Nanotechnology Lighting the Way for Gene Therapy in Ophthalmopathy: From Opportunities toward Applications
Abstract
:1. Introduction
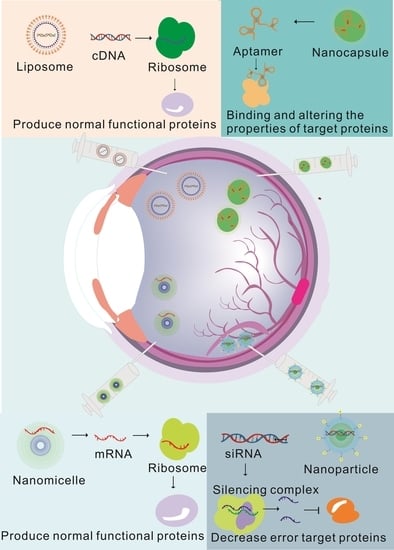
2. Classifications and Injection Schemes of NADs in Gene Therapy for Ophthalmopathy
2.1. Common NAD Types in Gene Therapy for Ophthalmopathy
2.2. Gene Selection Options of NADs in Gene Therapy for Ophthalmopathy
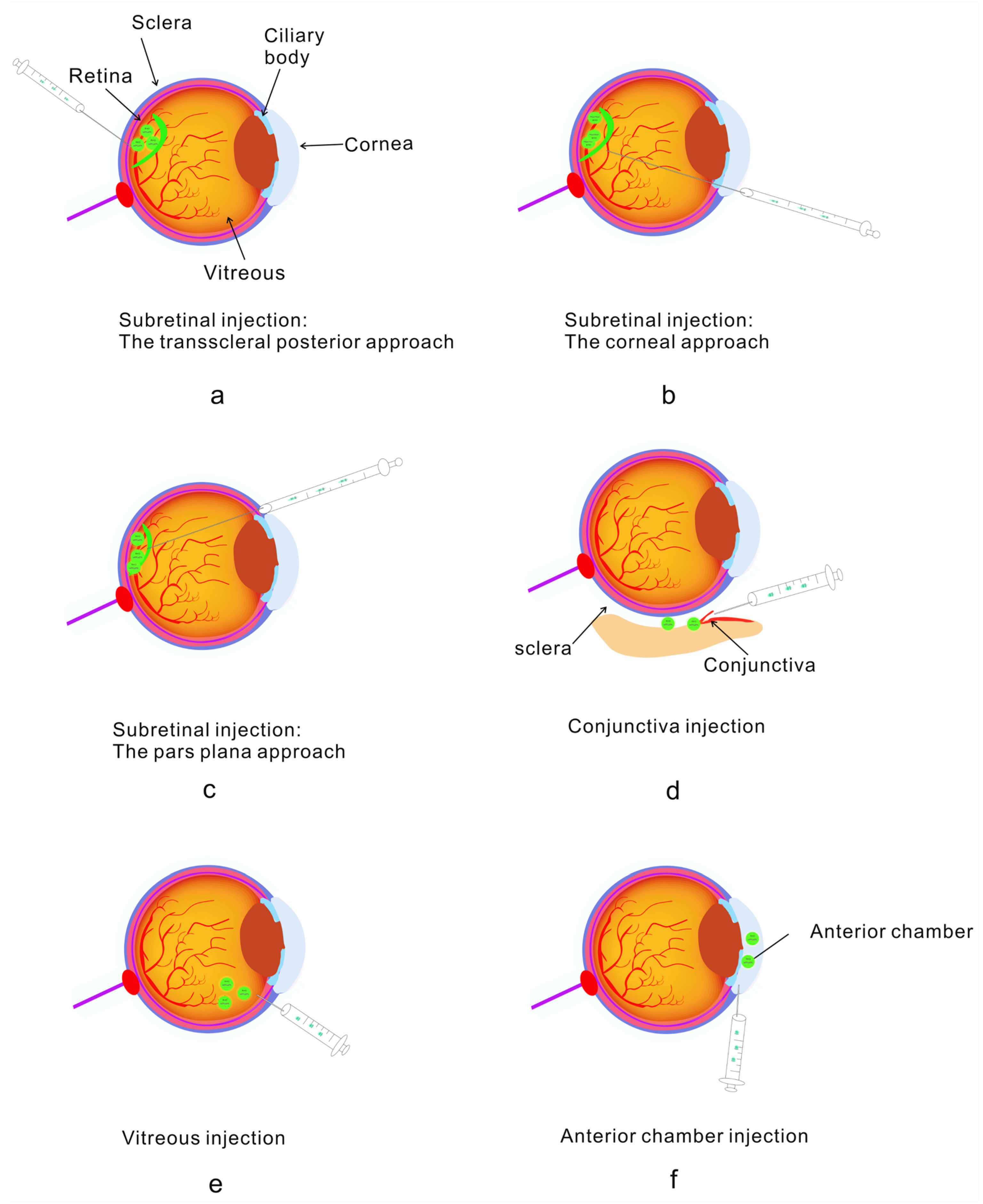
2.3. Diverse Delivery Strategies Overcoming Delivery Efficiency in Ophthalmopathy
3. The Application of Nanotechnology in Gene Therapy Can Help Improve Treatment
3.1. NAD Carriers in Gene Therapy for Eye Diseases
| Carrier | Advantages | Disadvantages | Target Tissue | References |
|---|---|---|---|---|
| Lentiviral carrier | Wide range of target cells and strong ability to carry foreign genes | Carcinogenic risk | Retinal ganglion cells, lens epithelial cells, corneal epithelial cells | [74,75,76] |
| Adenovirus carrier | Short expression time, High expression level of foreign genes | Strong immunogenicity | Photoreceptor cells, retinal pigment epithelial cells | [77] |
| Adeno-associated virus carrier | High infection efficiency | Strong immunogenicity | Photoreceptor cells, retinal pigment epithelial cells, retinal ganglion cells, lens epithelial cells | [78,79] |
| Nonviral Carrier | Advantages | Disadvantages | Target Tissue | References |
|---|---|---|---|---|
| Cationic lipid carrier | Increasing the local retention time of drugs, slow release of drugs, improving the bioavailability of drugs | Low transfection efficiency | Cornea, bulbar conjunctiva, sclera, retina, | [81,82] |
| Cationic polymer | Beneficial to endocytosis and will not be degraded by enzymes | High cytotoxicity | Retina | [83] |
| Lipid nanoparticles | High sustained release, high stability and low toxicity | Low transfection efficiency and hard to store | Cornea, retina | [84,85] |
| Inorganic nanoparticles | Easy to decorate, versatile | Poor biocompatibility | Cornea, retina | [86] |
3.2. Nanocarriers Can Improve the Targeting and Biological Distribution of NADs in the Eyes
3.3. Using Nanotechnology to Improve the Stability of NADs to Overcome Drug Instability
4. The Mechanisms and Advantages of NADs in Gene Therapy for Ophthalmopathy
4.1. Principles and Advantages of Using cDNA as Therapeutic Nucleic Acid
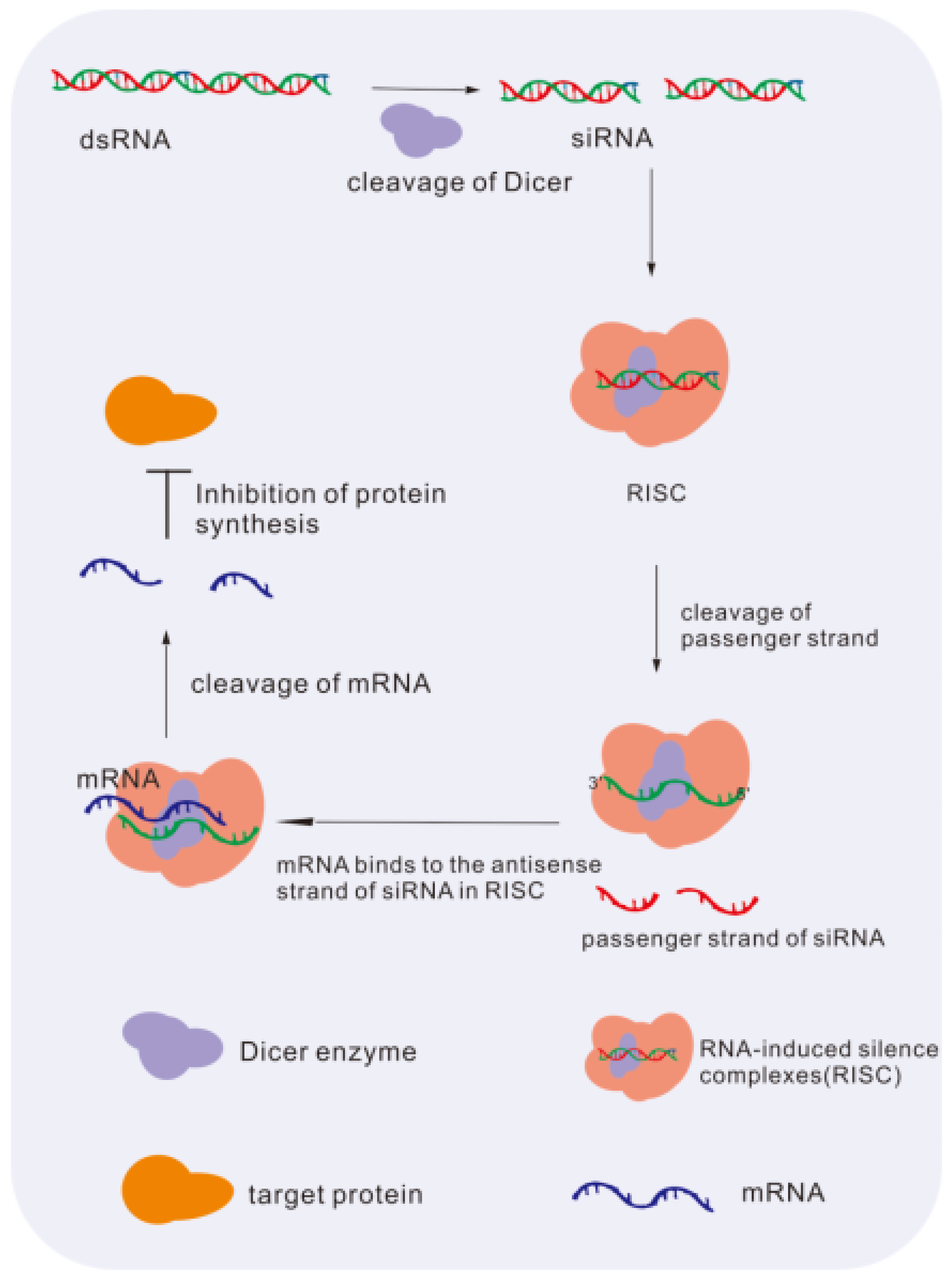
4.2. Principles and Advantages of Using Small Interfering RNA (siRNA) as Therapeutic Nucleic Acid
4.3. Principles and Advantages of Using microRNA (miRNA) as Therapeutic Nucleic Acid
4.4. Principles and Advantages of Using mRNA as Therapeutic Nucleic Acid
4.5. Principles and Advantages of Using Aptamer as Therapeutic Nucleic Acid
4.6. Principles and Advantages of Using Ribozymes as Therapeutic Nucleic Acid
5. Clinical Transformation and Application
| Drugs | Target Gene | Delivery System | Disease Type | Status | Clinical Trials Gov Identifier | Reference |
|---|---|---|---|---|---|---|
| rAAV2-CBSB-hRPE65(cDNA) | RPE65 | Recombinant adeno-associated virus serotype 2 (rAAV2) | Amaurosis of Leber | Phase 1 active, not recruiting | NCT00481546 | [7] |
| rAAV2-VMD2 (cDNA) | MERTK | rAAV2 | Retinitis pigmentosa | Phase 1 completed | NCT01482195 | [120] |
| RGX-314 (cDNA) | VEGF | rAAV2 | Neovascular AMD degeneration | Phase 1 | NCT03066258 | [121] |
| Pegaptanib (aptamer) | VEGF | Carrier-free | Wet AMD degeneration | Phase 3 | NCT01189461 | [119] |
| vMCO-I (cDNA) | MCO | rAAV2 | Retinal degeneration | Phase 1/2 | NCT04919473 | [122] |
| QR-1123 (ASO) | P23H | Water-based formulation | Retinal dystrophies | Phase 1/2 | NCT04123626 | [123] |
| SYL040012 (siRNA) | ADRB2 | Carrier-free | Glaucoma | Phase 1 | NCT00990743 | [124] |
| SYL1001 (siRNA) | TRPV1 | Carrier-free | Dry-eye disease | Phase 3 | NCT03108664 | [125] |
| AGN211745 (siRNA) | VEGF-1 | Carrier-free | Neovascular AMD | Phase 1/2 | NCT00363714 | [116] |
| ISTH0036 (ASO) | TGF-β2 | Water-based formulation | Primary open-angle glaucoma | Phase 1 | NCT02406833 | [117] |
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jackson, D.; Malka, S.; Harding, P.; Palma, J.; Dunbar, H.; Moosajee, M. Molecular diagnostic challenges for non-retinal developmental eye disorders in the United Kingdom. Am. J. Med. Genet. Part C Semin. Med. Genet. 2020, 184, 578–589. [Google Scholar] [CrossRef]
- Lin, T.-C.; Chung, Y.-C.; Hsu, T.-K.; Huang, H.-W.; Huang, Y.-M.; Chou, Y.-C.; Chao, C.-Y.; Tseng, P.-C. Therapeutic effect of simultaneous intravitreal dexamethasone and aflibercept on diabetic macular edema. Acta Diabetol. 2021, 59, 501–508. [Google Scholar] [CrossRef]
- Elnahry, A.G.; Talbet, J.H.; Elnahry, G.A. Methotrexate monotherapy for unilateral moderately active thyroid-related eye disease. Clin. Case Rep. 2021, 9, e04559. [Google Scholar] [CrossRef]
