Optimization of the Green Extraction of Red Araçá (Psidium catteyanum Sabine) and Application in Alginate Membranes for Use as Dressings
Abstract
:1. Introduction
2. Results and Discussion
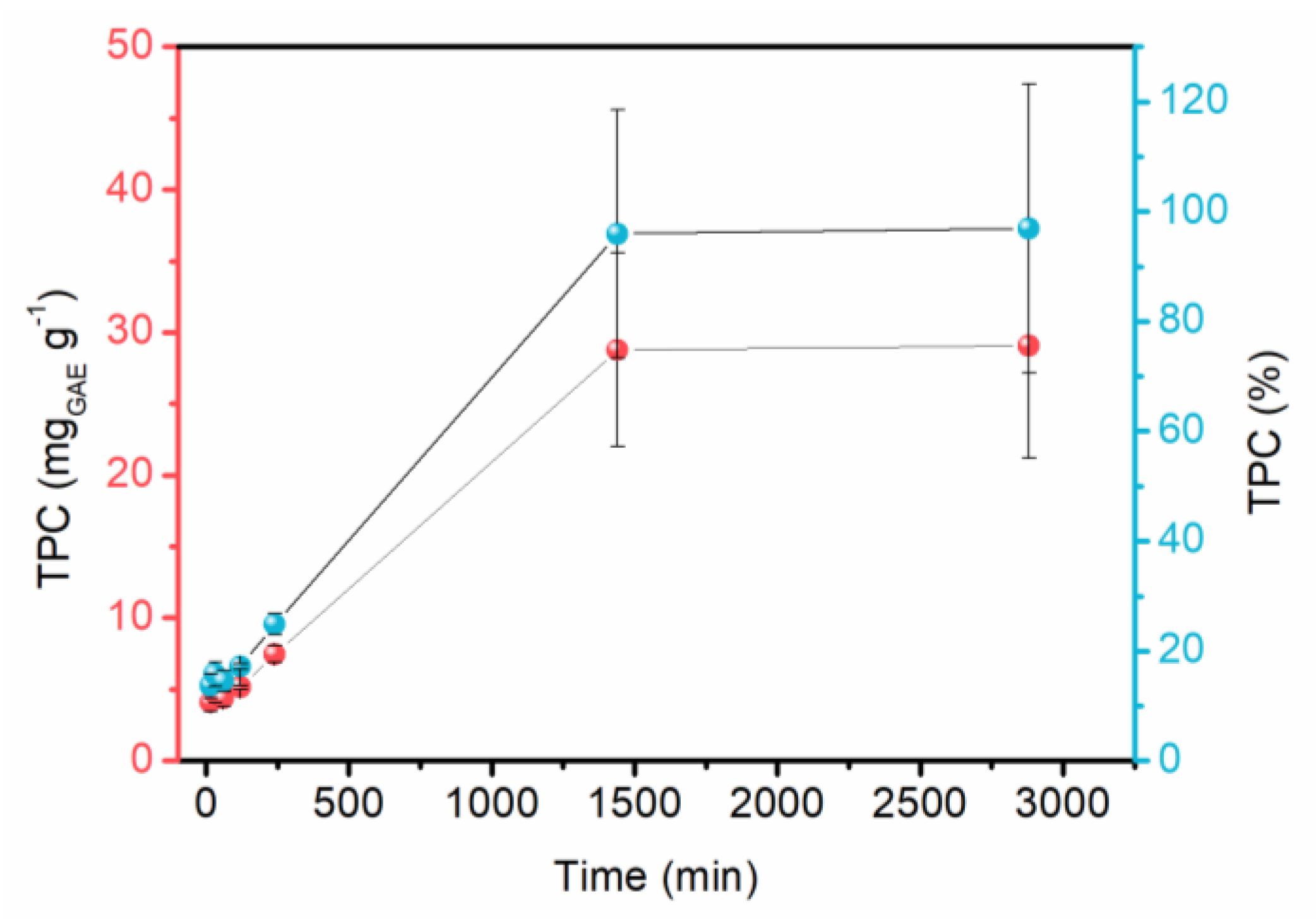
2.1. Optimization of Red Araçá Epicarp Extraction
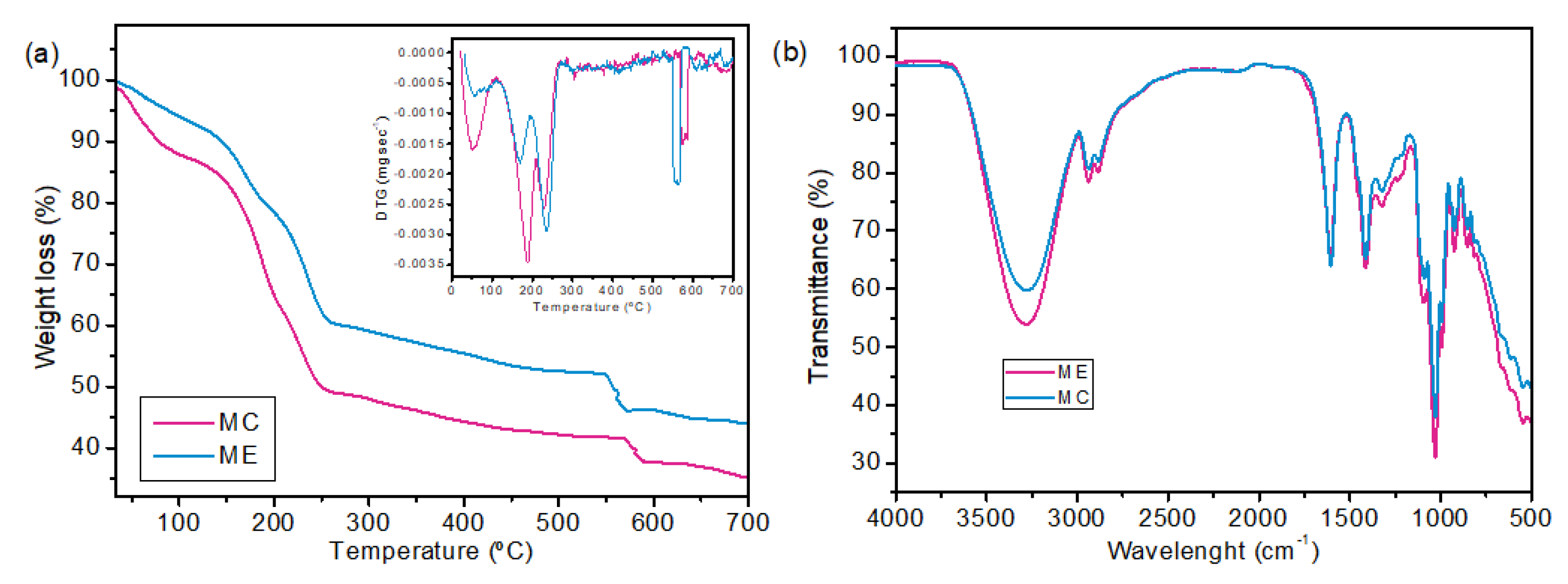
2.2. Membrane Characterization
3. Materials and Methods
3.1. Materials
3.2. Extraction Procedure
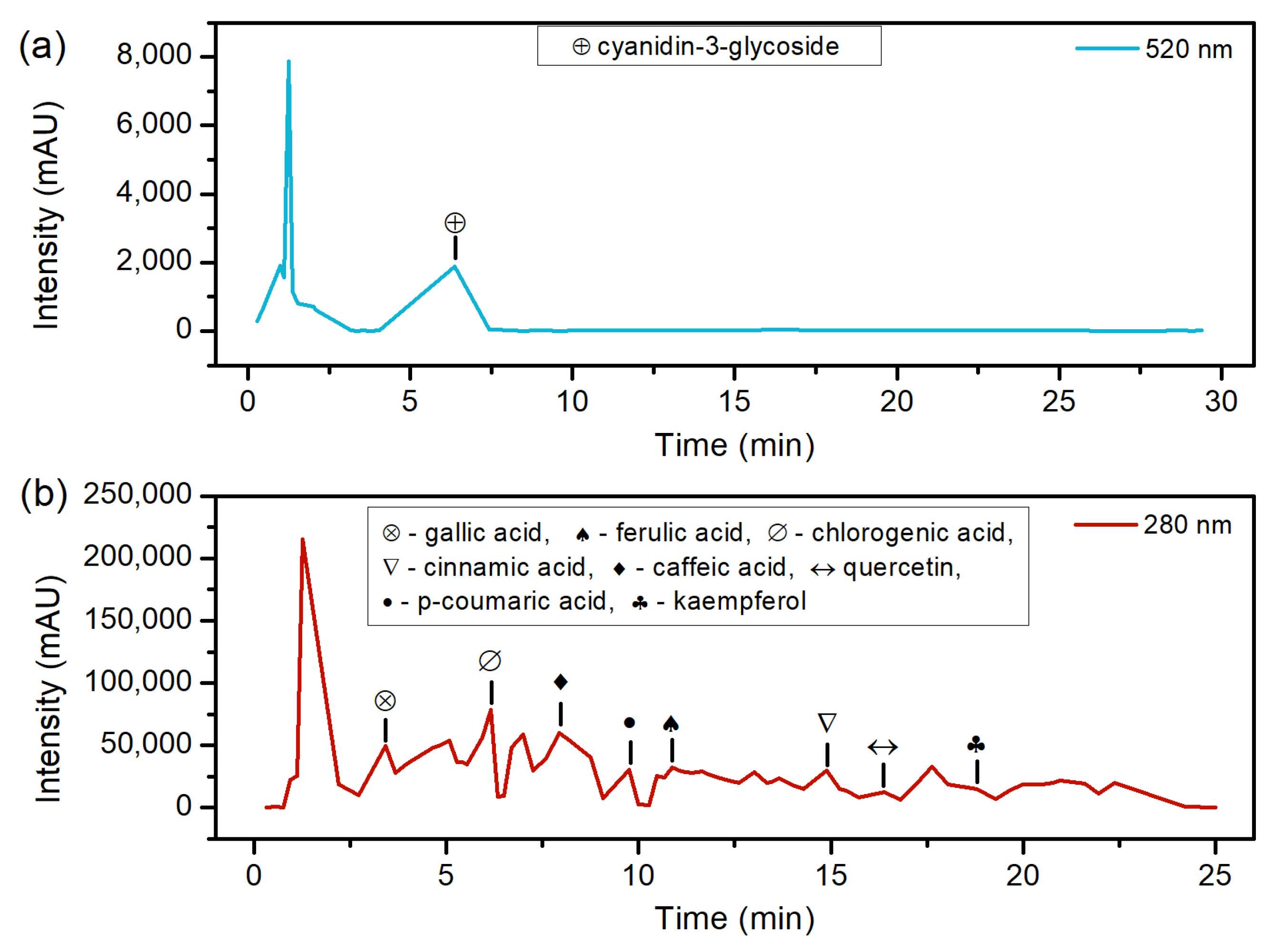
3.3. Extracts Characterization
3.4. Preparation and Characterization of Sodium Alginate Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kamoun, E.A.; Kenawy, E.R.S.; Chen, X. A Review on Polymeric Hydrogel Membranes for Wound Dressing Applications: PVA-Based Hydrogel Dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Şen, F.; Uzunsoy, İ.; Baştürk, E.; Kahraman, M.V. Antimicrobial Agent-Free Hybrid Cationic Starch/Sodium Alginate Polyelectrolyte Films for Food Packaging Materials. Carbohydr. Polym. 2017, 170, 264–270. [Google Scholar] [CrossRef]
- Saraiva, M.M.; Campelo, M.d.S.; Câmara Neto, J.F.; Lima, A.B.N.; Silva, G.d.A.; Dias, A.T.d.F.F.; Ricardo, N.M.P.S.; Kaplan, D.L.; Ribeiro, M.E.N.P. Alginate/Polyvinyl Alcohol Films for Wound Healing: Advantages and Challenges. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 220–233. [Google Scholar] [CrossRef]
- Valli, M.; Russo, H.M.; Bolzani, V.d.S. The Potential Contribution of the Natural Products from Brazilian Biodiversity to Bioeconomy. An. Acad. Bras. Cienc. 2018, 90, 763–778. [Google Scholar] [CrossRef]
- Schulz, M.; Seraglio, S.K.T.; Brugnerotto, P.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Composition and Potential Health Effects of Dark-Colored Underutilized Brazilian Fruits—A Review. Food Res. Int. 2020, 137, 109744. [Google Scholar] [CrossRef]
- Raks, V.; Al-Suod, H.; Buszewski, B. Isolation, Separation, and Preconcentration of Biologically Active Compounds from Plant Matrices by Extraction Techniques. Chromatographia 2018, 81, 189–202. [Google Scholar] [CrossRef]
- Das, A.B.; Goud, V.V.; Das, C. Extraction of Phenolic Compounds and Anthocyanin from Black and Purple Rice Bran (Oryza Sativa L.) Using Ultrasound: A Comparative Analysis and Phytochemical Profiling. Ind. Crops Prod. 2017, 95, 332–341. [Google Scholar] [CrossRef]
