Sea Conch Peptides Hydrolysate Alleviates DSS-Induced Colitis in Mice through Immune Modulation and Gut Microbiota Restoration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Conch Peptide Hydrolysate (CPH)
2.2. Molecular Mass Distribution of CPH (Liquid Chromatography-Mass Spectrometry)
2.3. Experimental Design and Animal Housing
2.4. Colitis Induction and Treatment Protocol
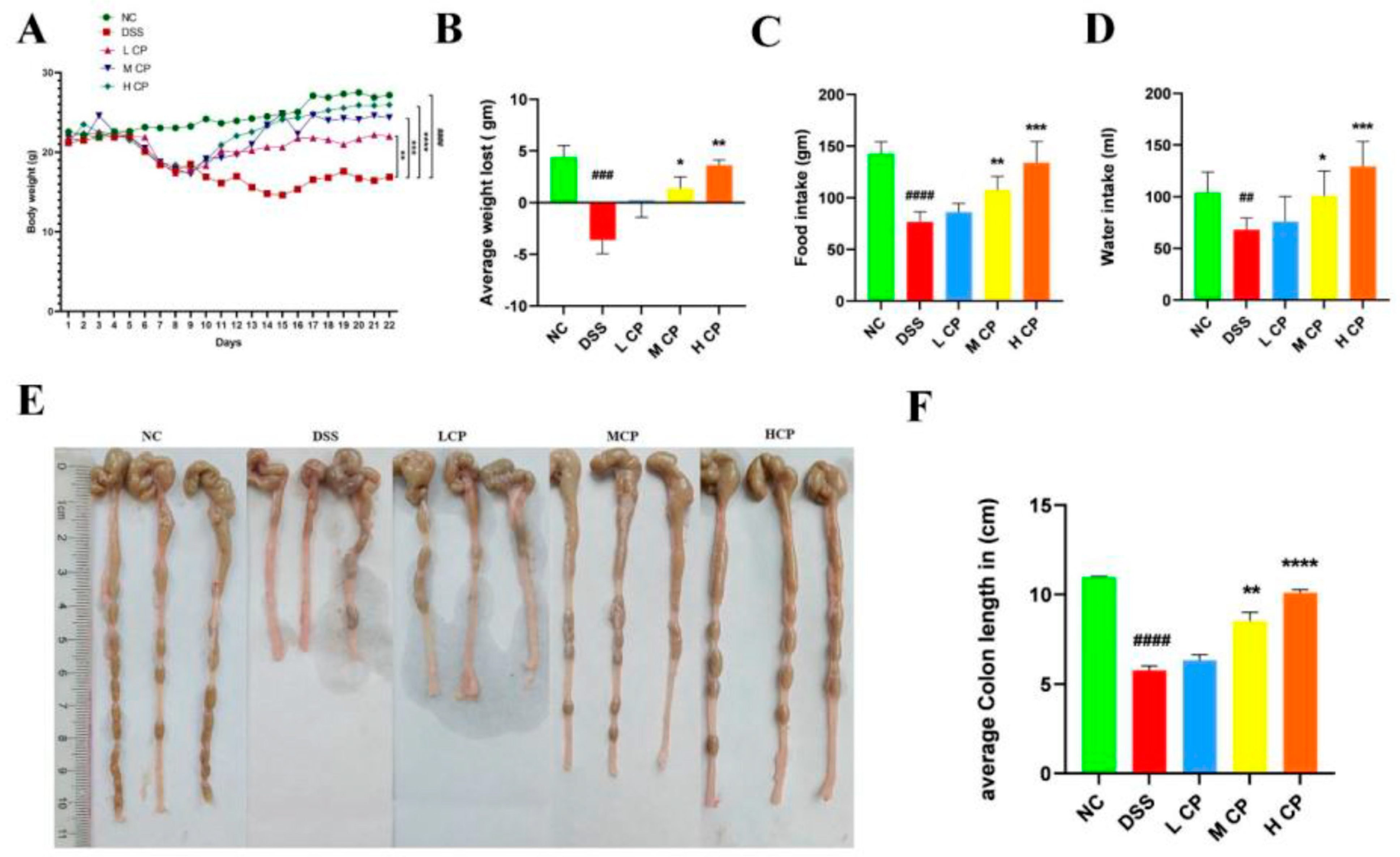
2.5. Disease Activity Index Measurement
2.6. Body Weight, Organ Indexing, Food, and Water Intake Determination
2.7. Histopathological Examination of the Colon
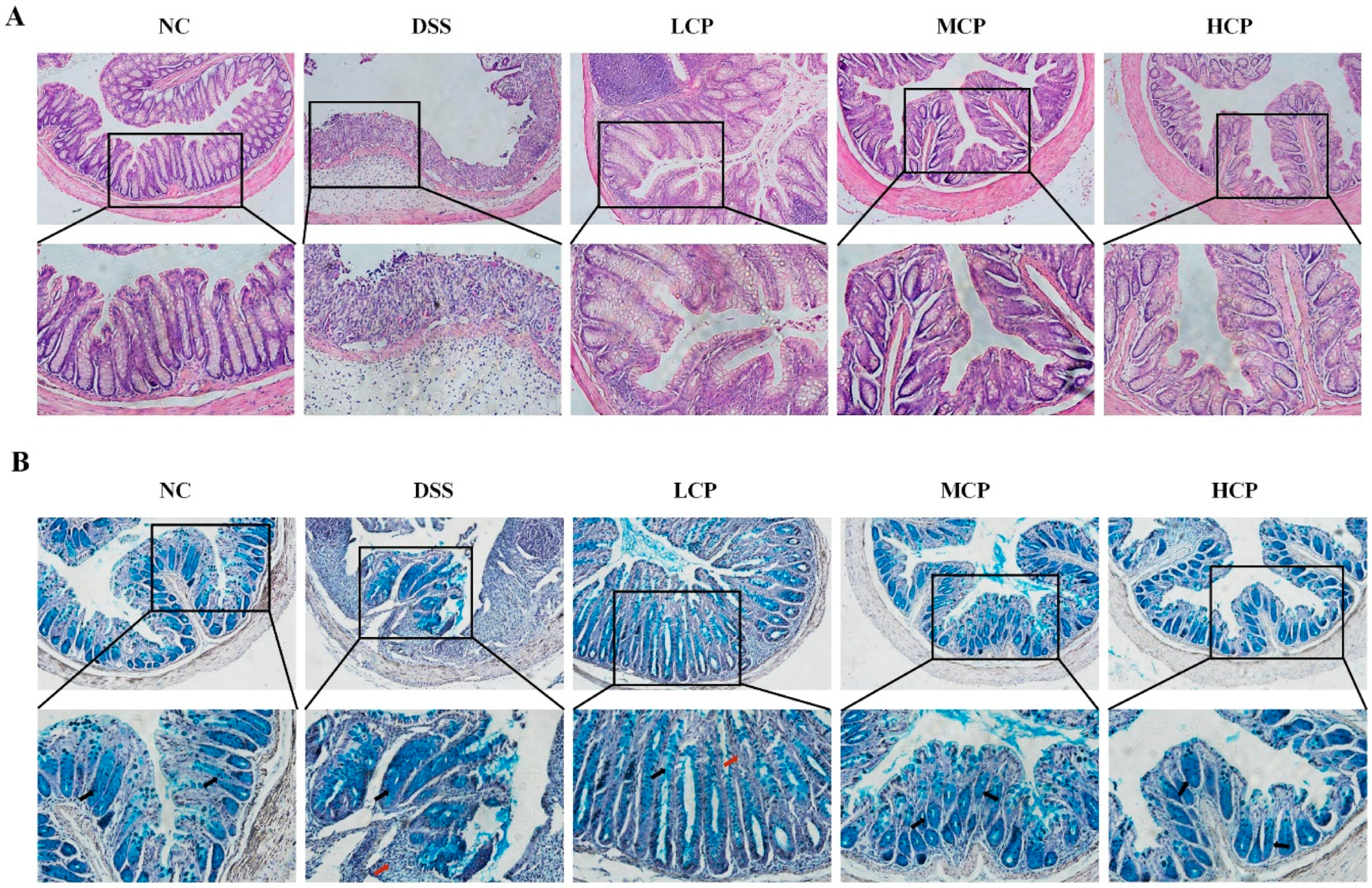
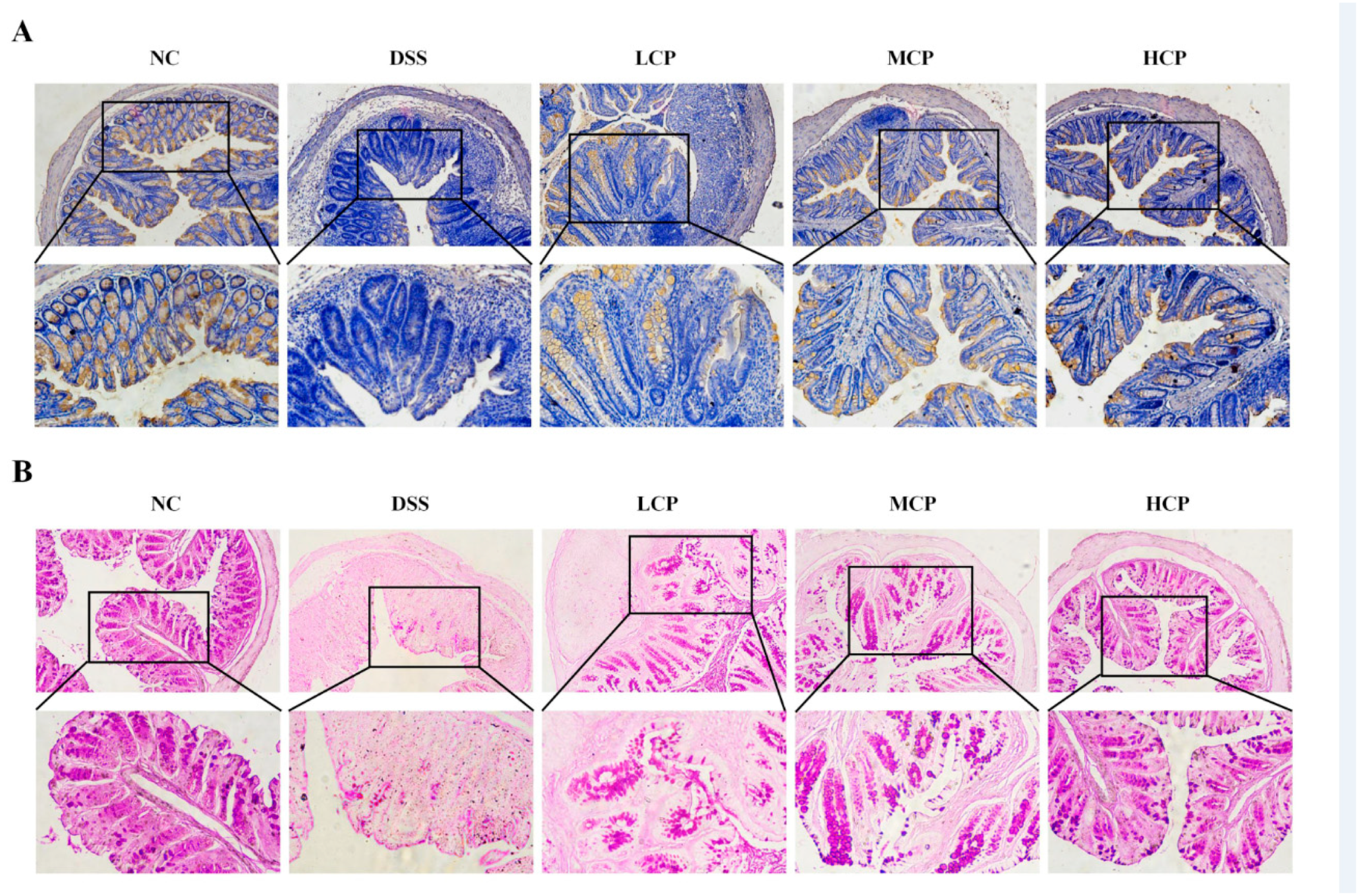
2.8. Mucin Analysis, Goblet Cells, and Mucus Layer Thickness
2.9. Myeloperoxidase Activity Assay
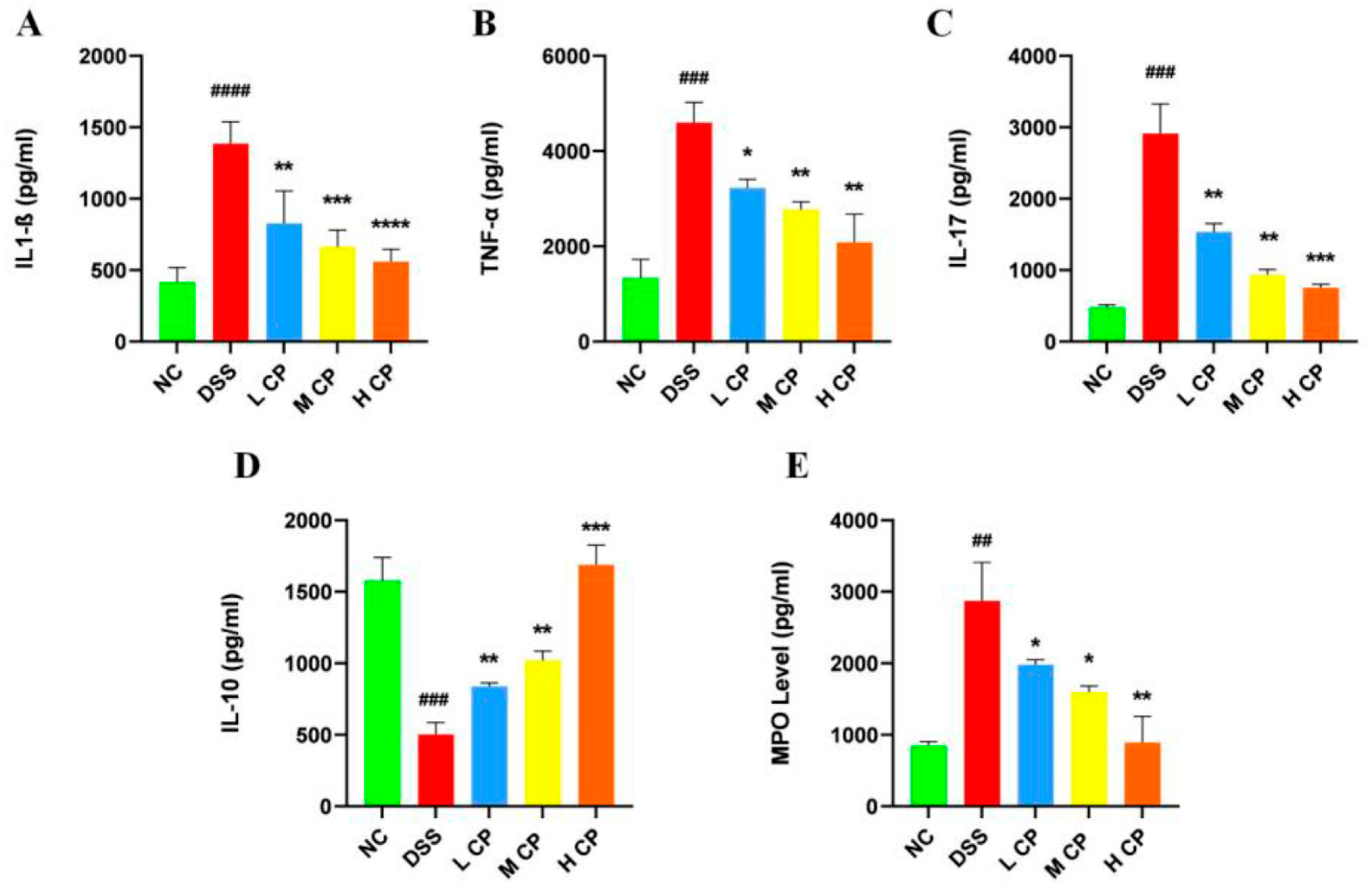
2.10. Measurement of Cytokine Level in the Colon by ELISA
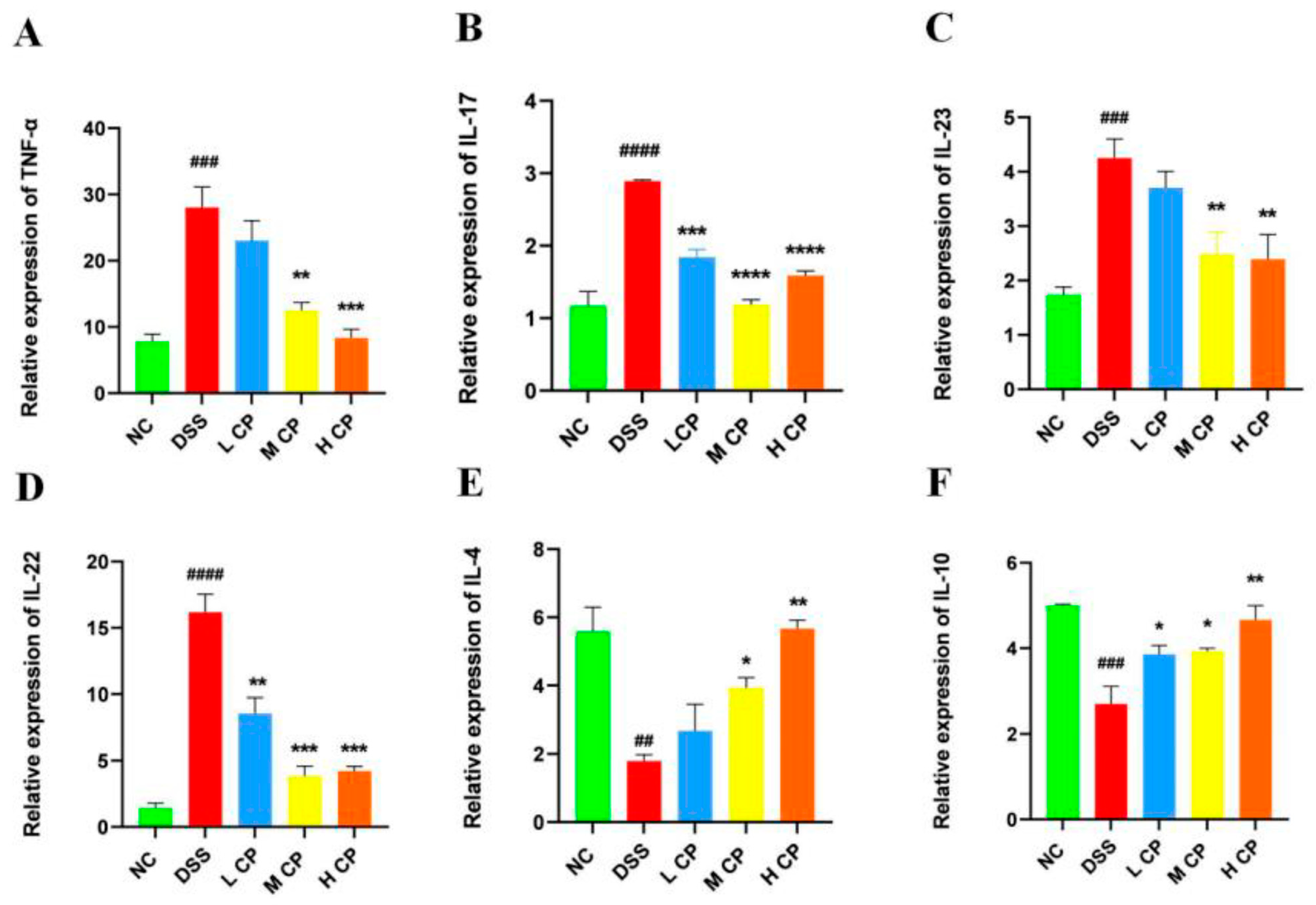
2.11. Determination of Intestinal mRNA
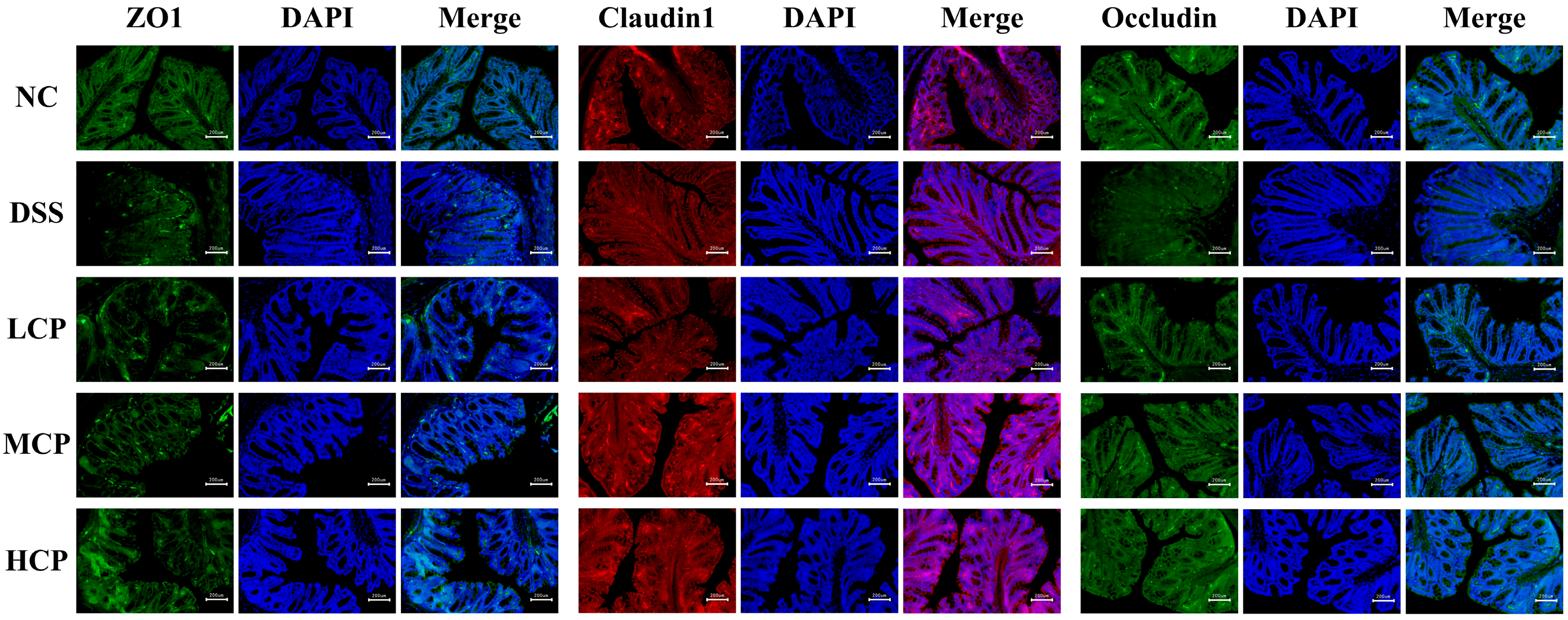
2.12. Immunofluorescent Staining for Tight Junction Protein
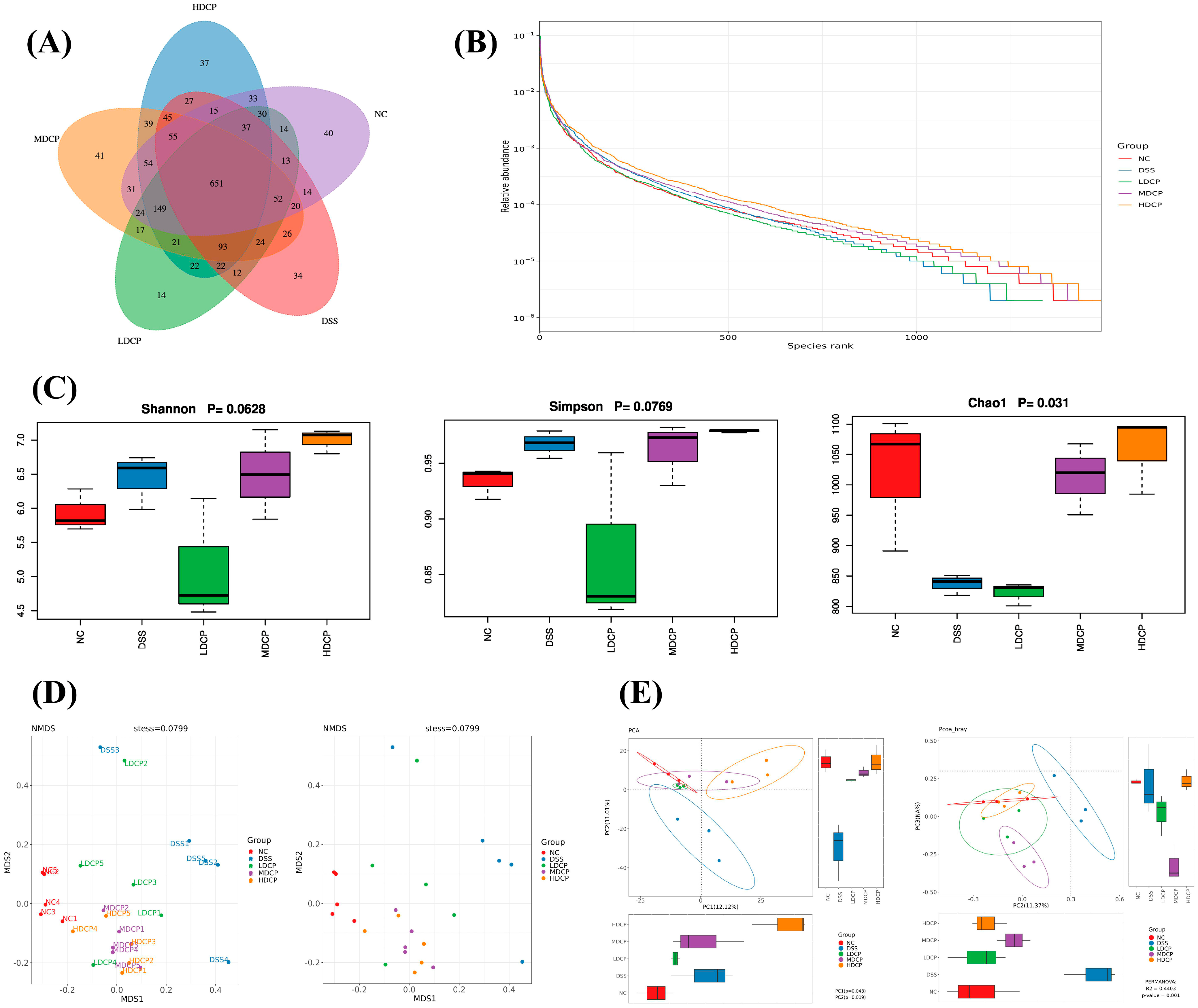
2.13. Stool DNA Extraction and 16s RNA Pyrosequencing
2.14. Statistical Analysis
3. Results
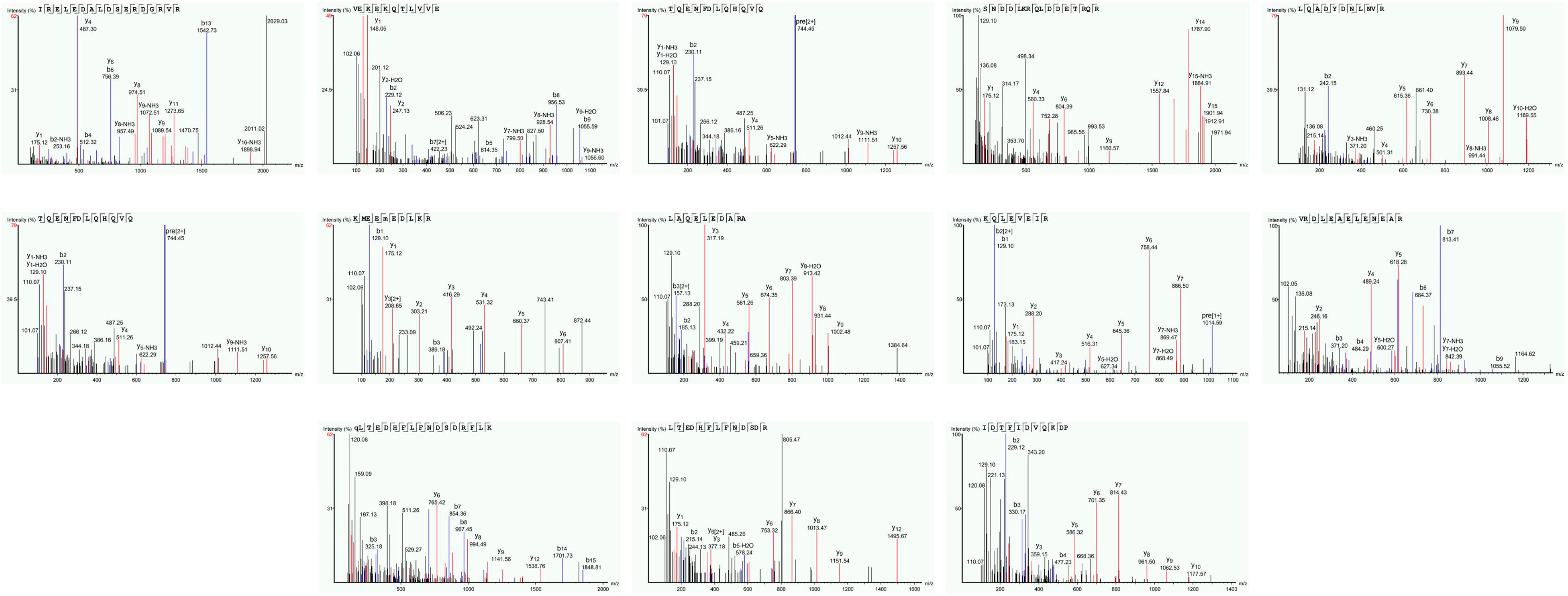
3.1. Molecular Mass Distribution of Conch Peptides Hydrolysate
3.2. Conch Peptides Hydrolysate Ameliorate Clinical Symptoms in DSS Induce Colitis Mice
3.3. CPH Improves Histological Changes, Goblet Cell and Mucin Production
3.4. CPH Regulate Leaky Gut Barrier and Intestinal Inflammation
3.5. CPH Treatment Regulates mRNA Expression in Colon
3.6. CPH Enhance the Expression of Tight Junction Proteins
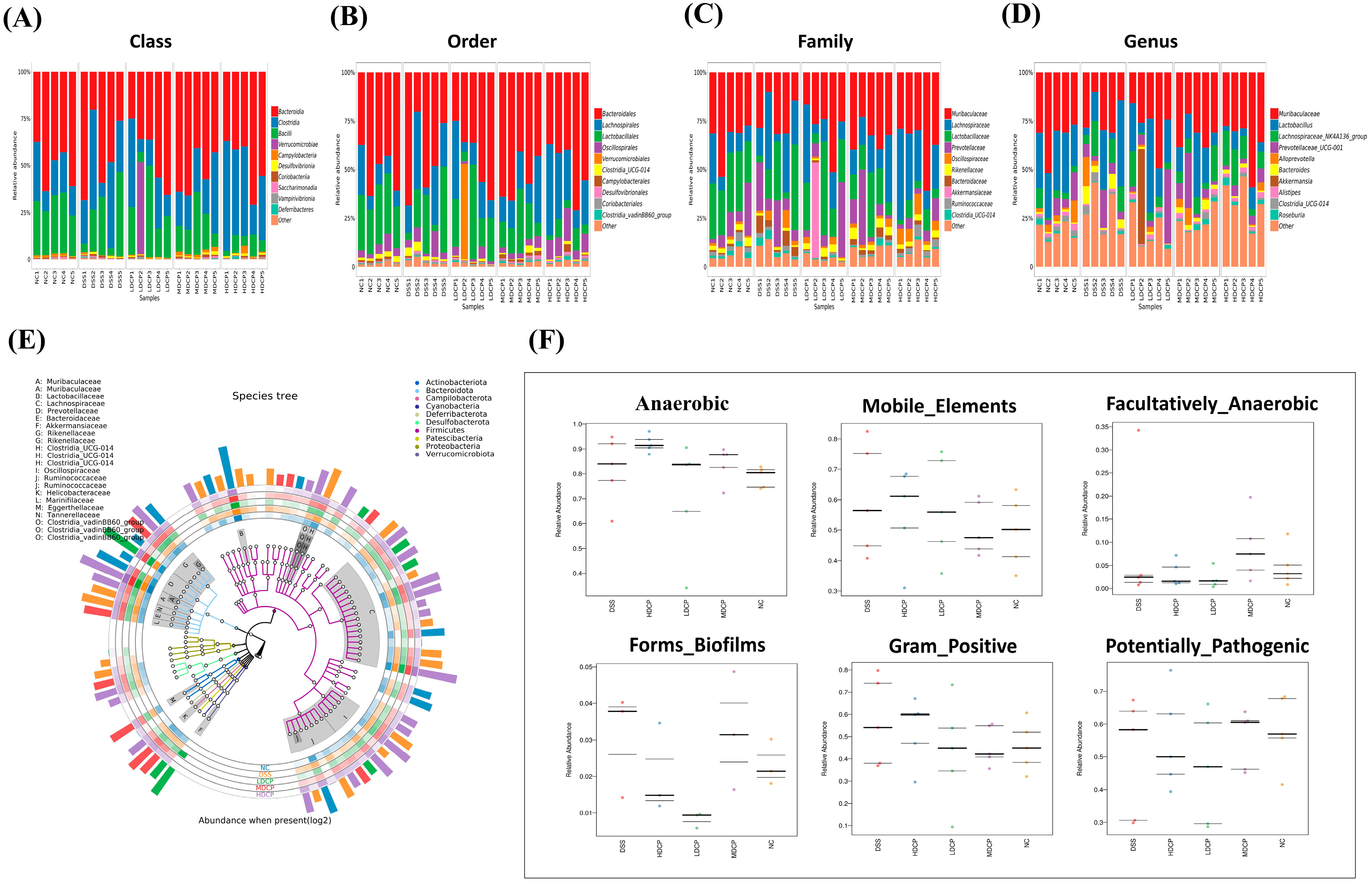
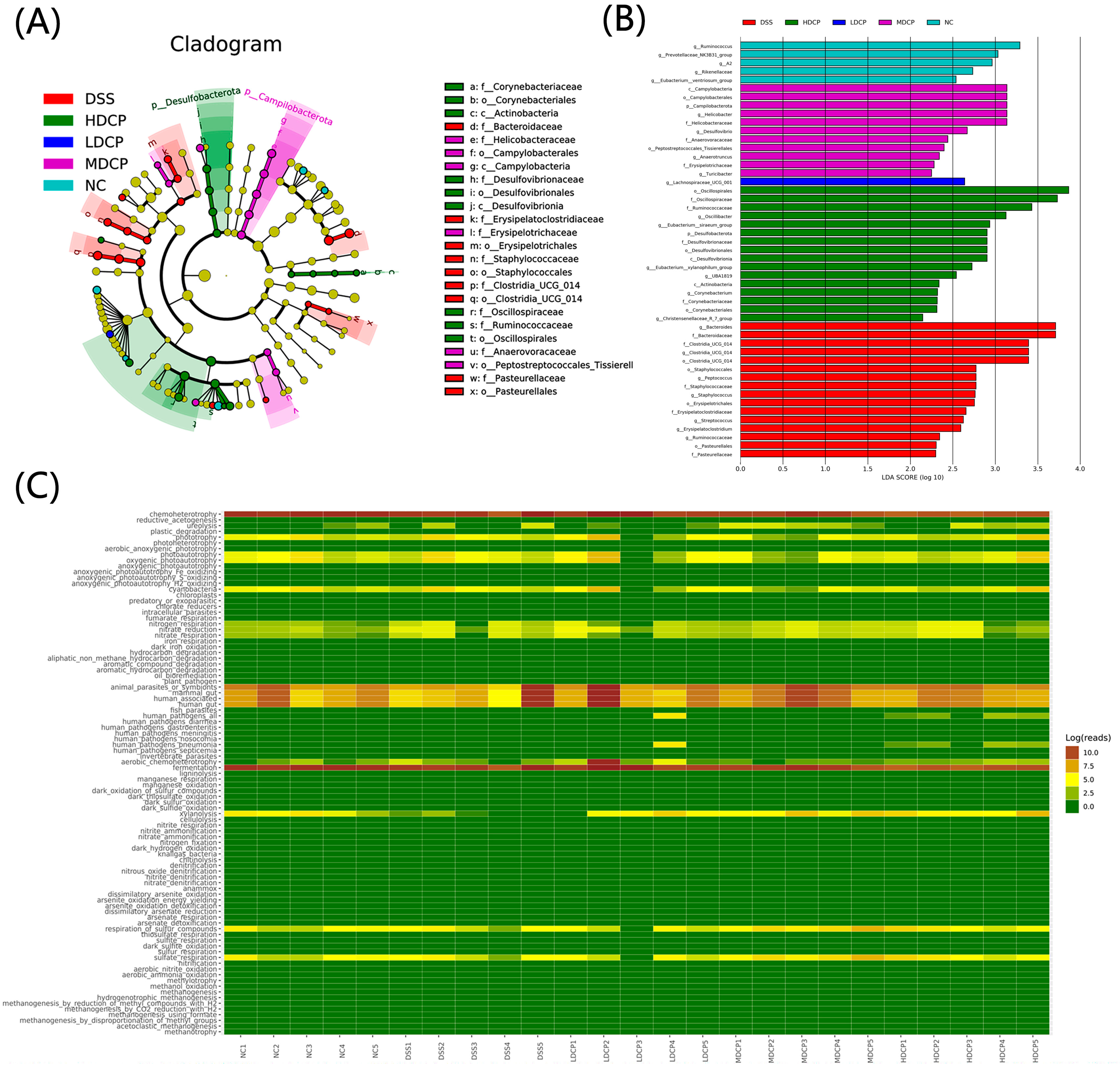
3.7. CPH Reinstate Gut Microbiota Dysbiosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Binder, V. Epidemiology of IBD during the twentieth century: An integrated view. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 463–479. [Google Scholar] [CrossRef] [PubMed]
