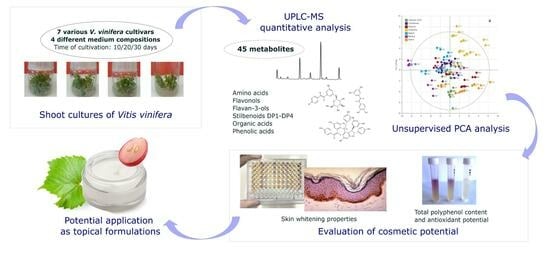
Shoot Cultures of Vitis vinifera (Vine Grape) Different Cultivars as a Promising Innovative Cosmetic Raw Material—Phytochemical Profiling, Antioxidant Potential, and Whitening Activity
Abstract
:1. Introduction
2. Results
2.1. Appearance and Biomass Output of Shoot Cultures
2.2. Metabolic Profiling and Relative Quantification of Metabolites
Quantitative Analysis of Metabolites
2.3. Biological Activities
2.3.1. Antioxidant Activity
2.3.2. Tyrosinase Inhibition Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. In Vitro Cultures Initiation
4.3. Experimental In Vitro Cultures
4.4. Metabolite Profiling
4.4.1. Extraction
4.4.2. UPLC–MS Analyses
4.4.3. Statistical Analysis
4.5. Evaluation of Biological Activity
4.5.1. Extraction
4.5.2. Antioxidant Activity
Free Radical Scavenging Activity
Ferrous Ion (Fe2+) Chelating Activity
4.5.3. Tyrosinase Inhibition Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sharafan, M.; Malinowska, M.A.; Ekiert, H.; Kwaśniak, B.; Sikora, E.; Szopa, A. Vitis vinifera (Vine Grape) as a Valuable Cosmetic Raw Material. Pharmaceutics 2023, 15, 1372. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en (accessed on 14 August 2023).
- Food and Drug Administration (FDA). Available online: https://www.fda.gov (accessed on 14 August 2023).
- European Food Safety Authority (EFSA). Available online: https://www.efsa.europa.eu/en (accessed on 14 August 2023).
- Cosmetic Ingredient Database (CosIng). Available online: www.ec.europa.eu (accessed on 14 August 2023).
- Esfahanian, Z.; Behbahani, M.; Shanehsaz, M.; Hessami, M.J.; Nejatian, M.A. Evaluation of anticancer activity of fruit and leave extracts from virus infected and healthy cultivars of Vitis vinifera. Cell J. 2013, 15, 116–123. [Google Scholar] [PubMed]
- Saleem, A.; Akhtar, M.F.; Sharif, A.; Akhtar, B.; Siddique, R.; Ashraf, G.M.; Alghamdi, B.S.; Alharthy, S.A. Anticancer, Cardio-Protective and Anti-Inflammatory Potential of Natural-Sources-Derived Phenolic Acids. Molecules 2022, 27, 7286. [Google Scholar]
- Sharma, S.K.; Vasudeva, N. Hepatoprotective activity of Vitis vinifera root extract against carbon tetrachloride-induced liver damage in rats. Acta Pol. Pharm. Drug Res. 2012, 69, 933–937. [Google Scholar]
- Lakshmi, B.V.S.; Sudhakar, M.; Anisha, M. Neuroprotective role of hydroalcoholic extract of Vitis vinifera against aluminium- induced oxidative stress in rat brain. Neurotoxicology 2014, 41, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Letsiou, S.; Kapazoglou, A.; Tsaftaris, A. Transcriptional and epigenetic effects of Vitis vinifera L. leaf extract on UV-stressed human dermal fibroblasts. Mol. Biol. Rep. 2020, 48, 5763–5772. [Google Scholar] [CrossRef]