- Denardin, C.C.; Hirsch, G.E.; Da Rocha, R.F.; Vizzotto, M.; Henriques, A.T.; Moreira, J.C.F.; Guma, F.T.C.R.; Emanuelli, T. Antioxidant Capacity and Bioactive Compounds of Four Brazilian Native Fruits. J. Food Drug Anal. 2015, 23, 387–398. [Google Scholar] [CrossRef]
- Scur, M.C.; Pinto, F.G.S.; Pandini, J.A.; Costa, W.F.; Leite, C.W.; Temponi, L.G. Atividade Antimicrobiana e Antioxidante Do Óleo Essencial e Diferentes Extratos Vegetais de Psidium cattleianum Sabine. Braz. J. Biol. 2016, 76, 101–108. [Google Scholar] [CrossRef]
- Pereira, E.d.S.; Vinholes, J.R.; Camargo, T.M.; Nora, F.R.; Crizel, R.L.; Chaves, F.; Nora, L.; Vizzotto, M. Characterization of Araçá Fruits (Psidium cattleianum Sabine): Phenolic Composition, Antioxidant Activity and Inhibition of α-Amylase and α-Glucosidase. Food Biosci. 2020, 37, 100665. [Google Scholar] [CrossRef]
- Mallmann, L.P.; Tischer, B.; Vizzotto, M.; Rodrigues, E.; Manfroi, V. Comprehensive Identification and Quantification of Unexploited Phenolic Compounds from Red and Yellow Araçá (Psidium cattleianum Sabine) by LC-DAD-ESI-MS/MS. Food Res. Int. 2020, 131, 108978. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.M.A.; Rey-Rico, A.; Oliveira, R.A.; Marceneiro, S.; Alvarez-Lorenzo, C.; Concheiro, A.; Júnior, R.N.C.; Braga, M.E.M.; De Sousa, H.C. Wound Dressings Loaded with an Anti-Inflammatory Jucá (Libidibia ferrea) Extract Using Supercritical Carbon Dioxide Technology. J. Supercrit. Fluids 2013, 74, 34–45. [Google Scholar] [CrossRef]
- Kharroubi, M.; Bellali, F.; Karrat, A.; Bouchdoug, M.; Jaouad, A. Preparation of Teucrium Polium Extract-Loaded Chitosan-Sodium Lauryl Sulfate Beads and Chitosan-Alginate Films for Wound Dressing Application. AIMS Public Health 2021, 8, 754–775. [Google Scholar] [CrossRef]
- Cirillo, G.; Curcio, M.; Oliviero Rossi, C.; De Filpo, G.; Baratta, M.; De Luca, M.; Iemma, F.; Nicoletta, F.P. Curcumin–Sodium Alginate and Curcumin–Chitosan Conjugates as Drug Delivery Systems: An Interesting Rheological Behaviour. Molecules 2023, 28, 5893. [Google Scholar] [CrossRef]
- Medina, A.L.; Haas, L.I.R.; Chaves, F.C.; Salvador, M.; Zambiazi, R.C.; Da Silva, W.P.; Nora, L.; Rombaldi, C.V. Araçá (Psidium cattleianum Sabine) Fruit Extracts with Antioxidant and Antimicrobial Activities and Antiproliferative Effect on Human Cancer Cells. Food Chem. 2011, 128, 916–922. [Google Scholar] [CrossRef]
