2-Pyridinecarboxaldehyde-Modified Chitosan–Silver Complexes: Optimized Preparation, Characterization, and Antibacterial Activity
Abstract
:1. Introduction
2. Results and Discussion
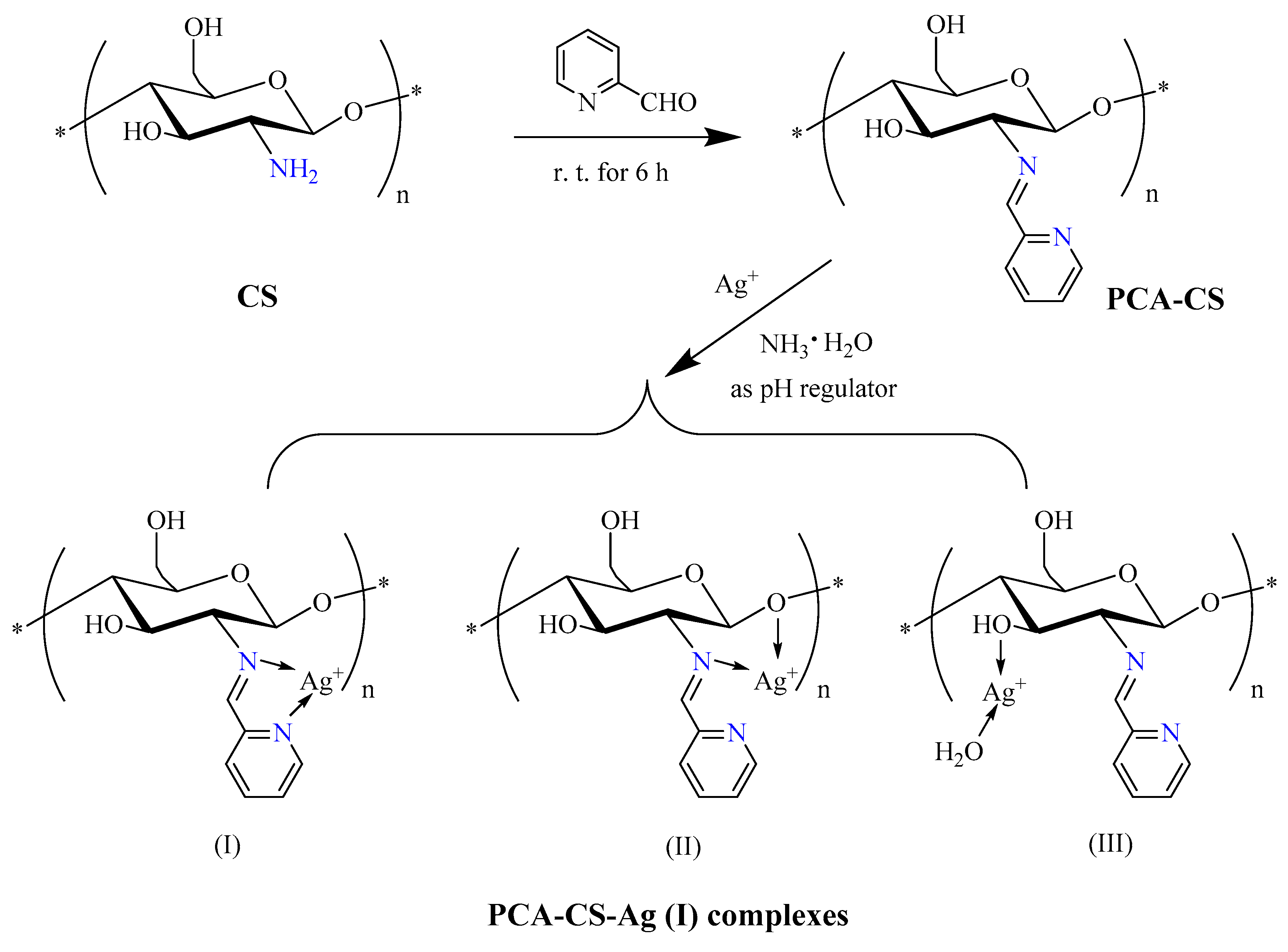
2.1. Preparation of PCA-CS-Ag Complex
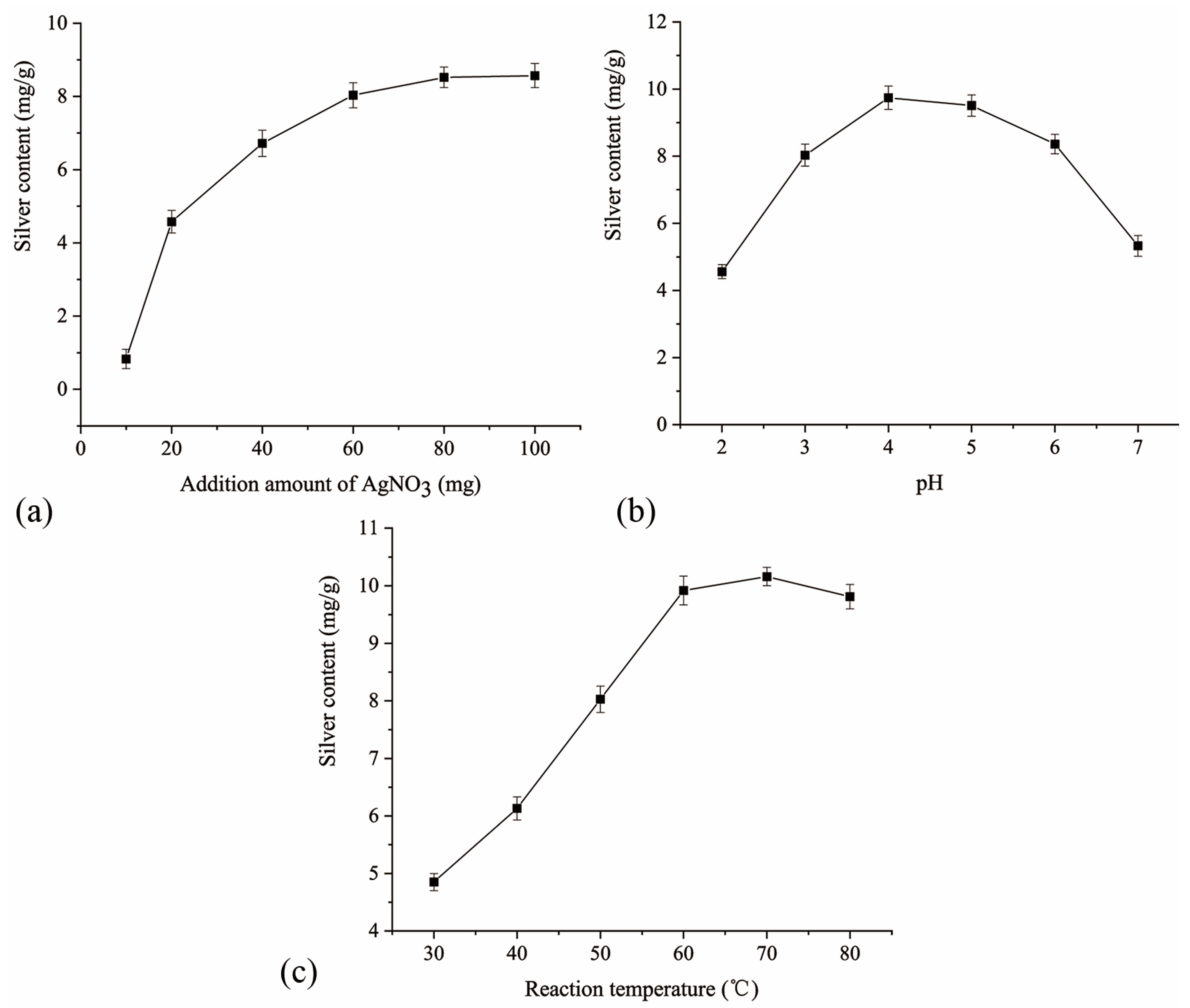
2.2. Single-Factor Experiment Results and Analysis
2.2.1. Effect of the Additional Amount of Silver Nitrate on the Silver Content
2.2.2. Effect of the Solution pH on the Silver Content
2.2.3. Effect of the Reaction Temperature on the Silver Content
2.3. Optimization Preparation of PCA-CS-Ag Complex
2.3.1. Experimental Design and Results
2.3.2. Regression Modeling and Analysis of Variance (ANOVA)
2.3.3. Optimization Validation
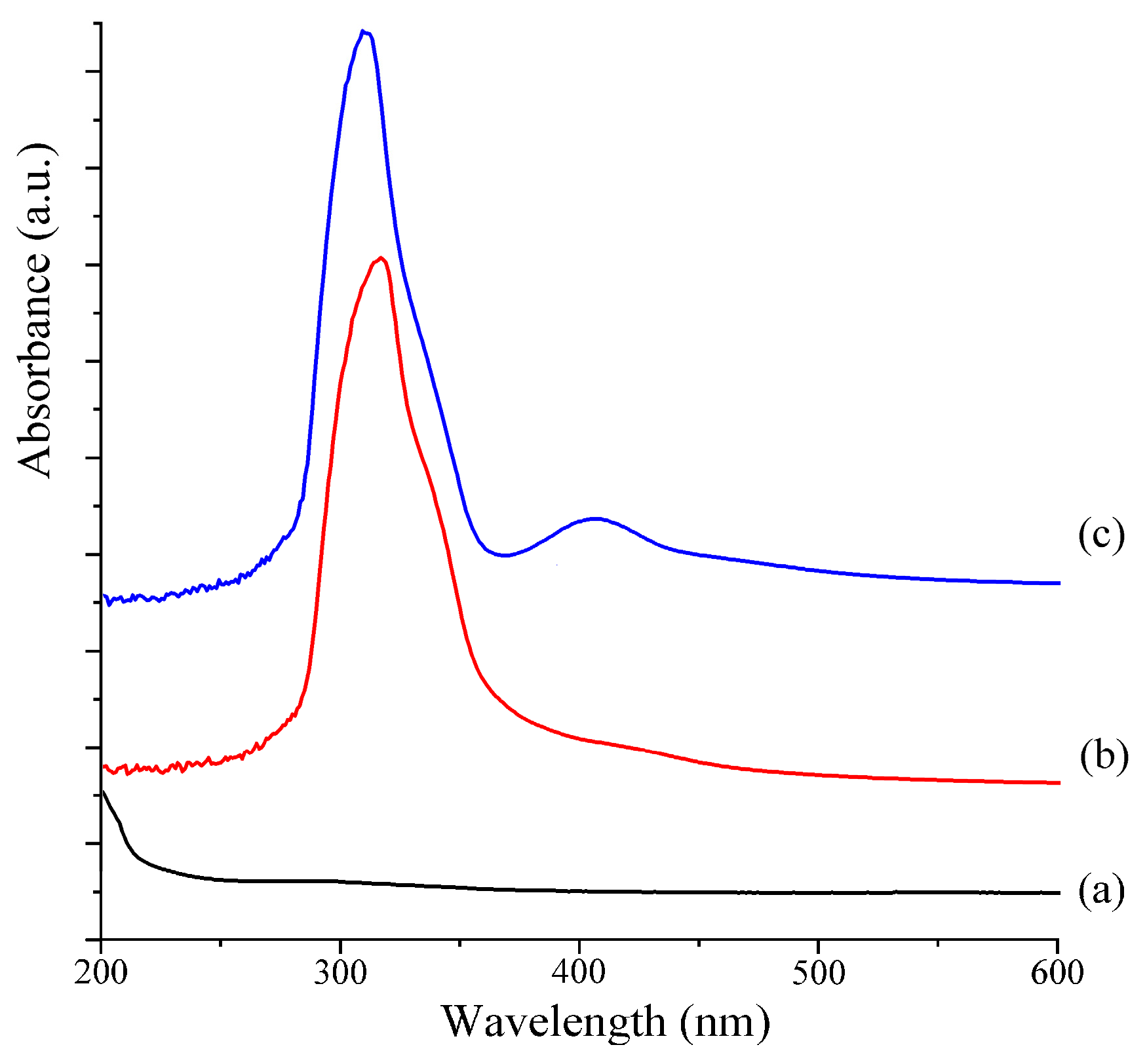
2.4. UV–Vis Analysis
2.5. FT-IR Analysis
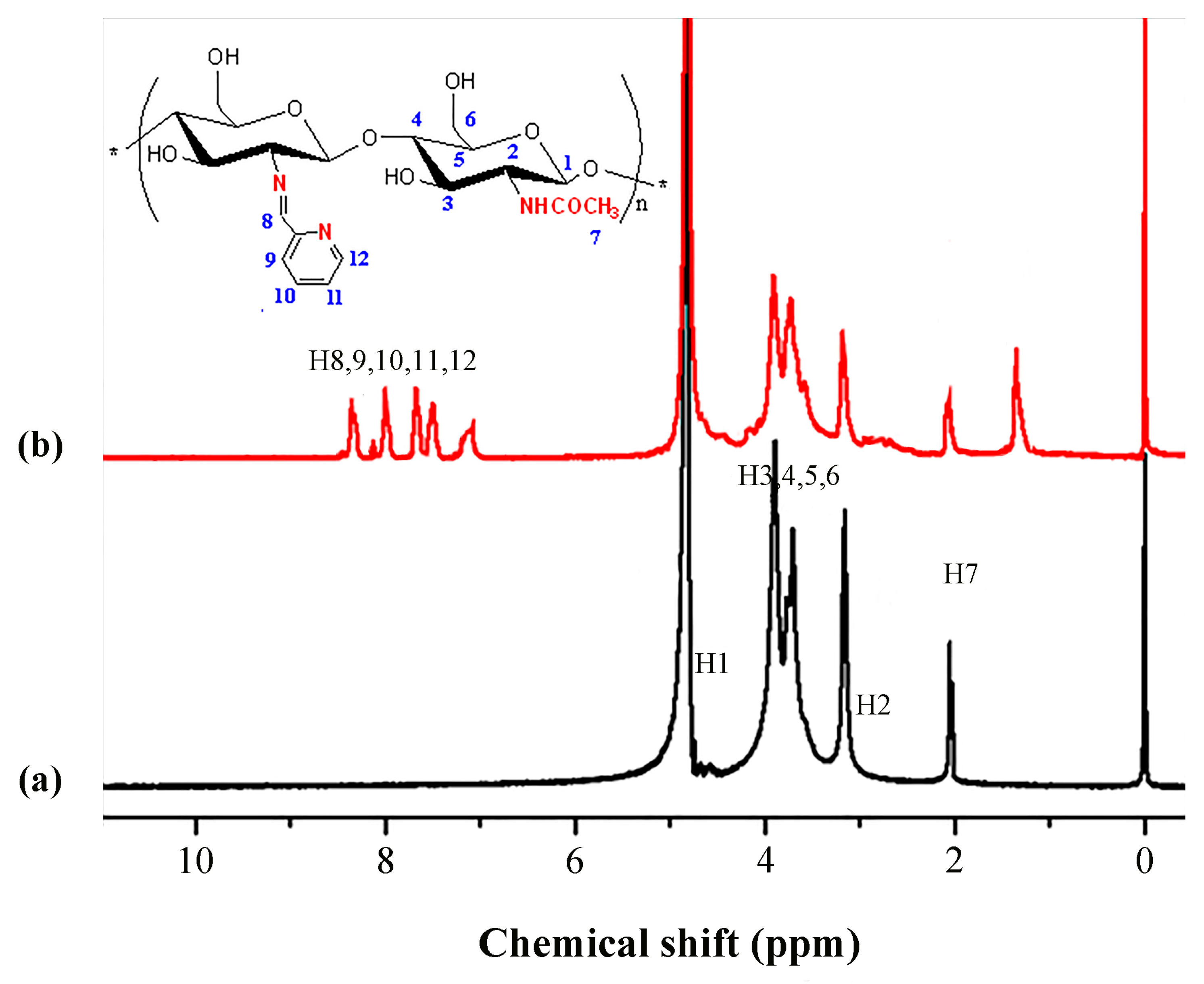
2.6. 1H NMR Analysis
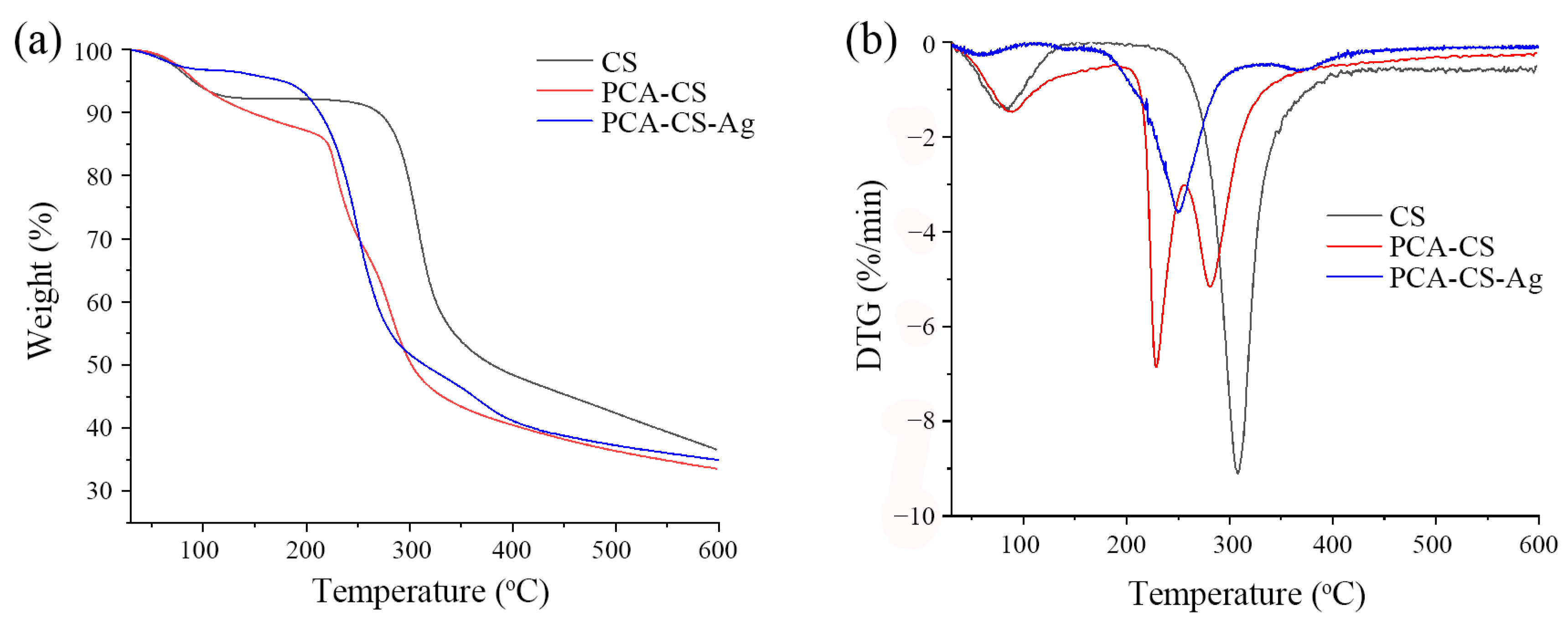
2.7. TG Analysis
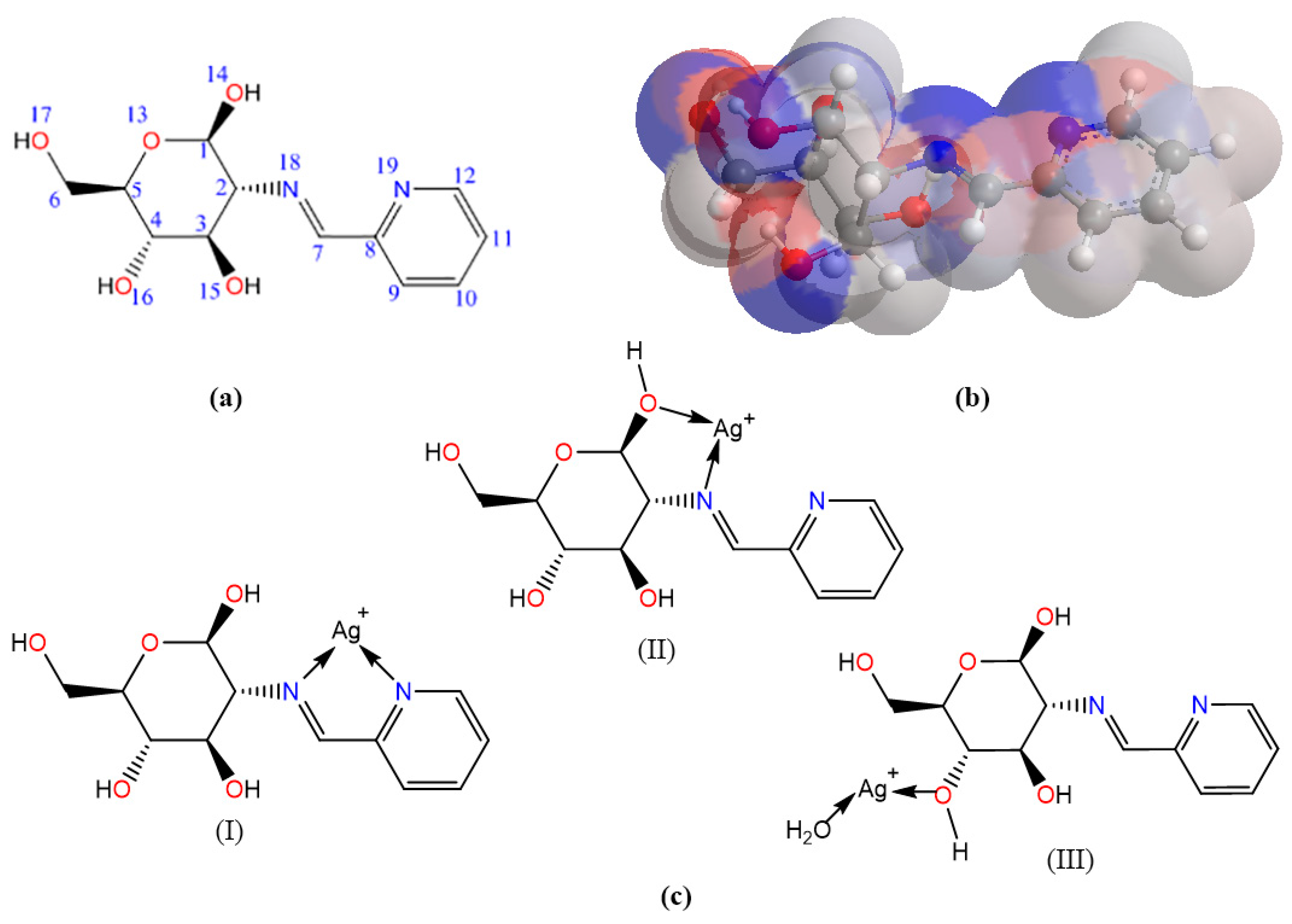
2.8. Molecular Computation Study
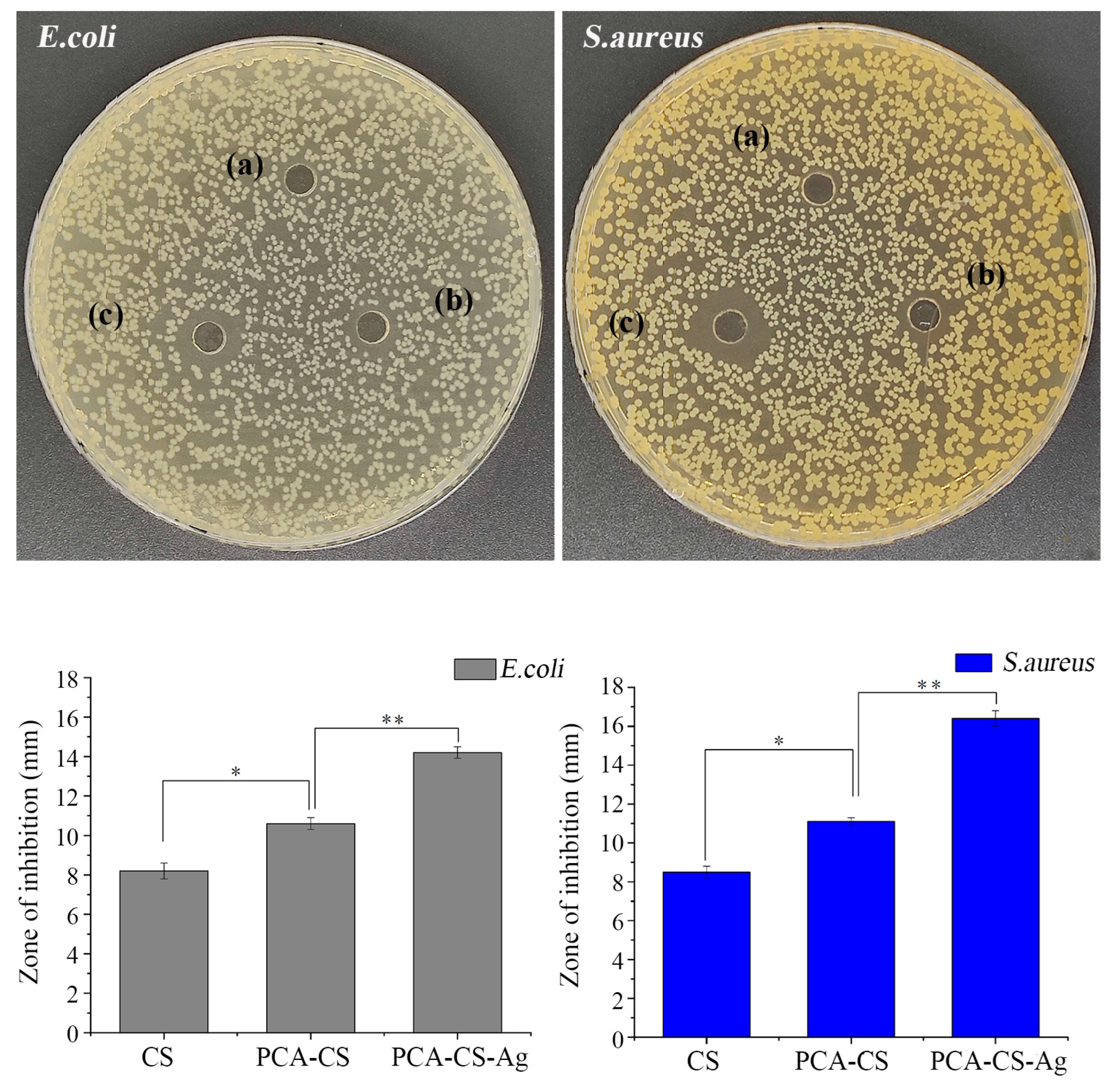
2.9. Antibacterial Activity
3. Materials and Methods
3.1. Materials
3.2. Synthesis of 2-Pyridinecarboxaldehyde-Modified Chitosan (PCA-CS)
3.3. Preparation of PCA-CS-Ag Complex
3.4. Investigation of the Influence of the Preparation Conditions
3.5. Box–Behnken Design
3.6. Ultraviolet–Visible (UV–Vis) Spectroscopy
3.7. Fourier-Transform Infrared (FT-IR) Spectroscopy
3.8. Proton Nuclear Magnetic Resonance (1H NMR) Spectroscopy
3.9. Thermogravimetric (TG) Analysis
3.10. Molecular Computation Study
3.11. Antibacterial Activity Determination
3.11.1. Zone of Inhibition (ZOI) Assay
3.11.2. Determination of Antibacterial Rate with Optical Density Method
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hu, F.; Xia, S.S.; He, Y.; Huang, Z.L.; Ke, H.; Liao, J.Z. Reactive organic radical-doped Ag(I)-based coordination compounds for highly efficient antibacterial wound therapy. Colloids Surf. B 2022, 213, 112425. [Google Scholar] [CrossRef] [PubMed]