- Fabre, M.; Mateo, L.; Lamaa, D.; Baillif, S.; Pagès, G.; Demange, L.; Ronco, C.; Benhida, R. Recent Advances in Age-Related Macular Degeneration Therapies. Molecules 2022, 27, 5089. [Google Scholar] [CrossRef]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Novack, G.D. Pharmacotherapy for the Treatment of Choroidal Neovascularization Due to Age-Related Macular Degeneration. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 61–78. [Google Scholar] [CrossRef]
- Bainbridge, J.W.; Mehat, M.S.; Sundaram, V.; Robbie, S.J.; Barker, S.E.; Ripamonti, C.; Georgiadis, A.; Mowat, F.M.; Beattie, S.G.; Gardner, P.J.; et al. Long-Term Effect of Gene Therapy on Leber’s Congenital Amaurosis. N. Engl. J. Med. 2015, 372, 1887–1897. [Google Scholar] [CrossRef] [Green Version]
- Rajesh, C.; Rao, M.D. Mechanisms and management of retinopathy of prematurity. N. Engl. J. Med. 2013, 368, 1160–1161. [Google Scholar]
- Weng, J.; Mata, N.L.; Azarian, S.M.; Tzekov, R.T.; Birch, D.G.; Travis, G.H. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell 1999, 98, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, J.A.; Witzigmann, D.; Thomson, S.B.; Chen, S.; Leavitt, B.R.; Cullis, P.R.; van der Meel, R. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 2021, 16, 630–643. [Google Scholar] [CrossRef]
- Petit, L.; Khanna, H.; Punzo, C. Advances in Gene Therapy for Diseases of the Eye. Hum. Gene Ther. 2016, 27, 563–579. [Google Scholar] [CrossRef] [Green Version]
- Wen, L.-Y.; Wan, L.; Lai, J.-N.; Chen, C.S.; Chen, J.J.-Y.; Wu, M.-Y.; Hu, K.-C.; Chiu, L.-T.; Tien, P.-T.; Lin, H.-J. Increased risk of Alzheimer’s disease among patients with age-related macular degeneration: A nationwide population-based study. PLoS ONE 2021, 16, e0250440. [Google Scholar] [CrossRef]
- Liu, K.C.; Gomez-Caraballo, M.; Challa, P.; Asrani, S.G. Recurrent Tube Erosions with Anti-Vascular Endothelial Growth Factor Therapy in Patients with Age-Related Macular Degeneration. Ophthalmol. Glaucoma 2020, 3, 295–300. [Google Scholar] [CrossRef]
- Foss, A.; Rotsos, T.; Empeslidis, T.; Chong, V. Development of Macular Atrophy in Patients with Wet Age-Related Macular Degeneration Receiving Anti-VEGF Treatment. Ophthalmologica 2021, 245, 204–217. [Google Scholar] [CrossRef]
- Pastuch-Gawołek, G.; Gillner, D.; Król, E.; Walczak, K.; Wandzik, I. Selected nucleos(t)ide-based prescribed drugs and their multi-target activity. Eur. J. Pharmacol. 2019, 865, 172747. [Google Scholar] [CrossRef]
- Zaneveld, S.A.; Eblimit, A.; Liang, Q.; Bertrand, R.; Wu, N.; Liu, H.; Nguyen, Q.; Zaneveld, J.; Wang, K.; Li, Y.; et al. Gene Therapy Rescues Retinal Degeneration in Receptor Expression-Enhancing Protein 6 Mutant Mice. Hum. Gene Ther. 2019, 30, 302–315. [Google Scholar] [CrossRef]
- Morrow, P.K.; Murthy, R.K.; Ensor, J.; Gordon, G.S.; Margolin, K.; Elias, A.D.; Urba, W.J.; Weng, D.E.; Rugo, H.S.; Hortobagyi, G.N. An open-label, phase 2 trial of RPI.4610 (angiozyme) in the treatment of metastatic breast cancer. Cancer 2012, 118, 4098–4104. [Google Scholar] [CrossRef]
- Hill, S.F.; Meisler, M.H. Antisense Oligonucleotide Therapy for Neurodevelopmental Disorders. Dev. Neurosci. 2021, 43, 247–252. [Google Scholar] [CrossRef]
- Ni, Z.; Hui, P. Emerging Pharmacologic Therapies for Wet Age-Related Macular Degeneration. Ophthalmologica 2009, 223, 401–410. [Google Scholar] [CrossRef]
- Borrás, T. Recent developments in ocular gene therapy. Exp. Eye Res. 2003, 76, 643–652. [Google Scholar] [CrossRef]
- Villanueva, M.T. An mRNA universal vaccine for influenza. Nat. Rev. Drug Discov. 2023, 22, 98. [Google Scholar] [CrossRef]
- Seyednejad, S.A.; Sartor, G.C. Noncoding RNA therapeutics for substance use disorder. Adv. Drug Alcohol Res. 2022, 2, 10807. [Google Scholar] [CrossRef]
- Ashwath, P.; Somanath, D.; Sannejal, A.D. CRISPR and Antisense RNA Technology: Exploiting Nature’s Tool to Restrain Virulence in Tenacious Pathogens. Mol. Biotechnol. 2022, 65, 17–27. [Google Scholar] [CrossRef]
- Rostamighadi, M.; Mehta, V.; Khan, R.H.; Moses, D.; Salavati, R. Hammerhead ribozyme-based U-insertion and deletion RNA editing assays for multiplexing in HTS applications. RNA 2022, 29, 252–261. [Google Scholar] [CrossRef]
- Zhou, Q.-H.; Zhang, Q.; Yang, R.-L.; Yuan, G.-R.; Wang, J.-J.; Dou, W. RNAi-mediated knockdown of juvenile hormone acid O-methyltransferase disrupts larval development in the oriental fruit fly, Bactrocera dorsalis (Hendel). Pestic. Biochem. Physiol. 2022, 188, 105285. [Google Scholar] [CrossRef]
- Kamenova, S.; Sharapkhanova, A.; Akimniyazova, A.; Kuzhybayeva, K.; Kondybayeva, A.; Rakhmetullina, A.; Pyrkova, A.; Ivashchenko, A. piRNA and miRNA Can Suppress the Expression of Multiple Sclerosis Candidate Genes. Nanomaterials 2022, 13, 22. [Google Scholar] [CrossRef]
- Liu, X.; Hu, J.; Ning, Y.; Xu, H.; Cai, H.; Yang, A.; Shi, Z.; Li, Z. Aptamer Technology and Its Applications in Bone Diseases. Cell Transplant. 2023, 32. [Google Scholar] [CrossRef]
- Srilekha, S.; Rao, B.; Rao, D.M.; Sudha, D.; Chandrasekar, S.P.; Pandian, A.; Soumittra, N.; Sripriya, S. Strategies for Gene Mapping in Inherited Ophthalmic Diseases. Asia Pac. J. Ophthalmol. 2016, 5, 282–292. [Google Scholar] [CrossRef]
