N-Acetylcysteine Displaces Glutathionyl-Moieties from Hg2+ and MeHg+ to Form More Hydrophobic Complexes at Near-Physiological Conditions
Abstract
1. Introduction
2. Results and Discussion
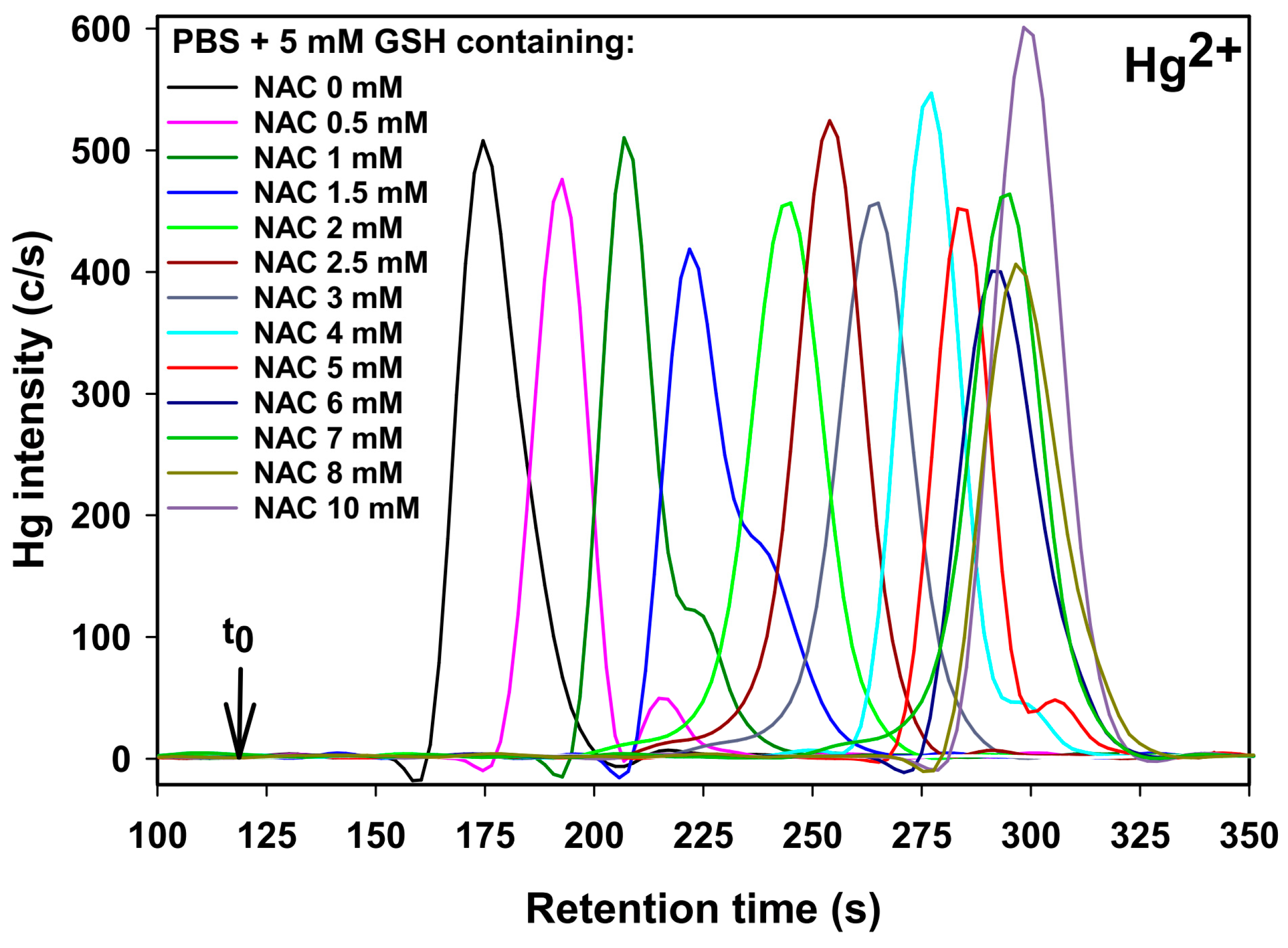
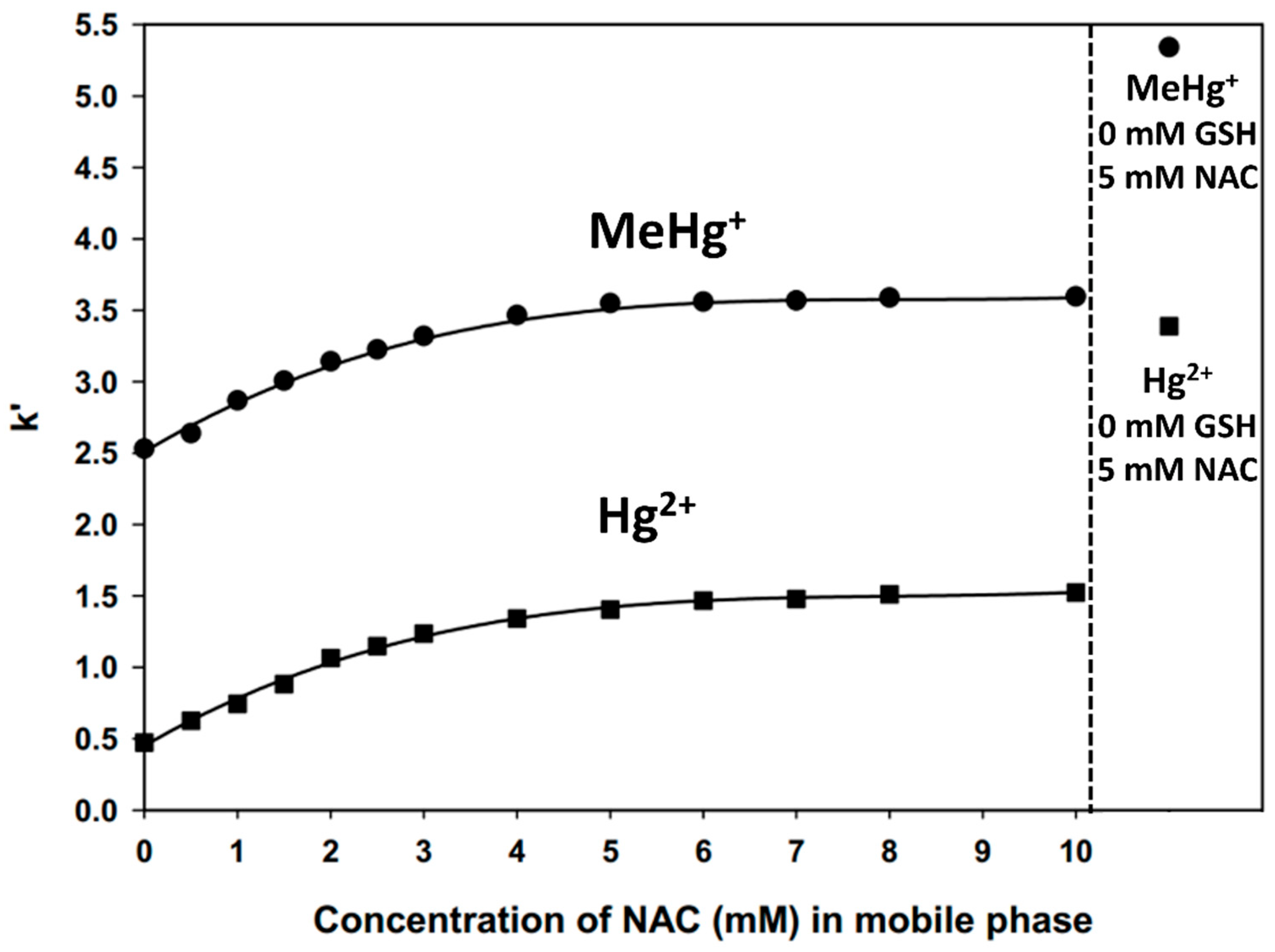
2.1. Retention Behavior of Hg2+ as a Function of the NAC Mobile Phase Concentration
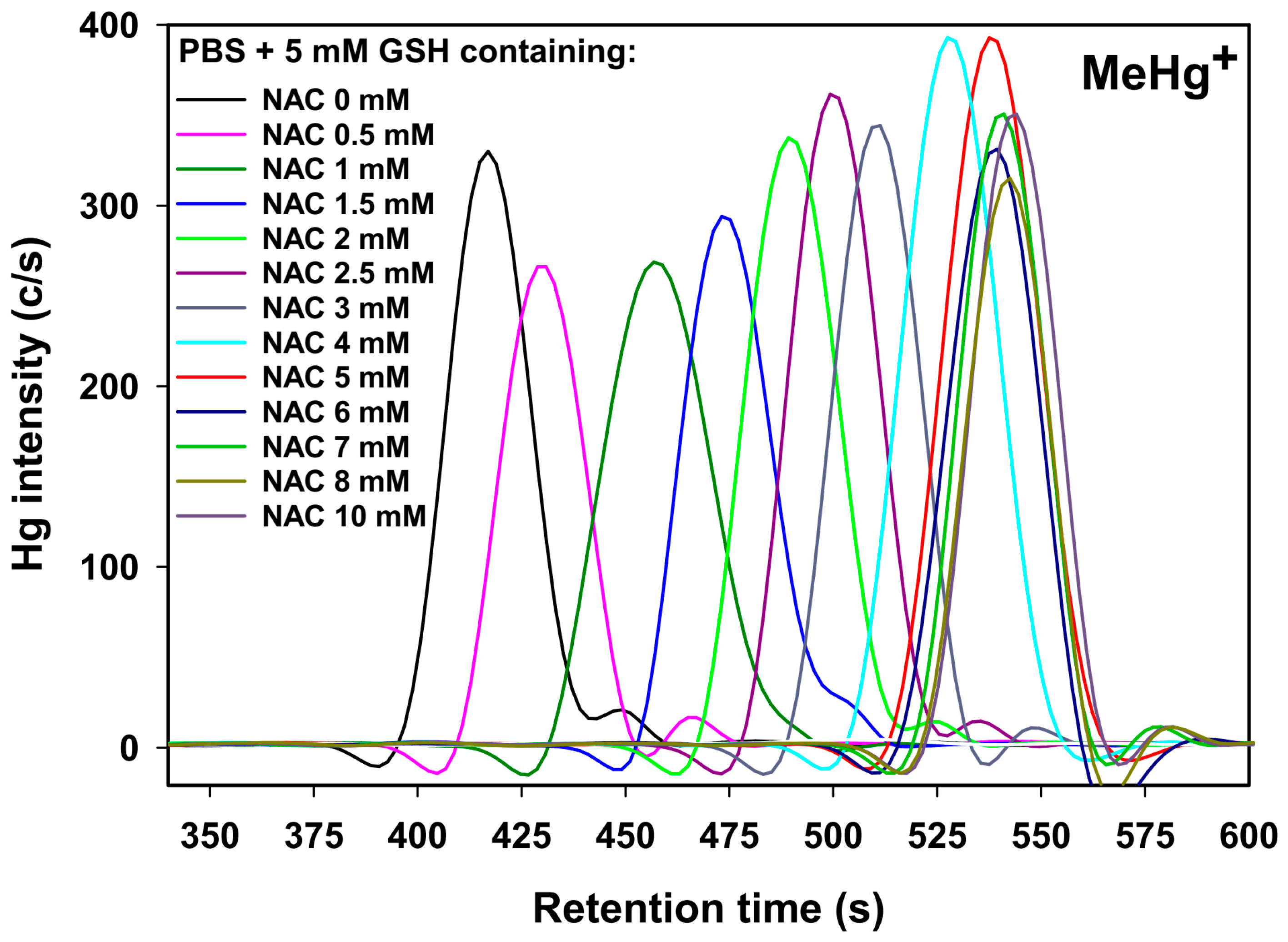
2.2. Retention Behavior of MeHg+ as a Function of the NAC Mobile Phase Concentration
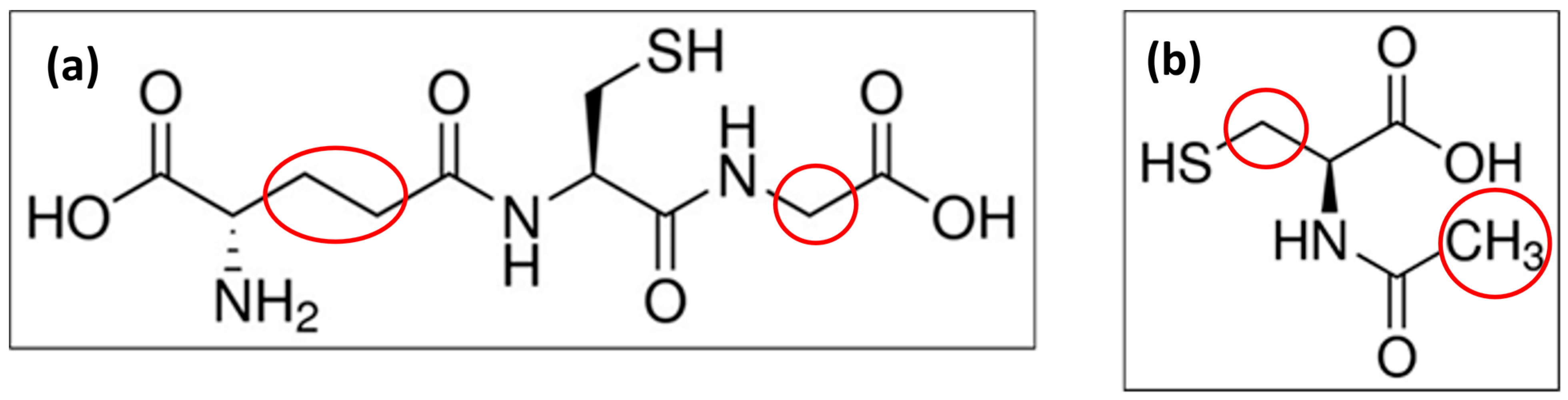
2.3. Comparison of NAC Results to Cys-Related Chromatographic Results
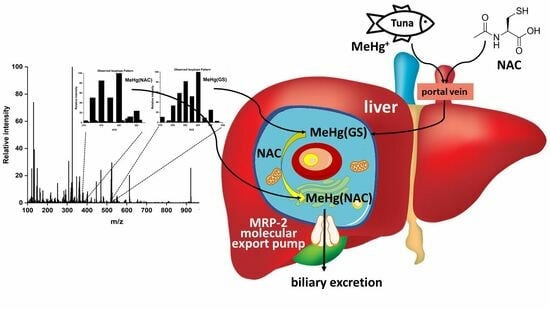
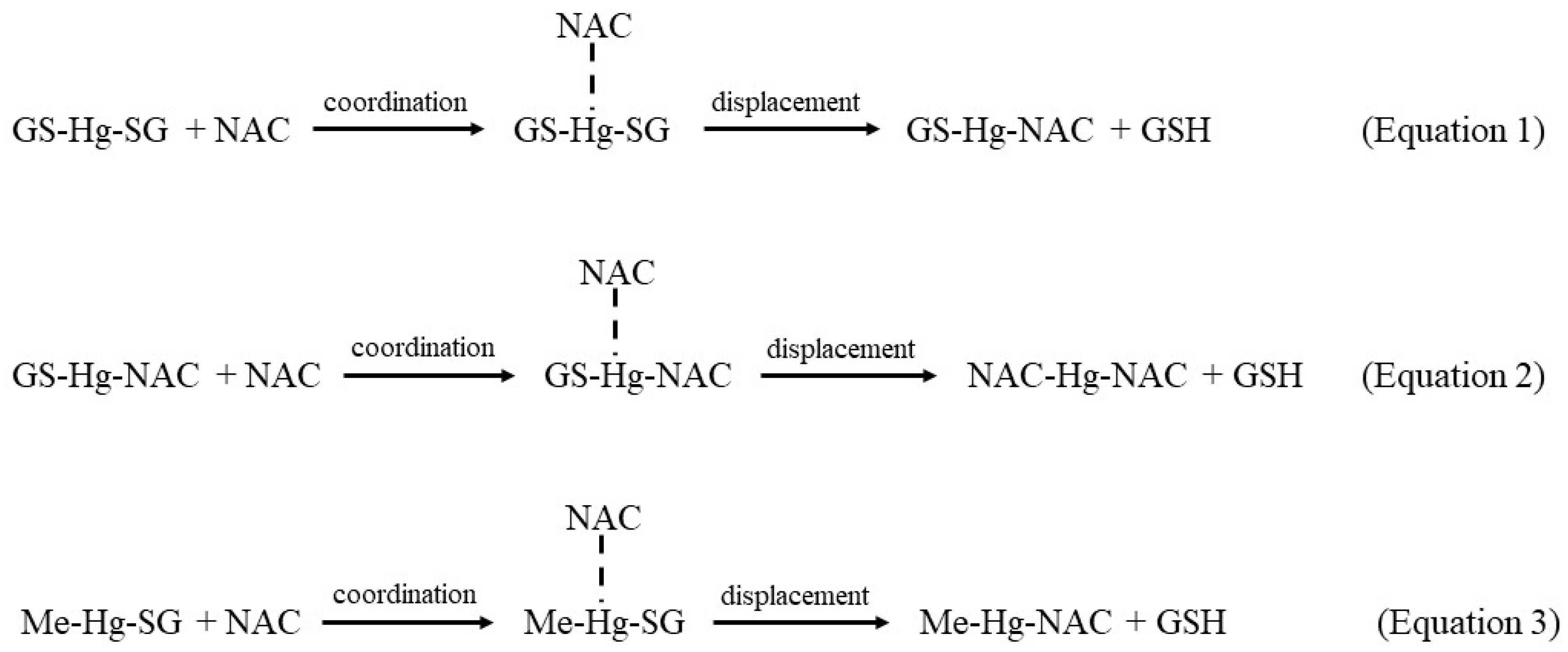
2.4. Potential Bioinorganic Implications of the Findings
3. Materials and Methods
3.1. Chemicals
3.2. Solutions and Mobile Phases
3.3. RP-HPLC-ICP-AES
3.4. Electrospray Ionization Mass Spectrometry (ESI-MS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Pacyna, J.M.; Pacyna, E.G. An assessment of global and regional emissions of trace metals to the atmosphere from anthropogenic sources worldwide. Environ. Res. 2001, 9, 269–298. [Google Scholar] [CrossRef]
- Kasozi, K.I.; Otim, E.O.; Ninsiima, H.I.; Zirintunda, G.; Tamale, A.; Ekou, J.; Musoke, G.H.; Muyinda, R.; Matama, K.; Mujinga, R.; et al. An analysis of heavy metals contamination and estimating the daily intakes of vegetables in Uganda. Toxicol. Res. Appl. 2021, 5, 1–15. [Google Scholar] [CrossRef]
- Eagles-Smith, C.A.; Wiener, J.G.; Eckley, C.S.; Willacker, J.J.; Evers, D.C.; Marvin-DiPasquale, M.; Obrist, D.; Fleck, J.A.; Aiken, J.M.; Lepak, J.M. Mercury in western North America: A synthesis of environmental contamination, fluxes, bioaccummulation, and risk to fish and wildlife. Sci. Total Environ. 2016, 568, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Yorifuji, T.; Tsuda, T.; Inoue, S.; Takao, S.; Harada, M. Long-term exposure to methylmercury and psychiatric symptoms in residents of Minamata. Environ. Int. 2011, 37, 907–913. [Google Scholar] [CrossRef]
