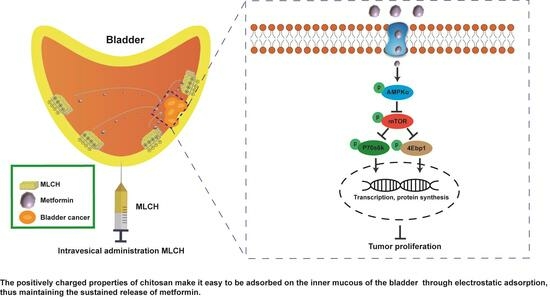
Metformin-Loaded Chitosan Hydrogels Suppress Bladder Tumor Growth in an Orthotopic Mouse Model via Intravesical Administration
Abstract
:1. Introduction
2. Results
2.1. Freeze-Drying Improves the Microstructure of Cs and Induces the Formation of Colloidal Hydrogels
2.2. MLCH Exhibits Favorable Physical and Chemical Properties and Can Be Administered Intravesically
2.3. MLCH Shows a Robust Inhibitory Effect against BC Cell Growth In Vitro
2.4. Intravesical Treatment of MLCH Possesses Potent Anticancer Effects In Vivo
2.5. MLCH Increases Phosphorylation of AMPKα in BC Tissues
2.6. MLCH Exhibits No Detectable Toxic Effects and Remains Localized in the Bladder
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of Freeze-Dried Cs Powder
4.3. Physicochemical Characterization
4.4. Cell Viability Assay
4.5. Clonogenic Assay
4.6. Orthotopic Implantation and Intravesical Treatment
4.7. Histological Analysis
4.8. Western Blotting
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Xiao, D.; Hu, X.; Peng, M.; Deng, J.; Zhou, S.; Xu, S.; Wu, J.; Yang, X. Inhibitory role of proguanil on the growth of bladder cancer via enhancing EGFR degradation and inhibiting its downstream signaling pathway to induce autophagy. Cell Death Dis. 2022, 13, 499. [Google Scholar] [CrossRef]
- Yu, C.; Wang, S.; Lai, W.F.; Zhang, D. The Progress of Chitosan-Based Nanoparticles for Intravesical Bladder Cancer Treatment. Pharmaceutics 2023, 15, 211. [Google Scholar] [CrossRef]
- Xiao, D.; Xu, S.; Zhou, X.; Li, D.; Peng, M.; Chu, X.; Zhang, Z.; Peng, Y.; Chen, A.F.; Yang, X. Synergistic augmentation of osimertinib-induced autophagic death by proguanil or rapamycin in bladder cancer. MedComm 2023, 4, e236. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Kitson, S.J.; Maskell, Z.; Sivalingam, V.N.; Allen, J.L.; Ali, S.; Burns, S.; Gilmour, K.; Latheef, R.; Slade, R.J.; Pemberton, P.W.; et al. PRE-surgical Metformin In Uterine Malignancy (PREMIUM): A Multi-Center, Randomized Double-Blind, Placebo-Controlled Phase III Trial. Clin. Cancer Res. 2019, 25, 2424–2432. [Google Scholar] [CrossRef] [PubMed]
- Tsakiridis, T.; Pond, G.R.; Wright, J.; Ellis, P.M.; Ahmed, N.; Abdulkarim, B.; Roa, W.; Robinson, A.; Swaminath, A.; Okawara, G.; et al. Metformin in Combination With Chemoradiotherapy in Locally Advanced Non-Small Cell Lung Cancer: The OCOG-ALMERA Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, L.; Wang, Y.; Zhao, Y.; Zhang, X.J.; Wu, G.; Zhou, X.; Sun, J.; Bai, J.; Ren, B.; et al. Combination of Metformin and Gefitinib as First-Line Therapy for Nondiabetic Advanced NSCLC Patients with EGFR Mutations: A Randomized, Double-Blind Phase II Trial. Clin. Cancer Res. 2019, 25, 6967–6975. [Google Scholar] [CrossRef]
- Deng, J.; Peng, M.; Zhou, S.; Xiao, D.; Hu, X.; Xu, S.; Wu, J.; Yang, X. Metformin targets Clusterin to control lipogenesis and inhibit the growth of bladder cancer cells through SREBP-1c/FASN axis. Signal Transduct. Target. Ther. 2021, 6, 98. [Google Scholar] [CrossRef]