- Meregalli, M.M.; Puton, B.M.S.; Camera, F.D.M.; Amaral, A.U.; Zeni, J.; Cansian, R.L.; Mignoni, M.L.; Backes, G.T. Conventional and Ultrasound-Assisted Methods for Extraction of Bioactive Compounds from Red Araçá Peel (Psidium cattleianum Sabine). Arab. J. Chem. 2020, 13, 5800–5809. [Google Scholar] [CrossRef]
- Castañeda-Valbuena, D.; Ayora-Talavera, T.; Luján-Hidalgo, C.; Álvarez-Gutiérrez, P.; Martínez-Galero, N.; Meza-Gordillo, R. Ultrasound Extraction Conditions Effect on Antioxidant Capacity of Mango By-Product Extracts. Food Bioprod. Process. 2021, 127, 212–224. [Google Scholar] [CrossRef]
- Reungoat, V.; Gaudin, M.; Flourat, A.L.; Isidore, E.; Mouterde, L.M.M.; Allais, F.; Ducatel, H.; Ioannou, I. Optimization of an Ethanol/Water-Based Sinapine Extraction from Mustard Bran Using Response Surface Methodology. Food Bioprod. Process. 2020, 122, 322–331. [Google Scholar] [CrossRef]
- Avila, L.B.; Fontes, M.R.V.; Zavareze, E.d.R.; Moraes, C.C.; Morais, M.M.; da Rosa, G.S. Recovery of Bioactive Compounds from Jaboticaba Peels and Application into Zein Ultrafine Fibers Produced by Electrospinning. Polymers 2020, 12, 2916. [Google Scholar] [CrossRef]
- Favaro, G.; De Leo, D.; Pastore, P.; Magno, F.; Ballardin, A. Quantitative Determination of Chlorophenols in Leather by Pressurized Liquid Extraction and Liquid Chromatography with Diode-Array Detection. J. Chromatogr. A 2008, 1177, 36–42. [Google Scholar] [CrossRef]
- Filho, A.V.; Avila, L.B.; Lacorte, D.H.; Martiny, T.R.; Rosseto, V.; Moraes, C.C.; Dotto, G.L.; Carreno, N.L.V.; da Rosa, G.S. Brazilian Agroindustrial Wastes as a Potential Resource of Bioative Compounds and Their Antimicrobial and Antioxidant Activities. Molecules 2022, 27, 6876. [Google Scholar] [CrossRef] [PubMed]
- Tuncel, N.B.; Yılmaz, N. Optimizing the Extraction of Phenolics and Antioxidants from Feijoa (Feijoa sellowiana, Myrtaceae). J. Food Sci. Technol. 2015, 52, 141–150. [Google Scholar] [CrossRef]
- Ribeiro, A.B.; Chisté, R.C.; Freitas, M.; Da Silva, A.F.; Visentainer, J.V.; Fernandes, E. Psidium cattleianum Fruit Extracts Are Efficient in vitro Scavengers of Physiologically Relevant Reactive Oxygen and Nitrogen Species. Food Chem. 2014, 165, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and Anti-Inflammatory Activities of Quercetin and Its Derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Yong-Bing, X.; Gui-Lin, C.; Ming-Quan, G. Antioxidant and Anti-Inflammatory Activities of the Crude Extracts of Moringa Oleifera from Kenya and Their Correlations with Flavonoids. Antioxidants 2019, 8, 296. [Google Scholar] [CrossRef]