- Clarkson, T.W.; Strain, J.J. Methylmercury: Loaves versus fishes. Reprod. Neurotoxicol. 2020, 81, 282–287. [Google Scholar] [CrossRef]
- Bridges, C.C.; Zalups, R.K. Mechanisms involved in the transport of mercuric ions in target tissues. Arch. Toxicol. 2017, 91, 63–81. [Google Scholar] [CrossRef]
- Ralston, N.V.C.; Raymond, L.J. Mercury’s neurotoxicity is characterized by its disruption of selenium biochemistry. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2405–2416. [Google Scholar] [CrossRef]
- James, A.K.; Dolgova, N.V.; Nehzati, S.; Korbas, M.; Cotelesage, J.J.H.; Sokaras, D.; Kroll, T.; O’Donoghue, J.L.; Watson, G.E.; Myers, G.J.; et al. Molecular fates of organometallic mercury in human brain. ACS Chem. Neurosci. 2022, 13, 1756–1768. [Google Scholar] [CrossRef]
- Bridle, T.G.; Kumarathasan, P.; Gailer, J. Toxic metal species and ‘endogenous’ metalloproteins at the blood-organ interface: Analytical and bioinorganic aspects. Molecules 2021, 26, 3408. [Google Scholar] [CrossRef]
- Zalups, R.K. Molecular interactions with mercury in the kidney. Pharmacol. Rev. 2000, 52, 113–143. [Google Scholar]
- Hazelhoff, M.H.; Torres, A.M. Gender differences in mercury-induced hepatotoxicity: Potential mechanisms. Chemosphere 2018, 202, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Ajsuvakova, O.P.; Tinkov, A.A.; Aschner, M.; Rocha, J.B.T.; Michalke, B.; Skalnaya, M.G.; Skalny, A.V.; Butnariu, M.; Dadar, M.; Sarac, I.; et al. Sulfhydryl groups as targets of mercury toxicity. Coord. Chem. Rev. 2020, 417, 213343. [Google Scholar] [CrossRef] [PubMed]
- Bridle, T.G.; Doroudian, M.; White, W.; Gailer, J. Physiologically relevant hCys concentrations mobilize MeHg from rabbit serum albumin to form MeHg-hCys complexes. Metallomics 2022, 14, mfac010. [Google Scholar] [CrossRef]