- Bouma, G.; Strober, W. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 2003, 3, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Coskun, M. Intestinal epithelium in inflammatory bowel disease. Front. Med. 2014, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Lee, B.; Lee, H.-S.; Bae, E.-A.; Lee, H.; Ahn, Y.-T.; Lim, K.-S.; Huh, C.-S.; Kim, D.-H. Lactobacillus suntoryeus inhibits pro-inflammatory cytokine expression and TLR-4-linked NF-κB activation in experimental colitis. Int. J. Color. Dis. 2009, 24, 231–237. [Google Scholar] [CrossRef]
- Mar, J.S.; Nagalingam, N.A.; Song, Y.; Onizawa, M.; Lee, J.W.; Lynch, S.V. Amelioration of DSS-induced murine colitis by VSL# 3 supplementation is primarily associated with changes in ileal microbiota composition. Gut Microbes 2014, 5, 494–503. [Google Scholar]
- Baumgart, D.C.; Sandborn, W.J. Crohn’s disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef]
- DeRoche, T.C.; Xiao, S.-Y.; Liu, X. Histological evaluation in ulcerative colitis. Gastroenterol. Rep. 2014, 2, 178–192. [Google Scholar] [CrossRef]
- Dethlefsen, L.; Huse, S.; Sogin, M.L.; Relman, D.A. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008, 6, e280. [Google Scholar] [CrossRef]
- Engel, M.A.; Neurath, M.F. New pathophysiological insights and modern treatment of IBD. J. Gastroenterol. 2010, 45, 571–583. [Google Scholar] [CrossRef]
- Marehbian, J.; Arrighi, M.H.; Hass, S.; Tian, H.; Sandborn, W.J. Adverse events associated with common therapy regimens for moderate-to-severe Crohn’s disease. Off. J. Am. Coll. Gastroenterol. 2009, 104, 2524–2533. [Google Scholar] [CrossRef]
- Van Assche, G.; Dignass, A.; Panes, J.; Beaugerie, L.; Karagiannis, J.; Allez, M.; Ochsenkühn, T.; Orchard, T.; Rogler, G.; Louis, E.; et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J. Crohn’s Colitis 2010, 4, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-T.; Lai, H.-C.; Chen, Y.-B.; Chen, L.-G.; Wu, Y.-H.; Ko, Y.-F.; Lu, C.-C.; Chang, C.-J.; Wu, C.-Y.; Martel, J.; et al. cis-Resveratrol produces anti-inflammatory effects by inhibiting canonical and non-canonical inflammasomes in macrophages. J. Endotoxin Res. 2014, 20, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Diling, C.; Xin, Y.; Chaoqun, Z.; Jian, Y.; Xiaocui, T.; Jun, C.; Ou, S.; Yizhen, X. Extracts from Hericium erinaceus relieve inflammatory bowel disease by regulating immunity and gut microbiota. Oncotarget 2017, 8, 85838. [Google Scholar] [CrossRef]