- Rodrigues, L.M.; Romanini, E.B.; Silva, E.; Pilau, E.J.; da Costa, S.C.; Madrona, G.S. Camu-Camu Bioactive Compounds Extraction by Ecofriendly Sequential Processes (Ultrasound Assisted Extraction and Reverse Osmosis). Ultrason. Sonochem 2020, 64, 105017. [Google Scholar] [CrossRef]
- Nora, C.D.; Müller, C.D.R.; de Bona, G.S.; Rios, A.d.O.; Hertz, P.F.; Jablonski, A.; De Jong, E.V.; Flôres, S.H. Effect of Processing on the Stability of Bioactive Compounds from Red Guava (Psidium cattleyanum Sabine) and Guabiju (Myrcianthes pungens). J. Food Compos. Anal. 2014, 34, 18–25. [Google Scholar] [CrossRef]
- Fernandes-Negreiros, M.M.; Batista, L.A.N.C.; Viana, R.L.S.; Sabry, D.A.; Paiva, A.A.O.; Paiva, W.S.; Machado, R.I.A.; de Sousa Junior, F.L.; Pontes, D.d.L.; Vitoriano, J.d.O.; et al. Gallic Acid-Laminarin Conjugate Is a Better Antioxidant than Sulfated or Carboxylated Laminarin. Antioxidants 2020, 9, 1192. [Google Scholar] [CrossRef]
- Qie, X.; Chen, W.; Zeng, M.; Wang, Z.; Chen, J.; Goff, H.D.; He, Z. Interaction between β-Lactoglobulin and Chlorogenic Acid and Its Effect on Antioxidant Activity and Thermal Stability. Food Hydrocoll. 2021, 121, 107059. [Google Scholar] [CrossRef]
- Otan Özden, F.; Lütfioğlu, M.; Demir, E.; Bilgici, B. Antioxidant Effect of Caffeic Acid Phenethyl Ester in Experimentally Induced Periodontitis. Clin. Oral. Investig. 2021, 25, 4959–4966. [Google Scholar] [CrossRef]
- Babaeenezhad, E.; Nouryazdan, N.; Nasri, M.; Ahmadvand, H.; Moradi Sarabi, M. Cinnamic Acid Ameliorate Gentamicin-Induced Liver Dysfunctions and Nephrotoxicity in Rats through Induction of Antioxidant Activities. Heliyon 2021, 7, e07465. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the Anti-Inflammatory and Antioxidant Activities of Luteolin, Kaempferol, Apigenin and Quercetin. S. Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Jaiswal, V.; Park, M.; Lee, H.J. Comparative Transcriptome Analysis of the Expression of Antioxidant and Immunity Genes in the Spleen of a Cyanidin 3-o-Glucoside-Treated Alzheimer’s Mouse Model. Antioxidants 2021, 10, 1435. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.S.; da Silva, T.B.; Tomoda, B.T.; de Moraes, M.A. Evaluation of Diclofenac Sodium Incorporation in Alginate Membranes as Potential Drug Release System. Materialia 2020, 12, 100827. [Google Scholar] [CrossRef]
