Electroassisted Incorporation of Ferrocene within Sol–Gel Silica Films to Enhance Electron Transfer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Electrochemical Performance of Bare ITO Electrodes in Ferrocenium Solutions
2.2. Electrochemical Performance of Silica-Modified Electrodes
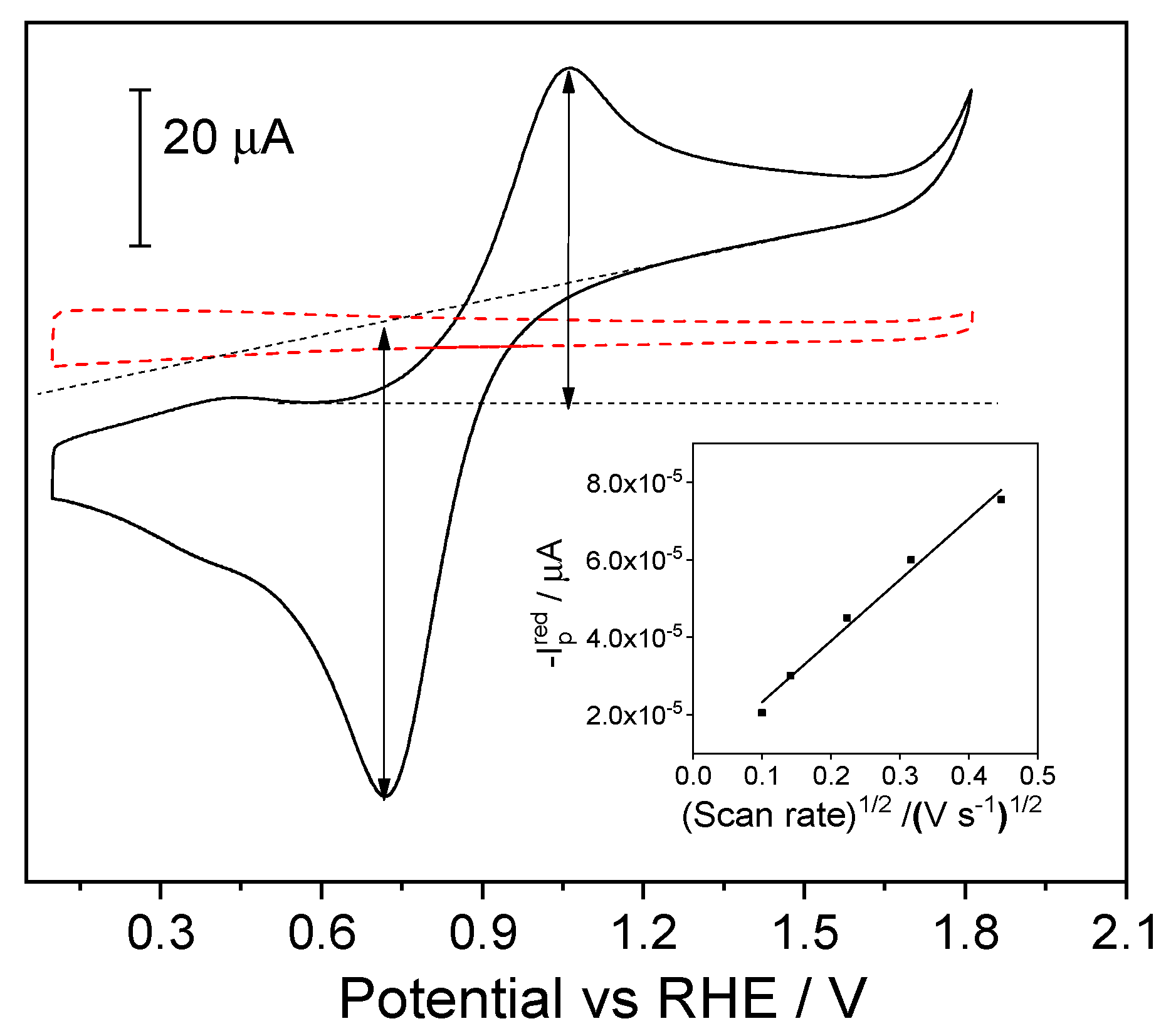
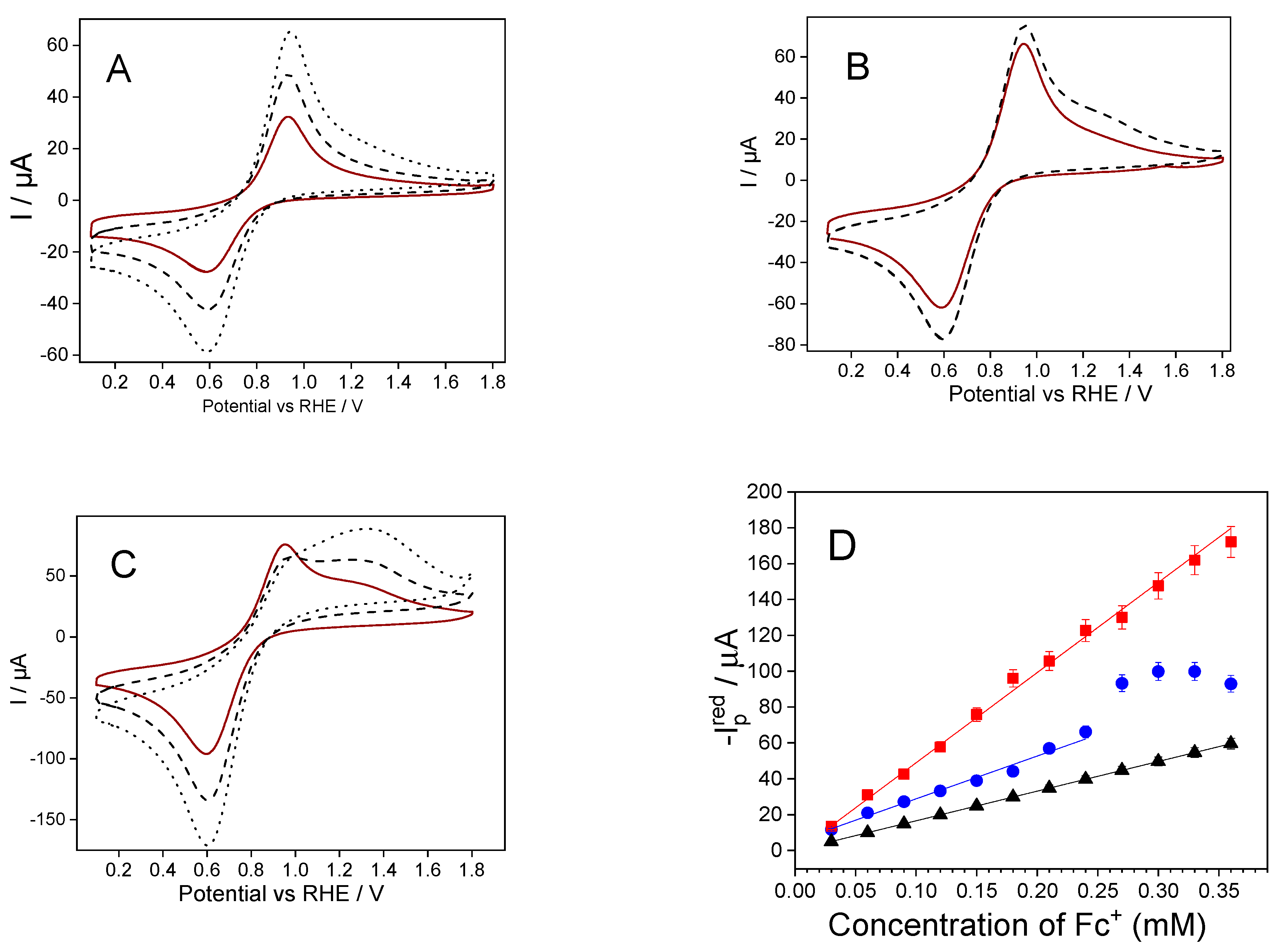
2.3. Electrochemical Performance of Fc@Silica-Modified Electrodes
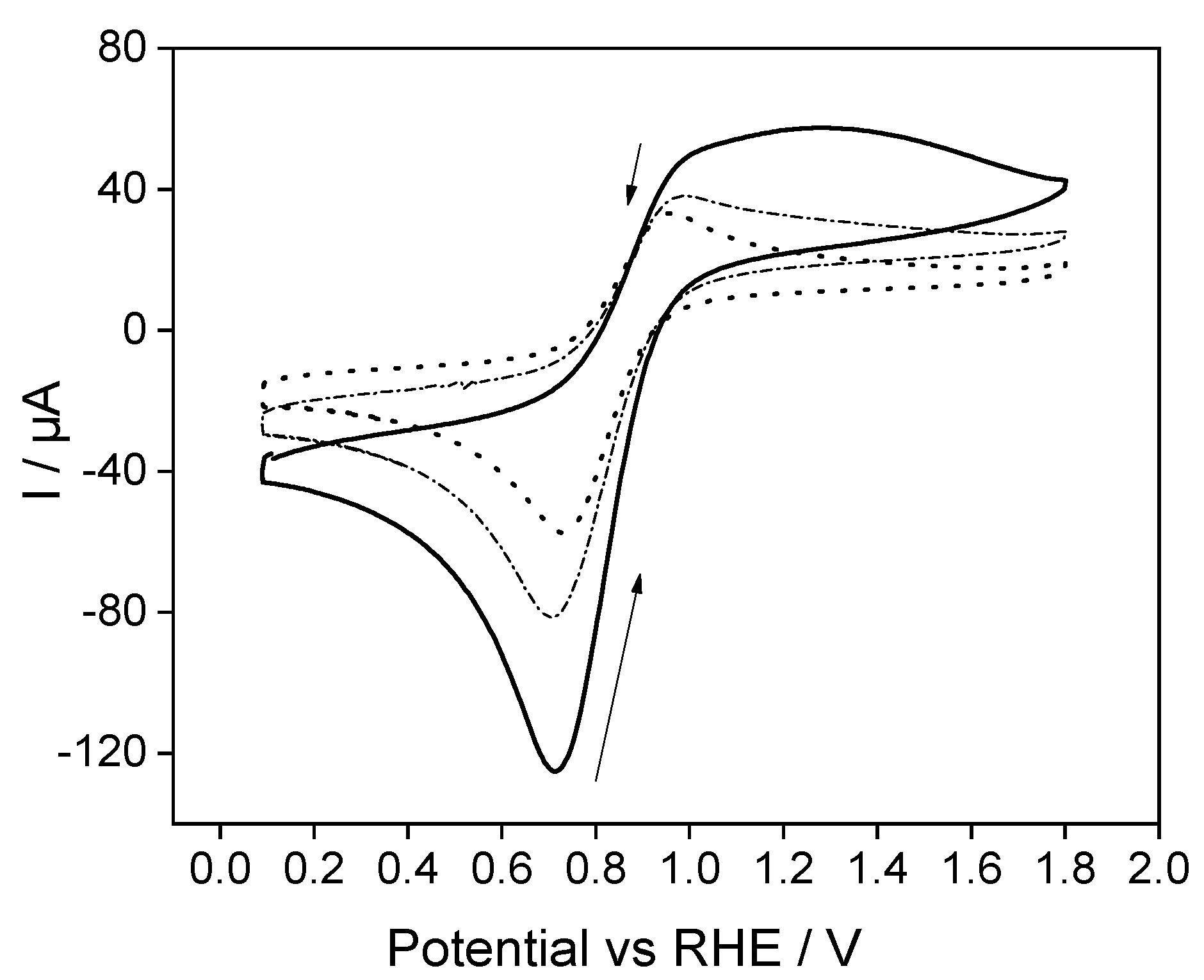
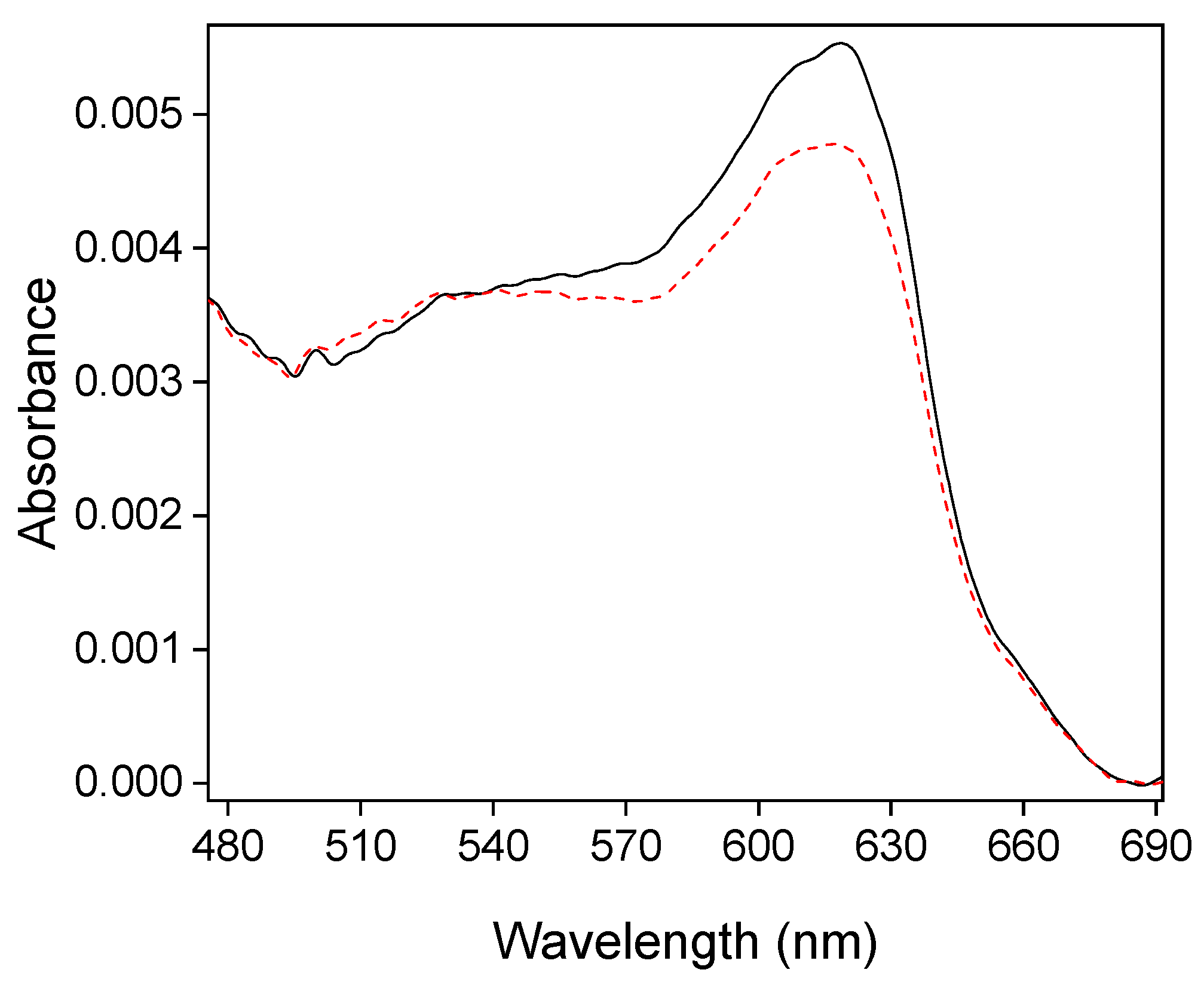
2.4. Electrochemical Performance of Fc@Silica-Modified Electrodes for Electron Transfer to Cyt c
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peled, Y.; Shamir, D.; Marks, V.; Kornweitz, H.; Albo, Y.; Yakhin, E.; Meyerstein, D.; Burg, A. Sol-gel matrices for the separation of uranyl and other heavy metals. J. Environ. Chem. Eng. 2022, 10, 108142. [Google Scholar] [CrossRef]
- Gamero-Quijano, A.; Dossot, M.; Walcarius, A.; Scanlon, M.D.; Herzog, G. Electrogeneration of a Free-Standing Cytochrome c—Silica Matrix at a Soft Electrified Interface. Langmuir 2021, 37, 4033–4041. [Google Scholar] [CrossRef]
- Shekarriz, M.; Khadivi, R.; Taghipoor, S.; Eslamian, M. Systematic synthesis of high surface area silica nanoparticles in the sol-gel condition by using the central composite design (CCD) method. Can. J. Chem. Eng. 2014, 92, 828–834. [Google Scholar] [CrossRef]
- Schäfer, H.; Milow, B.; Ratke, L. Synthesis of inorganic aerogels via rapid gelation using chloride precursors. RSC Adv. 2013, 3, 15263–15272. [Google Scholar] [CrossRef]