- Gibson, M.A.; Sarpong-Kumankomah, S.; Nehzati, S.; George, G.N.; Gailer, J. Remarkable differences in the biochemical fate of Cd2+, Hg2+, CH3Hg+ and thimerosal in red blood cell lysate. Metallomics 2017, 9, 1060–1072. [Google Scholar] [CrossRef]
- Rabenstein, D.L.; Isab, A.A. A proton nuclear magnetic resonance study of the interaction of mercury with intact human erythrocytes. Biochim. Biophys. Acta 1982, 721, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Rabenstein, D.L.; Isab, A.A.; Reid, R.S. A proton nuclear magnetic resonance study of the binding of methylmercury in human erythrocytes. Biochim. Biophys. Acta 1982, 696, 53–64. [Google Scholar] [CrossRef]
- Ballatori, N.; Clarkson, T.W. Inorganic mercury secretion into bile as a low molecular weight complex. Biochem. Pharmacol. 1984, 33, 1087–1092. [Google Scholar] [CrossRef]
- Dutczak, W.J.; Ballatori, N. Transport of the glutathione-methylmercury complex across liver canalicular membranes on reduced glutathione carriers. J. Biol. Chem. 1994, 269, 9746–9751. [Google Scholar] [CrossRef]
- Bjorklund, G.; Mutter, J.; Aaseth, J. Metal chelators and neurotoxicity: Lead, mercury and arsenic. Arch. Toxicol. 2017, 91, 3787–3797. [Google Scholar] [CrossRef]
- Aposhian, H.V.; Bruce, D.C.; Alter, W.; Dart, R.C.; Hurlbut, K.M.; Aposhian, M.M. Urinary mercury after administration of 2,3-dimercaptopropane-1sulfonic acid: Correlation with dental amalgam score. FASEB J. 1992, 6, 2472–2476. [Google Scholar] [CrossRef][Green Version]
- Bjorklund, G.; Crisponi, G.; Nurchi, V.M.; Cappai, R.; Djordjevic, A.B.; Aaseth, J. A review on coordination properties of thiol-containing chjelating agents towards mercury, cadmium, and lead. Molecules 2019, 24, 3247. [Google Scholar] [CrossRef] [PubMed]