- Marangoni Júnior, L.; Jamróz, E.; Gonçalves, S.d.Á.; da Silva, R.G.; Alves, R.M.V.; Vieira, R.P. Preparation and Characterization of Sodium Alginate Films with Propolis Extract and Nano-SiO2. Food Hydrocoll. Health 2022, 2, 100094. [Google Scholar] [CrossRef]
- Sobczyk, A.d.E.; Luchese, C.L.; Faccin, D.J.L.; Tessaro, I.C. Influence of Replacing Oregano Essential Oil by Ground Oregano Leaves on Chitosan/Alginate-Based Dressings Properties. Int. J. Biol. Macromol. 2021, 181, 51–59. [Google Scholar] [CrossRef]
- Rhim, J.W. Physical and Mechanical Properties of Water Resistant Sodium Alginate Films. LWT 2004, 37, 323–330. [Google Scholar] [CrossRef]
- Mahcene, Z.; Khelil, A.; Hasni, S.; Akman, P.K.; Bozkurt, F.; Birech, K.; Goudjil, M.B.; Tornuk, F. Development and Characterization of Sodium Alginate Based Active Edible Films Incorporated with Essential Oils of Some Medicinal Plants. Int. J. Biol. Macromol. 2020, 145, 124–132. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Harte, B.R. Physical Properties and Antioxidant Activity of an Active Film from Chitosan Incorporated with Green Tea Extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Kaczmarek, B. Improving Sodium Alginate Films Properties by Phenolic Acid Addition. Materials 2020, 13, 2895. [Google Scholar] [CrossRef]
- Lamke, L.-O.; Nilsson, G.E.; Reithner, H.L. The Evaporative Water Loss from Burns and the Water-Vapor Permeability of Grafts and Artificial Membranes Used in the Treatment of Burns. Burns 1977, 3, 159–165. [Google Scholar] [CrossRef]
- Sutar, T.; Bangde, P.; Dandekar, P.; Adivarekar, R. Herbal Hemostatic Biopolymeric Dressings of Alginate/Pectin Coated with Croton Oblongifolius Extract. Carbohydr. Polym. Technol. Appl. 2021, 2, 100025. [Google Scholar] [CrossRef]
- Türkoğlu, G.C.; Sark, A.M.; Karavana, S.Y. Development of Textile-Based Sodium Alginate and Chitosan Hydrogel Dressings. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 916–925. [Google Scholar] [CrossRef]
