A Novel Method for γ-Aminobutyric Acid Biosynthesis Using Glutamate Decarboxylase Entrapped in Polyvinyl Alcohol–Sodium Alginate Capsules
Abstract
:1. Introduction
2. Results and Discussion
2.1. GAD Immobilization
2.1.1. Effects of Synthesis Conditions on the PVA/SA–GADMs Structure
2.1.2. Characterization of the PVA/SA GAD Microspheres
2.1.3. Effects of Synthesis Conditions on the Properties of the PVA/SA–GADMs Catalysis
2.2. PVA/SA–GADMs Enzyme Properties and Applications
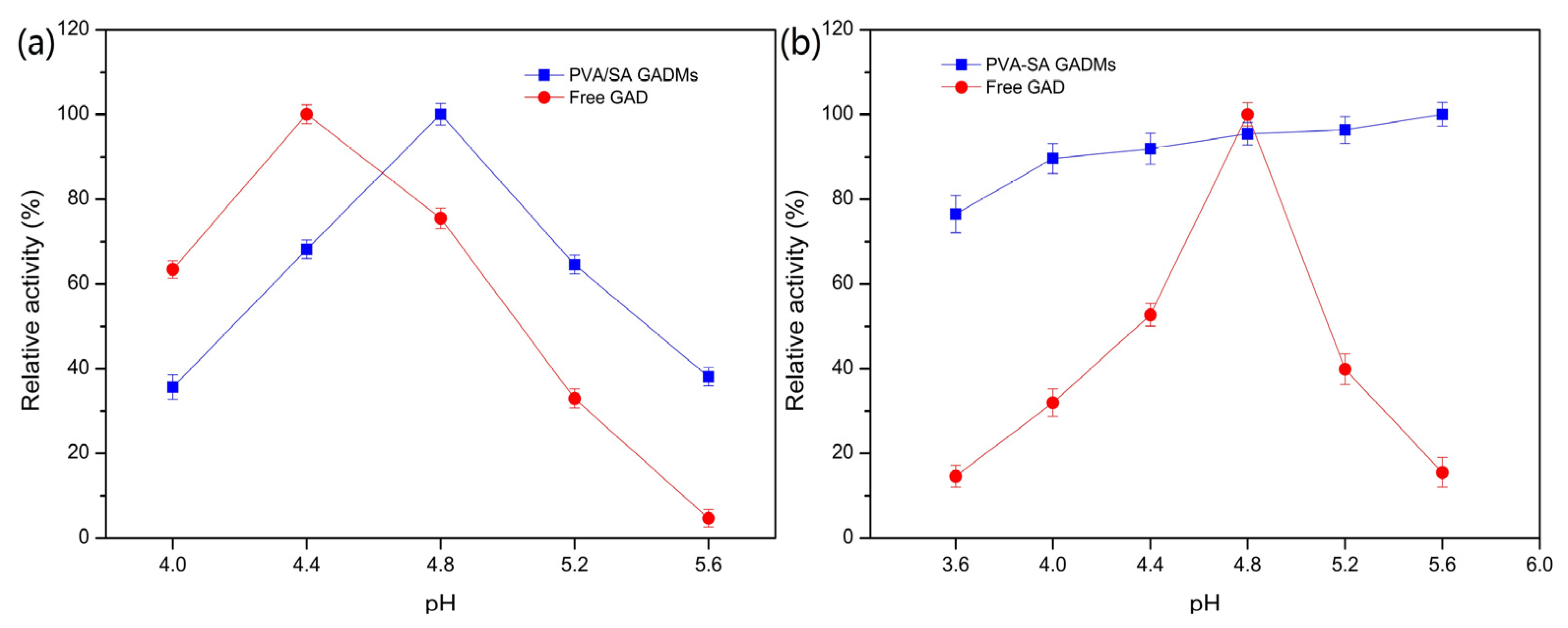
2.2.1. Effects of pH on the PVA/SA–GADMs Enzyme Activity and Stability
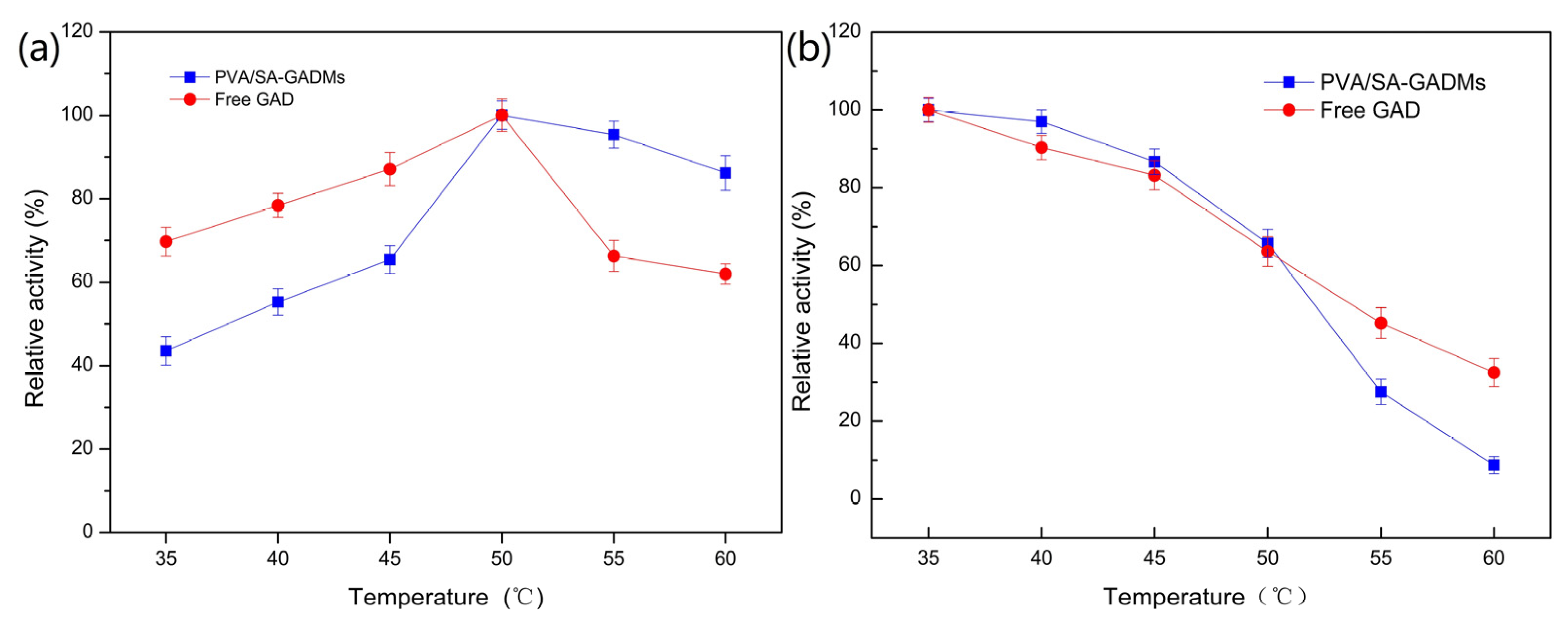
2.2.2. Effects of Temperature on PVA/SA–GADMs Enzyme Activity and Stability
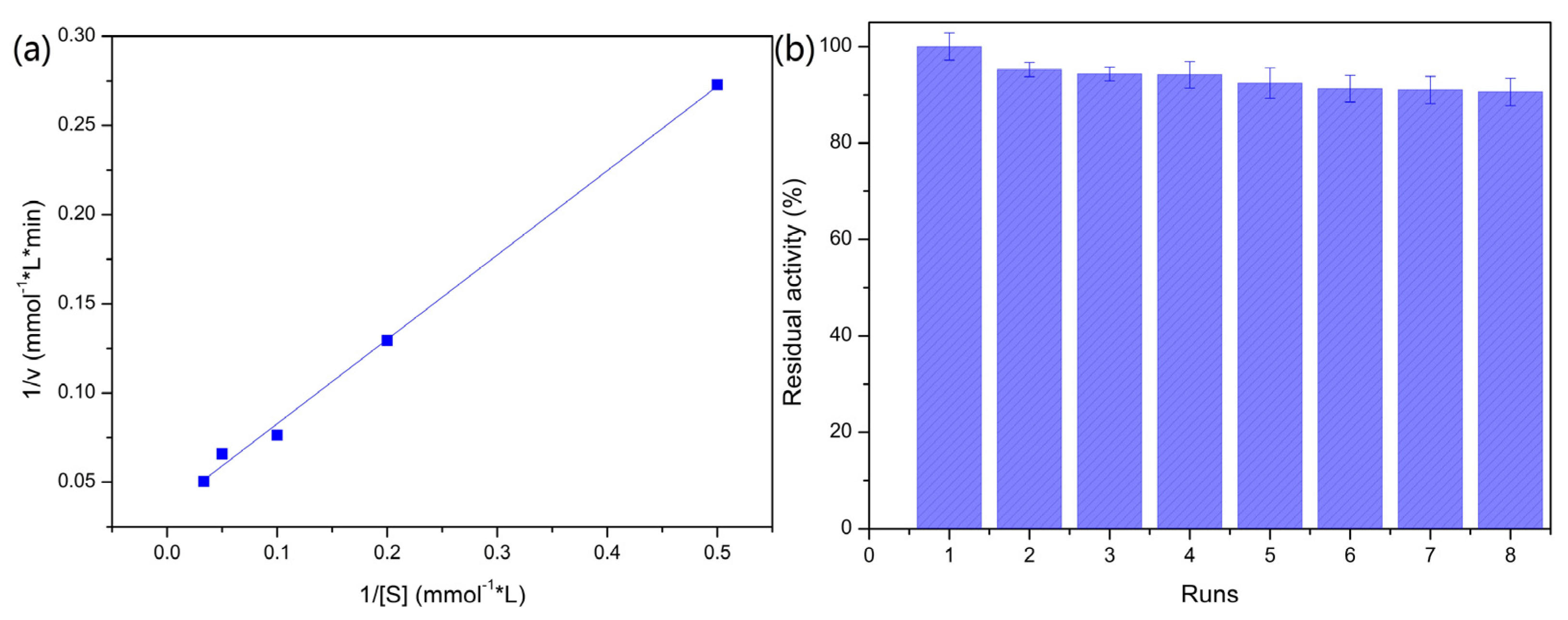
2.2.3. Kinetic Study of the PVA/SA–GADMs
2.2.4. Operation Stability for the PVA/SA–GADMs Recycling Batch
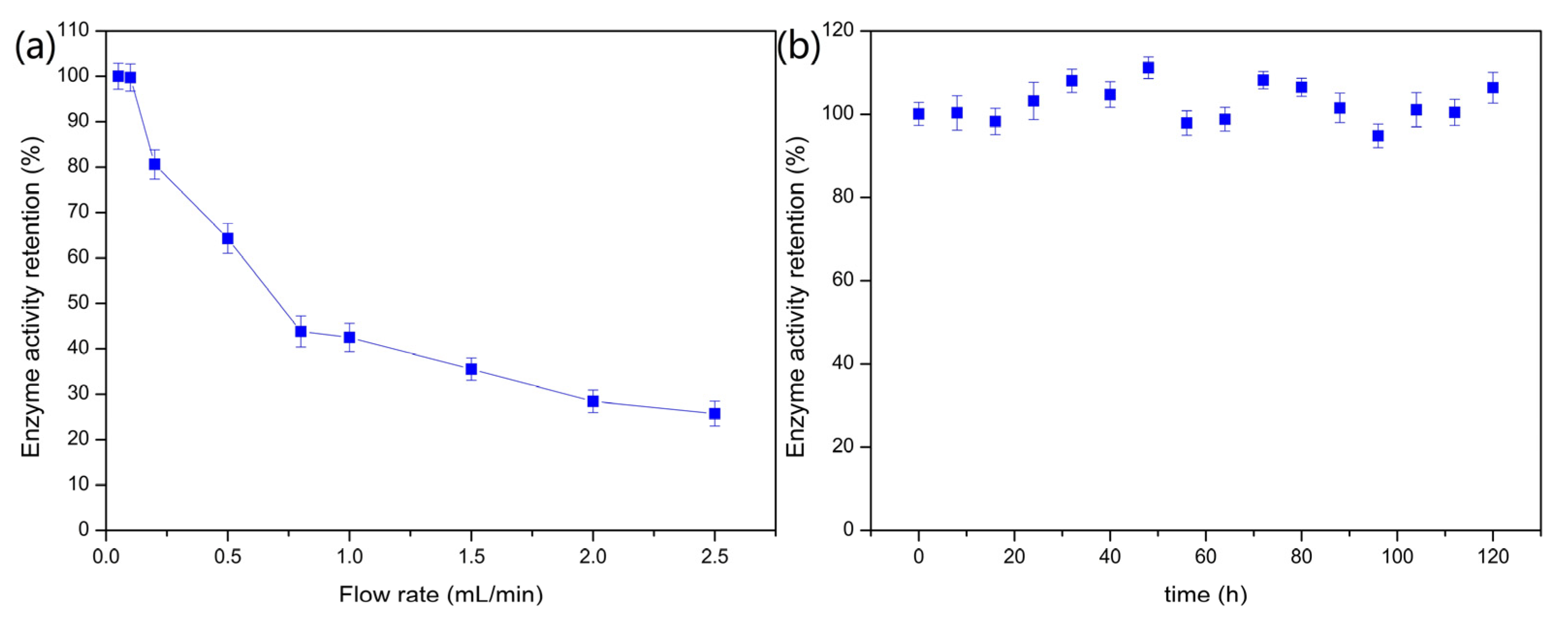
2.2.5. Continuous Fixed-Bed Column Catalyst Operation for PVA/SA–GADMs
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. GAD Immobilization and Optimization
3.2.2. Characterization of PVA/SA–GADMs
3.2.3. Enzyme Activity Assays
3.2.4. Enzymatic Properties and Kinetic Parameter Measurements
3.2.5. PVA/SA–GADMs Batch Recycling Operation
3.2.6. PVA/SA–GADMs Continuous Fixed-Bed Column Catalyst Operation
3.2.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Roberts, E. gamma-aminobutyric acid and nervous system function—A perspective. Biochem. Pharmacol. 1974, 23, 2637–2649. [Google Scholar] [CrossRef] [PubMed]
- Storm-Mathisen, J. GABA as a transmitter in the central nervous system of vertebrates. J. Neural Transm. 1974, 11, 227–253. [Google Scholar]
- Powers, M.E.; Yarrow, J.F.; Mccoy, S.C.; Borst, S.E. Growth hormone isoform responses to GABA ingestion at rest and after exercise. Med. Sci. Sports Exerc. 2008, 40, 104–110. [Google Scholar] [CrossRef]
- Bazil, C.W.; Battista, J.; Basner, R.C. Gabapentin improves sleep in the presence of alcohol. J. Clin. Sleep Med. 2005, 3, 284–287. [Google Scholar] [CrossRef]
- Inoue, K.; Shirai, T.; Ochiai, H.; Kasao, M.; Hayakawa, K.; Kimura, M.; Sansawa, H. Blood-pressure-lowering effect of a novel fermented milk containing γ-aminobutyric acid (GABA) in mild hypertensives. Eur. J. Clin. Nutr. 2003, 57, 490–495. [Google Scholar] [CrossRef]