- Rooney, J.P.K. The role of thiols, dithiols, nutritional factors and interacting ligands in the toxicology of mercury. Toxicology 2007, 234, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, G.F.; Megson, I.L. Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014, 141, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.S.; Jain, K.; Panghal, A.; Patwa, J. Chemistry, pharmacology, and toxicology of monoisoamyldimercaptosuccinic aicd: A chelating agent for chronic metal poisoning. Chem. Res. Toxicol. 2022, 35, 10. [Google Scholar] [CrossRef]
- Ballatori, N.; Lieberman, M.W.; Wang, W. N-acetylcysteine as an antidote in methylmercury poisoning. Environ. Health Perspect. 1998, 106, 267–271. [Google Scholar] [CrossRef]
- Koh, A.S.; Simmons-Willis, T.A.; Pritchard, J.B.; Grassl, S.M.; Ballatori, N. Identification of a mechanism by which the methylmercury antidotes N-acetylcysteine and dimercaptopropanesulfonate enhance urinary metal excretion: Transport by the renal organic anion transporter-1. Mol. Pharmacol. 2002, 62, 921–926. [Google Scholar] [CrossRef]
- Meers, J.D.; Jahromi, E.Z.; Heyne, B.; Gailer, J. Improved RP-HPLC separation of Hg2+ and CH3Hg+ using a mixture of thiol-based mobile phase additives. J. Environ. Sci. Health 2012, 47, 149–154. [Google Scholar] [CrossRef]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The chemistry and biological activities of M-acetylcysteine. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 4117–4129. [Google Scholar]
- Ballatori, N.; Clarkson, T.W. Dependence of biliary secretion of inorganic mercury on the biliary transport of glutathione. Biochem. Pharmacol. 1984, 33, 1093–1098. [Google Scholar] [CrossRef]
- Pei, K.L.; Sooriyaarachchi, M.; Sherrell, D.A.; George, G.N.; Gailer, J. Probing the coordination behavior of Hg2+, CH3Hg+, and Cd2+ towards mixtures of two biological thiols by HPLC-ICP-AES. J. Inorg. Biochem. 2011, 105, 375–381. [Google Scholar]