- Junaid, M.; Thirapanmethee, K.; Khuntayaporn, P.; Chomnawang, M.T. CRISPR-based gene editing in acinetobacter baumannii to combat antimicrobial resistance. Pharmaceuticals 2023, 16, 920. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Liu, Y.; Zhu, X.; Chen, X.; Liu, J.; Zhou, Y.; Qin, X.; Liu, J. Bacteriaresponsive biomimetic selenium nanosystem for multidrug-resistant bacterial infection detection and inhibition. ACS Nano 2019, 13, 13965–13984. [Google Scholar] [CrossRef] [PubMed]
- Politano, A.D.; Campbell, K.T.; Rosenberger, L.H.; Sawyer, R.G. Use of silver in the prevention and treatment of infections: Silver review. Surg. Infect. 2013, 14, 8–20. [Google Scholar] [CrossRef]
- Liang, X.; Luan, S.; Yin, Z.; He, M.; He, C.; Yin, L.; Zou, Y.; Yuan, Z.; Li, L.; Song, X.; et al. Recent advances in the medical use of silver complex. Eur. J. Med. Chem. 2018, 157, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Ulu, Ö.D.; Kuruçay, A.; Ateş, B.; Özdemir, İ. Synthesis, characterization, in vitro antibacterial, and anticancer studies of Ag(I)-N-heterocyclic carbene (NHC) complexes. Chem. Pap. 2023, 77, 423–435. [Google Scholar] [CrossRef]
- Chakraborty, I.; Pinto, M.; Stenger-Smith, J.; Martinez-Gonzalez, J.; Mascharak, P.K. Synthesis, structures and antibacterial properties of Cu(II) and Ag(I) complexes derived from 2,6-bis(benzothiazole)-pyridine. Polyhedron 2019, 172, 1–7. [Google Scholar] [CrossRef]
- Ibrahim, F.M.; El-Hawary, Y.M.; Butler, I.S.; Mostafa, S.I. Bone repair stimulation in rat mandible by new chitosan silver(I) complexes. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 846–858. [Google Scholar] [CrossRef]
- Leone, G.; Pepi, S.; Consumi, M.; Mahdizadeh, F.F.; Lamponi, S.; Magnani, A. Phosphorylated xanthan gum-Ag(I) complex as antibacterial viscosity enhancer for eye drops formulation. Carbohydr. Polym. 2021, 267, 118196. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Dong, Q.; Xu, C.; Deng, S.; Kang, Y.; Fan, M.; Li, L. Application of functionalized chitosan in food. Int. J. Biol. Macromol. 2023, 235, 123716. [Google Scholar] [CrossRef]
- Fan, P.; Zeng, Y.; Zaldivar-Silva, D.; Agüero, L.; Wang, S. Chitosan-based hemostatic hydrogels: The concept, mechanism, application, and prospects. Molecules 2023, 28, 1473. [Google Scholar] [CrossRef]
