Ultra-High Cycling Stability of 3D Flower-like Ce(COOH)3 for Supercapacitor Electrode via a Facile and Scalable Strategy
Abstract
:1. Introduction
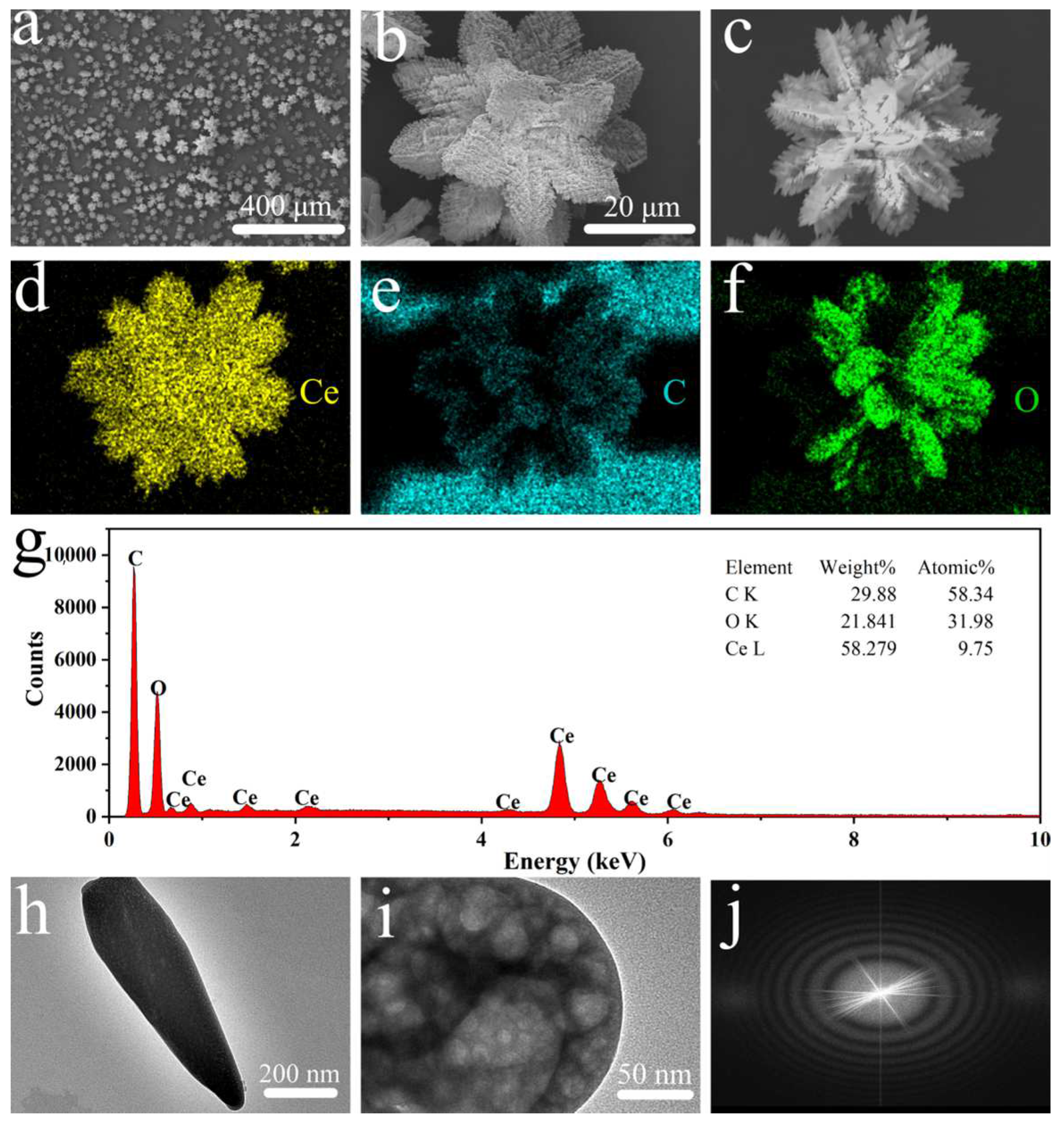
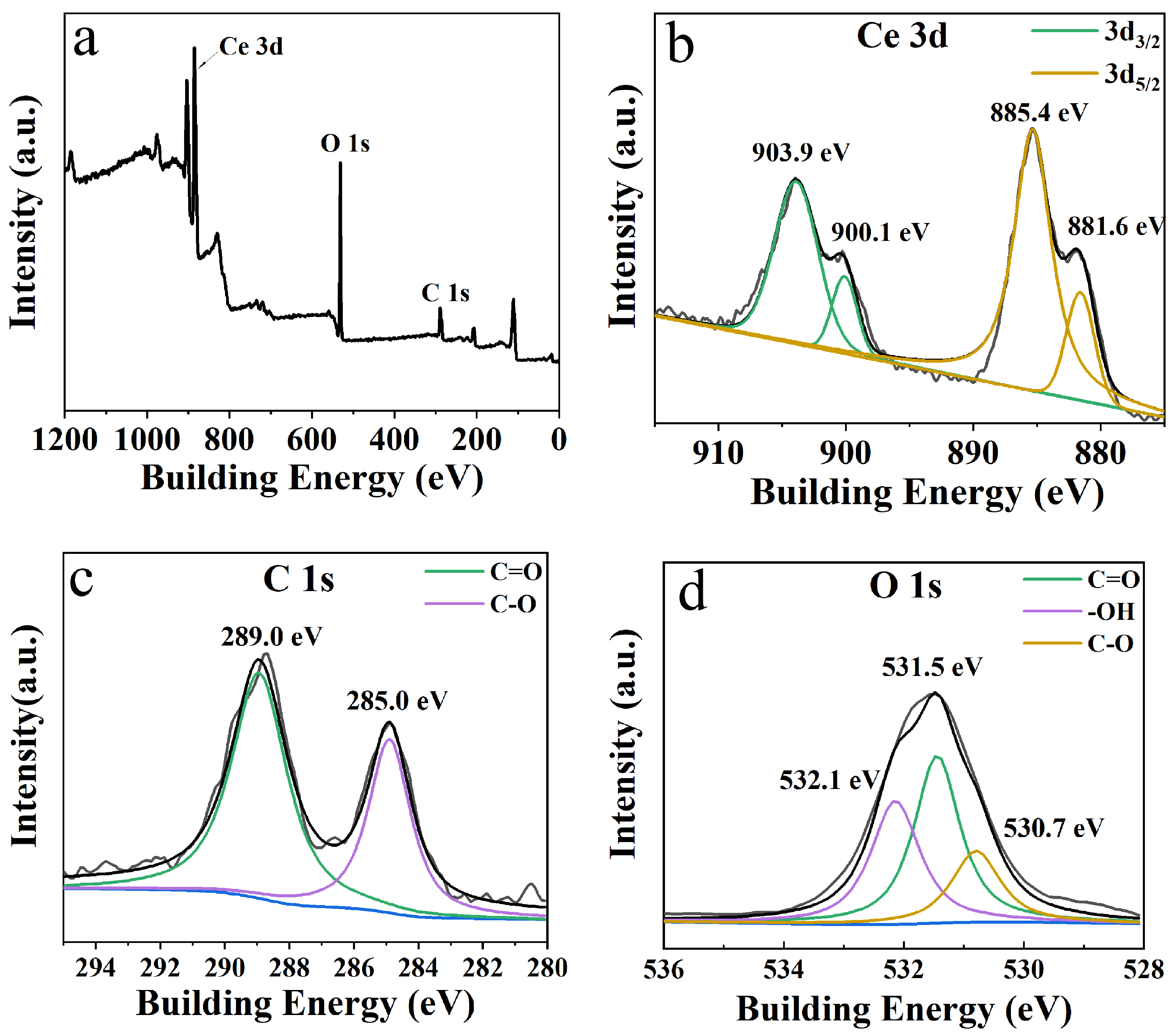
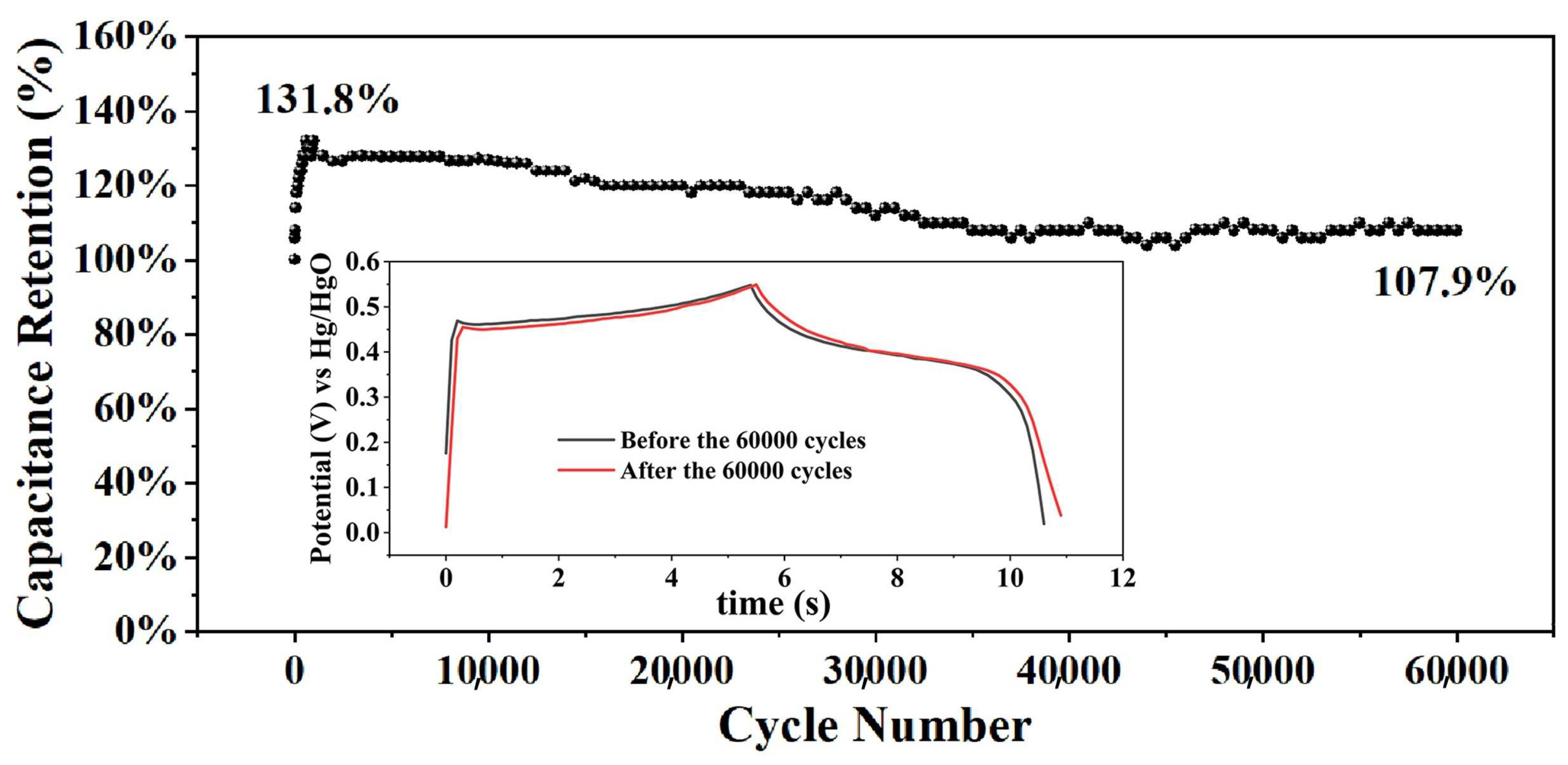
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of Ce(COOH)3
3.3. Characterization
3.4. Electrochemical Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Turner, J.M. The matter of a clean energy future. Science 2022, 376, 1361. [Google Scholar] [CrossRef]
- Editorial. Clean energy can fuel the future—And make the world healthier. Nature 2023, 620, 245. [Google Scholar] [CrossRef] [PubMed]
- Xia, G. The Chemistry of Sustainable Energy Conversion and Storage. Molecules 2022, 27, 3731. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, Y.; Holze, R. Ag(e)ing and Degradation of Supercapacitors: Causes, Mechanisms, Models and Countermeasures. Molecules 2023, 28, 5028. [Google Scholar] [CrossRef] [PubMed]
- Fares, A.M.; Kippke, M.; Rashed, M.; Klumpner, C.; Bozhko, S. Development of a smart supercapacitor energy storage system for aircraft electric power systems. Energies 2021, 14, 8056. [Google Scholar] [CrossRef]
- Guo, L.; Hu, P.; Wei, H. Development of supercapacitor hybrid electric vehicle. J. Energy Storage 2023, 65, 107269–107277. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Wang, L.L.; Zhao, L.J.; Wang, K.; Zheng, Y.Q.; Yuan, Z.Y.; Wang, D.Y.; Fu, X.Y.; Shen, G.Z.; Han, W. Flexible self-powered integrated sensing system with 3D periodic ordered black phosphorus@ MXene thin-films. Adv. Mater. 2021, 33, 2007890–2007901. [Google Scholar] [CrossRef]
- Zhan, C.; Lian, C.; Zhang, Y.; Thompson, M.W.; Xie, Y.; Wu, J.Z.; Kent, P.R.C.; Cummings, P.T.; Jiang, D.E.; Wesolowski, D.J. Computational insights into materials and interfaces for capacitive energy storage. Adv. Sci. 2017, 4, 1700059–1700086. [Google Scholar] [CrossRef]
