Zhu, K.; Deng, Y.; Huang, C. 3D printing and osteogenesis of loofah-like hydroxyapatite bone scaffolds. Ceram. Int. 2021, 47, 20352–20361. [Google Scholar] [CrossRef]
- Lim, D.-J.; Vines, J.B.; Park, H.; Lee, S.-H. Microneedles: A versatile strategy for transdermal delivery of biological molecules. Int. J. Biol. Macromol. 2018, 110, 30–38. [Google Scholar] [CrossRef]
- Darvishha, S.; Amiri, S. (Trans) dermal insulin delivery based on polymeric systems. Int. J. Polym. Mater. Polym. Biomater. 2018, 68, 1118–1132. [Google Scholar] [CrossRef]
- Kulkarni, D.; Damiri, F.; Rojekar, S.; Zehravi, M.; Ramproshad, S.; Dhoke, D.; Musale, S.; Mulani, A.A.; Modak, P.; Paradhi, R.; et al. Recent Advancements in Microneedle Technology for Multifaceted Biomedical Applications. Pharmaceutics 2022, 14, 1097. [Google Scholar] [CrossRef]
- Xu, J.; Xu, D.; Xuan, X.; He, H. Advances of Microneedles in Biomedical Applications. Molecules 2021, 26, 5912. [Google Scholar] [CrossRef]
- Jyoung, J.-Y.; Shim, B.-S.; Hwang, I.-S.; Cho, D.-E. Iontophoretic transdermal delivery of alendronate in hairless mouse skin. Polymer 2009, 33, 237–242. [Google Scholar]
- Tas, C.; Mansoor, S.; Kalluri, H.; Zarnitsyn, V.G.; Choi, S.-O.; Banga, A.K.; Prausnitz, M.R. Delivery of salmon calcitonin using a microneedle patch. Int. J. Pharm. 2012, 423, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.J.; Kang, N.W.; Jeong, H.R.; Sohn, S.Y.; Jeon, Y.E.; Yu, N.Y.; Hwang, Y.; Kim, S.; Kim, D.D.; Park, J.H. The Relationship between the Drug Delivery Properties of a Formulation of Teriparatide Microneedles and the Pharmacokinetic Evaluation of Teriparatide Administration in Rats. Pharm. Res. 2022, 39, 989–999. [Google Scholar] [CrossRef]
- Daddona, P.E.; Matriano, J.A.; Mandema, J.; Maa, Y.-F. Parathyroid Hormone (1-34)-Coated Microneedle Patch System: Clinical Pharmacokinetics and Pharmacodynamics for Treatment of Osteoporosis. Pharm. Res. 2011, 28, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Hattersley, G.; Harris, A.; Jamal, S.; Hamed, E.; Brown, K.; Dohmeier, D.; Moseman, J.; Zhang, Y.; Dick, L. Formulations of Abalo-paratide, Transdermal Patches Thereof, and Uses Thereof. U.S. Patent No. 10,568,937, 25 February 2020. [Google Scholar]
- Katsumi, H.; Tanaka, Y.; Hitomi, K.; Liu, S.; Quan, Y.-S.; Kamiyama, F.; Sakane, T.; Yamamoto, A. Efficient Transdermal Delivery of Alendronate, a Nitrogen-Containing Bisphosphonate, Using Tip-Loaded Self-Dissolving Microneedle Arrays for the Treatment of Osteoporosis. Pharmaceutics 2017, 9, 29. [Google Scholar] [CrossRef]
- Sultana, N.; Ali, A.; Waheed, A.; Jabi, B.; Yaqub Khan, M.; Mujeeb, M.; Sultana, Y.; Aqil, M. Dissolving microneedle transdermal patch loaded with Risedronate sodium and Ursolic acid bipartite nanotransfersomes to combat osteoporosis: Optimization, characterization, in vitro and ex vivo assessment. Int. J. Pharm. 2023, 644, 123335. [Google Scholar] [CrossRef]
- Naito, C.; Katsumi, H.; Suzuki, T.; Quan, Y.-S.; Kamiyama, F.; Sakane, T.; Yamamoto, A. Self-Dissolving Microneedle Arrays for Transdermal Absorption Enhancement of Human Parathyroid Hormone (1-34). Pharmaceutics 2018, 10, 215. [Google Scholar] [CrossRef]
- Vemulapalli, V.; Bai, Y.; Kalluri, H.; Herwadkar, A.; Kim, H.; Davis, S.P.; Friden, P.M.; Banga, A.K. In Vivo Iontophoretic Delivery of Salmon Calcitonin Across Microporated Skin. J. Pharm. Sci. 2012, 101, 2861–2869. [Google Scholar] [CrossRef]