- Chen, J.; Wu, A.; Yang, M.; Ge, Y.; Pristijono, P.; Li, J.; Xu, B.; Mi, H. Characterization of Sodium Alginate-Based Films Incorporated with Thymol for Fresh-Cut Apple Packaging. Food Control 2021, 126, 108063. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, J.; Zhang, R.; Kong, R.; Lu, W.; Wang, X. Characterization and Application of the Microencapsulated Carvacrol/Sodium Alginate Films as Food Packaging Materials. Int. J. Biol. Macromol. 2019, 141, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, A.J.; Ghazali, C.M.R.; Mat Amin, K.A. Sodium Alginate/Ageratum Conyzoides Extract Film for Wound Dressing Materials. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012087. [Google Scholar] [CrossRef]
- Rezvanian, M.; Mohd Amin, M.C.I.; Ng, S.F. Development and Physicochemical Characterization of Alginate Composite Film Loaded with Simvastatin as a Potential Wound Dressing. Carbohydr. Polym. 2016, 137, 295–304. [Google Scholar] [CrossRef]
- Avila, L.B.; Barreto, E.R.C.; Moraes, C.C.; Morais, M.M.; da Rosa, G.S. Promising New Material for Food Packaging: An Active and Intelligent Carrageenan Film with Natural Jaboticaba Additive. Foods 2022, 11, 792. [Google Scholar] [CrossRef]
- Chi, W.; Cao, L.; Sun, G.; Meng, F.; Zhang, C.; Li, J.; Wang, L. Developing a Highly PH-Sensitive ĸ-Carrageenan-Based Intelligent Film Incorporating Grape Skin Powder via a Cleaner Process. J. Clean. Prod. 2020, 244, 118862. [Google Scholar] [CrossRef]
- Hassan, A.; Niazi, M.B.K.; Hussain, A.; Farrukh, S.; Ahmad, T. Development of Anti-Bacterial PVA/Starch Based Hydrogel Membrane for Wound Dressing. J. Polym. Environ. 2018, 26, 235–243. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Sohail, M.; Minhas, M.U.; Khaliq, T.; Kousar, M.; Khan, S.; Hussain, Z.; Munir, A. Bioinspired Sodium Alginate Based Thermosensitive Hydrogel Membranes for Accelerated Wound Healing. Int. J. Biol. Macromol. 2020, 155, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, V.; Bajpai, S.K. Moisture Uptake Behavior, Antibacterial Property, and Heat of Sorption of Nano Silver-Loaded Calcium Alginate Films. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 119–127. [Google Scholar] [CrossRef]
- Salisu, A.; Musa Yar’, U.; Sanagi, M.M.; Naim, A.A.; Juhanni, K.; Karim, A. Graft Copolymerization of Methyl Methacrylate onto Alginate Using Benzoyl Peroxide Initiator. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1408–1416. [Google Scholar]
- Wei, J.; Xu, D.; Zhang, X.; Yang, J.; Wang, Q. Evaluation of Anthocyanins in Aronia Melanocarpa/BSA Binding by Spectroscopic Studies. AMB Express 2018, 8, 72. [Google Scholar] [CrossRef]