- Riaz Rajoka, M.S.; Mehwish, H.M.; Wu, Y.; Zhao, L.; Arfat, Y.; Majeed, K.; Anwaar, S. Chitin/chitosan derivatives and their interactions with microorganisms: A comprehensive review and future perspectives. Crit. Rev. Biotechnol. 2020, 40, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Panahi, H.K.S.; Dehhaghi, M.; Amiri, H.; Guillemin, G.J.; Gupta, V.K.; Rajaei, A.; Yang, Y.; Peng, W.; Pan, J.; Aghbashlo, M.; et al. Current and emerging applications of saccharide-modified chitosan: A critical review. Biotechnol. Adv. 2023, 66, 108172. [Google Scholar]
- Chen, L.; Tang, J.; Wu, S.; Wang, S.; Ren, Z. Selective removal of Au(III) from wastewater by pyridine-modified chitosan. Carbohydr. Polym. 2022, 286, 119307. [Google Scholar] [CrossRef] [PubMed]
- Piegat, A.; Żywicka, A.; Niemczyk, A.; Goszczyńska, A. Antibacterial activity of N,O-acylated chitosan derivative. Polymers 2020, 13, 107. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Yang, X.; Zhang, W.; Jiang, M.; Wen, T.; Wang, J.; Guo, R.; Liu, H. Preparation and application of quaternized chitosan- and AgNPs-base synergistic antibacterial hydrogel for burn wound healing. Molecules 2021, 26, 4037. [Google Scholar] [CrossRef]
- Drozd, N.; Lunkov, A.; Shagdarova, B.; Il’ina, A.; Varlamov, V. New N-methylimidazole-functionalized chitosan derivatives: Hemocompatibility and antibacterial properties. Biomimetics 2023, 8, 302. [Google Scholar] [CrossRef]
- Guo, W.L.; Shi, F.F.; Li, L.; Xu, J.X.; Chen, M.; Wu, L.; Hong, J.L.; Qian, M.; Bai, W.D.; Liu, B.; et al. Preparation of a novel Grifola frondosa polysaccharide-chromium (III) complex and its hypoglycemic and hypolipidemic activities in high fat diet and streptozotocin-induced diabetic mice. Int. J. Biol. Macromol. 2019, 131, 81–88. [Google Scholar] [CrossRef]
- Adewuyi, S.; Kareem, K.T.; Atayese, A.O.; Amolegbe, S.A.; Akinremi, C.A. Chitosan-cobalt(II) and nickel(II) chelates as antibacterial agents. Int. J. Biol. Macromol. 2011, 48, 301–303. [Google Scholar] [CrossRef]
- Chi, Y.; Li, Y.; Zhang, G.; Gao, Y.; Ye, H.; Gao, J.; Wang, P. Effect of extraction techniques on properties of polysaccharides from Enteromorpha prolifera and their applicability in iron chelation. Carbohydr. Polym. 2018, 181, 616–623. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Z.; Pan, Y.; Gao, X.; Chen, H. Anti-diabetic effects of Inonotus obliquus polysaccharides-chromium (III) complex in type 2 diabetic mice and its sub-acute toxicity evaluation in normal mice. Food Chem. Toxicol. 2017, 108, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zhang, H.; Wang, Z.; Liu, S.; Regenstein, J.M. Response surface methodology for the synthesis of an Auricularia auriculajudae polysaccharides-CDDP complex. Int. J. Biol. Macromol. 2016, 93, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Addo, P.W.; Sagili, S.U.K.R.; Bilodeau, S.E.; Gladu-Gallant, F.A.; MacKenzie, D.A.; Bates, J.; McRae, G.; MacPherson, S.; Paris, M.; Raghavan, V.; et al. Microwave- and ultrasound-assisted extraction of Cannabinoids and Terpenes from Cannabis using response surface methodology. Molecules 2022, 27, 8803. [Google Scholar] [CrossRef] [PubMed]
- Alarfaj, N.A.; Altamimi, S.A.; El-Tohamy, M.F.; Almahri, A.M. Exploitation of localized surface plasmon resonance of silver/gold nanoparticles for the fluorescence quantification of angiotensin II receptor antagonists in their tablets and bio-samples. New J. Chem. 2019, 43, 492–503. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Q.; Yang, H.; Shi, H.; Dong, A.; Wang, L.; Yu, S. Progress in infrared spectroscopy as an efficient tool for predicting protein secondary structure. Int. J. Biol. Macromol. 2022, 206, 175–187. [Google Scholar] [CrossRef]
- Ladan, M.K.; Glavač, N.K. Statistical FT-IR spectroscopy for the characterization of 17 vegetable oils. Molecules 2022, 27, 3190. [Google Scholar] [CrossRef]
- Fan, L.; Zou, S.; Ge, H.; Xiao, Y.; Wen, H.; Feng, H.; Liu, M.; Nie, M. Preparation and characterization of hydroxypropyl chitosan modified with collagen peptide. Int. J. Biol. Macromol. 2016, 93, 636–643. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, J.; Mi, Y.; Li, Q.; Guo, Z. Synthesis and characterization of α-lipoic acid grafted chitosan derivatives with antioxidant activity. React. Funct. Polym. 2022, 172, 105205. [Google Scholar] [CrossRef]
- Anush, S.M.; Vishalakshi, B.; Kalluraya, B.; Manju, N. Synthesis of pyrazole-based schiff bases of chitosan: Evaluation of antimicrobial activity. Int. J. Biol. Macromol. 2018, 119, 446–452. [Google Scholar] [CrossRef]
