A High Crystalline Perylene-Based Hydrogen-Bonded Organic Framework for Enhanced Photocatalytic H2O2 Evolution
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Photocatalysts

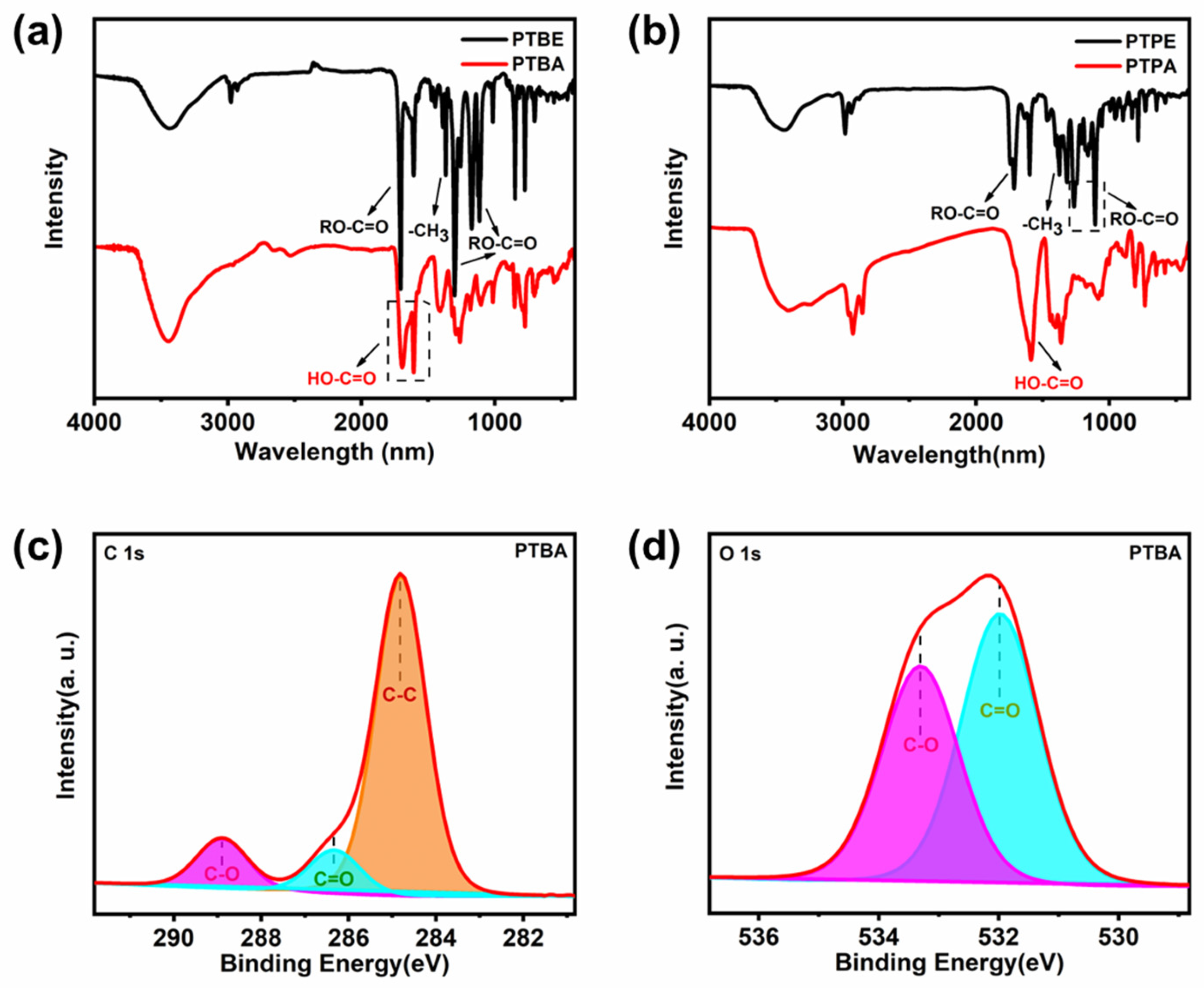
2.2. Structural Characterization and Morphology Analysis
2.3. Photochemical and Photoelectrochemical Properties
2.4. Photocatalytic Performance and Mechanism Discussion
3. Materials and Methods
3.1. Synthesis of MBP, PTBE, PTPE, PTBA, and PTPA
3.2. Characterization of PTBA and PTPA
3.3. Photocatalytic H2O2 Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dias, I.M.; Mourão, L.C.; Andrade, L.A.; Souza, G.B.M.; Viana, J.C.V.; Oliveira, S.B.; Alonso, C.G. Degradation of antibiotic amoxicillin from pharmaceutical industry wastewater into a continuous flow reactor using supercritical water gasification. Water Res. 2023, 234, 119826. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yan, S.; Ouyang, C.; Qiu, L.; Liu, J.; Ren, L. A new hydrogel to promote healing of bacteria infected wounds: Enzyme-like catalytic activity based on MnO2 nanocrystal. Chem. Eng. J. 2023, 470, 143986. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, H.; Huang, W.; Sui, Y.; Sa, R.; Chen, W.; Zhou, G.; Li, X.; Li, D.; Wen, M.; et al. The construction of conjugated organic polymers containing phenanthrenequinone redox centers for visible-light-driven H2O2 production from H2O and O2 without any additives. Chem. Eng. J. 2023, 454, 139929. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Sun, B.; Yuan, Q.; Li, H.; Ma, Z.; Xu, T.; Chen, X.; Fu, M. Boosting photocatalytic H2O2 production in pure water over a plasmonic photocatalyst with polyethylenimine modification. J. Mater. Chem. A 2023, 11, 1503–1510. [Google Scholar] [CrossRef]
- Miao, W.; Yao, D.; Chu, C.; Liu, Y.; Huang, Q.; Mao, S.; Ostrikov, K. Highly-efficient photocatalytic H2O2 evolution using hydrothermal carbons with donor-acceptor furan couples. Appl. Catal. B Environ. 2023, 332, 122770. [Google Scholar] [CrossRef]
- Wang, P.; Fan, S.; Li, X.; Duan, J.; Zhang, D. Modulating the molecular structure of graphitic carbon nitride for identifying the impact of the piezoelectric effect on photocatalytic H2O2 production. ACS Catal. 2023, 13, 9515–9523. [Google Scholar] [CrossRef]
- Chu, C.; Li, Q.; Miao, W.; Qin, H.; Liu, X.; Yao, D.; Mao, S. Photocatalytic H2O2 production driven by cyclodextrin-pyrimidine polymer in a wide pH range without electron donor or oxygen aeration. Appl. Catal. B Environ. 2022, 314, 121485. [Google Scholar] [CrossRef]
- Li, H.; Zhu, B.; Cheng, B.; Luo, G.; Xu, J.; Cao, S. Single-atom Cu anchored on N-doped graphene/carbon nitride heterojunction for enhanced photocatalytic H2O2 production. J. Mater. Sci. Technol. 2023, 161, 192–200. [Google Scholar] [CrossRef]