- Lin, Z.; Goikolea, E.; Balducci, A.; Naoi, K.; Taberna, P.L.; Salanne, M.; Simon, P. Materials for supercapacitors: When Li-ion battery power is not enough. Mater. Today 2018, 21, 419–436. [Google Scholar] [CrossRef]
- Ji, D.; Park, J.M.; Oh, M.S.; Nguyen, T.L.; Shin, H.; Kim, J.S.; Kim, D.; Park, H.S.; Kim, J. Superstrong, superstiff, and conductive alginate hydrogels. Nat. Commun. 2022, 13, 3019–3029. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, J.; Nie, Y.; Zhao, Y.; Wang, L.; Li, Y.; Duan, H. Integrating flexible ultralight 3D Ni micromesh current collector with NiCo bimetallic hydroxide for smart hybrid supercapacitors. Adv. Funct. Mater. 2021, 31, 2100290–2100300. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, G.; Yang, H.; Bi, W.; Liang, X.; Zhang, Y.; Zhang, G.; Wu, G. Controlled synthesis of V2O5/MWCNT core/shell hybrid aerogels through a mixed growth and self-assembly methodology for supercapacitors with high capacitance and ultralong cycle life. J. Mater. Chem. A 2015, 3, 15692–15699. [Google Scholar] [CrossRef]
- Bi, W.; Gao, G.; Wu, Y.; Yang, H.; Wang, J.; Zhang, Y.; Liang, X.; Liu, Y.; Wu, G. Novel three-dimensional island-chain structured V2O5/graphene/MWCNT hybrid aerogels for supercapacitors with ultralong cycle life. RSC Adv. 2017, 7, 7179–7187. [Google Scholar] [CrossRef]
- Chen, B.L.; Xu, L.L.; Xie, Z.Y.; Wong, W.Y. Supercapacitor electrodes based on metal-organic compounds from the first transition metal series. EcoMat 2021, 3, e12106–e12160. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, J.L.; Shi, Y.X.; Xu, Y.; Mao, Y.J.; Lv, R.G.; Huang, B.; Sun, Y.Z.; Zhao, Z.Y.; Chang, Y.N.; et al. Latest advances of metal-organic frameworks-based materials for supercapacitors. Sustain. Mater. Technol. 2023, 36, e00588–e00611. [Google Scholar] [CrossRef]
- Sheberla, D.; Bachman, J.C.; Elias, J.S.; Sun, C.J.; Shao-Horn, Y.; Dinca, M. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat. Mater. 2017, 16, 220–224. [Google Scholar] [CrossRef]