- Lin, Z.; Zheng, K.; Zhong, J.; Zheng, X. Advances in microneedle-based therapy for bone disorders. Biomed. Pharmacother. 2023, 165, 115013. [Google Scholar] [CrossRef]
- Bushra, F. Delivery of Denosumab via Hollow Microneedle. Ph.D. Thesis, Brac University, Dhaka, Bangladesh, 2020. [Google Scholar]
- Sabri, A.; Ogilvie, J.; McKenna, J.; Segal, J.; Scurr, D.; Marlow, M. Intradermal Delivery of an Immunomodulator for Basal Cell Carcinoma; Expanding the Mechanistic Insight into Solid Microneedle-Enhanced Delivery of Hydrophobic Molecules. Mol. Pharm. 2020, 17, 2925–2937. [Google Scholar] [CrossRef]
- Long, L.-Y.; Zhang, J.; Yang, Z.; Guo, Y.; Hu, X.; Wang, Y. Transdermal delivery of peptide and protein drugs: Strategies, advantages and disadvantages. J. Drug Deliv. Sci. Technol. 2020, 60, 102007. [Google Scholar] [CrossRef]
- Li, S.; Li, W.; Prausnitz, M. Individually coated microneedles for co-delivery of multiple compounds with different properties. Drug Deliv. Transl. Res. 2018, 8, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Ita, K. Modulation of transdermal drug delivery with coated microneedles. J. Drug Deliv. Sci. Technol. 2018, 45, 203–212. [Google Scholar] [CrossRef]
- Sharma, S.; Hatware, K.; Bhadane, P.; Sindhikar, S.; Mishra, D.K. Recent advances in microneedle composites for biomedical applications: Advanced drug delivery technologies. Mater. Sci. Eng. C 2019, 103, 109717. [Google Scholar] [CrossRef] [PubMed]
- Fakhraei Lahiji, S.; Jang, Y.; Huh, I.; Yang, H.; Jang, M.; Jung, H. Exendin-4–encapsulated dissolving microneedle arrays for efficient treatment of type 2 diabetes. Sci. Rep. 2018, 8, 1170. [Google Scholar] [CrossRef]
- Katsumi, H.; Liu, S.; Tanaka, Y.; Hitomi, K.; Hayashi, R.; Hirai, Y.; Kusamori, K.; Quan, Y.-S.; Kamiyama, F.; Sakane, T.; et al. Development of a Novel Self-Dissolving Microneedle Array of Alendronate, a Nitrogen-Containing Bisphosphonate: Evaluation of Transdermal Absorption, Safety, and Pharmacological Effects After Application in Rats. J. Pharm. Sci. 2012, 101, 3230–3238. [Google Scholar] [CrossRef]
- Ren, Y.; Li, J.; Chen, Y.; Wang, J.; Chen, Y.; Wang, Z.; Zhang, Z.; Chen, Y.; Shi, X.; Cao, L.; et al. Customized flexible hollow microneedles for psoriasis treatment with reduced-dose drug. Bioeng. Transl. Med. 2023, 8, e10530. [Google Scholar] [CrossRef]
- Vora, L.K.; Moffatt, K.; Tekko, I.A.; Paredes, A.J.; Volpe-Zanutto, F.; Mishra, D.; Peng, K.; Raj Singh Thakur, R.; Donnelly, R.F. Mi-croneedle array systems for long-acting drug delivery. Eur. J. Pharm. Biopharm. 2021, 159, 44–76. [Google Scholar] [CrossRef]
- Ma, G.; Wu, C. Microneedle, bio-microneedle and bio-inspired microneedle: A review. J. Control. Release 2017, 251, 11–23. [Google Scholar] [CrossRef]
- Al-Japairai, K.A.S.; Mahmood, S.; Almurisi, S.H.; Venugopal, J.R.; Hilles, A.R.; Azmana, M.; Raman, S. Current trends in polymer microneedle for transdermal drug delivery. Int. J. Pharm. 2020, 587, 119673. [Google Scholar] [CrossRef]
- Cárcamo-Martínez, Á.; Mallon, B.; Domínguez-Robles, J.; Vora, L.K.; Anjani, Q.K.; Donnelly, R.F. Hollow microneedles: A per-spective in biomedical applications. Int. J. Pharm. 2021, 599, 120455. [Google Scholar] [CrossRef]