- Thavikulwat, A.T.; Edward, D.P.; AlDarrab, A.; Vajaranant, T.S. Pathophysiology and management of glaucoma associated with phakomatoses. J. Neurosci. Res. 2018, 97, 57–69. [Google Scholar] [CrossRef] [Green Version]
- Khandhadia, S.; Hakobyan, S.; Heng, L.Z.; Gibson, J.; Adams, D.H.; Alexander, G.J.; Gibson, J.M.; Martin, K.R.; Menon, G.; Nash, K.; et al. Age-related Macular Degeneration and Modification of Systemic Complement Factor H Production Through Liver Transplantation. Ophthalmology 2013, 120, 1612–1618. [Google Scholar] [CrossRef]
- May, A.; Su, F.; Dinh, B.; Ehlen, R.; Tran, C.; Adivikolanu, H.; Shaw, P.X. Ongoing controversies and recent insights of the ARMS2-HTRA1 locus in age-related macular degeneration. Exp. Eye Res. 2021, 210, 108605. [Google Scholar] [CrossRef]
- Chakravarthy, U.; McKay, G.; de Jong, P.T.; Rahu, M.; Seland, J.; Soubrane, G.; Tomazzoli, L.; Topouzis, F.; Vingerling, J.R.; Vioque, J.; et al. ARMS2 Increases the Risk of Early and Late Age-related Macular Degeneration in the European Eye Study. Ophthalmology 2013, 120, 342–348. [Google Scholar] [CrossRef]
- Heiferman, M.J.; Fawzi, A.A. Progression of subclinical choroidal neovascularization in age-related macular degeneration. PLoS ONE 2019, 14, e0217805. [Google Scholar] [CrossRef] [Green Version]
- Courtaut, F.; Scagliarini, A.; Aires, V.; Cornebise, C.; de Barros, J.-P.P.; Olmiere, C.; Delmas, D. VEGF-R2/Caveolin-1 Pathway of Undifferentiated ARPE-19 Retina Cells: A Potential Target as Anti-VEGF-A Therapy in Wet AMD by Resvega, an Omega-3/Polyphenol Combination. Int. J. Mol. Sci. 2021, 22, 6590. [Google Scholar] [CrossRef]
- Yuan, D.; Yan, T.; Luo, S.; Huang, J.; Tan, J.; Zhang, J.; Zhang, V.W.; Lan, Y.; Hu, T.; Guo, J.; et al. Identification and Functional Characterization of a Novel Nonsense Variant in ARR3 in a Southern Chinese Family with High Myopia. Front. Genet. 2021, 12, 765503. [Google Scholar] [CrossRef]
- Jiang, D.; Li, J.; Xiao, X.; Li, S.; Jia, X.; Sun, W.; Guo, X.; Zhang, Q. Detection of Mutations in LRPAP1, CTSH, LEPREL1, ZNF644, SLC39A5, and SCO2 in 298 Families with Early-Onset High Myopia by Exome Sequencing. Investig. Opthalmol. Vis. Sci. 2014, 56, 339–345. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-M.; Lu, S.-Y.; Zhang, X.-J.; Chen, L.-J.; Pang, C.-P.; Yam, J.C. Myopia Genetics and Heredity. Children 2022, 9, 382. [Google Scholar] [CrossRef]
- Cai, X.-B.; Zheng, Y.-H.; Chen, D.-F.; Zhou, F.-Y.; Xia, L.-Q.; Wen, X.-R.; Yuan, Y.-M.; Han, F.; Piao, S.-Y.; Zhuang, W.; et al. Expanding the Phenotypic and Genotypic Landscape of Nonsyndromic High Myopia: A Cross-Sectional Study in 731 Chinese Patients. Investig. Opthalmol. Vis. Sci. 2019, 60, 4052–4062. [Google Scholar] [CrossRef] [Green Version]
- Hafler, B.P.; Comander, J.; DiFranco, C.W.; Place, E.M.; Pierce, E.A. Course of Ocular Function in PRPF31 Retinitis Pigmentosa. Semin. Ophthalmol. 2016, 31, 49–52. [Google Scholar] [CrossRef]
- DuPont, M.; Jones, E.M.; Xu, M.; Chen, R. Investigating the disease association of USH2A p.C759F variant by leveraging large retinitis pigmentosa cohort data. Ophthalmic Genet. 2017, 39, 291–292. [Google Scholar] [CrossRef]
- Da Palma, M.M.; Martin, D.; Salles, M.V.; Motta, F.L.T.; Abujamra, S.; Sallum, J.M.F. Retinal dystrophies and variants in PRPH2. Arq. Bras. De Oftalmol. 2019, 82, 158–160. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Chen, Q.; Zhao, K.; Wang, L.; Wang, L.; Traboulsi, E.I. Update on the molecular genetics of retinitis pigmentosa. Ophthalmic Genet. 2001, 22, 133–154. [Google Scholar] [CrossRef]
- Katoli, P.; Godbole, A.; Romanowski, M.J.; Clark, K.; Meredith, E.; Saenz-Vash, V.; Wang, Y.K.; Lewicki, N.; Nguyen, A.A.; Lynch, J.M. Full-length myocilin protein is purified from mammalian cells as a dimer. Protein Expr. Purif. 2018, 147, 38–48. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, D.; Jiao, X.; Wang, T.; Fan, M.; Wang, Y.; Hejtmancik, J.F.; Liu, X. Novel compound heterozygous mutations in CYP1B1 identified in a Chinese family with developmental glaucoma. Mol. Med. Rep. 2021, 24, 803. [Google Scholar] [CrossRef]
- Lin, Z.; Zhu, M.; Deng, H. A Pedigree Report of a Rare Case of Weill–Marchesani Syndrome with New Compound Heterozygous LTBP2 Mutations. Risk Manag. Health Policy 2021, 14, 1785–1789. [Google Scholar] [CrossRef]
- Huang, L.; Chi, J.; Berry, F.B.; Footz, T.K.; Sharp, M.W.; Walter, M.A. Human p32 Is a Novel FOXC1-Interacting Protein That Regulates FOXC1 Transcriptional Activity in Ocular Cells. Investig. Opthalmol. Vis. Sci. 2008, 49, 5243–5249. [Google Scholar] [CrossRef]
- Jin, X.; Liu, W.; Qv, L.H.; Huang, H.B. A novel variant in PAX6 as the cause of aniridia in a Chinese family. BMC Ophthalmol. 2021, 21, 225. [Google Scholar] [CrossRef]
- Safonova, T.N.; Surnina, Z.V.; Zaitseva, G.V.; Burdennyi, A.M.; Loginov, V.I. The Role of Polymorphic Markers rs1478604, rs2292305, and rs2228262 in THBS1 Gene in the Development of Autoimmune Dry Eye Syndrome. Bull. Exp. Biol. Med. 2020, 169, 707–709. [Google Scholar] [CrossRef]