- Li, P.; Zhao, J.; Chen, Y.; Cheng, B.; Yu, Z.; Zhao, Y.; Yan, X.; Tong, Z.; Jin, S. Preparation and characterization of chitosan physical hydrogels with enhanced mechanical and antibacterial properties. Carbohydr. Polym. 2017, 157, 1383–1392. [Google Scholar] [CrossRef]
- Shen, J.; Nada, A.A.; Abou-Zeid, N.Y.; Hudson, S.M. Synthesis of chitosan iodoacetamides via carbodiimide coupling reaction: Effect of degree of substitution on the hemostatic properties. Carbohydr. Polym. 2020, 229, 115522. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Dang, Q.; Liu, C.; Zhang, Y.; Wang, Y.; Zhu, W.; Chang, G.; Sun, H.; Cha, D.; Fan, B. Characterization and antibacterial mechanism of poly(aminoethyl) modified chitin synthesized via a facile one-step pathway. Carbohydr. Polym. 2018, 195, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, X.; Zhang, S.; Wang, Z.; Gao, F.; Li, H. Simple and prompt protonation of new dyes containing double conjugated imine bonds to strengthen the protection of copper in aggressive sulfuric acid solution. J. Mol. Liq. 2021, 341, 117402. [Google Scholar] [CrossRef]
- Rangel Rangel, E.; Maya, E.M.; Sánchez, F.; de la Campa, J.G.; Iglesias, M. Palladium-heterogenized porous polyimide materials as effective and recyclable catalysts for reactions in water. Green Chem. 2015, 17, 466–473. [Google Scholar] [CrossRef]
- Nina, M.; Fathana, H.; Iqhrammullah, M. Preparation and characterization of new magnetic chitosan-glycine-PEGDE (Fe3O4/Ch-G-P) beads for aqueous Cd(II) removal. J. Water Process Eng. 2022, 45, 102493. [Google Scholar] [CrossRef]
- Foroughnia, A.; Khalaji, A.D.; Kolvari, E.; Koukabi, N. Synthesis of new chitosan Schiff base and its Fe2O3 nanocomposite: Evaluation of methyl orange removal and antibacterial activity. Int. J. Biol. Macromol. 2021, 177, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, H.F.G.; Cavalheiro, É.T.G. The influence of reaction parameters on complexation of Zn(II) complexes with biopolymeric Schiff bases prepared from chitosan and salicylaldehyde. Int. J. Biol. Macromol. 2019, 121, 1179–1185. [Google Scholar] [CrossRef]