- Kanwal, S.; Joseph, T.P.; Owusu, L.; Xiaomeng, R.; Meiqi, L.; Yi, X. A polysaccharide isolated from Dictyophora indusiata promotes recovery from antibiotic-driven intestinal dysbiosis and improves gut epithelial barrier function in a mouse model. Nutrients 2018, 10, 1003. [Google Scholar] [CrossRef]
- Ashby, M.; Petkova, A.; Gani, J.; Mikut, R.; Hilpert, K. Use of peptide libraries for identification and optimization of novel antimicrobial peptides. Curr. Top. Med. Chem. 2017, 17, 537–553. [Google Scholar] [CrossRef]
- Moiola, M.; Memeo, M.G.; Quadrelli, P. Stapled peptides—A useful improvement for peptide-based drugs. Molecules 2019, 24, 3654. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, R.R.; Popovic-Nikolic, M.R.; Nikolic, K.; Uliassi, E.; Bolognesi, M.L. A perspective on multi-target drug discovery and design for complex diseases. Clin. Transl. Med. 2018, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Garattini, S.; La Vecchia, C. Perspectives in cancer chemotherapy. Eur. J. Cancer 2001, 37, 128–147. [Google Scholar] [CrossRef]
- Ang, K.; Holmes, M.; Kara, U. Immune-mediated parasite clearance in mice infected with Plasmodium berghei following treatment with manzamine A. Parasitol. Res. 2001, 87, 715–721. [Google Scholar] [CrossRef]
- Sawadogo, W.R.; Boly, R.; Cerella, C.; Teiten, M.H.; Dicato, M.; Diederich, M. A survey of marine natural compounds and their derivatives with anti-cancer activity reported in 2012. Molecules 2015, 20, 7097–7142. [Google Scholar] [CrossRef]
- Butler, M.S. The role of natural product chemistry in drug discovery. J. Nat. Prod. 2004, 67, 2141–2153. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural products as sources of new drugs over the period 1981−2002. J. Nat. Prod. 2003, 66, 1022–1037. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- White, J. Drug Addiction: From Basic Research to Therapy; Wiley Online Library: Hoboken, NJ, USA, 2009. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Drugs and drug candidates from marine sources: An assessment of the current “state of play”. Planta Med. 2016, 82, 775–789. [Google Scholar] [CrossRef]