- Abdou, A.M.; Higashiguchi, S.; Horie, K.; Kim, M.; Hatta, H.; Yokogoshi, H. Relaxation and immunity enhancement effects of γ-aminobutyric acid (GABA) administration in humans. Biofactors 2006, 26, 201–208. [Google Scholar] [CrossRef]
- Oh, S.H.; Moon, Y.J.; Soh, J.R.; Cha, Y.S. Effect of water extract of germinated brown rice on adiposity and obesity indices in mice fed a high fat diet. J. Food Sci. Nutr. 2005, 10, 251–256. [Google Scholar] [CrossRef]
- Leventhal, A.G.; Wang, Y.; Pu, M.; Zhou, Y.; Ma, Y. GABA and its agonists improved visual cortical function in senescent monkeys. Science 2003, 300, 812–815. [Google Scholar] [CrossRef]
- Krishnan, S.; Laskowski, K.; Shukla, V.; Merewitz, E.B. Mitigation of drought stress damage by exogenous application of a non-protein amino acid γ–aminobutyric acid on perennial ryegrass. J. Am. Soc. Hortic. Sci. 2013, 138, 358–366. [Google Scholar] [CrossRef]
- Vijayakumari, K.; Jos, T. γ-aminobutyric acid (GABA) priming enhances the osmotic stress tolerance in Piper nigrum Linn. plants subjected to PEG-induced stress. Plant Growth Regul. 2016, 78, 57–67. [Google Scholar] [CrossRef]
- Li, H.; Qiu, T.; Huang, G.; Cao, Y. Production of gamma-aminobutyric acid by Lactobacillus brevis NCL912 using fed-batch fermentation. Microb. Cell Factories 2010, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cao, Y. Lactic acid bacterial cell factories for gamma-aminobutyric acid. Amino Acids 2010, 39, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ma, Z.W.Y.; Wen-xia, Y.; Li, C.Z.Y.; Xiao-ting, X.; Hong-jie, Z. Determination and comparison of γ-aminobutyric acid (GABA) content in Pu-erh and other types of Chinese tea. J. Agric. Food Chem. 2011, 59, 3641–3648. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xuanhui, H.; Yan Liu, J.L.; Qingyong, H.; Cuiying, Z.; Benjun, W.; Ye, Z.; Jie, W. Extraction, purification and anti-fatigue activity of γ-aminobutyric acid from mulberry (Morus alba L.) leaves. Sci. Rep. 2016, 6, 18933. [Google Scholar] [CrossRef]