- Ma, F.; Li, P.; Zhang, B.; Wang, Z. The facile synthesis of a chitosan Cu(II) complex by solution plasma process and evaluation of their antioxidant activities. Int. J. Biol. Macromol. 2017, 103, 501–507. [Google Scholar] [CrossRef]
- Mukred, R.A.; Yehya, F.B.; Al-Gabr, H.M.; Al Fakih, M.A. New bioactive Co (II) coordination polymers with morphline and carboxylate ligands; Synthesis, structures, spectroscopic and thermal properties. J. Polym. Res. 2022, 29, 377. [Google Scholar] [CrossRef]
- Adhikari, J.; Bhattarai, A.; Chaudhary, N.K. Synthesis, characterization, physicochemical studies, and antibacterial evaluation of surfactant-based Schiff base transition metal complexes. Chem. Pap. 2022, 76, 2549–2566. [Google Scholar] [CrossRef]
- Higazy, A.; Hashem, M.; ElShafei, A.; Shaker, N.; Hady, M.A. Development of antimicrobial jute packaging using chitosan and chitosan-metal complex. Carbohydr. Polym. 2010, 79, 867–874. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Jiang, S.; Yu, H.J. Study on novel antibacterial polymer materials (I) preparation of zeolite antibacterial agents and antibacterial polymer composite and their antibacterial properties. J. Polym. Mater. 2003, 20, 279–284. [Google Scholar]
- Ardean, C.; Davidescu, C.M.; Nemeş, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Omer, A.M.; Abbas, E.; Baset, W.M.A.; Tamer, T.M. Preparation, physicochemical characterization and antimicrobial activities of novel two phenolic chitosan Schiff base derivatives. Sci. Rep. 2018, 8, 11416. [Google Scholar] [CrossRef] [PubMed]
- Severino, R.; Ferrari, G.; Vu, K.D.; Donsì, F.; Salmieri, S.; Lacroix, M. Antimicrobial effects of modified chitosan based coating containing nanoemulsion of essential oils, modified atmosphere packaging and gamma irradiation against Escherichia coli O157:H7 and Salmonella Typhimurium on green beans. Food Control 2015, 50, 215–222. [Google Scholar] [CrossRef]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, D.; Wu, D.; Cui, Y.; Ren, G.; Wang, Y.; Wang, J.; Peng, C. Chitosan-Based Biomaterial Scaffolds for the Repair of Infected Bone Defects. Front. Bioeng. Biotechnol. 2022, 10, 899760. [Google Scholar] [CrossRef]
- Tan, H.; Ma, R.; Lin, C.; Liu, Z.; Tang, T. Quaternized chitosan as an antimicrobial agent: Antimicrobial activity, mechanism of action and biomedical applications in orthopedics. Int. J. Mol. Sci. 2013, 14, 1854–1869. [Google Scholar] [CrossRef]