- Nobili, S.; Lippi, D.; Witort, E.; Donnini, M.; Bausi, L.; Mini, E.; Capaccioli, S. Natural compounds for cancer treatment and prevention. Pharmacol. Res. 2009, 59, 365–378. [Google Scholar] [CrossRef]
- Bhanot, A.; Sharma, R.; Noolvi, M.N. Natural sources as potential anti-cancer agents: A review. Int. J. Phytomed. 2011, 3, 9–26. [Google Scholar]
- Qian, B.; Zhao, X.; Yang, Y.; Tian, C. Antioxidant and anti-inflammatory peptide fraction from oyster soft tissue by enzymatic hydrolysis. Food Sci. Nutr. 2020, 8, 3947–3956. [Google Scholar] [CrossRef]
- Merdzhanova, A.; Panayotova, V.; Dobreva, D.A.; Stancheva, R.; Peycheva, K. Lipid composition of raw and cooked Rapana venosa from the Black Sea. Ovidius Univ. Ann. Chem. 2018, 29, 49–55. [Google Scholar] [CrossRef]
- Leontowicz, M.; Leontowicz, H.; Namiesnik, J.; Apak, R.; Barasch, D.; Nemirovski, A.; Moncheva, S.; Goshev, I.; Trakhtenberg, S.; Gorinstein, S. Rapana venosa consumption improves the lipid profiles and antioxidant capacities in serum of rats fed an atherogenic diet. Nutr. Res. 2015, 35, 592–602. [Google Scholar] [CrossRef]
- Dolashka, P.; Nesterova, N.; Zagorodnya, S.; Dolashki, A.; Baranova, G.; Golovan, A.; Voelter, W. Antiviral activity of hemocyanin Rapana venosa and its isoforms against Epstein-Barr virus. Glob. J. Pharmacol. 2014, 8, 206–212. [Google Scholar]
- Dolashka, P.; Moshtanska, V.; Borisova, V.; Dolashki, A.; Stevanovic, S.; Dimanov, T.; Voelter, W. Antimicrobial proline-rich peptides from the hemolymph of marine snail Rapana venosa. Peptides 2011, 32, 1477–1483. [Google Scholar] [CrossRef]
- Benkendorff, K.; Rudd, D.; Devi Nongmaithem, B.; Liu, L.; Young, F.; Edwards, V.; Avila, C.; Abbott, C.A. Are the traditional medical uses of Muricidae molluscs substantiated by their pharmacological properties and bioactive compounds? Mar. Drugs 2015, 13, 5237–5275. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, S.; Fan, F.; Tu, M.; Xu, Z.; Du, M. Identification and molecular mechanism of antithrombotic peptides from oyster proteins released in simulated gastro-intestinal digestion. Food Funct. 2019, 10, 5426–5435. [Google Scholar] [CrossRef]