- Nampi, P.P.; Mohan, V.S.; Sinha, A.K.; Varma, H. High surface area sol-gel nano silica as a novel drug carrier substrate for sustained drug release. Mater. Res. Bull. 2012, 47, 1379–1384. [Google Scholar] [CrossRef]
- Hubbard, P.J.; Benzie, J.W.; Bakhmutov, V.I.; Blümel, J. Ferrocene Adsorbed on Silica and Activated Carbon Surfaces: A Solid-State NMR Study of Molecular Dynamics and Surface Interactions. ACS Appl. Mater. Interfaces 2020, 39, 1080–1091. [Google Scholar] [CrossRef]
- Vilà, N.; André, E.; Ciganda, R.; Ruiz, J.; Astruc, D.; Walcarius, A. Molecular Sieving with Vertically Aligned Mesoporous Silica Films and Electronic Wiring through Isolating Nanochannels. Chem. Mater. 2016, 28, 2511–2514. [Google Scholar] [CrossRef]
- Gamero-Quijano, A.; Huerta, F.; Salinas-Torres, D.; Morallón, E.; Montilla, F. Electrocatalytic Performance of SiO2-SWCNT Nanocomposites Prepared by Electroassisted Deposition. Electrocatalysis 2013, 4, 259–266. [Google Scholar] [CrossRef]
- Gamero-Quijano, A.; Huerta, F.; Morallón, E.; Montilla, F.; Morallon, E.; Montilla, F. Modulation of the silica sol-gel composition for the promotion of direct electron transfer to encapsulated cytochrome c. Langmuir 2014, 30, 10531–10538. [Google Scholar] [CrossRef]
- Celebanska, A.; Tomaszewska, D.; Lesniewski, A.; Opallo, M. Film electrode prepared from oppositely charged silicate submicroparticles and carbon nanoparticles for selective dopamine sensing. Biosens. Bioelectron. 2011, 26, 4417–4422. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic-inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Yui, T.; Kobayashi, Y.; Yamada, Y.; Yano, K.; Fukushima, Y.; Torimoto, T.; Takagi, K. Photoinduced electron transfer between the anionic porphyrins and viologens in titania nanosheets and monodisperse mesoporous silica hybrid films. ACS Appl. Mater. Interfaces 2011, 3, 931–935. [Google Scholar] [CrossRef]
- Vancea, A.; Kirkpatrick, I.; Worrall, D.R.; Williams, S.L. Energy and electron transfer reactions on silica gel and titania–silica mixed oxide surfaces. Res. Chem. Intermed. 2019, 45, 4205–4223. [Google Scholar] [CrossRef]
- Worrall, D.R.; Williams, S.L.; Wilkinson, F. Electron transfer reactions of anthracene adsorbed on silica gel. J. Phys. Chem. B 1997, 101, 4709–4716. [Google Scholar] [CrossRef]
- Karman, C.; Vilà, N.; Walcarius, A. Amplified Charge Transfer for Anionic Redox Probes through Oriented Mesoporous Silica Thin Films. ChemElectroChem 2016, 3, 2130–2137. [Google Scholar] [CrossRef]
- Doherty, W.J.; Armstrong, N.R.; Saavedra, S.S. Erratum: Conducting polymer growth in porous sol-gel thin films: Formation of Nanoelectrode arrays and mediated electron transfer to sequestered macromolecules (Chemistry of Materials (2005) 17 (3652–3660)). Chem. Mater. 2005, 17, 6842. [Google Scholar] [CrossRef]
- Lad, U.; Kale, G.M.; Bryaskova, R. Sarcosine Oxidase Encapsulated Polyvinyl Alcohol-Silica-AuNP Hybrid Films for Sarcosine Sensing Electrochemical Bioelectrode. J. Electrochem. Soc. 2014, 161, B98–B101. [Google Scholar] [CrossRef]
- Kumar, A.; Hsu, L.H.H.; Kavanagh, P.; Barrière, F.; Lens, P.N.L.; Lapinsonnière, L.; Lienhard, J.H.; Schröder, U.; Jiang, X.; Leech, D. The ins and outs of microorganism-electrode electron transfer reactions. Nat. Rev. Chem. 2017, 1, 24. [Google Scholar] [CrossRef]