- Zhou, P.; Luo, Y.; Liu, X.; Fan, L. Down-regulation and CpG island hypermethylation of CRYAA in age-related nuclear cataract. FASEB J. 2012, 26, 4897–4902. [Google Scholar] [CrossRef]
- Colin, E.; Sentilhes, L.; Sarfati, A.; Miné, M.; Guichet, A.; Ploton, C.; Boussion, F.; Delorme, B.; Tournier-Lasserve, E.; Bonneau, D. Fetal intracerebral hemorrhage and cataract: Think COL4A1. J. Perinatol. 2013, 34, 75–77. [Google Scholar] [CrossRef]
- Shi, X.; Cui, B.; Wang, Z.; Weng, L.; Xu, Z.; Ma, J.; Xu, G.; Kong, X.; Hu, L. Removal of Hsf4 leads to cataract development in mice through down-regulation of gamma S-crystallin and Bfsp expression. BMC Mol. Biol. 2009, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Hou, S.; Li, N.; Liao, X.; Kijlstra, A.; Yang, P. Uveitis genetics. Exp. Eye Res. 2020, 190, 107853. [Google Scholar] [CrossRef]
- Lv, Y.; Xu, W.-Q.; Dong, W.-G.; Li, M.-H.; Chang, T.-F.; Sun, J.-X.; Sun, L.-J.; Pan, X.-Y.; Li, H.; Dou, G.-R.; et al. Integrin alpha5beta1 promotes BMCs mobilization and differentiation to exacerbate choroidal neovascularization. Exp. Eye Res. 2020, 193, 107991. [Google Scholar] [CrossRef]
- Zong, H.; Ward, M.; Stitt, A.W. AGEs, RAGE, and diabetic retinopathy. Curr. Diab. Rep. 2011, 11, 244–252. [Google Scholar] [CrossRef]
- Cheung, A.K.; Fung, M.K.; Lo, A.C.; Lam, T.T.; So, K.F.; Chung, S.S.; Chung, S.K. Aldose Reductase Deficiency Prevents Diabetes-Induced Blood-Retinal Barrier Breakdown, Apoptosis, and Glial Reactivation in the Retina of db/db Mice. Diabetes 2005, 54, 3119–3125. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Sheng, Y.; Shi, Y.; Du, M.; Guo, Y.; Li, S. The Efficacy of Simultaneous Injection of Dexamethasone Implant and Ranibizumab into Vitreous Cavity on Macular Edema Secondary to Central Retinal Vein Occlusion. Front. Pharmacol. 2022, 13, 842805. [Google Scholar] [CrossRef]
- Olufsen, M.E.; Spindler, L.; Sørensen, N.B.; Christiansen, A.T.; Alberti, M.; Heegaard, S.; Kiilgaard, J.F. Controlled Subretinal Injection Pressure Prevents Damage in Pigs. Ophthalmologica 2022, 245, 285–294. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Espina, M.; Doktorovova, S.; Souto, E.B.; Garcia, M.L. Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye—Part I—Barriers and determining factors in ocular delivery. Eur. J. Pharm. Biopharm. 2017, 110, 70–75. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, W. Kinetic analysis of the effects of target structure on siRNA efficiency. J. Chem. Phys. 2012, 137, 225102. [Google Scholar] [CrossRef]
- Bramsen, J.B.; Kjems, J. Development of Therapeutic-Grade Small Interfering RNAs by Chemical Engineering. Front. Genet. 2012, 3, 154. [Google Scholar] [CrossRef] [Green Version]
- Mustafina, K.; Nomura, Y.; Rotrattanadumrong, R.; Yokobayashi, Y. Circularly-Permuted Pistol Ribozyme: A Synthetic Ribozyme Scaffold for Mammalian Riboswitches. ACS Synth. Biol. 2021, 10, 2040–2048. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, X.; Ma, W.; Mao, C.; Shao, X.; Lin, Y. Multi-targeted Antisense Oligonucleotide Delivery by a Framework Nucleic Acid for Inhibiting Biofilm Formation and Virulence. Nano Micro Lett. 2020, 12, 74. [Google Scholar] [CrossRef] [Green Version]
- Dehghani, S.; Alibolandi, M.; Tehranizadeh, Z.A.; Oskuee, R.K.; Nosrati, R.; Soltani, F.; Ramezani, M. Self-assembly of an aptamer-decorated chimeric peptide nanocarrier for targeted cancer gene delivery. Colloids Surf. B Biointerfaces 2021, 208, 112047. [Google Scholar] [CrossRef]
- Zhan, P.; Peil, A.; Jiang, Q.; Wang, D.; Mousavi, S.; Xiong, Q.; Shen, Q.; Shang, Y.; Ding, B.; Lin, C.; et al. Recent Advances in DNA Origami-Engineered Nanomaterials and Applications. Chem. Rev. 2023, 123, 3976–4050. [Google Scholar] [CrossRef]
- Bollhorst, T.; Rezwan, K.; Maas, M. Colloidal capsules: Nano- and microcapsules with colloidal particle shells. Chem. Soc. Rev. 2017, 46, 2091–2126. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Yang, D.; Sun, Y.; Liu, T.; Wang, W.; Fu, J.; Wang, Q.; Bai, X.; Quan, G.; Pan, X.; et al. In Situ Self-Assembly Nanomicelle Microneedles for Enhanced Photoimmunotherapy via Autophagy Regulation Strategy. ACS Nano 2021, 15, 3387–3401. [Google Scholar] [CrossRef]
- Si, Y.; Chen, M.; Wu, L. Syntheses and biomedical applications of hollow micro-/nano-spheres with large-through-holes. Chem. Soc. Rev. 2016, 45, 690–714. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef]
- Chen, L.; Doyle, P.S. Design and Use of a Thermogelling Methylcellulose Nanoemulsion to Formulate Nanocrystalline Oral Dosage Forms. Adv. Mater. 2021, 33, e2008618. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, C.; Wang, C.; Jankovic, K.E.; Dong, Y. Lipids and Lipid Derivatives for RNA Delivery. Chem. Rev. 2021, 121, 12181–12277. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Huang, D.; Norat, P.; Grannonico, M.; Cooper, R.C.; Gui, Q.; Chow, W.N.; Liu, X.; Yang, H. Nano-in-Nano dendrimer gel particles for efficient topical delivery of antiglaucoma drugs into the eye. Chem. Eng. J. 2021, 425, 130498. [Google Scholar] [CrossRef]