- Cefali, L.C.; Ataide, J.A.; Sousa, I.M.O.; Figueiredo, M.C.; Ruiz, A.L.T.G.; Foglio, M.A.; Mazzola, P.G. In vitro solar protection factor, antioxidant activity, and stability of a topical formulation containing Benitaka grape (Vitis vinifera L.) peel extract. Nat. Prod. Res. 2020, 34, 2677–2682. [Google Scholar] [CrossRef]
- Zielonka-Brzezicka, J.; Florkowska, K.; Nowak, A.; Muzykiewicz-Szyma´nska, A.; Klimowicz, A. The effect of thawing on the antioxidant activity of the leaves and fruit of the grapevine (Vitis vinifera). Pomer. J. Life Sci. 2021, 67, 63–70. [Google Scholar]
- Oliveira, D.A.; Salvador, A.A.; Smânia, A., Jr.; Smânia, E.F.A.; Maraschin, M.; Ferreira, S.R.S. Antimicrobial activity and composition profile of grape (Vitis vinifera) pomace extracts obtained by supercritical fluids. J. Biotechnol. 2013, 164, 423–432. [Google Scholar] [CrossRef]
- Yadav, D.; Kumar, A.; Kumar, P.; Mishra, D. Antimicrobial properties of black grape (Vitis vinifera L.) peel extracts against antibiotic-resistant pathogenic bacteria and toxin producing molds. Indian J. Pharmacol. 2015, 47, 663–667. [Google Scholar] [CrossRef]
- Chopra, A.; Geetha, R.V. In vitro Anti-inflammatory Activity of Vitis vinifera Seed Extract Using Albumin Denaturation Assay. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 33–37. [Google Scholar]
- Sangiovanni, E.; Di Lorenzo, C.; Piazza, S.; Manzoni, Y.; Brunelli, C.; Fumagalli, M.; Magnavacca, A.; Martinelli, G.; Colombo, F.; Casiraghi, A.; et al. Vitis vinifera L. Leaf Extract Inhibits In Vitro Mediators of Inflammation and Oxidative Stress Involved in Inflammatory-Based Skin Diseases. Antioxidants 2019, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-S.; Chen, H.-J.; Huang, J.-P.; Lee, P.-C.; Tsai, C.-R.; Hsu, T.-F.; Huang, W.-Y. Kinetics of Tyrosinase Inhibitory Activity Using Vitis vinifera Leaf Extracts. BioMed Res. Int. 2017, 2017, 5232680. [Google Scholar] [CrossRef]
- Malinowska, M.A.; Billet, K.; Drouet, S.; Munsch, T.; Unlubayir, M.; Tungmunnithum, D.; Giglioli-Guivarc’h, N.; Hano, C.; Lanoue, A. Grape Cane Extracts as Multifunctional Rejuvenating Cosmetic Ingredient: Evaluation of Sirtuin Activity, Tyrosinase Inhibition and Bioavailability Potential. Molecules 2020, 25, 2203. [Google Scholar] [CrossRef]
- Billet, K.; Houillé, B.; Dugé de Bernonville, T.; Besseau, S.; Oudin, A.; Courdavault, V.; Delanoue, G.; Guérin, L.; Clastre, M.; Giglioli-Guivarc’h, N.; et al. Field-Based Metabolomics of Vitis vinifera L. Stems Provides New Insights for Genotype Discrimination and Polyphenol Metabolism Structuring. Front. Plant Sci. 2018, 9, 798. [Google Scholar] [CrossRef]
- Ferrier, M.; Billet, K.; Drouet, S.; Tungmunnithum, D.; Malinowska, M.A.; Marchal, C.; Dedet, S.; Giglioli-Guivarc’h, N.; Hano, C.; Lanoue, A. Identifying Major Drivers of Antioxidant Activities in Complex Polyphenol Mixtures from Grape Canes. Molecules 2022, 27, 4029. [Google Scholar] [CrossRef]