- Kanwal, S.; Joseph, T.P.; Aliya, S.; Song, S.; Saleem, M.Z.; Nisar, M.A.; Wang, Y.; Meyiah, A.; Ma, Y.; Xin, Y. Attenuation of DSS induced colitis by Dictyophora indusiata polysaccharide (DIP) via modulation of gut microbiota and inflammatory related signaling pathways. J. Funct. Foods 2020, 64, 103641. [Google Scholar] [CrossRef]
- He, X.; Wei, Z.; Wang, J.; Kou, J.; Liu, W.; Fu, Y.; Yang, Z. Alpinetin attenuates inflammatory responses by suppressing TLR4 and NLRP3 signaling pathways in DSS-induced acute colitis. Sci. Rep. 2016, 6, 28370. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.-D.; Lim, S.; Kim, D.-H. Oleanolic acid ameliorates dextran sodium sulfate-induced colitis in mice by restoring the balance of Th17/Treg cells and inhibiting NF-κB signaling pathway. Int. Immunopharmacol. 2015, 29, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.-P.; Sun, X.-N.; Cui, L.-J.; Cao, Q.-F.; Zhuang, G.-F.; Deng, T.-Z.; Zhang, D.-Y. Suppressive effect of pectic polysaccharides extracted from Rauwolfia verticillata (Lour.) Baill. var. hainanensis Tsiang on inflammation by regulation of NF–κ B pathway and interleukin–17 in mice with dextran sulphatesodium–induced ulcerative colitis. Asian Pac. J. Trop. Med. 2015, 8, 147–152. [Google Scholar] [CrossRef]
- Kitts, D.D.; Weiler, K. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr. Pharm. Des. 2003, 9, 1309–1323. [Google Scholar] [CrossRef]
- Najafian, L.; Babji, A. A review of fish-derived antioxidant and antimicrobial peptides: Their production, assessment, and applications. Peptides 2012, 33, 178–185. [Google Scholar] [CrossRef]
- Khan, A.I.; Rehman, A.U.; Farooqui, N.A.; Siddiqui, N.Z.; Ayub, Q.; Ramzan, M.N.; Wang, L.; Xin, Y. Effects of shrimp peptide hydrolysate on intestinal microbiota restoration and immune modulation in cyclophosphamide-treated mice. Molecules 2022, 27, 1720. [Google Scholar] [CrossRef]
- Chandrashekar, P.M.; Venkatesh, Y.P. Fructans from aged garlic extract produce a delayed immunoadjuvant response to ovalbumin antigen in BALB/c mice. Immunopharmacol. Immunotoxicol. 2012, 34, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Kraus, M.D. Splenic histology and histopathology: An update. Semin. Diagn. Pathol. 2003, 20, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-Q.; Li, H.-S.; Nie, S.