- Sha, P.; Huang, L.; Zhao, J.; Wu, Z.; Wang, Q.; Li, L.; Bu, D.; Huang, S. Carbon nitrides with grafted dual-functional ligands as electron acceptors and active sites for ultra-stable photocatalytic H2O2 production. ACS Catal. 2023, 13, 10474–10486. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, J.; Zhu, B.; Fedin, M.V.; Cheng, B.; Yu, J.; Zhang, L. ZnO/COF S-scheme heterojunction for improved photocatalytic H2O2 production performance. Chem. Eng. J. 2022, 444, 136584. [Google Scholar] [CrossRef]
- Yao, D.; Miao, W.; Chu, C.; Chen, Z.; Qin, H.; Mao, S. One-step pyrolysis conversion of glucose and urea into melanoidin for highly efficient photocatalytic H2O2 production. Chem. Eng. J. 2023, 467, 143550. [Google Scholar] [CrossRef]
- Mendori, D.; Hiroya, T.; Ueda, M.; Sanyoushi, M.; Nagai, K.; Abe, T. Novel photocatalytic material of organic p–n bilayer responsive to near-infrared energy. Appl. Catal. B Environ. 2017, 205, 514–518. [Google Scholar] [CrossRef]
- Yu, B.; Meng, T.; Ding, X.; Liu, X.; Wang, H.; Chen, B.; Zheng, T.; Li, W.; Zeng, Q.; Jiang, J. Hydrogen-bonded organic framework ultrathin nanosheets for efficient visible-light photocatalytic CO2 reduction. Angew. Chem. Int. Ed. 2022, 61, e202211482. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Das, S.; Saeki, A.; Praveen, V.K.; Seki, S.; Ajayaghosh, A. A hybrid organogel of a low band gap diketopyrrolopyrrole with PC71BM: Phase separated morphology and enhanced photoconductivity. ChemNanoMat 2018, 4, 831–836. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, Y.-P.; Xu, H.; Gong, X.-q.; Hua, J. Heteroatom bay-annulated perylene imides/g-C3N4 heterojunctions for efficient photocatalytic H2O2 evolution. J. Alloys Compd. 2023, 938, 168500. [Google Scholar] [CrossRef]
- Dai, W.; Jiang, L.; Wang, J.; Pu, Y.; Zhu, Y.; Wang, Y.; Xiao, B. Efficient and stable photocatalytic degradation of tetracycline wastewater by 3D polyaniline/perylene diimide organic heterojunction under visible light irradiation. Chem. Eng. J. 2020, 397, 125476. [Google Scholar] [CrossRef]
- Ning, L.; Chen, X.; Wang, Z.; Xu, J. High-efficiency pollutant degradation, disinfection and H2O2 production activities of magnetically separable Co-imbedded N-doped carbonaceous framework/supramolecular perylene diimide photocatalyst. Appl. Catal. B Environ. 2023, 324, 122282. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Wang, H.; Cheng, H.; Wu, X.; Zhang, Q.; Xu, H. Forming electron traps deactivates self-assembled crystalline organic nanosheets toward photocatalytic overall water splitting. Sci. Bull. 2021, 66, 265–274. [Google Scholar] [CrossRef]