- Ehrhardt, C.; Arapitsas, P.; Stefanini, M.; Flick, G.; Mattivi, F. Analysis of the phenolic composition of fungus-resistant grape varieties cultivated in Italy and Germany using UHPLC-MS/MS. J. Mass Spectrom. 2014, 49, 860–869. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Sroka, P.; Satora, P.; Jurasz, E. Polish wines: Characteristics of cool-climate wines. J. Food Compos. Anal. 2010, 23, 463–468. [Google Scholar] [CrossRef]
- Pink, M. Poland as a wine country? From traditions to emerging opportunities. Probl. Small Agric. Hold. 2015, 2, 37–56. [Google Scholar] [CrossRef]
- Zeghad, N.; Ahmed, E.; Belkhiri, A.; Heyden, Y.V.; Demeyer, K. Antioxidant activity of Vitis vinifera, Punica granatum, Citrus aurantium and Opuntia ficus indica fruits cultivated in Algeria. Heliyon 2019, 5, e01575. [Google Scholar] [CrossRef]
- Llobera, A. Study on the Antioxidant Activity of Grape Stems (Vitis vinifera). A Preliminary Assessment of Crude Extracts. Food Nutr. Sci. 2012, 3, 500–504. [Google Scholar]
- Jin, Y.J.; Lin, C.C.; Lu, T.M.; Li, J.H.; Chen, I.S.; Kuo, Y.H.; Ko, H.H. Chemical constituents derived from Artocarpus xanthocarpus as inhibitors of melanin biosynthesis. Phytochemistry 2015, 117, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, J.H.; Baek, S.H.; Seo, J.H.; Kho, Y.H.; Oh, T.K.; Lee, C.H. Enhancement of tyrosinase inhibition of the extract of Veratrum patulum using cellulase. Biotechnol. Bioeng. 2004, 87, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.B.; Kim, S.D.; Kim, T.B.; Jeong, E.J.; Kim, Y.C.; Sung, J.H.; Sung, S.H. Tyrosinase Inhibitory Constituents of Morus bombycis Cortex. Nat. Prod. Sci. 2011, 17, 198–201. [Google Scholar]
- Ben Khadher, T.; Aydi, S.; Mars, M.; Bouajila, J. Study on the Chemical Composition and the Biological Activities of Vitis vinifera Stem Extracts. Molecules 2022, 27, 3109. [Google Scholar] [CrossRef] [PubMed]
- Soural, I.; Vrchotová, N.; Tříska, J.; Balík, J.; Horník, S.; Cuřínová, P.; Sýkora, J. Various extraction methods for obtaining stilbenes from grape cane of Vitis vinifera L. Molecules 2015, 20, 6093–6112. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Montealegre, R.; Romero Peces, R.; Chacón Vozmediano, J.L.; Martínez Gascueña, J.; García Romero, E. Phenolic compounds in skins and seeds of ten grape Vitis vinifera varieties grown in a warm climate. J. Food Compos. Anal. 2006, 19, 687–693. [Google Scholar] [CrossRef]
- Burin, V.M.; Ferreira-Lima, N.E.; Panceri, C.P.; Bordignon-Luiz, M.T. Bioactive compounds and antioxidant activity of Vitis vinifera and Vitis labrusca grapes: Evaluation of different extraction methods. Microchem. J. 2014, 114, 155–163. [Google Scholar] [CrossRef]
- Halford, N.G. Plant biotechnology: Current and future applications of genetically modified crops. J. Wiley 2006, 1–328. [Google Scholar]