- Gelain, F.; Luo, Z.; Zhang, S. Self-Assembling Peptide EAK16 and RADA16 Nanofiber Scaffold Hydrogel. Chem. Rev. 2020, 120, 13434–13460. [Google Scholar] [CrossRef]
- Hornof, M.; Toropainen, E.; Urtti, A. Cell culture models of the ocular barriers. Eur. J. Pharm. Biopharm. 2005, 60, 207–225. [Google Scholar] [CrossRef]
- Stewart, S.A.; Dykxhoorn, D.M.; Palliser, D.; Mizuno, H.; Yu, E.Y.; An, D.S.; Sabatini, D.M.; Chen, I.S.; Hahn, W.C.; Sharp, P.A.; et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA 2003, 9, 493–501. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.-P.; Zhang, S.-B.; Wang, F.; Liu, H.; Zhang, W.; Song, B.; Liu, Z.-Y.; Xiong, L.; Fan, Y.-Z.; Liao, D.-Y. Effects of lentiviral RNA interference-mediated downregulation of integrin-linked kinase on biological behaviors of human lens epithelial cells. Int. J. Ophthalmol. 2016, 9, 21–28. [Google Scholar] [CrossRef]
- De Oliveira, L.A.; Kim, C.; De Sousa, L.B.; Schwab, I.R.; Rosenblatt, M.I. Gene transfer to primary corneal epithelial cells with an integrating lentiviral vector. Arq. Bras. De Oftalmol. 2010, 73, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Cashman, S.M.; Bowman, L.; Christofferson, J.; Kumar-Singh, R. Inhibition of Choroidal Neovascularization by Adenovirus-Mediated Delivery of Short Hairpin RNAs Targeting VEGF as a Potential Therapy for AMD. Investig. Opthalmol. Vis. Sci. 2006, 47, 3496–3504. [Google Scholar] [CrossRef]
- Gong, Y.; Chang, Z.-P.; Ren, R.-T.; Wei, S.-H.; Zhou, H.-F.; Chen, X.-F.; Hou, B.-K.; Jin, X.; Zhang, M.-N. Protective Effects of Adeno-associated Virus Mediated Brain-derived Neurotrophic Factor Expression on Retinal Ganglion Cells in Diabetic Rats. Cell. Mol. Neurobiol. 2012, 32, 467–475. [Google Scholar] [CrossRef]
- Ruan, X.; Yuan, Z.; Du, Y.; Yang, G.; Wang, Q. Recombinant adeno-associated virus delivered human thioredoxin-PR39 prevents hypoxia-induced apoptosis of ECV304 cells. Neural Regen. Res. 2012, 7, 708–713. [Google Scholar] [CrossRef]
- Schnabolk, G.; Parsons, N.; Obert, E.; Annamalai, B.; Nasarre, C.; Tomlinson, S.; Lewin, A.; Rohrer, B. Delivery of CR2-fH Using AAV Vector Therapy as Treatment Strategy in the Mouse Model of Choroidal Neovascularization. Mol. Ther. Methods Clin. Dev. 2017, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Andrade, L.M.; Rocha, K.A.D.; De Sá, F.A.P.; Marreto, R.N.; Lima, E.M.; Gratieri, T.; Taveira, S.F. Voriconazole-Loaded Nanostructured Lipid Carriers for Ocular Drug Delivery. Cornea 2016, 35, 866–871. [Google Scholar] [CrossRef]
- Peeters, L.; Sanders, N.N.; Braeckmans, K.; Boussery, K.; Van De Voorde, J.; De Smedt, S.C.; Demeester, J. Vitreous: A Barrier to Nonviral Ocular Gene Therapy. Investig. Opthalmol. Vis. Sci. 2005, 46, 3553–3561. [Google Scholar] [CrossRef]
- Sasaki, H.; Karasawa, K.; Hironaka, K.; Tahara, K.; Tozuka, Y.; Takeuchi, H. Retinal drug delivery using eyedrop preparations of poly-l-lysine-modified liposomes. Eur. J. Pharm. Biopharm. 2013, 83, 364–369. [Google Scholar] [CrossRef]
- Attama, A.A.; Reichl, S.; Müller-Goymann, C.C. Diclofenac sodium delivery to the eye: In vitro evaluation of novel solid lipid nanoparticle formulation using human cornea construct. Int. J. Pharm. 2008, 355, 307–313. [Google Scholar] [CrossRef]
- Wang, Y.; Rajala, A.; Cao, B.; Ranjo-Bishop, M.; Agbaga, M.-P.; Mao, C.; Rajala, R.V. Cell-Specific Promoters Enable Lipid-Based Nanoparticles to Deliver Genes to Specific Cells of the Retina In Vivo. Theranostics 2016, 6, 1514–1527. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, L.; Fang, L.; Cao, F. Multifunctional carboxymethyl chitosan derivatives-layered double hydroxide hybrid nanocomposites for efficient drug delivery to the posterior segment of the eye. Acta Biomater. 2020, 104, 104–114. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, X.; Hirano, Y.; Tyagi, P.; Barabás, P.; Uehara, H.; Miya, T.R.; Singh, N.; Archer, B.; Qazi, Y.; et al. Targeted Intraceptor Nanoparticle Therapy Reduces Angiogenesis and Fibrosis in Primate and Murine Macular Degeneration. ACS Nano 2013, 7, 3264–3275. [Google Scholar] [CrossRef]
- Chew, E.Y.; Glassman, A.R.; Beck, R.W.; Bressler, N.M.; Fish, G.E.; Ferris, F.; Kinyoun, J.L. Ocular Side Effects Associated with Peribulbar Injections of Triamcinolone Acetonide for Diabetic Macular Edema. Retina 2011, 31, 284–289. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Jiao, J.; Niu, M.; Gao, X.; Zhang, G.; Yu, H.; Yang, X.; Liu, L. Ten Years of Knowledge of Nano-Carrier Based Drug Delivery Systems in Ophthalmology: Current Evidence, Challenges, and Future Prospective. Int. J. Nanomed. 2021, 16, 6497–6530. [Google Scholar] [CrossRef]