- Lu, S.; Xu, L.; Kang, E.T.; Mahendran, R.; Chiong, E.; Neoh, K.G. Co-delivery of peptide-modified cisplatin and doxorubicin via mucoadhesive nanocapsules for potential synergistic intravesical chemotherapy of non-muscle-invasive bladder cancer. Eur. J. Pharm. Sci. 2016, 84, 103–115. [Google Scholar] [CrossRef]
- Jhaveri, J.; Raichura, Z.; Khan, T.; Momin, M.; Omri, A. Chitosan Nanoparticles-Insight into Properties, Functionalization and Applications in Drug Delivery and Theranostics. Molecules 2021, 26, 272. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hafez, S.M.; Hathout, R.M.; Sammour, O.A. Towards better modeling of chitosan nanoparticles production: Screening different factors and comparing two experimental designs. Int. J. Biol. Macromol. 2014, 64, 334–340. [Google Scholar] [CrossRef]
- Yu, Y.; Huo, M.; Fu, Y.; Xu, W.; Cai, H.; Yao, L.; Chen, Q.; Mu, Y.; Zhou, J.; Yin, T. N-Deoxycholic acid-N,O-hydroxyethyl Chitosan with a Sulfhydryl Modification To Enhance the Oral Absorptive Efficiency of Paclitaxel. Mol. Pharm. 2017, 14, 4539–4550. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef]
- John, B.A.; Said, N. Insights from animal models of bladder cancer: Recent advances, challenges, and opportunities. Oncotarget 2017, 8, 57766–57781. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Huang, Y.; Tao, T.; Peng, C.Y.; Su, Q.; Xu, W.; Darko, K.O.; Tao, X.; Yang, X. Metformin and gefitinib cooperate to inhibit bladder cancer growth via both AMPK and EGFR pathways joining at Akt and Erk. Sci. Rep. 2016, 6, 28611. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Tao, T.; Tang, L.; Deng, J.; Darko, K.O.; Zhou, S.; Peng, M.; He, S.; Zeng, Q.; Chen, A.F.; et al. Down-regulation of PKM2 enhances anticancer efficiency of THP on bladder cancer. J. Cell. Mol. Med. 2018, 22, 2774–2790. [Google Scholar] [CrossRef]
- Koga, T.; Kamiwatari, S.; Higashi, N. Preparation and Self-Assembly Behavior of β-Sheet Peptide-Inserted Amphiphilic Block Copolymer as a Useful Polymeric Surfactant. Langmuir 2013, 29, 15477–15484. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, L.; Shi, X.; Yang, L.; Hua, F.; Ma, J.; Zhu, W.; Liu, X.; Xuan, R.; Shen, Y.; et al. Metformin protects against myocardial ischemia-reperfusion injury and cell pyroptosis via AMPK/NLRP3 inflammasome pathway. Aging 2020, 12, 24270–24287. [Google Scholar] [CrossRef]
- Ahn, H.K.; Lee, Y.H.; Koo, K.C. Current Status and Application of Metformin for Prostate Cancer: A Comprehensive Review. Int. J. Mol. Sci. 2020, 21, 8540. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Xu, W.; Zhang, C.; Kong, W.J.E.; Medicine, T. Chitosan temperature-sensitive gel loaded with drug microspheres has excellent effectiveness, biocompatibility and safety as an ophthalmic drug delivery system. Exp. Ther. Med. 2018, 15, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Schaffhausen, N.; Tijsma, E.; Hissong, B. Injectable chitosan-based hydrogels for drug delivery after ear-nose-throat surgery. J. Control. Release 2008, 132, E47–E48. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Censi, R.; Martino, P.D.; Vermonden, T.; Hennink, W.E. Hydrogels for protein delivery in tissue engineering. J. Control. Release 2012, 161, 680–692. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, J.; Zhou, S.; Yang, N.; Duan, S.; Zhang, Z.; Su, J.; He, J.; Zhang, Z.; Lu, X.; et al. Mouse IP-10 Gene Delivered by Folate-modified Chitosan Nanoparticles and Dendritic/tumor Cells Fusion Vaccine Effectively Inhibit the Growth of Hepatocellular Carcinoma in Mice. Theranostics 2017, 7, 1942–1952. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.G.; Chen, W.M.; Yu, A.X.J.E.; Medicine, T. Efficacy of thermosensitive chitosan/βglycerophosphate hydrogel loaded with βcyclodextrincurcumin for the treatment of cutaneous wound infection in rats. Exp. Ther. Med. 2018, 15, 1304–1313. [Google Scholar]
- Wang, J.; Xu, M.; Cheng, X.; Kong, M.; Chen, X.J.C.P. Positive/negative surface charge of chitosan based nanogels and its potential influence on oral insulin delivery. Carbohydr. Polym. 2015, 136, 867–874. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Moran, H.B.T.; Turley, J.L.; Andersson, M.; Lavelle, E.C. Immunomodulatory properties of chitosan polymers. Biomaterials 2018, 184, 1–9. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Hathout, R.M.; Kassem, D.H. Positively Charged Electroceutical Spun Chitosan Nanofibers Can Protect Health Care Providers From COVID-19 Infection: An Opinion. Front. Bioeng. Biotechnol. 2020, 8, 885. [Google Scholar] [CrossRef] [PubMed]
- GuhaSarkar, S.; Banerjee, R. Intravesical drug delivery: Challenges, current status, opportunities and novel strategies. J. Control. Release 2010, 148, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Yang, X.; Kessler, E.; Su, L.-J.; Thorburn, A.; Frankel, A.E.; Li, Y.; La Rosa, F.G.; Shen, J.; Li, C.-Y.; Varella-Garcia, M.; et al. Diphtheria Toxin-Epidermal Growth Factor Fusion Protein DAB(389)EGF for the Treatment of Bladder Cancer. Clin. Cancer Res. 2013, 19, 148–157. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Hu, X.; Xie, Y.; Xie, L.; Chen, X.; Peng, M.; Li, D.; Deng, J.; Xiao, D.; Yang, X. Metformin-Loaded Chitosan Hydrogels Suppress Bladder Tumor Growth in an Orthotopic Mouse Model via Intravesical Administration. Molecules 2023, 28, 6720. https://doi.org/10.3390/molecules28186720
Zhang X, Hu X, Xie Y, Xie L, Chen X, Peng M, Li D, Deng J, Xiao D, Yang X. Metformin-Loaded Chitosan Hydrogels Suppress Bladder Tumor Growth in an Orthotopic Mouse Model via Intravesical Administration. Molecules. 2023; 28(18):6720. https://doi.org/10.3390/molecules28186720
Chicago/Turabian StyleZhang, Xingjian, Xin Hu, Yijun Xie, Lejing Xie, Xiangyi Chen, Mei Peng, Duo Li, Jun Deng, Di Xiao, and Xiaoping Yang. 2023. "Metformin-Loaded Chitosan Hydrogels Suppress Bladder Tumor Growth in an Orthotopic Mouse Model via Intravesical Administration" Molecules 28, no. 18: 6720. https://doi.org/10.3390/molecules28186720
APA StyleZhang, X., Hu, X., Xie, Y., Xie, L., Chen, X., Peng, M., Li, D., Deng, J., Xiao, D., & Yang, X. (2023). Metformin-Loaded Chitosan Hydrogels Suppress Bladder Tumor Growth in an Orthotopic Mouse Model via Intravesical Administration. Molecules, 28(18), 6720. https://doi.org/10.3390/molecules28186720