- Li, W.H.; Ding, K.; Tian, H.R.; Yao, M.S.; Nath, B.; Deng, W.H.; Wang, Y.B.; Xu, G. Conductive metal-organic framework nanowire array electrodes for high-performance solid-state supercapacitors. Adv. Funct. Mater. 2017, 27, 1702067–1702074. [Google Scholar] [CrossRef]
- Shen, C.H.; Chuang, C.H.; Gu, Y.J.; Ho, W.H.; Song, Y.D.; Chen, Y.C.; Wang, C.Y.; Kung, C.W. Cerium-based metal–organic framework nanocrystals interconnected by carbon nanotubes for boosting electrochemical capacitor performance. ACS Appl. Mater. Interfaces 2021, 13, 16418–16426. [Google Scholar] [CrossRef]
- Devic, T.; Serre, C. High valence 3p and transition metal based MOFs. Chem. Soc. Rev. 2014, 43, 6097–6115. [Google Scholar] [CrossRef]
- Han, J.J.; Yan, Q.R.; Chen, Z.W.; Wang, Z.; Chen, C. Application of Cr-metal organic framework (MOF) modified polyaniline/graphene oxide materials in supercapacitors. Ionics 2022, 28, 2349–2362. [Google Scholar] [CrossRef]
- Yoon, J.H.; Jinsoo, B.; Cho, I.; Vinodh, R.; Pollet, B.G.; Babu, R.S.; Kim, H.J. Novel supercapacitor electrode derived from one dimensional cerium hydrogen phosphate (1D-Ce (HPO4)2·xH2O). Molecules 2022, 27, 7691. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K. A review on the current research on microwave processing techniques applied to graphene-based supercapacitor electrodes: An emerging approach beyond conventional heating. J. Energy Chem. 2022, 74, 252–282. [Google Scholar] [CrossRef]
- Strauss, V.; Marsh, K.; Kowal, M.D.; El-Kady, M.; Kaner, R.B. A simple route to porous graphene from carbon nanodots for supercapacitor applications. Adv. Mater. 2018, 30, 1704449–1704459. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Tan, W.K.; Kawamura, G.; Matsuda, A.; Kar, K.K. Microwave-assisted thin reduced graphene oxide-cobalt oxide nanoparticles as hybrids for electrode materials in supercapacitor. J. Energy Storage 2021, 40, 102724–102733. [Google Scholar] [CrossRef]