- Maity, N.; Sharma, M.K.; Ghosh, S.; Huss-Hansen, M.K.; Roy, A.; Narayanan, R.; Knaapila, M.; Matsuda, W.; Seki, S.; Patil, S. Supramolecular self-assembly of diketopyrrolopyrrole with unprecedented photoconductivity. ACS Appl. Electron. Mater. 2023, 5, 5093–5102. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Hou, B.; Huang, X.; Wang, T.; Bao, Y.; Hao, H. Porous hydrogen-bonded organic frameworks (HOFs): From design to potential applications. Chem. Eng. J. 2020, 399, 125873. [Google Scholar] [CrossRef]
- Ghosh, S.; Tsutsui, Y.; Suzuki, K.; Kaji, H.; Honjo, K.; Uemura, T.; Seki, S. Impact of the position of the imine linker on the optoelectronic performance of π-conjugated organic frameworks. Mol. Syst. Des. Eng. 2019, 4, 325–331. [Google Scholar] [CrossRef]
- Yang, L.; Yuan, J.; Wang, G.; Cao, Q.; Zhang, C.; Li, M.; Shao, J.; Xu, Y.; Li, H.; Lu, J. Construction of tri-functional HOFs material for efficient selective adsorption and photodegradation of bisphenol A and hydrogen production. Adv. Funct. Mater. 2023, 33, 2300954. [Google Scholar] [CrossRef]
- Lin, R.-B.; He, Y.; Li, P.; Wang, H.; Zhou, W.; Chen, B. Multifunctional porous hydrogen-bonded organic framework materials. Chem. Soc. Rev. 2019, 48, 1362–1389. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, T.; Chen, X.; Chen, Q.; Hao, J.; Song, W.; Zeng, Y.; Xue, P. Guest-regulated luminescence and force-stimuli response of a hydrogen-bonded organic framework. ACS Appl. Mater. Interfaces 2021, 13, 32270–32277. [Google Scholar] [CrossRef] [PubMed]
- Di Nunzio, M.R.; Hisaki, I.; Douhal, A. HOFs under light: Relevance to photon-based science and applications. J. Photochem. Photobiol. C Photochem. Rev. 2021, 47, 100418. [Google Scholar] [CrossRef]
- Yu, B.; Li, L.; Liu, S.; Wang, H.; Liu, H.; Lin, C.; Liu, C.; Wu, H.; Zhou, W.; Li, X.; et al. Robust biological hydrogen-bonded organic framework with post-functionalized Rhenium(I) sites for efficient heterogeneous visible-light-driven CO2 reduction. Angew. Chem. Int. Ed. 2021, 60, 8983–8989. [Google Scholar] [CrossRef]
- Wu, P.; Yin, X.; Zhao, Y.; Li, F.; Yang, Y.; Liu, N.; Liao, J.; Lan, T. Porphyrin-based hydrogen-bonded organic framework for visible light driven photocatalytic removal of U(VI) from real low-level radioactive wastewater. J. Hazard. Mater. 2023, 459, 132179. [Google Scholar] [CrossRef]
- Huang, W.; Yuan, H.; Yang, H.; Tong, L.; Gao, R.; Kou, X.; Wang, J.; Ma, X.; Huang, S.; Zhu, F.; et al. Photodynamic hydrogen-bonded biohybrid framework: A photobiocatalytic cascade nanoreactor for accelerating diabetic wound therapy. JACS Au 2022, 2, 2048–2058. [Google Scholar] [CrossRef]
- Ghosh, S.; Prasanthkumar, S.; Das, S.; Saeki, A.; Seki, S.; Ajayaghosh, A. Structurally directed thienylenevinylene self-assembly for improved charge carrier mobility: 2D sheets vs. 1D fibers. Chem. Commun. 2022, 58, 6837–6840. [Google Scholar] [CrossRef]
- Tran, L.D.; Ma, J.; Wong-Foy, A.G.; Matzger, A.J. A perylene-based microporous coordination polymer interacts selectively with electron-poor aromatics. Chem. Eur. J. 2016, 22, 5509–5513. [Google Scholar] [CrossRef]