-P.; Shen, M.-Y.; Hu, J.-L.; Xie, M.-Y. Tea polysaccharide prevents colitis-associated carcinogenesis in mice by inhibiting the proliferation and invasion of tumor cells. Int. J. Mol. Sci. 2018, 19, 506. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-W.; Lee, S.-J.; Kim, Y.-S.; Kim, E.-K.; Ahn, C.-B.; Jeon, Y.-J.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Purification and characterization of a novel peptide with inhibitory effects on colitis induced mice by dextran sulfate sodium from enzymatic hydrolysates of Crassostrea gigas. Fish Shellfish. Immunol. 2012, 33, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.11–15.25.14. [Google Scholar] [CrossRef]
- Poritz, L.S.; Garver, K.I.; Green, C.; Fitzpatrick, L.; Ruggiero, F.; Koltun, W.A. Loss of tight junction Protein ZO-1 in dextran sulfate sodium induced colitis. J. Surg. Res. 2007, 140, 12–19. [Google Scholar] [CrossRef]
- Erben, U.; Loddenkemper, C.; Spieckermann, S.; Heimesaat, M.M.; Siegmund, B.; Kuehl, A.A. Histomorphology of intestinal inflammation in inflammatory bowel diseases (IBD) mouse models and its relevance for IBD in men. Int. J. Clin. Exp. Pathol. 2016, 9, 408–442. [Google Scholar]
- Fina, D.; Pallone, F. What is the role of cytokines and chemokines in IBD? Inflamm. Bowel Dis. 2008, 14, S117–S118. [Google Scholar] [CrossRef]
- Randhawa, P.K.; Singh, K.; Singh, N.; Jaggi, A.S. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J. Physiol. Pharmacol. 2014, 18, 279. [Google Scholar] [CrossRef]
- Perše, M.; Cerar, A. Dextran sodium sulphate colitis mouse model: Traps and tricks. BioMed Res. Int. 2012, 2012, 718617. [Google Scholar] [CrossRef]
- Carlsson, A.H.; Yakymenko, O.; Olivier, I.; Håkansson, F.; Postma, E.; Keita, Å.V.; Söderholm, J.D. Faecalibacterium prausnitzii supernatant improves intestinal barrier function in mice DSS colitis. Scand. J. Gastroenterol. 2013, 48, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.E.; Zheng, B.; Koelink, P.J.; van de Kant, H.J.; Haazen, L.C.; van Roest, M.; Garssen, J.; Folkerts, G.; Kraneveld, A.D. New perspective on dextran sodium sulfate colitis: Antigen-specific T cell development during intestinal inflammation. PLoS ONE 2013, 8, e69936. [Google Scholar] [CrossRef] [PubMed]