- Yoshimura, M.; Toyoshi, T.; Sano, A.; Izumi, T.; Fujii, T.; Konishi, C.; Inai, S.; Matsukura, C.; Fukuda, N.; Ezura, H.; et al. Antihypertensive effect of a γ-aminobutyric acid rich tomato cultivar ‘DG03-9’ in spontaneously hypertensive rats. J. Agric. Food Chem. 2010, 58, 615–619. [Google Scholar] [CrossRef]
- Aoki, H.; Uda, I.; Tagami, K.; Furuya, Y.; Endo, Y.; Fujimoto, K. The production of a new Tempeh-like fermented soybean containing a high level of γ-aminobutyric acid by anaerobic incubation with Rhizopus. Biosci. Biotechnol. Biochem. 2003, 67, 1018–1023. [Google Scholar] [CrossRef]
- Hong, J.; Kim, K. Crystal structure of γ-aminobutyrate aminotransferase in complex with a PLP-GABA adduct from Corynebacterium glutamicum. Biochem. Biophys. Res. Commun. 2019, 514, 601–606. [Google Scholar] [CrossRef]
- Zhang, L.; Yue, Y.; Wang, X.; Dai, W.; Piao, C.; Yu, H. Optimization of fermentation for γ-aminobutyric acid (GABA) production by yeast Kluyveromyces marxianus C21 in okara (soybean residue). Bioprocess Biosyst. Eng. 2022, 45, 1111–1123. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, X.; Fu, J.; Wang, S.; Chen, Y.; Chang, K.; Li, H. Substrate sustained release-based high efficacy biosynthesis of GABA by Lactobacillus brevis NCL912. Microb. Cell Factories 2018, 17, 80. [Google Scholar] [CrossRef]
- Diana, M.; Tres, A.; Quílez, J.; Llombart, M.; Rafecas, M. Spanish cheese screening and selection of lacticacid bacteria with high gamma-aminobutyric acid production. LWT-Food Sci. Technol. 2014, 56, 351–355. [Google Scholar] [CrossRef]
- Gangaraju, D.S.; Murty, V.R.; Prapulla, S.G. Probiotic-mediated biotransformation of monosodium glutamate to γ-aminobutyric acid: Differential production in complex and minimal media and kinetic modelling. Ann. Microbiol. 2014, 64, 229–237. [Google Scholar] [CrossRef]
- Siragusa, S.; de Angelis, M.; di Cagno, R.; Rizzello, C.G.; Coda, R.; Gobbetti, M. Synthesis of γ-aminobutyric acid by lactic acid bacteria isolated from a variety of Italian cheeses. Appl. Environ. Microbiol. 2007, 73, 7283–7290. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Li, D.; Qin, H. Molecular cloning, expression, and immobilization of glutamate decarboxylase from Lactobacillus fermentum YS2. Electron. J. Biotechnol. 2017, 27, 8–13. [Google Scholar] [CrossRef]