- Kuang, H.F.; Zhang, H.Q.; Liu, X.H.; Chen, Y.D.; Zhang, W.G.; Chen, H.; Ling, Q.D. Microwave-assisted synthesis of NiCo-LDH/graphene nanoscrolls composite for supercapacitor. Carbon 2022, 190, 57–67. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Yang, N.; Yang, L.; Wang, W.L.; Fang, X.; He, Q. NiCo-MOF Nanospheres Created by the Ultra-Fast Microwave Method for Use in High-Performance Supercapacitors. Molecules 2023, 28, 5613. [Google Scholar] [CrossRef]
- Younas, W.; Naveed, M.; Cao, C.B.; Zhu, Y.Q.; Du, C.L.; Ma, X.L.; Mushtaq, N.; Tahir, M.; Naeem, M. Facile one-step microwave-assisted method to synthesize nickel selenide nanosheets for high-performance hybrid supercapacitor. J. Colloid Interface Sci. 2022, 608, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Ni, D.; Yang, X.W.; Liu, C.C.; Yin, J.L.; Cai, K.F. Microwave-assisted synthesis of honeycomblike hierarchical spherical Zn-doped Ni-MOF as a high-performance battery-type supercapacitor electrode material. Electrochim. Acta 2018, 278, 114–123. [Google Scholar] [CrossRef]
- He, Q.; He, R.; Zia, A.; Gao, G.H.; Liu, Y.F.; Neupane, M.; Wang, M.; Benedict, Z. Self-Promoting Energy Storage in Balsa Wood-Converted Porous Carbon Coupled with Carbon Nanotubes. Small 2022, 18, 2200272–2200282. [Google Scholar] [CrossRef]
- Wang, D.G.; Liang, Z.B.; Gao, S.; Qu, C.; Zou, R.G. Metal-organic framework-based materials for hybrid supercapacitor application. Coord. Chem. Rev. 2020, 404, 213093–213115. [Google Scholar] [CrossRef]
- Rabani, I.; Karuppasamy, K.; Vikraman, D.; Ulhaq, Z.; Kim, H.S.; Seo, Y.S. Hierarchical structured nano-polyhedrons of CeO2@ ZIF-8 composite for high performance supercapacitor applications. J. Alloys Compd. 2021, 875, 160074–160086. [Google Scholar] [CrossRef]
- Khan, U.A.; Iqbal, N.; Noor, T.; Ahmad, R.; Ahmad, A.; Gao, J.K.; Amjad, Z.; Wahab, A. Cerium based metal organic framework derived composite with reduced graphene oxide as efficient supercapacitor electrode. J. Energy Storage 2021, 41, 102999–103008. [Google Scholar] [CrossRef]
- Yuan, N.C.; Mei, Y.X.; Liu, Y.W.; Xie, Y.T.; Lin, B.N.; Zhou, Y.H. Fabrication of UiO-66-NH2/Ce (HCOO)3 heterojunction with enhanced photocatalytic reduction of CO2 to CH4. J. CO2 Util. 2022, 64, 102151–102162. [Google Scholar] [CrossRef]