- Brayden, D.; Hill, T.; Fairlie, D.; Maher, S.; Mrsny, R. Systemic delivery of peptides by the oral route: Formulation and medicinal chemistry approaches. Adv. Drug Deliv. Rev. 2020, 157, 2–36. [Google Scholar] [CrossRef] [PubMed]
- Sturmer, A.; Mehta, N.; Giacchi, J.; Cagatay, T.; Tavakkol, R.; Mitta, S.; Fitzpatrick, L.; Wald, J.; Trang, J. Pharmacokinetics of oral recombinant human parathyroid hormone [rhPTH(1-31)NH2] in postmenopausal women with osteoporosis. Clin. Pharmacokinet. 2013, 52, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Hamza, A.; Saramet, G. Actualities in Endocrine Pharmacology: Advances in the Development of Oral Formulations for Calci-tonin and Semaglutide. Acta Endocrinol. 2020, 16, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Subramony, J.A. Chapter Seven—Clinical translation of oral peptide delivery technologies. In Oral Delivery of Thera-peutic Peptides and Proteins; Tyagi, P., Subramony, J.A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 225–250. [Google Scholar]
- Naot, D.; Musson, D.S.; Cornish, J. The Activity of Peptides of the Calcitonin Family in Bone. Physiol. Rev. 2019, 99, 781–805. [Google Scholar] [CrossRef]
- Lavrador, P.; Gaspar, V.M.; Mano, J.F. Stimuli-responsive nanocarriers for delivery of bone therapeutics—Barriers and progresses. J. Control. Release 2018, 273, 51–67. [Google Scholar] [CrossRef]


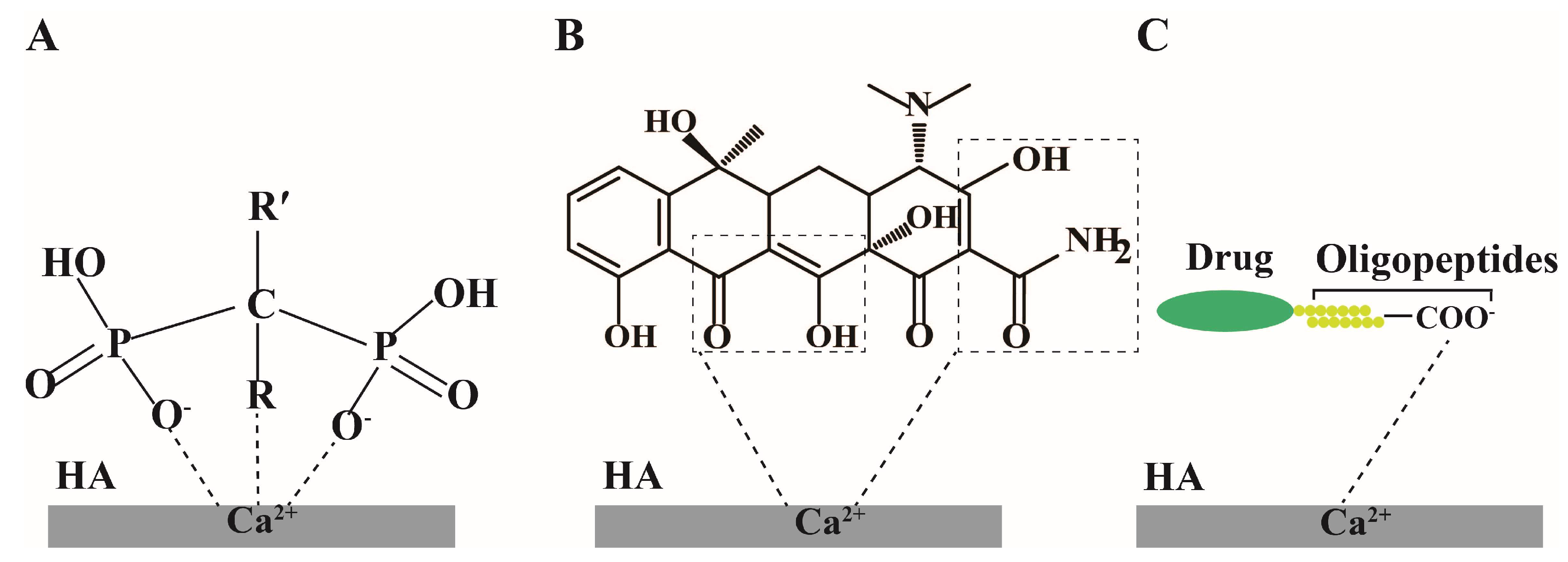
| Classification | Drug | Dosage Form | Mechanism of Action |
|---|---|---|---|
| Bisphosphonates | Alendronate | Tablets | Specifically binds to hydroxyapatite in bone and inhibits osteoclast activity, inhibiting bone resorption. |
| Risedronate | Tablets | ||
| Zoledronate | Injections | ||
| Hormone | Calcitonin | Injections/nasal sprays | Binds to calcitonin receptors on osteoclasts and inhibits osteoclast activity to reduce serum calcium levels. |
| Selective estrogen receptor modulators | Raloxifene | Tablets | Activates or inhibits estrogen receptor-mediated cytokine responses, inhibiting bone resorption. |
| Bazedoxifene | Tablets | ||
| RANKL inhibitors | Denosumab | Injections | Interfering with the binding of RANKL to its receptor RANK, specifically inhibiting the differentiation and maturation of osteoclasts. |
| Strontium ranelate | Dry suspensions | Strontium ions can enhance the DNA synthesis of pre-osteoblasts and promote the multiplication of osteoblasts, increases the expression of osteoprotegerin in osteoblasts, and inhibits bone resorption. | |
| Parathyroid hormone analogs | Teriparatide | Injections | Selectively activate the parathyroid hormone type 1 receptor’s signaling pathway and stimulate osteoblast-mediated bone formation. |
| Abaloparatide | Injections | ||
| Sclerostin inhibitors | Romosozumab | Injections | Inhibit the activity of osteosclerosin, promote bone formation, and inhibit bone resorption. |
| Nutritional supplements | Calcium | Tablets/capsules | The first messenger in the signal transduction pathway, indirectly promoting bone formation. |
| Vitamin D | |||
| Calcitriol |
| Strategies | Advantages | Limitations | Ref. |
|---|---|---|---|
| Hydroxyapatite nanoparticles | Biocompatibility, stable mechanical properties, bone affinity. | Poor biodegradability. | [82] |