- Matkowski, A. Plant in vitro culture for the production of antioxidants—A review. Biotechnol. Adv. 2008, 26, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Sharma, K.; Dahiya, R.; Bera, T. Modern Applications of Plant Biotechnology in Pharmaceutical Sciences; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 1–439. [Google Scholar]
- Kumar, P.; Srivastava, D.K. Biotechnological applications in in vitro plant regeneration studies of broccoli (Brassica oleracea L. var. italica), an important vegetable crop. Biotechnol. Lett. 2016, 38, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Verpoorte, R.; Contin, A.; Memelink, J. Biotechnology for the production of plant secondary metabolites. Phytochem. Rev. 2002, 1, 13–25. [Google Scholar] [CrossRef]
- Szopa, A.; Ekiert, H. In vitro cultures of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine)—A potential biotechnological rich source of therapeutically important phenolic acids. Appl. Biochem. Biotechnol. 2012, 166, 1941–1948. [Google Scholar] [CrossRef]
- Billet, K.; Malinowska, M.A.; Munsch, T.; Unlubayir, M.; Adler, S.; Delanoue, G.; Lanoue, A. Semi-Targeted Metabolomics to Validate Biomarkers of Grape Downy Mildew Infection Under Field Conditions. Plants 2020, 9, 1008. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Vrhovsek, U.; Masuero, D.; Gasperotti, M.; Franceschi, P.; Caputi, L.; Viola, R.; Mattivi, F. A versatile targeted metabolomics method for the rapid quantification of multiple classes of phenolics in fruits and beverages. J. Agric. Food Chem. 2012, 60, 8831–8840. [Google Scholar] [CrossRef] [PubMed]
- Rzeppa, S.; Von Bargen, C.; Bittner, K.; Humpf, H.-U. Analysis of Flavan-3-ols and Procyanidins in Food Samples by Reversed Phase High-Performance Liquid Chromatography Coupled to Electrospray Ionization Tandem Mass Spectrometry (RP-HPLC-ESI-MS/MS). J. Agric. Food Chem. 2011, 59, 10594–10603. [Google Scholar] [CrossRef] [PubMed]
- Narduzzi, L.; Stanstrup, J.; Mattivi, F. Comparing Wild American Grapes with Vitis vinifera: A Metabolomics Study of Grape Composition. J. Agric. Food Chem. 2015, 63, 6823–6834. [Google Scholar] [CrossRef] [PubMed]
- Mulinacci, N.; Innocenti, M.; Santamaria, A.R.; la Marca, G.; Pasqua, G. High-performance liquid chromatography/electrospray ionization tandem mass spectrometric investigation of stilbenoids in cell cultures of Vitis vinifera L., cv. Malvasia. Rapid Commun. Mass Spectrom. 2010, 24, 2065–2073. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.; Mao, Q.; Taylor, D.; Saucier, C. Investigation of monomeric and oligomeric wine stilbenoids in red wines by ultra-high-performance liquid chromatography/electrospray ionization quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 1815–1827. [Google Scholar] [CrossRef] [PubMed]