- Luettig, J.; Rosenthal, R.; Barmeyer, C.; Schulzke, J. Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers 2015, 3, e977176. [Google Scholar] [CrossRef] [PubMed]
- Gkouskou, K.K.; Deligianni, C.; Tsatsanis, C.; Eliopoulos, A.G. The gut microbiota in mouse models of inflammatory bowel disease. Front. Cell. Infect. Microbiol. 2014, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, B.M.; Becker, A.; Ayiseh, R.B.; Hildebrand, F.; Raes, J.; Huys, G.; Vandenabeele, P. Gut microbiota affects sensitivity to acute DSS-induced colitis independently of host genotype. Inflamm. Bowel Dis. 2013, 19, 2560–2567. [Google Scholar] [CrossRef] [PubMed]
- Andoh, A.; Sakata, S.; Koizumi, Y.; Mitsuyama, K.; Fujiyama, Y.; Benno, Y. Terminal restriction fragment length polymorphism analysis of the diversity of fecal microbiota in patients with ulcerative colitis. Inflamm. Bowel Dis. 2007, 13, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Krogius-Kurikka, L.; Lyra, A.; Malinen, E.; Aarnikunnas, J.; Tuimala, J.; Paulin, L.; Mäkivuokko, H.; Kajander, K.; Palva, A. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. 2009, 9, 95. [Google Scholar] [CrossRef]
- Frank, D.N.; St. Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef]

| Score | Weight Loss % | Stool Consistency | Rectal Bleeding |
|---|---|---|---|
| 0 | None | Normal | Negative |
| 1 | 1–5 | ||
| 2 | 5–10 | Loose stool | Positive |
| 3 | 10–20 | Diarrhea | Positive |
| 4 | >20 | Diarrhea | Gross bleeding |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| TNF-α | GGTGCCTATGTCTCAGCCTCTT | GCCATAGAACTGATGAGAGGGAG |
| IL 23 | GAGCAACTTCACACCTCCCT | TAGAACTCAGGCTGGGCATC |
| IL-22 | TCCAACTTCCAGCAGCCATACATC | GGTAGCACTGATCCTTAGCACTGAC |
| IL-17 | GACTCTCCACCGCAATGAAGAC | CTCTTCAGGACCAGGATCTCTTG |
| IL-4 | ACCAGGAGCCATATCCACGGATG | GGTGTTCTTCGTTGCTGTGAGGAC |
| IL-10 | CGGGAAGACAATAACTGCACCC | CGGTTAGCAGTATGTTGTCCAGC |
| Β-actin | ATCGCTGCGCTGGTCG | GTCCTTCTGACCCATTCCC |
| Serial No. | Protein Accession Number | Peptides Detected in MS Spectra | Mol. Weight (Da) | Length | m/z |
|---|---|---|---|---|---|
| 1 | QPB41107.1 | R.IRELEDALDSERDGRVR.A | 2028.035 | 17 | 508.0164 |