- Sonar, P.A.; Sanjeevagol, S.G.; Manjanna, J.; Patake, V.D.; Nitin, S. Electrochemical behavior of cerium (III) hydroxide thin-film electrode in aqueous and non-aqueous electrolyte for supercapacitor applications. J. Mater. Sci.-Mater 2022, 33, 25787–25795. [Google Scholar] [CrossRef]
- Chen, K.; Xue, D. In-situ electrochemical route to aerogel electrode materials of graphene and hexagonal CeO2. J. Colloid Interface Sci. 2015, 446, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Xuan, W.; Zhao, C.; Leng, X.; Sun, D.; Luo, D.; Wang, F. Enhanced electrochemical properties of cerium metal–organic framework based composite electrodes for high-performance supercapacitor application. RSC Adv. 2018, 8, 3462–3469. [Google Scholar] [CrossRef]
- Hunpratub, S.; Chullaphan, T.; Chumpolkulwong, S.; Chanlek, N.; Phokha, S. Characterization and electrochemical properties of Carbon/CeO2 composites prepared using a hydrothermal method. Mater. Chem. Phys. 2023, 303, 127820–127832. [Google Scholar] [CrossRef]
- Singh, D.L.; Ghosh, T.K.; Mishra, V.; Ramasamy, S.; Sahoo, M.K.; Gangavarapu, R.R. Three-dimensional lanthanide-based nanoporous metal–organic frameworks for high-performance supercapacitors. ACS Appl. Nano Mater. 2022, 5, 15237–15249. [Google Scholar] [CrossRef]
- Chang, Y.L.; Tsai, M.D.; Shen, C.H.; Huang, C.W.; Wang, Y.C.; Kung, C.W. Cerium-based metal–organic framework-conducting polymer nanocomposites for supercapacitors. Mater. Today Sustain. 2023, 23, 100449–100459. [Google Scholar] [CrossRef]
- Fatemi, S.; Ganjali, M.R.; Mohammad, R.G. Fabrication and comparison of composites of cerium metal-organic framework/reduced graphene oxide as the electrode in supercapacitor application. J. Energy Storage 2022, 55, 105545–105554. [Google Scholar]
- Kumaresan, L.; Hanamantrao, D.P.; Raj, S.L.S.; Chenrayan, S.; Rangasamy, B.; Vediappan, K. Spherically Structured Ce-Metal-Organic Frameworks with Rough Surfaces and Carbon-Coated Cerium Oxide as Potential Electrodes for Lithium Storage and Supercapacitors. Chem. Sel. 2023, 8, e202204759–e202204769. [Google Scholar] [CrossRef]