| Liposomes | Solubilization, biocompatibility, low immunogenicity, biodegradable, drug protection against external environment degradation, side effects reduction. | Low encapsulation rate, difficulty in scaling up production, high production costs, susceptibility to recognition by the mononuclear phagocytosis system. | [83] |
| Emulsions | Solubilization, reduction of adverse effects, drugs protection against degradation, better bioavailability. | Low stability, use of surfactants that can be cytotoxic. | [84,85] |
| Dendrimers | High drug loading capacity, easy surface modification, biodegradable. | Cytotoxicity. | [86] |
| Micelles | Solubilization, low toxicity, simple preparation. | Sensitive to environmental changes, low stability. | [87] |
| Polymeric nanoparticles | Easy to chemically modify, drug protection against gastrointestinal environment. | Low physical and chemical stability. | [88] |
| Scaffolds | Localized drug delivery. | High cost, sudden release, risk of infection. | [79,80] |
| Microneedles | Fast onset of action, good patient compliance. | Skin irritation, skin sensitization. | [89] |
| Vehicle | API | Drug Loading Method | Outcomes | Ref. |
|---|---|---|---|---|
| HA-NPs | HA | HA was made into nanoparticles with chitosan or silver. | Reducing serum bone alkaline phosphatase and salivary protein levels. | [91] |
| HA-NPs | HA | Composite nanoparticles composed of HA-NPs and iron oxides. | Improving the viability of osteoblasts, promoting the expression of Runx2, and inhibiting the activity of osteoclasts. | [93] |
| HA-NPs | Simvastatin | Simvastatin was loaded in poly(N-isopropylacrylamide)-modified mesoporous HA-NPs. | Osteogenic differentiation of BMSCs was promoted and bone formation in OVX rats was improved. | [94] |
| HA-NPs | Calcitonin | Calcitonin was loaded on HA-NPs by ion complexation. | The relative bioavailability of calcitonin-HA-NPs administered in sublingual mucosa was 15% compared with subcutaneous injection. | [96] |
| Liposomes | Zoledronic acid | HA was modified on the surface of liposomes, and the drug was encapsulated in a hydrophilic core. | Prolonged release of the drug. | [100] |
| Liposomes | Icariin | PPi-TEG-Chol is modified on the surface of liposomes to increase their targeting ability. | The bone strength of OVX rats was improved and bone resorption was inhibited to a certain extent. | [104] |
| Liposomes | Calcitonin | Thioglycolic acid and 6,6′-dithionicotinamide modified chitosan adorn the liposome surface. | The oral bioavailability of calcitonin was 8.2-fold higher than that of free calcitonin solutions. | [106] |
| Liposomes | Anti-mir-132 | The drug was encapsulated in (AspSer)6 peptide-modified cationic liposomes. | Liposomes successfully targeted the bone and silenced the expression of miRNA-132-3p in BMSCs, thereby reversing OP. | [108] |
| Emulsions | Tocotrienols | Self-emulsifying drug delivery system consisting of Cremophor® EL, Labrasol®, Captex® 355 and corn oil. | The plasma levels of δ-tocopherols and antioxidant enzymes in the test group were significantly higher than those in the free drug group, which improved the cortical bone thickness and bone strength of OVX rats. | [110] |
| Emulsions | Raloxifene | Raloxifene nanoemulsions were loaded in a hydrogel composed of poloxamer 407 and carbomer 934 to prolong the adhesion time of milk droplets on the nasal mucosa. | The bioavailability of raloxifene in latex was 7.4 times higher than that of commercially available tablets, and rabbit bone density in the latex group increased by 162% compared to those given oral tablets. | [112] |