- Woraharn, S.; Lailerd, N.; Sivamaruthi, B.S.; Wangcharoen, W.; Sirisattha, S.; Peerajan, S.; Chaiyasut, C. Evaluation of factors that influence the L-glutamic and γ-aminobutyric acid production during Hericium erinaceus fermentation by lactic acid bacteria. CyTA-J. Food 2016, 14, 47–54. [Google Scholar] [CrossRef]
- Zhuang, K.; Jiang, Y.; Feng, X.; Li, L.; Dang, F.; Zhang, W.; Man, C. Transcriptomic response to GABA-producing Lactobacillus plantarum CGMCC 1.2437T induced by L-MSG. PLoS ONE 2018, 13, e0199021. [Google Scholar] [CrossRef]
- Franciosi, E.; Carafa, I.; Nardin, T.; Schiavon, S.; Poznanski, E.; Cavazza, A.; Larcher, R.; Tuohy, K.M. Biodiversity and γ-aminobutyric acid production by lactic acid bacteria isolated from traditional alpine raw cow’s milk cheeses. BioMed Res. Int. 2015, 2015, 625740. [Google Scholar] [CrossRef]
- Katsimpouras, C.; Stephanopoulos, G. Enzymes in biotechnology: Critical platform technologies for bioprocess development. Curr. Opin. Biotechnol. 2021, 69, 91–102. [Google Scholar] [CrossRef]
- Deng, X.; Zheng, X.; Jia, F.; Cao, C.; Song, H.; Jiang, Y.; Liu, Y.; Liu, G.; Li, S.; Wang, L. Unspecific peroxygenases immobilized on Pdloaded three-dimensional ordered macroporous (3DOM) titaniaphotocatalyst for photo-enzyme integrated catalysis. Appl. Catal. 2023, 330, 122622. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, M.; Yan, X.; Zhang, G.; Xia, J.; Zeng, Z.; Yu, P.; Deng, Q.; Gong, D. Green synthesis of polydopamine functionalized magnetic mesoporous biochar for lipase immobilization and its application in interesterification for novel structured lipids production. Food Chem. 2022, 379, 132148. [Google Scholar] [CrossRef]
- Boudrant, J.; Woodley, J.M.; Fernandez-Lafuente, R. Parameters necessary to define an immobilized enzyme preparation. Process Biochem. 2020, 90, 66–80. [Google Scholar] [CrossRef]
- Morellon-Sterling, R.; Carballares, D.; Arana-Peña, S.; Siar, E.H.; Braham, S.A.; Fernandez-Lafuente, R. Advantages of Supports Activated with Divinyl Sulfone in Enzyme Coimmobilization: Possibility of Multipoint Covalent Immobilization of the Most Stable Enzyme and Immobilization via Ion Exchange of the Least Stable Enzyme. ACS Sustain. Chem. Eng. 2021, 9, 7508–7518. [Google Scholar] [CrossRef]