- Mattivi, F.; Vrhovsek, U.; Malacarne, G.; Masuero, D.; Zulini, L.; Stefanini, M.; Moser, C.; Velasco, R.; Guella, G. Profiling of resveratrol oligomers, important stress metabolites, accumulating in the leaves of hybrid Vitis vinifera (Merzling × Teroldego) genotypes infected with Plasmopara viticola. J. Agric. Food Chem. 2011, 59, 5364–5375. [Google Scholar] [CrossRef]
- Püssa, T.; Floren, J.; Kuldkepp, P.; Raal, A. Survey of grapevine Vitis vinifera stem polyphenols by liquid chromatography-diode array detection-tandem mass spectrometry. J. Agric. Food Chem. 2006, 54, 7488–7494. [Google Scholar] [CrossRef]
- Kubica, P.; Kokotkiewicz, A.; Malinowska, M.A.; Synowiec, A.; Gniewosz, M.; Hussain, S.; Yaqoob, M.; Bonn, G.K.; Jakschitz, T.; Mahmoud, E.A. Phenylpropanoid Glycoside and Phenolic Acid Profiles and Biological Activities of Biomass Extracts from Different Types of Verbena officinalis Microshoot Cultures and Soil-Grown Plant. Antioxidants 2022, 11, 409. [Google Scholar] [CrossRef]
- Teoh, E.S. Secondary Metabolites of Plants. In Medicinal Orchids of Asia; Springer: Cham, Switzerland, 2015; pp. 59–73. [Google Scholar]
- Houillé, B.; Papon, N.; Boudesocque, L.; Bourdeaud, E.; Besseau, S.; Courdavault, V.; Enguehard-Gueiffier, C.; Delanoue, G.; Guérin, L.; Bouchara, J.-P.; et al. Antifungal activity of resveratrol derivatives against Candida species. J. Nat. Prod. 2014, 77, 1658–1662. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Schenk, R.U.; Hildebrandt, A.C. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Chidambara Murthy, K.N.; Singh, R.P.; Jayaprakasha, G.K. Antioxidant activities of grape (Vitis vinifera) pomace extracts. J. Agric. Food Chem. 2002, 21, 5909–5914. [Google Scholar] [CrossRef]


| V. vinifera cvs. | Medium Variant | |||
|---|---|---|---|---|
| W1 | W2 | W3 | W4 | |
| Johanniter | 89.95 ± 1.37 | 91.83 ± 0.03 | 88.03 ± 4.40 | 88.44 ± 1.57 |
| Chardonnay | 87.77 ± 6.95 | 95.89 ± 4.79 | 91.85 ± 4.58 | 92.73 ± 1.98 |
| Riesling | 84.85 ± 5.45 | 94.99 ± 6.78 | 95.26 ± 8.55 | 94.95 ± 3.89 |
| Cabernet Cortis | 86.05 ± 8.55 | 92.24 ± 5.12 | 84.44 ± 6.45 | 91.10 ± 9.43 |
| Regent | 85.67 ± 2.47 | 73.78 ± 4.78 | 89.60 ± 3.42 | 90.36 ± 7.14 |
| Hibernal | 81.60 ± 7.58 | 90.94 ± 3.80 | 81.86 ± 4.21 | 89.75 ± 7.61 |
| Solaris | 82.98 ± 3.36 | 86.71 ± 2.74 | 83.17 ± 3.33 | 89.50 ± 5.49 |
| ID Metabolite | Metabolic Class | RT | Name | Authentication |
|---|---|---|---|---|
| m1 | Amino acids | 1.07 | L-Proline | Standard |
| m2 | 1.37 | L-Tyrosine | Standard | |
| m4 | 1.54 | L-Isoleucine | Standard | |
| m5 | 1.67 | L-Leucine | Standard | |
| m7 | 2.45 | L-Phenylalanine | Standard | |
| m9 | 3.56 | L-Tryptophan | Standard | |
| m3 | Organic acids | 1.38 | Citric acid | Standard |
| m6 | Phenolic acids | 1.85 | Gallic acid | Standard |
| m8 | 3.36 | Caftaric acid | Standard | |
| m12 | 4.29 | Coutaric acid | Standard | |