- Cai, X.; Conley, S.M.; Nash, Z.; Fliesler, S.J.; Cooper, M.J.; Naash, M.I. Gene delivery to mitotic and postmitotic photoreceptors Via compacted DNA nanoparticles results in improved phenotype in a mouse model of retinitis pigmentosa. FASEB J. 2009, 24, 1178–1191. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Gan, L.; Zhu, C.; Dong, Y.; Liu, J.; Gan, Y. Cationic core-shell liponanoparticles for ocular gene delivery. Biomaterials 2012, 33, 7621–7630. [Google Scholar] [CrossRef]
- Rossi, J.J. Ribozymes to the rescue: Repairing genetically defective mRNAs. Trends Genet. 1998, 14, 295–298. [Google Scholar] [CrossRef]
- Hendry, P.; McCall, M.J.; Stewart, T.S.; Lockett, T.J. Redesigned and chemically-modified hammerhead ribozymes with improved activity and serum stability. BMC Chem. Biol. 2004, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsova, M.; Novopashina, D.; Repkova, M.; Venyaminova, A.; Vlassov, V. Binary Hammerhead Ribozymes with High Cleavage Activity. Nucl. Nucl. Nucleic Acids 2004, 23, 1037–1042. [Google Scholar] [CrossRef]
- Pierce, E.A.; Bennett, J. The Status of RPE65 Gene Therapy Trials: Safety and Efficacy. Cold Spring Harb. Perspect. Med. 2015, 5, a017285. [Google Scholar] [CrossRef] [Green Version]
- Redmond, T.M.; Yu, S.; Lee, E.; Bok, D.; Hamasaki, D.; Chen, N.; Goletz, P.; Ma, J.-X.; Crouch, R.K.; Pfeifer, K. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat. Genet. 1998, 20, 344–351. [Google Scholar] [CrossRef]
- Pennesi, M.E.; Weleber, R.G.; Yang, P.; Whitebirch, C.; Thean, B.; Flotte, T.R.; Humphries, M.; Chegarnov, E.; Beasley, K.N.; Stout, J.T.; et al. Results at 5 Years After Gene Therapy for RPE65-Deficient Retinal Dystrophy. Hum. Gene Ther. 2018, 29, 1428–1437. [Google Scholar] [CrossRef]
- Levin, A.A. Treating Disease at the RNA Level with Oligonucleotides. N. Engl. J. Med. 2019, 380, 57–70. [Google Scholar] [CrossRef]
- Guzman-Aranguez, A.; Loma, P.; Pintor, J. Small-interfering RNAs (siRNAs) as a promising tool for ocular therapy. Br. J. Pharmacol. 2013, 170, 730–747. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, F.; Pan, H.; Huang, X.; Yu, J.; Liu, X.; Zhang, Q.; Xiao, C.; Zhang, H.; Zhang, L. Targeted OUM1/PTPRZ1 silencing and synergetic CDT/enhanced chemical therapy toward uveal melanoma based on a dual-modal imaging-guided manganese metal-organic framework nanoparticles. J. Nanobiotechnol. 2022, 20, 472. [Google Scholar] [CrossRef]
- Chan, P.Y.; Phillips, M.M.; Ellis, S.; Johnston, A.; Feng, X.; Arora, A.; Hay, G.; Cohen, V.M.L.; Sagoo, M.S.; Bomalaski, J.S.; et al. A Phase 1 study of ADI-PEG20 (pegargiminase) combined with cisplatin and pemetrexed in ASS1-negative metastatic uveal melanoma. Pigment Cell Melanoma Res. 2022, 35, 461–470. [Google Scholar] [CrossRef]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef] [Green Version]
- Antonetti, D.A.; Silva, P.S.; Stitt, A.W. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat. Rev. Endocrinol. 2021, 17, 195–206. [Google Scholar] [CrossRef]
- Öhnell, H.M.; Andreasson, S.; Gränse, L. Dexamethasone Eye Drops for the Treatment of Retinopathy of Prematurity. Ophthalmol. Retin. 2021, 6, 181–182. [Google Scholar] [CrossRef]
- McKenzie, L.K.; El-Khoury, R.; Thorpe, J.D.; Damha, M.J.; Hollenstein, M. Recent progress in non-native nucleic acid modifications. Chem. Soc. Rev. 2021, 50, 5126–5164. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Liu, C.-H.; Wu, A.-L.; Chen, H.-C.; Hsueh, Y.-J.; Chen, K.-J.; Lai, C.-C.; Huang, C.-Y.; Wu, W.-C. MicroRNA-126 inhibits pathological retinal neovascularization via suppressing vascular endothelial growth factor expression in a rat model of retinopathy of prematurity. Eur. J. Pharmacol. 2021, 900, 174035. [Google Scholar] [CrossRef]
- Mukwaya, A.; Jensen, L.; Peebo, B.; Lagali, N. MicroRNAs in the cornea: Role and implications for treatment of corneal neovascularization. Ocul. Surf. 2019, 17, 400–411. [Google Scholar] [CrossRef]
- Sahin, U.; Kariko, K.; Tureci, O. mRNA-based therapeutics—Developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef]
- Ling, S.; Yang, S.; Hu, X.; Yin, D.; Dai, Y.; Qian, X.; Wang, D.; Pan, X.; Hong, J.; Sun, X.; et al. Lentiviral delivery of co-packaged Cas9 mRNA and a Vegfa-targeting guide RNA prevents wet age-related macular degeneration in mice. Nat. Biomed. Eng. 2021, 5, 144–156. [Google Scholar] [CrossRef]
- Kowalski, P.S.; Rudra, A.; Miao, L.; Anderson, D.G. Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery. Mol. Ther. 2019, 27, 710–728. [Google Scholar] [CrossRef] [Green Version]
- Meng, H.-M.; Liu, H.; Kuai, H.; Peng, R.; Mo, L.; Zhang, X.-B. Aptamer-integrated DNA nanostructures for biosensing, bioimaging and cancer therapy. Chem. Soc. Rev. 2016, 45, 2583–2602. [Google Scholar] [CrossRef]
- Carrasquillo, K.G.; Ricker, J.A.; Rigas, I.K.; Miller, J.W.; Gragoudas, E.S.; Adamis, A.P. Controlled Delivery of the Anti-VEGF Aptamer EYE001 with Poly(lactic-co-glycolic)Acid Microspheres. Investig. Opthalmol. Vis. Sci. 2003, 44, 290–299. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, S.; Kashani-Sabet, M. Ribozymes in the age of molecular therapeutics. Curr. Mol. Med. 2004, 4, 489–506. [Google Scholar] [CrossRef]