| Emulsions | Teriparatide | Microemulsions consisting of Labrasol®, Crodamol GTCC, Solutol® HS 15, D-α-tocopheryl acetate, and aqueous phase (85:15, oil: water). | Bioavailability was 5.4% with oral administration and 12.0% when administered by ileal injection. | [113] |
| Dendrimers | — | Four amino acids rich in carboxylic acids were coupled to PAMAMs, respectively. | The amount of amino acid-modified PAMAM deposition in the bone was higher than that of unmodified PAMAM. | [47] |
| Dendrimers | Vitamin D | PAMAM was modified using CH6 aptamers and C11 peptides. | Vehicles were successfully targeted and accumulated in the bone within 24 h after administration. | [117] |
| Micelles | miR214 antagonist | Asp8 peptide was modified on the surface of polyurethane nanomicelles. | After administration, bone mass in OVX mice increased significantly, and osteoclast-associated genes (TRAP and CTSK) were downregulated. | [121] |
| Micelles | Atorvastatin | Use of tetracycline molecules to modify the surface of PEG-PLGA micelles. | Drug was continuously released from the micelles for more than 48 h, and the bone strength of the TC-PEG-PLGA micelle group significantly increased compared to that of the control group. | [124] |
| Micelles | Simvastatin | Use tetracycline molecules to modify the surface of PEG-PLGA micelles. | Micelles prolonged the systemic circulation time of simvastatin and preferentially cumulated in the bone tissue | [125] |
| Polymeric NPs | Risedronate sodium | PLGA nanoparticles were used as carriers. | The accumulative permeability of the drug in the nasal mucosa of pigs was 34.32 ± 2.64%. | [126] |
| Polymeric NPs | Alendronate sodium | PLGA nanoparticles with surface-modified chitosan and sodium alendronate were used as carriers. | The nanoparticles could continuously release sodium alendronate without obvious synaptic phenomena and had good biocompatibility with MC3T3-E1 cells. | [127] |
| Polymeric NPs | Calcitonin | Calcitonin and pueraria were encapsulated in chitosan nanoparticles. | The absolute oral bioavailability of calcitonin was up to 12.52 ± 1.83%, which was higher than that of the control group. | [67] |
| Polymeric NPs | Risedronate sodium | Risedronate was encapsulated in deacetylated chitosan nanoparticles. | The bone density of the rats in the treatment group was significantly improved, the microstructure of trabecular bone was significantly improved, and the cortical bone porosity was small. | [128] |
| Polymeric NPs | BMP2 | The BMP2 gene was encapsulated in chitosan-PEI nanoparticles. | MC3T3-E1 cells in the experimental group were significantly mineralized, and there was formation of new bone in rats with a significant increase in bone defects after administration. | [131] |
| Type/Technique | Drug | Formulation | Results | Ref. |
|---|---|---|---|---|
| Solid microneedles /Iontophoresis | Alendronate sodium | Glycerin, itaconate monobutyl ester, 3-sulfopropyl acrylate. | Increased the permeability of the drug. | [152] |
| Coated microneedles | Calcitonin | Low viscosity CMC-Na, trehalose, poloxamer 188. | There was no significant difference in bioavailability compared to subcutaneous injection, and it was 13 times that of nasal sprays. | [153] |