- Arana-Pena, S.; Carballares, D.; Morellon-Sterling, R.; Rocha-Martin, J.; Fernandez-Lafuente, R. The combination of covalent and ionic exchange immobilizations enables the coimmobilization on vinyl sulfone activated supports and the reuse of the most stable immobilized enzyme. Int. J. Biol. Macromol. 2022, 199, 51–60. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Nidetzky, B. The Microenvironment in Immobilized Enzymes: Methods of Characterization and Its Role in Determining Enzyme Performance. Molecules 2019, 24, 3460. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Rios, N.S.; Carballares, D.; Mendez-Sanchez, C.; Lokha, Y.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Effects of Enzyme Loading and Immobilization Conditions on the Catalytic Features of Lipase from Pseudomonas fluorescens Immobilized on Octyl-Agarose Beads. Front. Bioeng. Biotechnol. 2020, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Zaak, H.; Siar, E.H.; Kornecki, J.F.; Fernandez-Lopez, L.; Pedrero, S.G.; Virgen-Ortız, J.J.; Fernandez-Lafuente, R. Effect of immobilization rate and enzyme crowding on enzyme stability under different conditions. The case of lipase from Thermomyces lanuginosus immobilized on octyl agarose beads. Process Biochem. 2017, 56, 117–123. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. Enhanced mechanical, biomineralization, and cellular response of nanocomposite hydrogels by bioactive glass and halloysite nanotubes for bone tissue regeneration. Mater. Sci. Eng. C 2021, 128, 112236. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.S.B.; Gupta, A.; Alashwal, B.Y.; Sharma, S. Synthesis of PVA/PVP based hydrogel for biomedical applications: A review. Energy Sources A Recovery Util. Environ. Eff. 2018, 40, 2388–2393. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Fabrication of poly (vinyl alcohol)/ovalbumin/cellulose nanocrystals/nanohydroxyapatite based biocomposite scaffolds. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 191–201. [Google Scholar] [CrossRef]