- Takahashi, S.; Anzai, J. Recent Progress in Ferrocene-Modified Thin Films and Nanoparticles for Biosensors. Materials 2013, 6, 5742–5762. [Google Scholar] [CrossRef]
- Wang, B.; Anzai, J.I. A facile electrochemical detection of hypochlorite ion based on ferrocene compounds. Int. J. Electrochem. Sci. 2015, 10, 3260–3268. [Google Scholar] [CrossRef]
- Zhan, T.; Feng, X.Z.; An, Q.Q.; Li, S.; Xue, M.; Chen, Z.; Han, G.C.; Kraatz, H.B. Enzyme-free glucose sensors with efficient synergistic electro-catalysis based on a ferrocene derivative and two metal nanoparticles. RSC Adv. 2022, 12, 5072–5079. [Google Scholar] [CrossRef]
- Guven, N.; Apetrei, R.M.; Camurlu, P. Next step in 2nd generation glucose biosensors: Ferrocene-loaded electrospun nanofibers. Mater. Sci. Eng. C 2021, 128, 112270. [Google Scholar] [CrossRef]
- Cass, A.E.G.; Davis, G.; Francis, G.D.; Allen, H.; Hill, O.; Aston, W.J.; Higgins, I.J.; Plotkin, E.V.; Scott, L.D.L.; Turner, A.P.F. Ferrocene-Mediated Enzyme Electrode for Amperometric Determination of Glucose. Anal. Chem. 1984, 56, 667–671. [Google Scholar] [CrossRef]
- Delacote, C.; Bouillon, J.-P.; Walcarius, A. Voltammetric response of ferrocene-grafted mesoporous silica. Electrochim. Acta 2006, 51, 6373–6383. [Google Scholar] [CrossRef]
- Vilà, N.; Walcarius, A. Electrochemical response of vertically-aligned, ferrocene-functionalized mesoporous silica films: Effect of the supporting electrolyte. Electrochim. Acta 2015, 179, 304–314. [Google Scholar] [CrossRef]