- Madejczyk, M.S.; Aremu, D.A.; Simmons-Willis, T.A.; Clarkson, T.W.; Ballatori, N. Accelerated urinary excretion of methylmercury following administration of its antidote N-acetylcysteine requires Mrp2/Abcc2, the apical multidrug resistance-associated protein. J. Pharmacol. Exp. Ther. 2007, 322, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Zalups, R.K.; Ahmad, S. Transport of N-acetylcysteine S-conjugates of methylmercury in Madin-Darby canine kidney cells stably transfected with human isoform of organic anion transporter 1. J. Pharmacol. Exp. Ther. 2005, 314, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Bryan, S.E.; Lambert, C.; Hardy, K.J.; Simons, S. Intranuclear localization of mercury in vivo. Science 1974, 186, 832–833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, D.; Guo, X.; Qian, X.; Lu, Z.; Xu, Q.; TYang, Y.; Duan, L.; He, Y.; Feng, Z. Visible study of mercuric ion and its conjugate in living cells of mammals and plants. Chem. Res. Toxicol. 2005, 18, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Cheesman, B.V.; Arnold, A.P.; Rabenstein, D.L. Nuclear magnetic resonance studies on the solution chemistry of metal complexes. 25. Hg(thiol)3 complexes and Hg(II)-thiol ligand exchange kinetics. J. Am. Chem. Soc. 1988, 110, 6359–6364. [Google Scholar] [CrossRef]
- Ngu-Schwemlein, M.; Lin, X.; Rudd, B.; Bronson, M. Synthesis and ESI mass spectrometric analysis of the association of mercury(II) with multi-cysteinyl-peptides. J. Inorg. Biochem. 2014, 133, 8–23. [Google Scholar] [CrossRef]
- Seneque, O.; Rousselot-Pailley, P.; Pujol, A.; Boturyn, D.; Crouzy, S.; Proux, O.; Manceau, A.; Lebrun, C.; Delangle, P. Mercury trithiolate binding (HgS3) to a de novo designed cyclic decapeptide with three preoriented cysteine side chains. Inorg. Chem. 2018, 57, 2705–2713. [Google Scholar]
- Shoukry, M.M.; Cheesman, B.V.; Rabenstein, D.L. Polarimetric and nuclear magnetic resonance studies of the complexation of mercury by thiols. Can. J. Chem. 1988, 66, 3184–3189. [Google Scholar] [CrossRef]