| Coated microneedles | Teriparatide | CMC-Na/sucrose. | Sucrose allows the coating layer of teriparatide to dissolve quickly, while CMC-Na slows the drug release rate. | [154] |
| Coated microneedles | Teriparatide | Titanium, sucrose, hydrochloric acid, EDTA, polysorbate. | The phase II clinical trial found that the microneedle formulation can increase the bone density of the lumbar spine. | [155] |
| Coated microneedles | Abaloparatide | Zinc chloride | Phase II clinical trial showed a dose-dependent increase in bone density in the spine and hip, but the bone density was lower than with subcutaneous injection. | [156] |
| Dissolving microneedles | Alendronate sodium | Hyaluronic acid | The decrease in growth plate width and bone density were inhibited. | [157] |
| Dissolving microneedles | Risedronate sodium, ursolic acid | Gelatin | More than 80% drug release within 24 h in vitro permeation study | [158] |
| Dissolving microneedles | Teriparatide | Hyaluronic acid | It effectively prevented the reduction of bone density. | [159] |
| Dissolving microneedles /Iontophoresis | Calcitonin | Maltose | Increasing the blood concentration of the drug. | [160] |
| Dissolving microneedles | Calcitonin | Silk fibroin, hyaluronic acid. | Trabecular bone repair was better in the preparation group. | [161] |
| Hollow microneedles | Denosumab | 3D-printing technology. | It simulates the release profile of the subcutaneous injection group. | [162] |
| Drug | Delivery Strategy | Identifier | Phase | Status |
|---|---|---|---|---|
| Calcitonin | Citric acid acts as a pH adjuster | NCT00959764 | Phase III | Completed |
| Calcitonin | 8-[(5-chloro-2-hydroxybenzoyl) amino] octanoic acid (5-CNAC) as an absorption enhancer | NCT00525798 | Phase III | Completed |
| Teriparatide | Transdermal delivery via coated microneedles | NCT01011556 | Phase II | Completed |
| Teriparatide | - | NCT04003467 | Phase II | Completed |
| Teriparatide | Mucosal delivery via nasal sprays | NCT00624481 | Phase II | Withdrawn |
| Teriparatide | Long-acting preparations | CTR20181346 | Phase II | Active |
| rhPTH(1-31)NH2 | Citric acid acts as a pH adjuster | NCT01321723 | Phase II | Completed |
| Abaloparatide | Solid microstructured transdermal system | NCT04064411 | Phase III | Completed |
| Risedronate sodium | - | NCT02063854 | Phase III | Completed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, M.; Zeng, H.; Yang, P.; Xu, J.; Zhang, X.; He, W. Drug Delivery and Therapy Strategies for Osteoporosis Intervention. Molecules 2023, 28, 6652. https://doi.org/10.3390/molecules28186652
Ma M, Zeng H, Yang P, Xu J, Zhang X, He W. Drug Delivery and Therapy Strategies for Osteoporosis Intervention. Molecules. 2023; 28(18):6652. https://doi.org/10.3390/molecules28186652
Chicago/Turabian StyleMa, Mingyang, Huiling Zeng, Pei Yang, Jiabing Xu, Xingwang Zhang, and Wei He. 2023. "Drug Delivery and Therapy Strategies for Osteoporosis Intervention" Molecules 28, no. 18: 6652. https://doi.org/10.3390/molecules28186652
APA StyleMa, M., Zeng, H., Yang, P., Xu, J., Zhang, X., & He, W. (2023). Drug Delivery and Therapy Strategies for Osteoporosis Intervention. Molecules, 28(18), 6652. https://doi.org/10.3390/molecules28186652