- Audebert, P.; Miomandre, F.; Sadki, S.; Sallard, S. Analysis of the gelation process of hybrid silica–zirconia and silica–titania gels using two different grafted electroactive probes. J. Electroanal. Chem. 2006, 598, 15–21. [Google Scholar] [CrossRef]
- Rohlfing, D.F.; Rathouský, J.; Rohlfing, Y.; Bartels, O.; Wark, M. Functionalized mesoporous silica films as a matrix for anchoring electrochemically active guests. Langmuir 2005, 21, 11320–11329. [Google Scholar] [CrossRef]
- Abramczyk, H.; Brozek-Pluska, B.; Kopeć, M. Double face of cytochrome c in cancers by Raman imaging. Sci. Rep. 2022, 12, 2120. [Google Scholar] [CrossRef]
- Martinou, J.C.; Desagher, S.; Antonsson, B. Cytochrome c release from mitochondria: All or nothing. Nat. Cell Biol. 2000, 2, 41–43. [Google Scholar] [CrossRef]
- Manickam, P.; Kaushik, A.; Karunakaran, C.; Bhansali, S. Recent advances in cytochrome c biosensing technologies. Biosens. Bioelectron. 2017, 87, 654–668. [Google Scholar] [CrossRef]
- Ouyang, C.; Aoki, K.J.; Chen, J.; Nishiumi, T. Determination of concentration of saturated ferrocene in aqueous solution. Rep. Electrochem. 2013, 3, 17–23. [Google Scholar] [CrossRef]
- Wang, Y.; Rogers, E.I.; Compton, R.G. The measurement of the diffusion coefficients of ferrocene and ferrocenium and their temperature dependence in acetonitrile using double potential step microdisk electrode chronoamperometry. J. Electroanal. Chem. 2010, 648, 15–19. [Google Scholar] [CrossRef]