- Alzarea, A.I.; Alruwaili, N.K.; Ahmad, M.M.; Munir, M.U.; Butt, A.M.; Alrowaili, Z.A.; Shahari, M.S.B.; Almalki, Z.S.; Alqahtani, S.S.; Dolzhenko, A.V.; et al. Development and Characterization of Gentamicin-Loaded Arabinoxylan-Sodium Alginate Films as Antibacterial Wound Dressing. Int. J. Mol. Sci. 2022, 23, 2899. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, Q.; Du, Y. Alginate/Gelatin Blend Films and Their Properties for Drug Controlled Release. J. Memb. Sci. 2006, 280, 37–44. [Google Scholar] [CrossRef]
- Fata Moradali, M.; Donati, I.; Sims, I.M.; Ghods, S.; Rehm, B.H.A. Alginate Polymerization and Modification Are Linked in Pseudomonas aeruginosa. mBio 2015, 6, 1128. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Ravent6s, R.M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method. to Evaluate Antioxidant Activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- ASTM E96/E96M-16; tandard Test Methods for Water Vapor Transmission of Materials 1. ASTM: West Conshohocken, PA, USA, 2021. [CrossRef]
- Gontard, N.; Guilbert, S.; Cuq, J.-L. Edible Wheat Gluten Films: Influence of the Main Process Variables on Film Properties Using Response Surface Methodology. Food Sci. 1992, 57, 190–195. [Google Scholar] [CrossRef]
- ASTM-D882_2010_513100089384; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM: West Conshohocken, PA, USA, 2010.
- Liu, C.; Li, P.; Xu, Y.J.; Liu, Y.; Zhu, P. Synergistic Effects of Iron Alginate on Improving the Fire Safety and Mechanical Properties of Epoxy Resin/Ammonium Polyphosphate Composites. Macromol. Mater. Eng. 2023, 308, 2200516. [Google Scholar] [CrossRef]
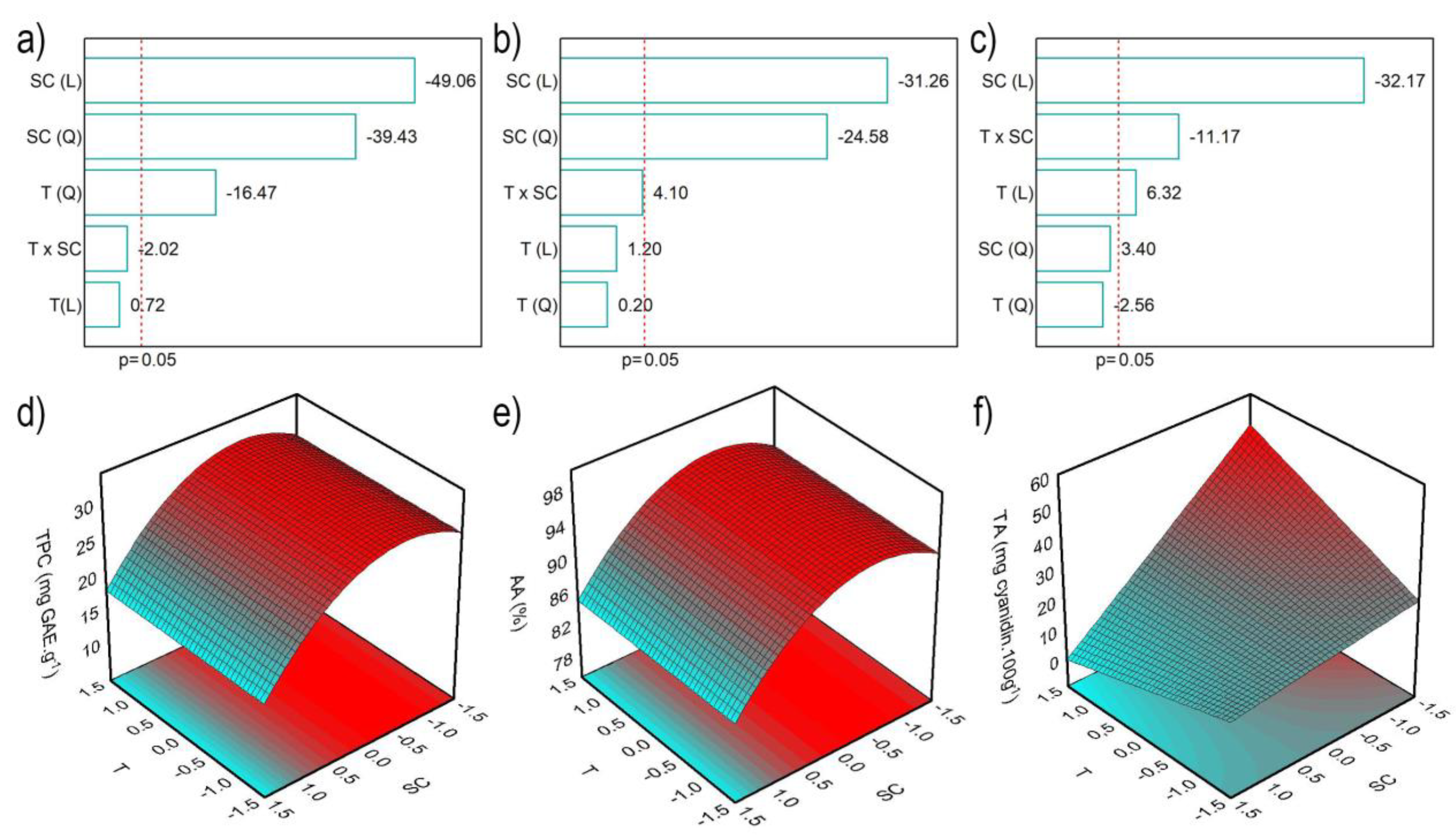
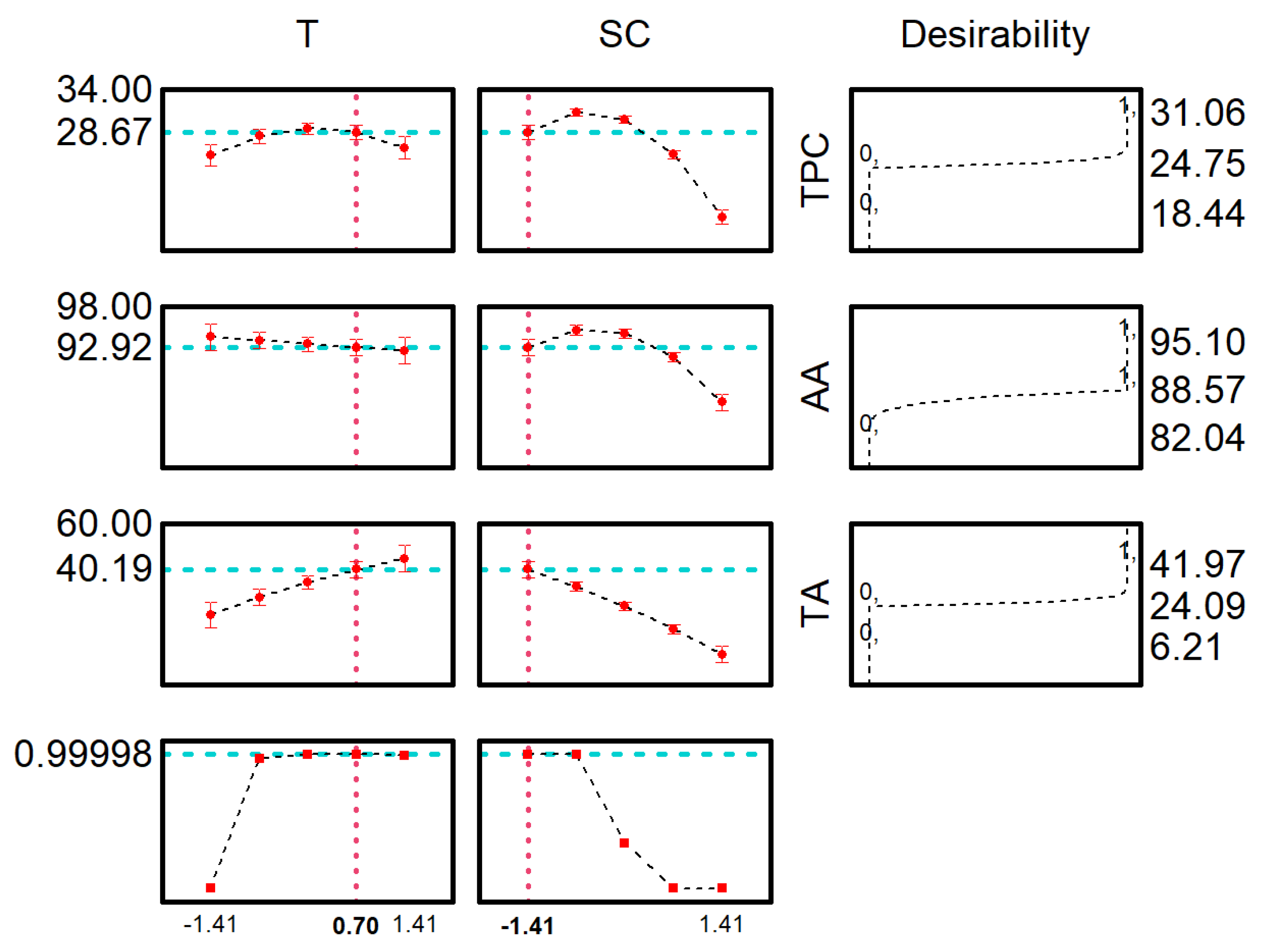

| Coded Values | Real Values | ||||||
|---|---|---|---|---|---|---|---|
| Run | T | SC | T (°C) | SC (%) | TPC (mgGAE g−1, d.b) | AA (% RSCDPPH) | TA (mgcn-3-glu 100 g−1, d.b) |
| 1 | −1 | −1 | 40 | −40 | 29.94 ± 1.21 | 94.25 ± 0.45 | 25.00 ± 0.15 |
| 2 | 1 | −1 | 70 | −40 | 31.06 ± 0.87 | 93.39 ± 0.27 | 41.97 ± 0.96 |
| 3 | −1 | 1 | 40 | 84 | 22.99 ± 0.61 | 91.42 ± 0.65 | 8.34 ± 0.06 |
| 4 | 1 | 1 | 70 | 84 | 23.27 ± 0.82 | 92.61 ± 1.12 | 6.21 ± 0.06 |
| 5 | 0 | 0 | 55 | 62 | 30.69 ± 0.89 | 94.33 ± 0.35 | 22.28 ± 0.70 |
| 6 | 0 | 0 | 55 | 62 | 31.06 ± 0.60 | 94.80 ± 0.11 | 23.79 ± 0.50 |
| 7 | 0 | 0 | 55 | 62 | 31.02 ± 0.03 | 31.02 ± 0.03 | 94.71 ± 0.08 |
| 8 | −1.41 | 0 | 34 | 62 | 27.65 ± 0.12 | 93.58 ± 0.23 | 22.02 ± 2.18 |