- Zhou, A.; Zhu, C.; Chen, W.; Wan, J.; Tao, T.; Zhang, T.C.; Xie, P. Phosphorus recovery from water by lanthanum hydroxide embedded interpenetrating network poly (vinyl alcohol)/sodium alginate hydrogel beads. Colloids Surf. A Physicochem. Eng. Asp. 2018, 554, 237–244. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Safa, Y.; Yakout, S.M.; Shair, O.H.; Iqbal, M.; Nazir, A. Efficient removal of dyes using carboxymethyl cellulose/alginate/polyvinyl alcohol/rice husk composite: Adsorption/desorption, kinetics and recycling studies. Int. J. Biol. Macromol. 2020, 150, 861–870. [Google Scholar] [CrossRef]
- Baigorria, E.; Cano, L.A.; Sanchez, L.M.; Alvarez, V.A.; Ollier, R.P. Bentonitecomposite polyvinyl alcohol/alginate hydrogel beads: Preparation, characterization and their use as arsenic removal devices. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100364. [Google Scholar]
- Wang, J.; Fan, Y.-C.; Chen, Y.-P. Nitrogen removal performance and characteristics of gel beads immobilized anammox bacteria under different PVA:SA ratios. Water Environ. Res. 2021, 93, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Candry, P.; Godfrey, B.J.; Wang, Z.; Sabba, F.; Dieppa, E.; Fudge, J.; Balogun, O.; Wells, G.; Winkler, M.K.H. Tailoring polyvinyl alcohol-sodium alginate (PVA-SA) hydrogel beads by controlling crosslinking pH and time. Sci. Rep. 2022, 12, 20822. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, N.S.; Khiabani, M.S.; Ghanbarzadeh, B.; Mokarram, R.R. Enhancement of biochemical aspects of lipase adsorbed on halloysite nanotubes and entrapped in a polyvinyl alcohol/alginate hydrogel: Strategies to reuse the most stable lipase. World J. Microbiol. Biotechnol. 2020, 36, 45. [Google Scholar] [CrossRef]
- Jawale, P.V.; Bhanage, B.M. Synthesis of propyl benzoate by solvent-free immobilized lipase-catalyzed transesterification:optimization and kinetic modeling. Bioprocess Biosyst. Eng. 2021, 44, 369–378. [Google Scholar] [CrossRef]
- Bonine, B.M.; Polizelli, P.P.; Bonilla-Rodriguez, G.O. Immobilization of a plant lipase from Pachira aquatica in alginate and alginate/PVA beads. Enzym. Res. 2014, 2014, 19–22. [Google Scholar] [CrossRef]