- Abdallah, M.; Alharbi, A.; Morad, M.; Hameed, A.M.; Al-Juaid, S.S.; Foad, N.; Mabrouk, E.M. Electrochemical studies and the electrode reaction mechanism of ferrocene and naphthoquinones in microemulsion Medium at GC electrode. Int. J. Electrochem. Sci. 2020, 15, 6522–6548. [Google Scholar] [CrossRef]
- Miecznikowski, K.; Cox, J.A. Electroanalysis based on stand-alone matrices and electrode-modifying films with silica sol-gel frameworks: A review. J. Solid State Electrochem. 2020, 24, 2617–2631. [Google Scholar] [CrossRef]
- Sipa, K.; Rudnicki, K.; Vilà, N.; Herzog, G.; Skrzypek, S.; Poltorak, L.; Walcarius, A. Switchable voltammetric response of electrodes modified with a mesoporous silica thin film and a polyelectrolyte multilayer. Electrochem. Commun. 2021, 132, 107142. [Google Scholar] [CrossRef]
- Walcarius, A. Silica-based electrochemical sensors and biosensors: Recent trends. Curr. Opin. Electrochem. 2018, 10, 88–97. [Google Scholar] [CrossRef]
- Brinker, C.F.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing, 1st ed.; Academic Press: Cambridge, MA, USA, 1990; ISBN 9780121349707. [Google Scholar]
- Moerz, S.T.; Huber, P. Protein adsorption into mesopores: A combination of electrostatic interaction, counterion release, and van der waals forces. Langmuir 2014, 30, 2729–2737. [Google Scholar] [CrossRef]
- Nasir, T.; Herzog, G.; Hébrant, M.; Despas, C.; Liu, L.; Walcarius, A. Mesoporous Silica Thin Films for Improved Electrochemical Detection of Paraquat. ACS Sens. 2018, 3, 484–493. [Google Scholar] [CrossRef]
- Fery-Forgues, S.; Delavaux-Nicot, B. Ferrocene and ferrocenyl derivatives in luminescent systems. J. Photochem. Photobiol. A Chem. 2000, 132, 137–159. [Google Scholar] [CrossRef]