| 9 | 1.41 | 0 | 76 | 62 | 26.96 ± 1.27 | 93.95 ± 0.30 | 22.32 ± 0.43 |
| 10 | 0 | −1.41 | 55 | 32 | 28.22 ± 2.54 | 95.10 ± 0.35 | 30.52 ± 0.10 |
| 11 | 0 | 1.41 | 55 | 92 | 18.44 ± 0.46 | 82.04 ± 1.43 | 12.61 ± 0.04 |
| Compounds | Retention Time (min) | Concentration (mg/g d.b.) |
|---|---|---|
| Cyanidin-3-glucoside | 6.37 | 0.904 ± 0.02 |
| Gallic acid | 2.7 | 0.136 ± 0.01 |
| Chlorogenic acid | 6.34 and 6.48 | 0.168 ± 0.004 |
| Caffeic acid | 7.26 | 0.118 ± 0.001 |
| P-coumaric acid | 9.08 | ND |
| Ferulic acid | 9.98 and 10.26 | ND |
| Cinnamic acid | 15.73 | 0.034 ± 0.001 |
| Quercetin | 16.38 and 16.8 | 0.657 ± 0.002 |
| Kaempferol | 19.28 | 0.355 ± 0.03 |
| MC | ME | |
|---|---|---|
| Thickness (mm) | 0.135 ± 0.022 a | 0.1172 ± 0.016 a |
| WVP (g/m.s.Pa) | 2.479 × 10−9 ± 5.940 × 10−11 a | 1.906 × 10−9 ± 2.244 × 10−10 b |
| WVPR (g/m².s) | 0.0642 ± 0.0015 a | 0.0568 ± 0.0066 a |
| Water solubility (%) | 50.18 ± 1.86 a | 41.43 ± 0.67 b |
| Tensile strength (MPa) | 8.468 ± 1.248 a | 6.676 ± 0.360 a |
| Elongation at break (%) | 15.372 ± 2.575 a | 8.190 ± 1.468 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacorte, D.H.; Valério Filho, A.; Carvalho, M.D.; Avila, L.B.; Moraes, C.C.; Rosa, G.S.d. Optimization of the Green Extraction of Red Araçá (Psidium catteyanum Sabine) and Application in Alginate Membranes for Use as Dressings. Molecules 2023, 28, 6688. https://doi.org/10.3390/molecules28186688
Lacorte DH, Valério Filho A, Carvalho MD, Avila LB, Moraes CC, Rosa GSd. Optimization of the Green Extraction of Red Araçá (Psidium catteyanum Sabine) and Application in Alginate Membranes for Use as Dressings. Molecules. 2023; 28(18):6688. https://doi.org/10.3390/molecules28186688
Chicago/Turabian StyleLacorte, Douglas Hardt, Alaor Valério Filho, Márcio Dantas Carvalho, Luisa Bataglin Avila, Caroline Costa Moraes, and Gabriela Silveira da Rosa. 2023. "Optimization of the Green Extraction of Red Araçá (Psidium catteyanum Sabine) and Application in Alginate Membranes for Use as Dressings" Molecules 28, no. 18: 6688. https://doi.org/10.3390/molecules28186688
APA StyleLacorte, D. H., Valério Filho, A., Carvalho, M. D., Avila, L. B., Moraes, C. C., & Rosa, G. S. d. (2023). Optimization of the Green Extraction of Red Araçá (Psidium catteyanum Sabine) and Application in Alginate Membranes for Use as Dressings. Molecules, 28(18), 6688. https://doi.org/10.3390/molecules28186688