- Bergamasco, J.; de Araujo, M.V.; de Vasconcellos, A.; Luizon Filho, R.A.; Hatanaka, R.R.; Giotto, M.V.; Aranda, D.A.G.; Nery, J.G. Enzymatic transesterification of soybean oil with ethanol using lipases immobilized on highly crystalline PVA microspheres. Biomass Bioenergy 2013, 59, 218–233. [Google Scholar] [CrossRef]
- Huang, J.; Fang, H.; Gai, Z.C.; Mei, J.Q.; Li, J.N.; Hu, S.; Lv, C.J.; Zhao, W.R.; Mei, L.H. Lactobacillus brevis CGMCC 1306 glutamate decarboxylase: Crystal structure and functional analysis. Biochem. Biophys. Res. Commun. 2018, 503, 1703–1709. [Google Scholar] [CrossRef]
- Huang, J.; Qiao, C.; Wang, X.; Gao, Y.; Zhao, J. The microsphere of sodium alginate-chitosan-Pichia kudriavzevii enhanced esterase activity to increase the content of esters in Baijiu solid-state fermentation. Food Chem. 2023, 407, 135154. [Google Scholar] [CrossRef]
- Bailey, J.E.; Ollis, D.F. Biochemical Engineering Fundamentals, 2nd ed.; McGraw-Hill: New York, NY, USA, 1986; pp. 181–189. [Google Scholar]
- Schule, G.E.; Schirmer, R.H. Priciples of Protein Stuecture; Springer: Berlin, Germany; New York, NY, USA, 1979; pp. 149–160. [Google Scholar]
- Rebroš, M.; Rosenberg, M.; Mlichová, Z.; Krištofíková, L. Hydrolysis of sucrose by invertase entrapped in polyvinyl alcohol hydrogel capsules. Food Chem. 2007, 102, 784–787. [Google Scholar] [CrossRef]
- Wilson, L.; Illanes, A.; Pessela, B.C.C.; Abian, O.; Fernández-Lafuente, R.; Guisán, J.M. Encapsulation of crosslinked penicillin G acylase aggregates in lentikats: Evaluation of a novel biocatalyst in organic media. Biotechnol. Bioeng. 2004, 86, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Hayakawa, K.; Takahashi, S.; Oda, K. Purification and characterization of glutamate decarboxylase from Lactobacillus brevis IFO 12005. Biosci. Biotechnol. Biochem. 1997, 61, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Mei, L.; Sheng, Q.; Yao, S.; Lin, D. Purification and characterization of glutamate decarboxyIase of Lactobacillus brevis CGMCC 1306 isolated from fresh milk. Chin. J. Chem. Eng. 2007, 15, 157–161. [Google Scholar] [CrossRef]