- Paparazzo, E. XPS and Auger Spectroscopy on mixtures of the oxides SiO2, Al2O3, Fe2O3, and Cr2O3. J. Electron Spectrosc. Relat. Phenom. 1987, 43, 97–112. [Google Scholar] [CrossRef]
- Singh, A.; Chowdhury, D.R.; Paul, A. A kinetic study of ferrocenium cation decomposition utilizing an integrated electrochemical methodology composed of cyclic voltammetry and amperometry. Analyst 2014, 139, 5747–5754. [Google Scholar] [CrossRef]
- Das, J.; Jo, K.; Jae, W.L.; Yang, H. Electrochemical immunosensor using p-aminophenol redox cycling by hydrazine combined with a low background current. Anal. Chem. 2007, 79, 2790–2796. [Google Scholar] [CrossRef]
- Akanda, M.R.; Tamilavan, V.; Park, S.; Jo, K.; Hyun, M.H.; Yang, H. Hydroquinone diphosphate as a phosphatase substrate in enzymatic amplification combined with electrochemical-chemical-chemical redox cycling for the detection of E. coli O157:H7. Anal. Chem. 2013, 85, 1631–1636. [Google Scholar] [CrossRef]
- Kajama, M.N. Hydrogen Permeation Using Nanostructured Silica Membranes. Sustain. Dev. Plan. VII 2015, 1, 447–456. [Google Scholar] [CrossRef]
- Choma, J.; Kloske, M.; Jaroniec, M. An Improved Methodology for Adsorption Characterization of Unmodified and Modified Silica Gels. J. Colloid Interface Sci. 2003, 266, 168–174. [Google Scholar] [CrossRef]
- Scott, D.R.; Becker, R.S. Comprehensive Investigation of the Electronic Spectroscopy and Theoretical Treatments of Ferrocene and Nickelocene. J. Chem. Phys. 1961, 35, 516–531. [Google Scholar] [CrossRef]