| m25 | Flavan-3-ols | 7.06 | Catechin gallate | [41] |
| m13 | 4.38 | Catechin | Standard | |
| m17 | 5.44 | Epicatechin | Standard | |
| m10 | 3.89 | Procyanidin B1 | Standard | |
| m11 | 4.21 | Procyanidin B3 | Standard | |
| m15 | 4.78 | Procyanidin B4 | Standard | |
| m16 | 4.91 | Procyanidin B2 | Standard | |
| m22 | 6.01 | Procyanidin dimer 5 | [42] | |
| m20 | 5.82 | Galloyl Procyanidin B a | [43] | |
| m21 | 5.97 | Galloyl Procyanidin B b | [43] | |
| m29 | 8.31 | Galloyl Procyanidin B c | [43] | |
| m14 | 4.44 | Procyanidin trimer | [42] | |
| m19 | 5.70 | Procyanidin C1 | Standard | |
| m18 | Flavonols | 5.65 | Quercetin-hexoside | [21] |
| m26 | 7.33 | Quercetin glucuronide | [21] | |
| m27 | 7.44 | Quercetin-3-O-glucoside | Standard | |
| m35 | Stilbenoids DP1 * | 10.79 | Z-Resveratrol | Standard |
| m32 | 9.39 | E-Resveratrol | Standard | |
| m24 | 7.01 | Piceid | Standard | |
| m23 | Stilbenoids DP2 * | 6.16 | Restrytisol 1 | [44] |
| m28 | 7.54 | Ampelopsin A | Standard | |
| m31 | 8.72 | Resveratrol dimer glycosylate | [45] | |
| m37 | 11.62 | Z-ε-Viniferin | [44] | |
| m38 | 11.86 | E-ε-Viniferin | Standard | |
| m41 | 12.82 | α-Viniferin | [46] | |
| m42 | 13.30 | ω-Viniferin | [47] | |
| m45 | 14.20 | δ-Viniferin | [44] | |
| m36 | Stilbenoids DP3 * | 11.05 | Resveratrol trimer 1 | [45] |
| m43 | 13.99 | Resveratrol trimer 2 | [45] | |
| m33 | Stilbenoids DP4 * | 10.39 | Hopeaphenol | Standard |
| m34 | 10.63 | Isohopeaphenol | [45] | |
| m39 | 12.01 | Resveratrol tetramer 3 | [45] | |
| m40 | 12.60 | Resveratrol tetramer 4 | [45] | |
| m44 | 14.14 | Vitisin B | Standard | |
| m46 | 14.52 | Resveratrol tetramer 6 | [45] |
| V. vinifera cvs. | Medium Variant * | Amino Acids | Organic Acids | Phenolic Acids | Flavan-3-ols | Flavonols | Stilbenoids DP1 | Stilbenoids DP2 | Stilbenoids DP3 | Stilbenoids DP4 | Total Metabolite Content |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Johanniter | W1 | 23.14 ± 0.60 | 0.58 ± 0.1 | 1.44 ± 0.08 | 7.04 ± 0.09 | 0.31 ± 0.02 | 2.17 ± 0.13 | 3.57 ± 0.06 | 0.49 ± 0.02 | 0.38 ± 0.00 | 39.1 ± 0.12 |
| W2 | 53.69 ± 0.45 | 0.55 ± 0.07 | 1.01 ± 0.02 | 10.07 ± 0.12 | 0.35 ± 0.01 | 2.53 ± 0.09 | 5.83 ± 0.04 | 0.54 ± 0.06 | 0.37 ± 0.01 | 74.93 ± 0.1 | |
| W3 | 16.09 ± 0.28 | 1.12 ± 0.13 | 1.04 ± 0.04 | 7.90 ± 0.07 | 0.28 ± 0.01 | 1.40 ± 0.07 | 4.53 ± 0.04 | 0.64 ± 0.04 | 1.38 ± 0.02 | 34.39 ± 0.07 | |
| W4 | 21.81 ± 0.51 | 1.34 ± 0.22 | 0.91 ± 0.05 | 7.53 ± 0.07 | 0.23 ± 0.01 | 2.42 ± 0.12 | 6.10 ± 0.09 | 0.76 ± 0.04 | 0.67 ± 0.02 | 41.76 ± 0.11 | |
| Chardonnay | W1 | 45.91 ± 0.84 | 0.76 ± 0.07 | 0.6 ± 0.00 | 5.43 ± 0.06 | 0.14 ± 0.01 | 1.57 ± 0.13 | 6.34 ± 0.05 | 0.62 ± 0.02 | 0.52 ± 0.01 | 61.89 ± 0.14 |
| W2 | 29.04 ± 0.19 | 0.67 ± 0.08 | 2.05 ± 0.04 | 3.69 ± 0.05 | 0.16 ± 0.01 | 1.35 ± 0.08 | 2.13 ± 0.05 | 0.33 ± 0.03 | 0.08 ± 0.00 | 39.49 ± 0.05 | |