- Jalilehvand, F.; Parmar, K.; Zielke, S. Mercury(II) complex formation with N-acetylcysteine. Metallomics 2013, 5, 1368–1376. [Google Scholar] [CrossRef]
- Watton, S.P.; Wright, J.G.; MacDonnel, F.M.; Bryson, J.W.; Sabat, M.; O’Halloran, T.V. Trigonal mercuric complex of an aliphatic thiolate: A spectroscopic and structural model for the receptor site in the Hg(II) biosensor MerR. J. Am. Chem. Soc. 1990, 112, 2824–2826. [Google Scholar] [CrossRef]
- Mah, V.; Jalilehvand, F. Glutathione complex formation with mercury(II) in aqueous solution at physiological pH. Chem. Res. Toxicol. 2010, 23, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- Stary, J.; Kratzer, K. Radiometric determination of stability constants of mercury species complexes with L-cysteine. J. Radioanal. Nucl. Chem. Lett. 1988, 126, 69–75. [Google Scholar] [CrossRef]
- Bryan, S.E. Heavy metals in the cell’s nucleus. In Metal Ions in Genetic Information Transfer; Eichhorn, G.L., Marzilli, L.G., Eds.; Elsevier: Amsterdam, The Netherlands, 1981; pp. 87–101. [Google Scholar]
- Francis, Y.M.; Karunakaran, B.; Ashfaq, F.; Qattan, M.Y.; Ahmad, I.; Alkhathami, A.G.; Khan, M.I.; Vraradhan, M.; Govindan, L.; Kasirajan, S.P. Mercuric chloride induced nephrotoxicity: Ameliorative effect of Carica papaya leaves confirmed by histopathology, immunohistochemistry, and gene expression studies. ACS Omega 2023, 8, 21696–21708. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, G. Drug transport by organic anion transporters (OATs). Pharmacol. Ther. 2012, 136, 106–130. [Google Scholar] [PubMed]
- Girardi, G.; Elias, M.M. Effectiveness of N-acetylcysteine in protecting against mercuric chloride-induced nephrotoxicity. Toxicology 1991, 67, 155–164. [Google Scholar] [CrossRef]