- Yang, J.; Chen, L.S.; Li, W.W.; Chen, G.R.; Wang, L.Z.; Zhao, S. A novel self-supported structure of Ce-UiO-66/TNF in a redox electrolyte with high supercapacitive performance. J. Colloid Interface Sci. 2020, 573, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.N.; Wang, X.K.; Zheng, S.H.; Sun, J.P.; Huang, Z.D.; Xu, B.; Fan, L.L.; Wang, R.M.; Sun, D.F.; Wu, Z.S. Toward high-performance and flexible all-solid-state micro-supercapacitors: MOF bulk vs. MOF nanosheets. Chem. Eng. J. 2021, 413, 127520–127528. [Google Scholar] [CrossRef]
- Sun, T.; Yue, L.; Wu, N.; Xu, M.; Yang, W.; Guo, H.; Yang, W. Isomorphism combined with intercalation methods to construct a hybrid electrode material for high-energy storage capacitors. J. Mater. Chem. A 2019, 7, 25120–25131. [Google Scholar] [CrossRef]
- Du, X.; Zhang, J.; Wang, H.; Huang, Z.; Guo, A.; Zhao, L.; Niu, Y.; Xiang, L.; Wu, B.; Liu, Y. Solid–solid interface growth of conductive metal–organic framework nanowire arrays and their supercapacitor application. Mater. Chem. Front. 2020, 4, 243–251. [Google Scholar] [CrossRef]
- Yan, Y.; Luo, Y.Q.; Ma, J.Y.; Li, B.; Xue, H.G.; Pang, H. Facile synthesis of vanadium metal-organic frameworks for high-performance supercapacitors. Small 2018, 14, 1801815–1801822. [Google Scholar] [CrossRef]
- Ojha, M.; Wu, B.; Deepa, M. Cost-effective MIL-53 (Cr) metal–organic framework-based supercapacitors encompassing fast-ion (Li+/H+/Na+) conductors. ACS Appl. Energ. Mater. 2021, 4, 4729–4743. [Google Scholar] [CrossRef]
- Shinde, P.A.; Seo, Y.; Lee, S.; Kim, H.; Pham, Q.N.; Won, Y.; Jun, S.C. Layered manganese metal-organic framework with high specific and areal capacitance for hybrid supercapacitors. Chem. Eng. J. 2020, 387, 122982–122992. [Google Scholar] [CrossRef]
- Liu, L.; Yan, Y.; Cai, Z.H.; Lin, S.X.; Hu, X.B. Growth-oriented Fe-based MOFs synergized with graphene aerogels for high-performance supercapacitors. Interfaces 2018, 5, 1701548–1701555. [Google Scholar] [CrossRef]