- Zhang, N.; Yin, Q.; Guo, S.; Chen, K.-K.; Liu, T.-F.; Wang, P.; Zhang, Z.-M.; Lu, T.-B. Hot-electron leading-out strategy for constructing photostable HOF catalysts with outstanding H2 evolution activity. Appl. Catal. B Environ. 2021, 296, 120337. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Zhang, H.; Liu, W.; Zhu, W.; Zhu, Y. A highly crystalline perylene imide polymer with the robust built-in electric field for efficient photocatalytic water oxidation. Adv. Mater. 2020, 32, 1907746. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Chai, Y.; Zhang, Z.; Zhu, Y. Efficient photocatalytic overall water splitting induced by the giant internal electric field of a g-C3N4/rGO/PDIP Z-scheme heterojunction. Adv. Mater. 2021, 33, 2007479. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, Q.; Nan, J.; Shi, W.; Cui, F.; Zhu, Y. Perylenetetracarboxylic acid nanosheets with internal electric fields and anisotropic charge migration for photocatalytic hydrogen evolution. Nat. Commun. 2022, 13, 2067. [Google Scholar] [CrossRef]
- Sheng, Y.; Li, W.; Xu, L.; Zhu, Y. High Photocatalytic oxygen evolution via strong built-in electric field induced by high crystallinity of perylene imide supramolecule. Adv. Mater. 2022, 34, 2102354. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Li, R.; Xu, X.; Huang, W.; He, Y.; Li, J.; Sun, M.; Chen, X.; Kang, L. Cobalt-intercalated one-dimensional nanocrystals of urea perylene imide polymer for enhanced visible-light photocatalytic water oxidation. Appl. Catal. B Environ. 2022, 309, 121293. [Google Scholar] [CrossRef]
- An, B.; Zhang, Q.-H.; Zheng, B.-S.; Li, M.; Xi, Y.-Y.; Jin, X.; Xue, S.; Li, Z.-T.; Wu, M.-B.; Wu, W.-T. Sulfone-decorated conjugated organic polymers activate oxygen for photocatalytic methane conversion. Angew. Chem. Int. Ed. 2022, 61, e202204661. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, M.; Wu, C.; Feng, S.; Hua, J. A High Crystalline Perylene-Based Hydrogen-Bonded Organic Framework for Enhanced Photocatalytic H2O2 Evolution. Molecules 2023, 28, 6850. https://doi.org/10.3390/molecules28196850
Hu M, Wu C, Feng S, Hua J. A High Crystalline Perylene-Based Hydrogen-Bonded Organic Framework for Enhanced Photocatalytic H2O2 Evolution. Molecules. 2023; 28(19):6850. https://doi.org/10.3390/molecules28196850
Chicago/Turabian StyleHu, Mengke, Chenxi Wu, Shufan Feng, and Jianli Hua. 2023. "A High Crystalline Perylene-Based Hydrogen-Bonded Organic Framework for Enhanced Photocatalytic H2O2 Evolution" Molecules 28, no. 19: 6850. https://doi.org/10.3390/molecules28196850
APA StyleHu, M., Wu, C., Feng, S., & Hua, J. (2023). A High Crystalline Perylene-Based Hydrogen-Bonded Organic Framework for Enhanced Photocatalytic H2O2 Evolution. Molecules, 28(19), 6850. https://doi.org/10.3390/molecules28196850