| 2 | QPB41107.1 | R. VEKEKQTLVVE.L | 1300.724 | 11 | 434.5822 |
| 3 | QPB41107.1 | L.TQENFDLQHQVQ.E | 1485.65 | 12 | 743.85 |
| 4 | QPB41107.1 | A.SNDDLKRQLDDETRQR.Q | 1987.967 | 16 | 497.9995 |
| 5 | QPB41107.1 | A. LQADYDNLNVR.L | 1319.647 | 11 | 660.8311 |
| 6 | QPB41107.1 | L.TQENFDLQHQVQ.E | 1485.685 | 12 | 743.85 |
| 7 | QPB41107.1 | L.KMEEM (+15.99)EDLKR.R | 1323.616 | 10 | 442.2125 |
| 8 | QPB41107.1 | A.LAQELEDARALLEGAER.A | 1882.975 | 17 | 628.666 |
| 9 | QPB41107.1 | R. KQLEVEIR.E | 1013.587 | 8 | 507.8008 |
| 10 | QPB41107.1 | R.VRDLEAELENEARRVR.E | 1954.034 | 16 | 489.5162 |
| 11 | ACT34361.1 | K.Q(-17.03) LTEDHFLFNDSDRFLK.A | 2107.001 | 17 | 703.342 |
| 12 | ACT34361.1 | Q. LTEDHFLFNDSDR.F | 1607.722 | 13 | 804.8658 |
| 13 | QPI34813.1 | K. IDTFIDVQKDP.V | 1289.65 | 11 | 645.8335 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, H.; Deng, T.; Ali, M.; Farooqui, N.A.; Alsholi, D.M.; Siddiqui, N.Z.; Rehman, A.U.; Ali, S.; Ilyas, M.; Wang, L.; et al. Sea Conch Peptides Hydrolysate Alleviates DSS-Induced Colitis in Mice through Immune Modulation and Gut Microbiota Restoration. Molecules 2023, 28, 6849. https://doi.org/10.3390/molecules28196849
Ullah H, Deng T, Ali M, Farooqui NA, Alsholi DM, Siddiqui NZ, Rehman AU, Ali S, Ilyas M, Wang L, et al. Sea Conch Peptides Hydrolysate Alleviates DSS-Induced Colitis in Mice through Immune Modulation and Gut Microbiota Restoration. Molecules. 2023; 28(19):6849. https://doi.org/10.3390/molecules28196849
Chicago/Turabian StyleUllah, Hidayat, Ting Deng, Muhsin Ali, Nabeel Ahmed Farooqui, Duaa M. Alsholi, Nimra Zafar Siddiqui, Ata Ur Rehman, Sharafat Ali, Muhammad Ilyas, Liang Wang, and et al. 2023. "Sea Conch Peptides Hydrolysate Alleviates DSS-Induced Colitis in Mice through Immune Modulation and Gut Microbiota Restoration" Molecules 28, no. 19: 6849. https://doi.org/10.3390/molecules28196849
APA StyleUllah, H., Deng, T., Ali, M., Farooqui, N. A., Alsholi, D. M., Siddiqui, N. Z., Rehman, A. U., Ali, S., Ilyas, M., Wang, L., & Xin, Y. (2023). Sea Conch Peptides Hydrolysate Alleviates DSS-Induced Colitis in Mice through Immune Modulation and Gut Microbiota Restoration. Molecules, 28(19), 6849. https://doi.org/10.3390/molecules28196849