- Shao, D.; Wang, L.; Lu, B.; Guo, J.; Zhang, S.; Lu, Y. A high N content cobalt-based metal organic framework with nanorod structure for supercapacitor electrode material. J. Electroanal. Chem. 2019, 847, 113188–113197. [Google Scholar] [CrossRef]



| Sample | Cycles | Capacitive Retention | Ref |
|---|---|---|---|
| Ce-H2L | 2000/4 a | 64.0% | Ref [38] |
| Ce-MOF-808/CNT | 2000/1 b | 71.0% | Ref [18] |
| Ce-MOF-808/PEDOT | 2000/0.5 b | 90.0% | Ref [39] |
| Ce-BTC (rod-like) | 4000/250 c | 52.8% | Ref [40] |
| Ce-MOF | 5000/4 a | 65.2% | Ref [41] |
| Ce-BTC (nanorod) | 5000/3 a | 83.0% | Ref [36] |
| Ce(HPO4)2.xH2O | 5000/3 a | 92.7% | Ref [21] |
| Ce-UiO-66/TNF | 10,000/50 b | 95.0% | Ref [42] |
| Ni3(HITP)2 | 10,000/1 b | 84.0% | Ref [16] |
| Co-MOF | 10,000/5 b | 96.0% | Ref [43] |
| Ni/Co-MOF | 12,000/10 a | 89.8% | Ref [44] |
| Cu3(HHTP)2 | 5000/5 a | 79.9% | Ref [45] |
| V-MOF | 10,000/1 a | 92.1% | Ref [46] |
| Cr-MOF | 10,000/0.5 a | 85.0% | Ref [47] |
| Mn-MOF | 10,000/10 a | 81.18% | Ref [48] |
| Fe-MOF | 10,000/1 a | 74.4% | Ref [49] |
| Ce(COOH)3 | 60,000/10 a | 107.9% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Wang, W.; Yang, N.; Chen, W.; Yang, X.; Fang, X.; Zhang, Y. Ultra-High Cycling Stability of 3D Flower-like Ce(COOH)3 for Supercapacitor Electrode via a Facile and Scalable Strategy. Molecules 2023, 28, 6806. https://doi.org/10.3390/molecules28196806
He Q, Wang W, Yang N, Chen W, Yang X, Fang X, Zhang Y. Ultra-High Cycling Stability of 3D Flower-like Ce(COOH)3 for Supercapacitor Electrode via a Facile and Scalable Strategy. Molecules. 2023; 28(19):6806. https://doi.org/10.3390/molecules28196806
Chicago/Turabian StyleHe, Qing, Wanglong Wang, Ning Yang, Wenmiao Chen, Xing Yang, Xing Fang, and Yuanxiang Zhang. 2023. "Ultra-High Cycling Stability of 3D Flower-like Ce(COOH)3 for Supercapacitor Electrode via a Facile and Scalable Strategy" Molecules 28, no. 19: 6806. https://doi.org/10.3390/molecules28196806