- Brandao, R.; Santos, F.W.; Zeni, G.; Rocha, J.B.T.; Nogueira, C.W. DMPS and N-acetylcysteine induced renal toxicity in mice exposed to mercury. Biometals 2006, 19, 389–398. [Google Scholar] [CrossRef]
- Kaplan, M.; Atakan, I.; Aydogdu, N.; Aktoz, T.; Oezpuyan, F.; Seren, G.; Tokuc, B.; Inci, O. Influence of N-acetylcysteine on renal toxicity of cadmium in rats. Pediatr. Nephrol. 2008, 23, 233–241. [Google Scholar] [CrossRef]
- Abdelrahman, A.; Al Salam, A.; Almahrugi, A.A.; Alhusseni, I.S.; Mansour, M.A.; Ali, B.H. N-acetylcysteine improves renal hemodynamics in rats with cisplatin-induced nephrotoxicity. J. Appl. Toxicol. 2010, 30, 15–21. [Google Scholar] [CrossRef]
- Dickey, D.T.; Muldoon, L.L.; Kraemer, D.F.; Neuwelt, E.A. Protection against cisplatin-induced ototoxicity by N-acetylcysteine in a rat model. Heart Res. 2004, 193, 25–30. [Google Scholar] [CrossRef]
- Doroudian, M.; Gailer, J. Integrative metallomics studies of toxic metal(loid) substances at the blood plasma-red blood cell-organ/tumor nexus. Inorganics 2022, 10, 200. [Google Scholar] [CrossRef]
- Marini, H.R.; Micali, A.; Squadrito, G.; Puzzolo, D.; Freni, J.; Antonuccio, P.; Minutoli, L. Nutraceuticals: A new challenge against cadmium-induced testicular injury. Nutrients 2022, 14, 663. [Google Scholar] [CrossRef] [PubMed]
- Pei, K.L.; Gailer, J. Probing the interaction of arsenobetaine with blood plasma constituents in vitro: An SEC-ICP-AES study. Metallomics 2009, 1, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Leslie, E.M.; Deely, R.G.; Cole, S.P.C. Toxicological relevance of the multidrug resistance protein 1, MRP1 (ABCC1) and related transporters. Toxicology 2001, 167, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Leslie, E.M. Arsenic-glutathione conjugate transport by the human multidrug resistance proteins (MRPs/ABCCs). J. Inorg. Biochem. 2012, 2012, 141–149. [Google Scholar]
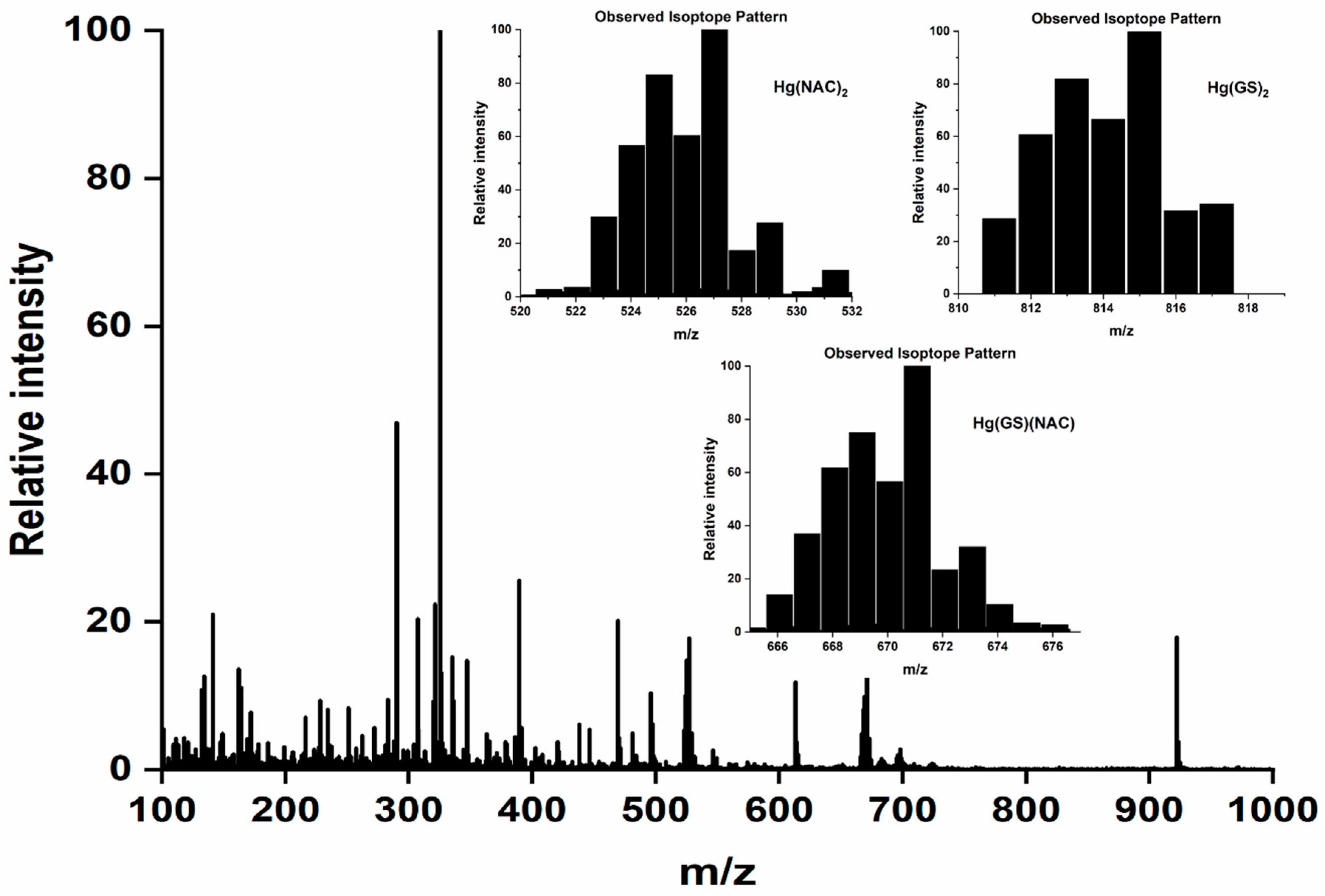
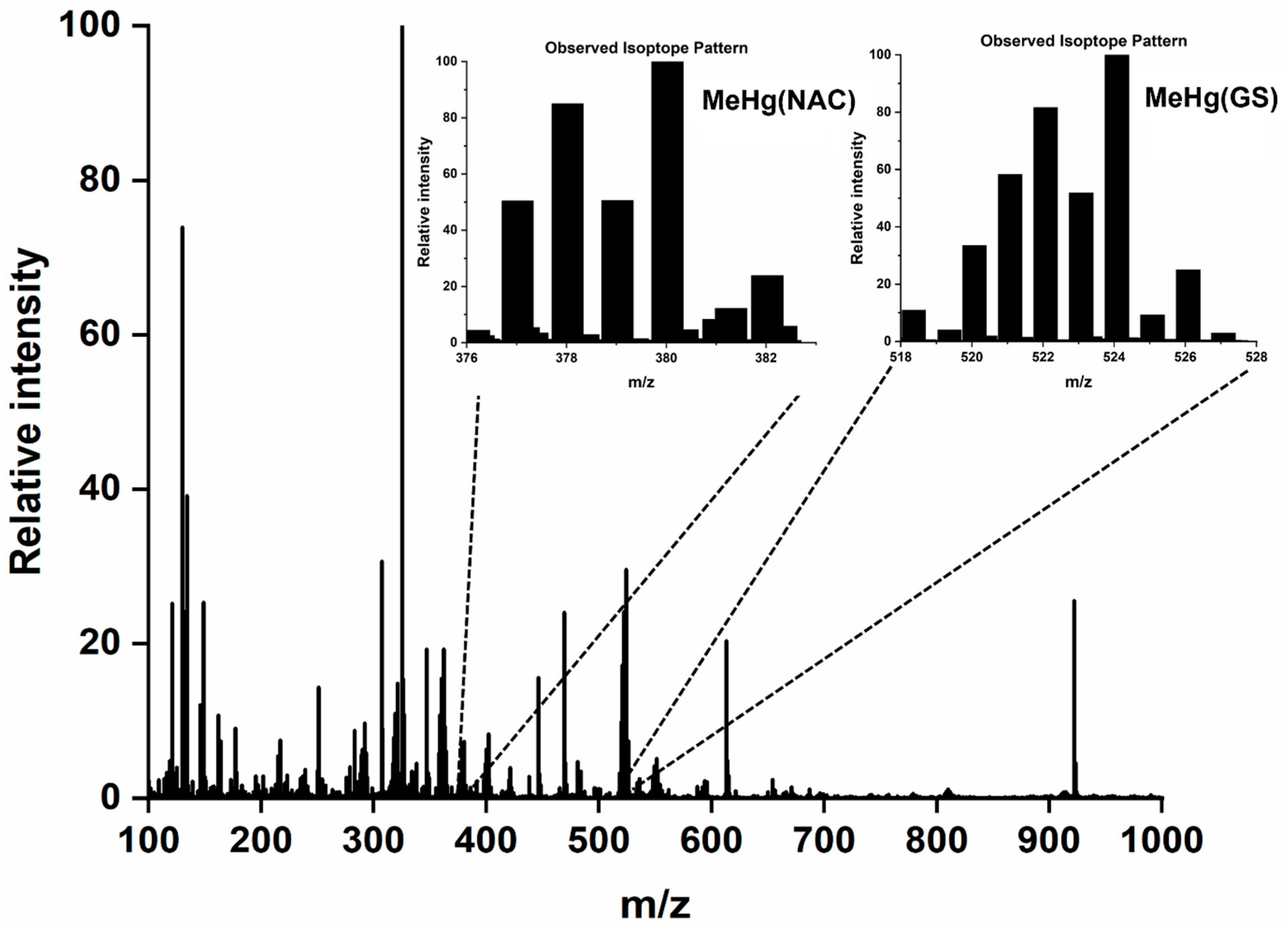