- Cardinaud, C.; Rhounna, A.; Turban, G.; Grolleau, B. Analyse XPS Des Surfaces de Si et SiO2 Exposées Aux Plasmas de CHF3 et CHF3—C2F6. Polymérisation et Gravure. Rev. Phys. Appliquée 1989, 24, 309–321. [Google Scholar] [CrossRef]
- Johansson, L.I.; Owman, F.; Mårtensson, P. High-Resolution Core-Level Study of 6H-SiC (0001). Phys. Rev. B 1996, 53, 13793. [Google Scholar] [CrossRef]
- Hollinger, G. Structures Chimique et Electronique de l’interface SiO2-Si. Appl. Surf. Sci. 1981, 8, 318–336. [Google Scholar] [CrossRef]
- Hristova, S.H.; Zhivkov, A.M. Isoelectric Point of Free and Adsorbed Cytochrome c Determined by Various Methods. Colloids Surf. B Biointerfaces 2019, 174, 87–94. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loughlani, R.-I.; Gamero-Quijano, A.; Montilla, F. Electroassisted Incorporation of Ferrocene within Sol–Gel Silica Films to Enhance Electron Transfer. Molecules 2023, 28, 6845. https://doi.org/10.3390/molecules28196845
Loughlani R-I, Gamero-Quijano A, Montilla F. Electroassisted Incorporation of Ferrocene within Sol–Gel Silica Films to Enhance Electron Transfer. Molecules. 2023; 28(19):6845. https://doi.org/10.3390/molecules28196845
Chicago/Turabian StyleLoughlani, Rayane-Ichrak, Alonso Gamero-Quijano, and Francisco Montilla. 2023. "Electroassisted Incorporation of Ferrocene within Sol–Gel Silica Films to Enhance Electron Transfer" Molecules 28, no. 19: 6845. https://doi.org/10.3390/molecules28196845
APA StyleLoughlani, R.-I., Gamero-Quijano, A., & Montilla, F. (2023). Electroassisted Incorporation of Ferrocene within Sol–Gel Silica Films to Enhance Electron Transfer. Molecules, 28(19), 6845. https://doi.org/10.3390/molecules28196845