- Xu, R.; Aotegen, B.; Zhong, Z. Synthesis, characterization and biological activity of C6-Schiff bases derivatives of chitosan. Int. J. Biol. Macromol. 2017, 105, 1563–1571. [Google Scholar] [CrossRef]
- Du, X.; Jiang, H.; Guo, X.; Chen, L.; Kang, T. Synthesis of ferrocene/chitosan-AgNPs films and application in plasmonic color-switching and antimicrobial materials. React. Funct. Polym. 2021, 169, 105061. [Google Scholar] [CrossRef]
- Zhou, P.; Xia, Z.; Qi, C.; He, M.; Yu, T.; Shi, L. Construction of chitosan/Ag nanocomposite sponges and their properties. Int. J. Biol. Macromol. 2021, 192, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.W.; Lee, J.Y.; Rezk, A.I.; Park, C.H.; Kim, C.S. In-situ cellulose-framework templates mediated monodispersed silver nanoparticles via facile UV-light photocatalytic activity for anti-microbial functionalization. Carbohydr. Polym. 2021, 269, 118255. [Google Scholar] [CrossRef] [PubMed]
- Alharthi, S.S.; Gomathi, T.; John Joseph, J.; Rakshavi, J.; Annie Kamala Florence, J.; Sudha, P.N.; Rajakumar, G.; Thiruvengadam, M. Biological activities of chitosan-salicylaldehyde schiff base assisted silver nanoparticles. J. King Saud Univ. Sci. 2022, 34, 102177. [Google Scholar] [CrossRef]



| No. | A: Additional Amount of AgNO3 (mg) | B: pH | C: Temperature (°C) | Silver Content (mg/g) |
|---|---|---|---|---|
| 1 | 0 (60) | −1 (3) | −1 (60) | 9.92 |
| 2 | 0 (60) | −1 (3) | 1 (80) | 9.61 |
| 3 | 0 (60) | 1 (5) | −1 (60) | 10.19 |
| 4 | 0 (60) | 1 (5) | 1 (80) | 8.92 |
| 5 | −1 (40) | 0 (4) | −1 (60) | 9.64 |
| 6 | −1 (40) | 0 (4) | 1 (80) | 8.83 |
| 7 | 1 (80) | 0 (4) | −1 (60) | 8.93 |
| 8 | 1 (80) | 0 (4) | 1 (80) | 8.64 |
| 9 | −1 (40) | −1 (3) | 0 (70) | 10.81 |
| 10 | −1 (40) | 1 (5) | 0 (70) | 12.42 |
| 11 | 1 (80) | −1 (3) | 0 (70) | 12.23 |
| 12 | 1 (80) | 1 (5) | 0 (70) | 9.38 |
| 13 | 0 (60) | 0 (4) | 0 (70) | 13.34 |
| 14 | 0 (60) | 0 (4) | 0 (70) | 12.91 |
| 15 | 0 (60) | 0 (4) | 0 (70) | 13.53 |
| 16 | 0 (60) | 0 (4) | 0 (70) | 13.31 |
| 17 | 0 (60) | 0 (4) | 0 (70) | 13.45 |
| Source of Variance | Sum of Square | Degree of Freedom | Mean Square | F-Value | p-Value 1 |