- Liu, J.; Timmers, A.M.; Lewin, A.S.; Hauswirth, W.W. Ribozyme knockdown of the gamma-subunit of rod cGMP phosphodiesterase alters the ERG and retinal morphology in wild-type mice. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3836–3844. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, S.G.; Cideciyan, A.V.; Ratnakaram, R.; Heon, E.; Schwartz, S.B.; Roman, A.J.; Peden, M.C.; Aleman, T.S.; Boye, S.L.; Sumaroka, A.; et al. Gene therapy for leber congenital amaurosis caused by RPE65 mutations: Safety and efficacy in 15 children and adults followed up to 3 years. Arch. Ophthalmol. 2012, 130, 9–24. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Zhang, X.; Tang, Y.; Li, S.; Chen, J. Progress on ocular siRNA gene-silencing therapy and drug delivery systems. Fundam. Clin. Pharmacol. 2020, 35, 4–24. [Google Scholar] [CrossRef]
- Pfeiffer, N.; Voykov, B.; Renieri, G.; Bell, K.; Richter, P.; Weigel, M.; Thieme, H.; Wilhelm, B.; Lorenz, K.; Feindor, M.; et al. First-in-human phase I study of ISTH0036, an antisense oligonucleotide selectively targeting transforming growth factor beta 2 (TGF-beta2), in subjects with open-angle glaucoma undergoing glaucoma filtration surgery. PLoS ONE 2017, 12, e0188899. [Google Scholar] [CrossRef] [Green Version]
- Sivaprasad, S.; Browning, R.; Starita, C. An open-label, one-year, noncomparative study to evaluate the safety and tolerability of intravitreal pegaptanib sodium in patients with diabetic macular edema. Clin. Ophthalmol. 2014, 8, 1565–1571. [Google Scholar] [CrossRef] [Green Version]
- Udaondo, P.; Garcia-Delpech, S.; Salom, D.; Garcia-Pous, M.; Diaz-Llopis, M. Intravitreal pegaptanib for refractory macular edema secondary to retinal vein occlusion. Clin. Ophthalmol. 2011, 5, 941–944. [Google Scholar] [CrossRef] [Green Version]
- Bucher, K.; Rodríguez-Bocanegra, E.; Dauletbekov, D.; Fischer, M.D. Immune responses to retinal gene therapy using adeno-associated viral vectors—Implications for treatment success and safety. Prog. Retin. Eye Res. 2021, 83, 100915. [Google Scholar] [CrossRef]
- Al-Khersan, H.; Hussain, R.M.; Ciulla, T.A.; Dugel, P.U. Innovative therapies for neovascular age-related macular degeneration. Expert Opin. Pharmacother. 2019, 20, 1879–1891. [Google Scholar] [CrossRef]
- Tchedre, K.T.; Batabyal, S.; Galicia, M.; Narcisse, D.; Mustafi, S.M.; Ayyagari, A.; Chavala, S.; Mohanty, S.K. Biodistribution of adeno-associated virus type 2 carrying multi-characteristic opsin in dogs following intravitreal injection. J. Cell. Mol. Med. 2021, 25, 8676–8686. [Google Scholar] [CrossRef]
- Gupta, A.; Kafetzis, K.N.; Tagalakis, A.D.; Yu-Wai-Man, C. RNA therapeutics in ophthalmology—Translation to clinical trials. Exp. Eye Res. 2021, 205, 108482. [Google Scholar] [CrossRef]
- Moreno-Montanes, J.; Sadaba, B.; Ruz, V.; Gómez-Guiu, A.; Zarranz, J.; González, M.V.; Pañeda, C.; Jimenez, A.I. Phase I clinical trial of SYL040012, a small interfering RNA targeting beta-adrenergic receptor 2, for lowering intraocular pressure. Mol. Ther. 2014, 22, 226–232. [Google Scholar] [CrossRef] [Green Version]
- Benitez-Del-Castillo, J.M.; Moreno-Montañés, J.; Jiménez-Alfaro, I.; Muñoz-Negrete, F.J.; Turman, K.; Palumaa, K.; Sádaba, B.; González, M.V.; Ruz, V.; Vargas, B.; et al. Safety and Efficacy Clinical Trials for SYL1001, a Novel Short Interfering RNA for the Treatment of Dry Eye Disease. Investig. Opthalmol. Vis. Sci. 2016, 57, 6447–6454. [Google Scholar] [CrossRef] [Green Version]
- Huynh, N.; Jeffrey, B.G.; Turriff, A.; Sieving, P.A.; Cukras, C.A. Sorting out Co-occurrence of Rare Monogenic Retinopathies: Stargardt Disease Co-existing with Congenital Stationary Night Blindness. Ophthalmic Genet. 2013, 35, 51–56. [Google Scholar] [CrossRef]
- Yang, M.; Li, S.; Liu, W.; Li, X.; He, Y.; Yang, Y.; Sun, K.; Zhang, L.; Tian, W.; Duan, L.; et al. The ER membrane protein complex subunit Emc3 controls angiogenesis via the FZD4/WNT signaling axis. Sci. China Life Sci. 2021, 64, 1868–1883. [Google Scholar] [CrossRef]
- Fenner, B.J.; Tan, T.-E.; Barathi, A.V.; Tun, S.B.B.; Yeo, S.W.; Tsai, A.S.H.; Lee, S.Y.; Cheung, C.M.G.; Chan, C.M.; Mehta, J.S.; et al. Gene-Based Therapeutics for Inherited Retinal Diseases. Front. Genet. 2022, 12, 794805. [Google Scholar] [CrossRef]
- Zorzi, G.K.; Schuh, R.S.; Maschio, V.J.; Brazil, N.T.; Rott, M.B.; Teixeira, H.F. Box Behnken design of siRNA-loaded liposomes for the treatment of a murine model of ocular keratitis caused by Acanthamoeba. Colloids Surf. B Biointerfaces 2019, 173, 725–732. [Google Scholar] [CrossRef]
- Xue, B.; Ge, M.; Fan, K.; Huang, X.; Yan, X.; Jiang, W.; Jiang, B.; Yang, Z. Mitochondria-targeted nanozymes eliminate oxidative damage in retinal neovascularization disease. J. Control. Release 2022, 350, 271–283. [Google Scholar] [CrossRef]
- Xue, B.; Wang, P.; Yu, W.; Feng, J.; Li, J.; Zhao, R.; Yang, Z.; Yan, X.; Duan, H. CD146 as a promising therapeutic target for retinal and choroidal neovascularization diseases. Sci. China Life Sci. 2021, 65, 1157–1170. [Google Scholar] [CrossRef]