- Marquez, F.J.; Quesada, A.R.; Sanchezjimenez, F.; Decastro, I.N. Determination of 27 dansyl amino-acid derivatives in biological-fluids by reversed-phase high-performance liquid-chromatography. J. Chromatogr. 1986, 380, 275–283. [Google Scholar] [CrossRef] [PubMed]
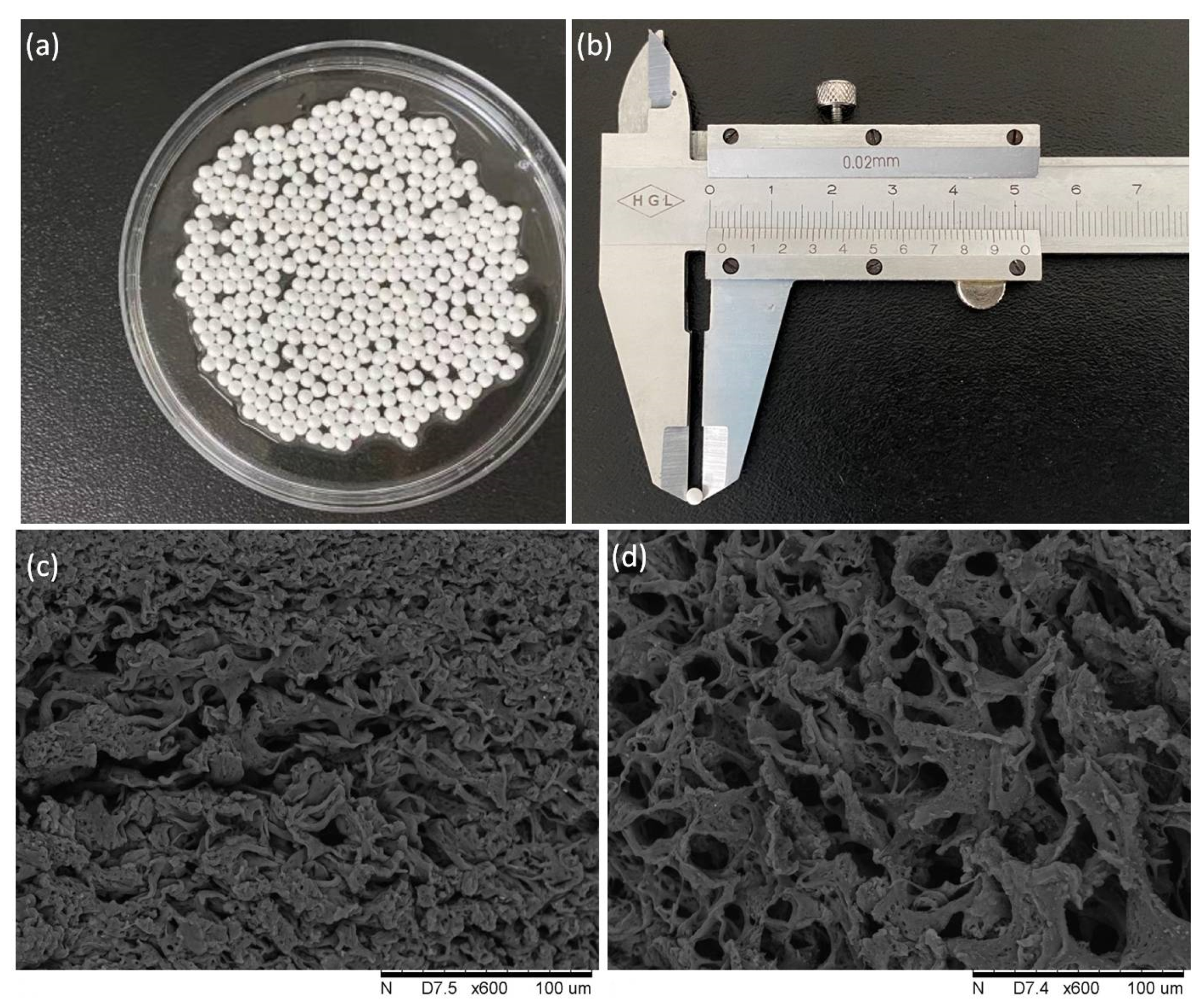


| PVA Concentration (w/v) | Spheronization |
|---|---|
| <6% | Microsphere not formed |
| 6–8% | Bad spheronization with tail |
| 8–10% | Good spheronization |
| >10% | High viscosity hard for dripping operation |
| PVA Concentration (w/v) | SA Concentration (w/v) | Diameter (mm) |
|---|---|---|
| 6% | 1% | 1.85 ± 0.05 |
| 2% | 1.78 ± 0.08 | |
| 3% | 1.76 ± 0.05 | |
| 8% | 1% | 1.90 ± 0.02 |
| 2% | 1.78 ± 0.05 | |
| 3% | 1.75 ± 0.05 | |
| 10% | 1% | 2.12 ± 0.05 |
| 2% | 2.08 ± 0.05 | |
| 3% | 1.95 ± 0.02 |
| PVA Concentration (w/v) | SA Concentration (w/v) | Mechanical Strength (N) |
|---|---|---|
| 6% | 1% | 1.35 ± 0.2 |
| 2% | 1.38 ± 0.2 | |
| 3% | 1.42 ± 0.2 | |
| 8% | 1% | 3.01 ± 0.2 |
| 2% | 3.08 ± 0.5 | |
| 3% | 3.32 ± 0.2 | |
| 10% | 1% | 5.40 ± 0.5 |
| 2% | 5.42 ± 0.2 | |
| 3% | 5.48 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, F.; Hu, S.; Zhao, W.; Mei, L. A Novel Method for γ-Aminobutyric Acid Biosynthesis Using Glutamate Decarboxylase Entrapped in Polyvinyl Alcohol–Sodium Alginate Capsules. Molecules 2023, 28, 6844. https://doi.org/10.3390/molecules28196844
Zhu F, Hu S, Zhao W, Mei L. A Novel Method for γ-Aminobutyric Acid Biosynthesis Using Glutamate Decarboxylase Entrapped in Polyvinyl Alcohol–Sodium Alginate Capsules. Molecules. 2023; 28(19):6844. https://doi.org/10.3390/molecules28196844
Chicago/Turabian StyleZhu, Fei, Sheng Hu, Weirui Zhao, and Lehe Mei. 2023. "A Novel Method for γ-Aminobutyric Acid Biosynthesis Using Glutamate Decarboxylase Entrapped in Polyvinyl Alcohol–Sodium Alginate Capsules" Molecules 28, no. 19: 6844. https://doi.org/10.3390/molecules28196844
APA StyleZhu, F., Hu, S., Zhao, W., & Mei, L. (2023). A Novel Method for γ-Aminobutyric Acid Biosynthesis Using Glutamate Decarboxylase Entrapped in Polyvinyl Alcohol–Sodium Alginate Capsules. Molecules, 28(19), 6844. https://doi.org/10.3390/molecules28196844