|---|---|---|---|---|---|
| Model | 57.09 | 9 | 6.34 | 109.22 | <0.0001 ** |
| A | 0.79 | 1 | 0.79 | 13.67 | 0.0077 ** |
| B | 0.34 | 1 | 0.34 | 5.93 | 0.0451 * |
| C | 0.90 | 1 | 0.90 | 15.46 | 0.0057 ** |
| AB | 4.97 | 1 | 4.97 | 85.63 | <0.001 |
| AC | 0.068 | 1 | 0.068 | 1.16 | 0.3164 |
| BC | 0.23 | 1 | 0.23 | 3.97 | 0.0867 |
| A2 | 7.95 | 1 | 7.95 | 136.87 | <0.0001 ** |
| B2 | 2.21 | 1 | 2.21 | 38.00 | 0.0005 ** |
| C2 | 36.00 | 1 | 36.00 | 619.86 | <0.0001 ** |
| Residual | 0.41 | 7 | 0.058 | — | — |
| Lack of fit | 0.18 | 3 | 0.059 | 1.03 | 0.4672 |
| Pure error | 0.23 | 4 | 0.057 | — | — |
| Cor. total | 57.50 | 16 | — | — | — |
| Energy (kcal/mol) | Monomer of PCA-CS | PCA-CS-Ag (Mode I) | PCA-CS-Ag (Mode II) | PCA-CS-Ag (Mode III) |
|---|---|---|---|---|
| Stretch | 1.2766 | 2.3763 | 17.8308 | 26.6365 |
| Bend | 8.9573 | 51.1380 | 62.5756 | 90.8197 |
| Stretch-Bend | 0.6058 | −0.0447 | −0.0597 | 0.4352 |
| Torsion | 5.3844 | 59.6525 | 29.8432 | 3.9841 |
| Non-1,4 VDW | −6.3229 | −6.8230 | −6.8841 | −18.7602 |
| 1,4 VDW | 18.3978 | 18.6322 | 21.9594 | 17.9010 |
| Dipole/Dipole | −1.6975 | −4.0458 | 0.1046 | 0.8527 |
| Total Energy | 26.6016 | 120.8853 | 125.3698 | 121.8691 |
| Sample | Antibacterial Rates (%) | |
|---|---|---|
| E. coli | S. aureus | |
| CS | 22.9 | 29.8 |
| PCA-CS | 45.7 ** | 51.6 ** |
| PCA-CS-Ag | 83.1 **## | 89.3 **## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhao, Y.; Hu, Z.; Si, Z.; Yang, Z. 2-Pyridinecarboxaldehyde-Modified Chitosan–Silver Complexes: Optimized Preparation, Characterization, and Antibacterial Activity. Molecules 2023, 28, 6777. https://doi.org/10.3390/molecules28196777
Zhang Z, Zhao Y, Hu Z, Si Z, Yang Z. 2-Pyridinecarboxaldehyde-Modified Chitosan–Silver Complexes: Optimized Preparation, Characterization, and Antibacterial Activity. Molecules. 2023; 28(19):6777. https://doi.org/10.3390/molecules28196777
Chicago/Turabian StyleZhang, Zhaoyu, Yurong Zhao, Zhang Hu, Zhenyu Si, and Ziming Yang. 2023. "2-Pyridinecarboxaldehyde-Modified Chitosan–Silver Complexes: Optimized Preparation, Characterization, and Antibacterial Activity" Molecules 28, no. 19: 6777. https://doi.org/10.3390/molecules28196777