- Miao, Y.-B.; Zhao, W.; Renchi, G.; Gong, Y.; Shi, Y. Customizing delivery nano-vehicles for precise brain tumor therapy. J. Nanobiotechnol. 2023, 21, 32. [Google Scholar] [CrossRef]
- Afarid, M.; Mahmoodi, S.; Baghban, R. Recent achievements in nano-based technologies for ocular disease diagnosis and treatment, review and update. J. Nanobiotechnol. 2022, 20, 361. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, M.; Guo, H. Modified mRNA as a Treatment for Myocardial Infarction. Int. J. Mol. Sci. 2023, 24, 4737. [Google Scholar] [CrossRef]
- Huang, H.; Yi, X.; Wei, Q.; Li, M.; Cai, X.; Lv, Y.; Weng, L.; Mao, Y.; Fan, W.; Zhao, M.; et al. Edible and cation-free kiwi fruit derived vesicles mediated EGFR-targeted siRNA delivery to inhibit multidrug resistant lung cancer. J. Nanobiotechnol. 2023, 21, 41. [Google Scholar] [CrossRef]
- Suarez-Torres, J.D.; Orozco, C.A.; Ciangherotti, C.E. The numerical probability of carcinogenicity to humans of some pharmaceutical drugs: Alkylating agents, topoisomerase inhibitors or poisons, and DNA intercalators. Fundam. Clin. Pharmacol. 2021, 35, 1069–1089. [Google Scholar] [CrossRef]
- Jerkins, G.W.; Pattar, G.R.; Kannarr, S.R. A Review of Topical Cyclosporine A Formulations—A Disease-Modifying Agent for Keratoconjunctivitis Sicca. Clin. Ophthalmol. 2020, 14, 481–489. [Google Scholar] [CrossRef] [Green Version]
- Khin, S.Y.; Soe, H.M.S.H.; Chansriniyom, C.; Pornputtapong, N.; Asasutjarit, R.; Loftsson, T.; Jansook, P. Development of Fenofibrate/Randomly Methylated beta-Cyclodextrin-Loaded Eudragit((R)) RL 100 Nanoparticles for Ocular Delivery. Molecules 2022, 27, 4755. [Google Scholar] [CrossRef]
- Li, J.; Fan, C.; Pei, H.; Shi, J.; Huang, Q. Smart Drug Delivery Nanocarriers with Self-Assembled DNA Nanostructures. Adv. Mater. 2013, 25, 4386–4396. [Google Scholar] [CrossRef]


| Types | Characteristics | Advantages | Disadvantages | Examples | References |
|---|---|---|---|---|---|
| cDNA | Long nucleotide sequences encoding specific proteins | High stability | Possibly inserted genome | rAAV8-Reep6.1 (rescuing Reep6 mutation via gene replacement therapy) | [24] |
| siRNA | Short nucleic acid double strand | Specific knockdown gene expression | Poor stability | AGN211745TM (Treatment of AMD, phase 2 clinical trial) | [25] |
| Antisense oligonucleotide | Hairpin structure | Precise regulation of gene expression | Poor stability | ISTH0036 (Treatment of glaucoma, phase 1 clinical trial) | [26] |
| Aptamers | Oligonucleotides specifically binding to DNA, RNA, proteins | Simple synthesis, low cost and wide range of action targets | Screening difficulty | ARC1905TM (Phase 1 clinical trial of combination therapy with Lucentis® 0.5 mg/eye for neovascular AMD) | [27] |
| Diseases | Disease-Causing Gene | Expression Location | References |
|---|---|---|---|
| Neovascular glaucoma (NVG) | GNAQ, CFH, Y402H | Retina | [29,30] |
| Age-related macular degeneration (AMD) | ARMS2/HTRA1, Y402H, ARMS2, VEGF-R, EST1 | Choriocapillaris | [31,32,33,34] |
| High myopia (HM) | ZNF644, P4HA2, SLC39A5, BSG, LRPAP1, LEPREL1, CTSH, OPN1, LW, ARR3 | Retina, retinal pigment epithelium | [35,36,37,38] |
| Retinitis pigmentosa (RP) | RHO, PRPF31, USH2A, Peripherin/RDS, NRL, RP1, RGR, ABCA4, RPE65, CNCG | Retina | [39,40,41,42] |
| Primary congenital glaucoma (PCG) | CYP1B1, MYOC, LTBP2, FOXC1 | Cornea, ciliary body, iris and retina | [43,44,45,46] |
| Congenital aniridia | PAX6 | Lens, iris | [47] |
| Xerophthalmia | TRP | Cornea | [48] |
| Cataract | CRYAA, COL4A1, BFSP | Lens | [49,50,51] |
| Uveitis | Peripherin/RDS, DRB1/DQA1, IL23R/C1orf141 ADO/ZNF365/EGR2 | Iris, lens, choroid | [52] |
| Choroidal neovascularization (CNV) | SDF-1, CXCR4, VEGF | Choriocapillaris | [53] |
| Diabetic retinopathy (DR) | VEGF, AR, AGE, RAGE, ACE, NOS | Vitreous vessels | [54,55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, W.; Duan, S.; Dai, C.; Xie, C.; Jiang, L.; Shi, Y. Nanotechnology Lighting the Way for Gene Therapy in Ophthalmopathy: From Opportunities toward Applications. Molecules 2023, 28, 3500. https://doi.org/10.3390/molecules28083500
Ren W, Duan S, Dai C, Xie C, Jiang L, Shi Y. Nanotechnology Lighting the Way for Gene Therapy in Ophthalmopathy: From Opportunities toward Applications. Molecules. 2023; 28(8):3500. https://doi.org/10.3390/molecules28083500
Chicago/Turabian StyleRen, Weiming, Suyang Duan, Chao Dai, Chunbao Xie, Lingxi Jiang, and Yi Shi. 2023. "Nanotechnology Lighting the Way for Gene Therapy in Ophthalmopathy: From Opportunities toward Applications" Molecules 28, no. 8: 3500. https://doi.org/10.3390/molecules28083500
APA StyleRen, W., Duan, S., Dai, C., Xie, C., Jiang, L., & Shi, Y. (2023). Nanotechnology Lighting the Way for Gene Therapy in Ophthalmopathy: From Opportunities toward Applications. Molecules, 28(8), 3500. https://doi.org/10.3390/molecules28083500