| Mobile Phase Composition | Identified Hg Species/Formula | Theoretical Mass of the Most Abundant Isotope (Hg202) Da | Experimental Mass of the Most Abundant Isotope (Hg202) Da | ΔMtheor-exp Da |
|---|---|---|---|---|
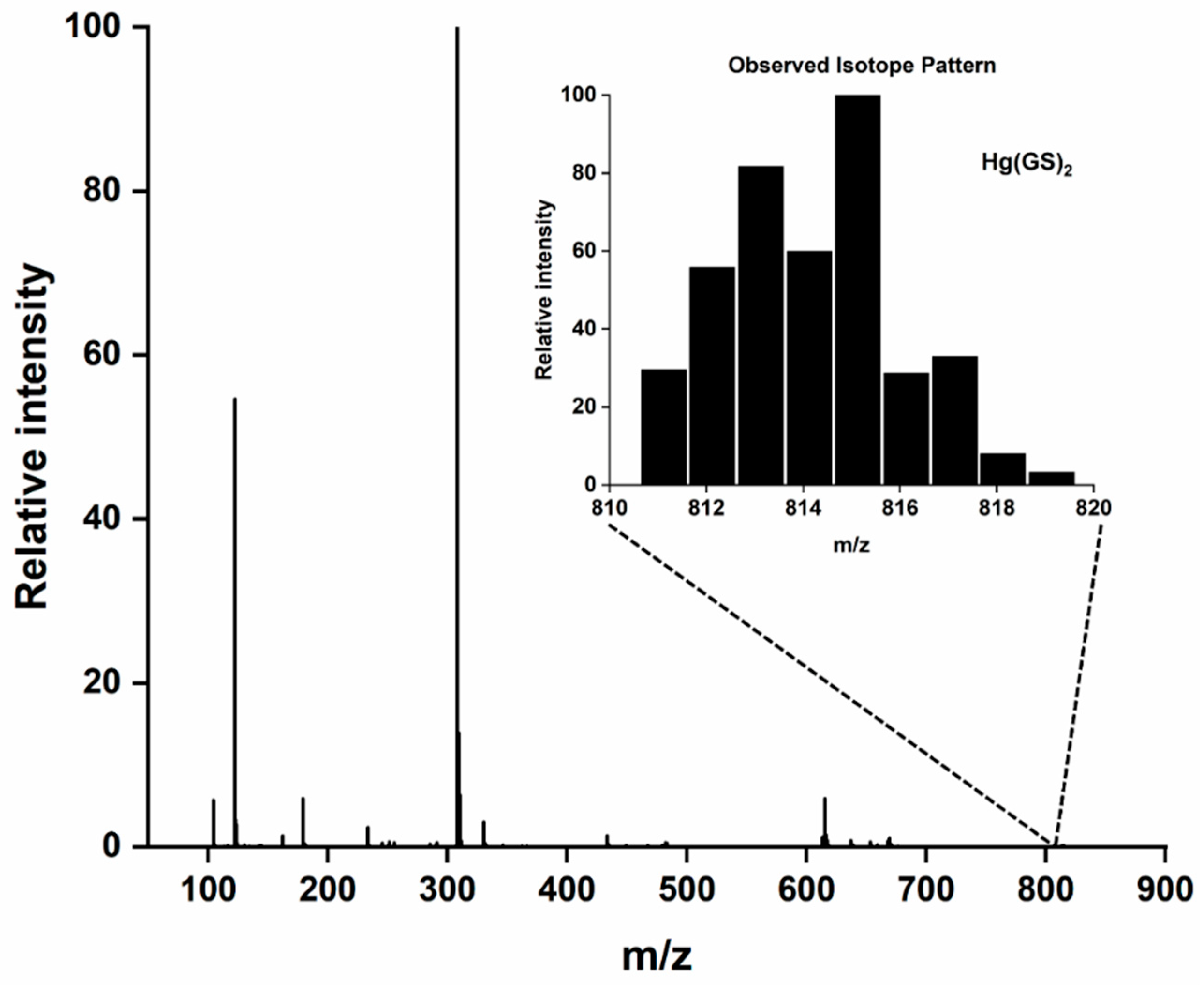
| 5.0 mM GSH | Hg(GS)2 C20H32N6O12S2 | 815.1299 | 815.1290 | 0.0009 |
| 5.0 mM GSH and 5.0 mM NAC | Hg(NAC)2 C10H16N2O6S2 | 527.0229 | 527.0226 | 0.003 |
| Hg(GS)2 C20H32N6O12S2 | 815.1299 | 815.1318 | −0.0001 | |
| Hg(NAC)(GS) C15H24N4O9S2 | 671.0764 | 671.0750 | 0.0014 | |
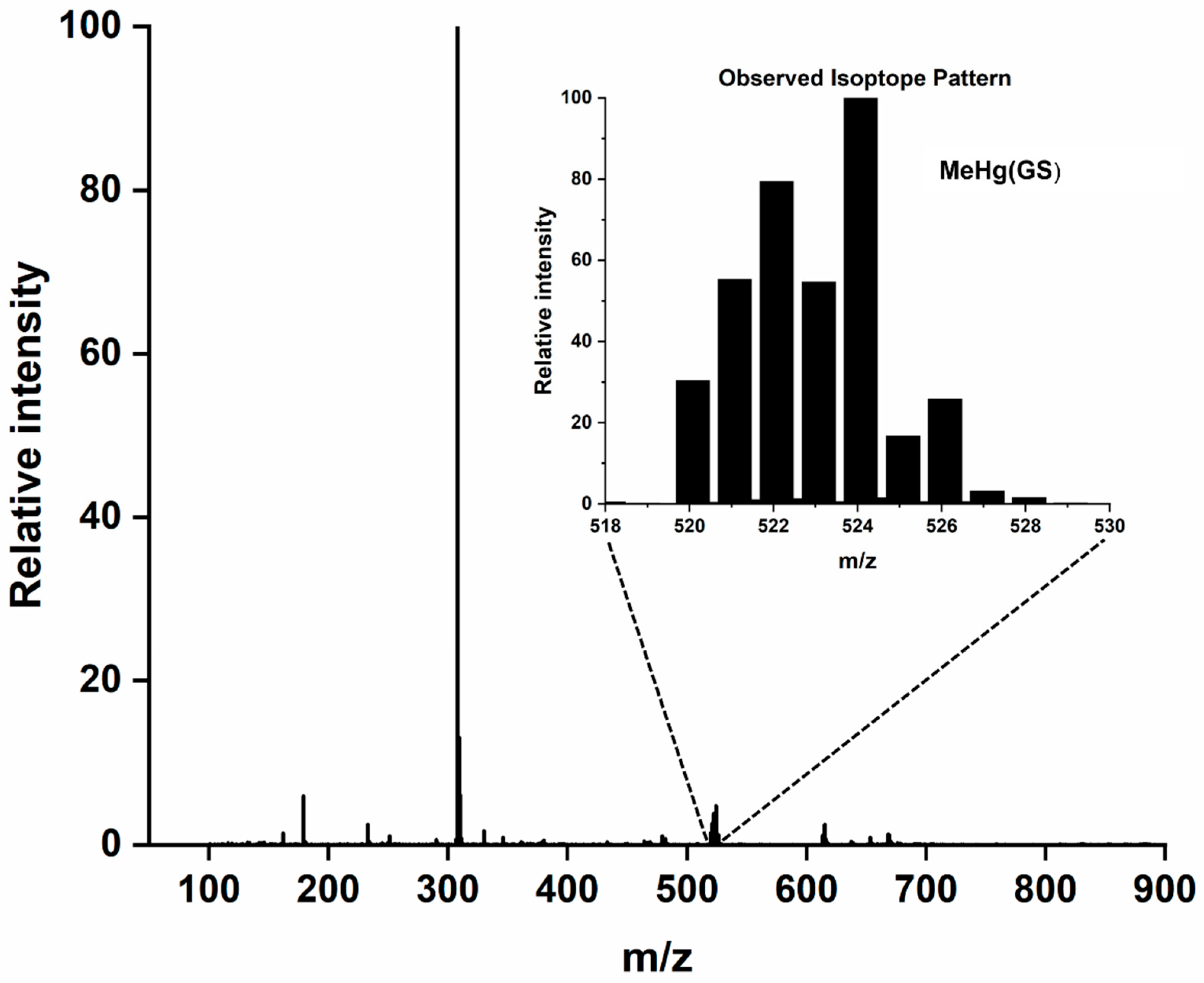
| 5.0 mM GSH | MeHg(GS) C11H19N3O6S1 | 524.0774 | 524.0774 | 0.0005 |
| 5.0 mM GSH and 5.0 mM NAC | MeHg(GS) C11H19N3O6S1 | 524.0774 | 524.0759 | 0.0015 |
| MeHg(NAC) C6H11N1O3S1 | 380.0239 | 380.0227 | 0.0012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doroudian, M.; Thibault, M.E.; Gailer, J. N-Acetylcysteine Displaces Glutathionyl-Moieties from Hg2+ and MeHg+ to Form More Hydrophobic Complexes at Near-Physiological Conditions. Molecules 2023, 28, 6762. https://doi.org/10.3390/molecules28196762
Doroudian M, Thibault ME, Gailer J. N-Acetylcysteine Displaces Glutathionyl-Moieties from Hg2+ and MeHg+ to Form More Hydrophobic Complexes at Near-Physiological Conditions. Molecules. 2023; 28(19):6762. https://doi.org/10.3390/molecules28196762
Chicago/Turabian StyleDoroudian, Maryam, Michelle E. Thibault, and Jürgen Gailer. 2023. "N-Acetylcysteine Displaces Glutathionyl-Moieties from Hg2+ and MeHg+ to Form More Hydrophobic Complexes at Near-Physiological Conditions" Molecules 28, no. 19: 6762. https://doi.org/10.3390/molecules28196762
APA StyleDoroudian, M., Thibault, M. E., & Gailer, J. (2023). N-Acetylcysteine Displaces Glutathionyl-Moieties from Hg2+ and MeHg+ to Form More Hydrophobic Complexes at Near-Physiological Conditions. Molecules, 28(19), 6762. https://doi.org/10.3390/molecules28196762