| W3 | 21.51 ± 0.45 | 1.68 ± 0.1 | 0.64 ± 0.03 | 4.20 ± 0.05 | 0.12 ± 0.01 | 0.87 ± 0.04 | 5.08 ± 0.05 | 0.69 ± 0.03 | 0.50 ± 0.01 | 35.29 ± 0.08 | |
| W4 | 28.59 ± 0.69 | 1.81 ± 0.34 | 0.64 ± 0.04 | 3.79 ± 0.04 | 0.17 ± 0.01 | 0.61 ± 0.03 | 3.54 ± 0.05 | 0.50 ± 0.02 | 0.49 ± 0.01 | 40.14 ± 0.11 | |
| Riesling | W1 | 25.41 ± 0.46 | 0.41 ± 0.07 | 0.38 ± 0.01 | 1.98 ± 0.02 | 0.20 ± 0.01 | 1.21 ± 0.06 | 15.06 ± 0.08 | 1.06 ± 0.07 | 1.68 ± 0.04 | 47.4 ± 0.09 |
| W2 | 40.73 ± 0.92 | 0.95 ± 0.08 | 0.76 ± 0.01 | 5.34 ± 0.05 | 0.18 ± 0.01 | 3.14 ± 0.09 | 4.00 ± 0.08 | 0.56 ± 0.04 | 0.31 ± 0.01 | 55.98 ± 0.15 | |
| W3 | 12.39 ± 0.18 | 1.03 ± 0.11 | 0.73 ± 0.04 | 3.04 ± 0.03 | 0.28 ± 0.02 | 1.20 ± 0.04 | 3.02 ± 0.05 | 0.32 ± 0.03 | 0.95 ± 0.02 | 22.96 ± 0.05 | |
| W4 | 18.13 ± 0.48 | 2.07 ± 0.29 | 0.34 ± 0.01 | 0.70 ± 0.01 | 0.19 ± 0.01 | 0.52 ± 0.01 | 2.69 ± 0.04 | 0.45 ± 0.04 | 0.56 ± 0.01 | 25.66 ± 0.08 | |
| Cabernet Cortis | W1 | 63.06 ± 1.57 | 0.7 ± 0.04 | 0.14 ± 0.00 | 3.60 ± 0.04 | 0.08 ± 0.01 | 0.69 ± 0.03 | 5.59 ± 0.04 | 0.63 ± 0.03 | 0.24 ± 0.01 | 74.73 ± 0.22 |
| W2 | 90.42 ± 2.27 | 0.85 ± 0.08 | 0.15 ± 0.00 | 3.59 ± 0.04 | 0.05 ± 0.00 | 0.80 ± 0.08 | 4.33 ± 0.03 | 0.47 ± 0.05 | 0.15 ± 0.01 | 100.81 ± 0.31 | |
| W3 | 23.14 ± 0.44 | 1.75 ± 0.25 | 0.3 ± 0.00 | 5.77 ± 0.04 | 0.10 ± 0.01 | 0.36 ± 0.02 | 4.58 ± 0.08 | 0.46 ± 0.02 | 0.68 ± 0.01 | 37.14 ± 0.08 | |
| W4 | 28.99 ± 0.78 | 1.96 ± 0.26 | 0.16 ± 0.00 | 8.33 ± 0.10 | 0.11 ± 0.01 | 0.93 ± 0.05 | 7.11 ± 0.11 | 1.09 ± 0.12 | 0.65 ± 0.02 | 49.34 ± 0.15 | |
| Hibernal | W1 | 33.96 ± 0.55 | 1.13 ± 0.17 | 0.24 ± 0.01 | 6.40 ± 0.04 | 0.18 ± 0.01 | 1.14 ± 0.05 | 6.48 ± 0.07 | 0.78 ± 0.04 | 0.24 ± 0.01 | 50.54 ± 0.1 |
| W2 | 51.62 ± 0.74 | 0.87 ± 0.04 | 0.25 ± 0.01 | 8.27 ± 0.08 | 0.17 ± 0.01 | 2.63 ± 0.16 | 5.42 ± 0.04 | 0.62 ± 0.04 | 0.26 ± 0.01 | 70.13 ± 0.14 | |
| W3 | 23.66 ± 0.37 | 1.93 ± 0.36 | 0.77 ± 0.03 | 5.44 ± 0.05 | 0.16 ± 0.01 | 1.19 ± 0.08 | 9.21 ± 0.06 | 1.44 ± 0.08 | 0.77 ± 0.01 | 44.58 ± 0.09 | |
| W4 | 29.58 ± 0.90 | 2.31 ± 0.35 | 0.35 ± 0.03 | 5.94 ± 0.08 | 0.16 ± 0.01 | 2.67 ± 0.23 | 6.53 ± 0.13 | 1.07 ± 0.11 | 0.43 ± 0.01 | 49.03 ± 0.19 | |
| Regent | W1 | 53.02 ± 1.55 | 0.76 ± 0.11 | 0.45 ± 0.00 | 5.33 ± 0.06 | 0.14 ± 0.01 | 1.57 ± 0.11 | 6.46 ± 0.06 | 0.83 ± 0.06 | 1.04 ± 0.02 | 69.6 ± 0.23 |
| W2 | 45.04 ± 0.65 | 1.79 ± 0.28 | 0.19 ± 0.01 | 3.52 ± 0.02 | 0.07 ± 0.00 | 1.54 ± 0.07 | 15.80 ± 0.08 | 1.73 ± 0.15 | 1.19 ± 0.02 | 70.87 ± 0.12 | |
| W3 | 32.86 ± 0.06 | 1.6 ± 0.35 | 0.69 ± 0.06 | 7.19 ± 0.09 | 0.23 ± 0.01 | 1.20 ± 0.11 | 5.73 ± 0.12 | 0.88 ± 0.06 | 1.46 ± 0.02 | 51.84 ± 0.07 | |
| W4 | 39.81 ± 0.57 | 1.73 ± 0.33 | 0.2 ± 0.01 | 7.45 ± 0.07 | 0.11 ± 0.00 | 1.82 ± 0.12 | 9.97 ± 0.09 | 1.48 ± 0.05 | 1.13 ± 0.01 | 63.71 ± 0.12 | |
| Solaris | W1 | 47.32 ± 1.50 | 1.03 ± 0.19 | 0.12 ± 0.01 | 4.99 ± 0.05 | 0.10 ± 0.00 | 1.44 ± 0.06 | 3.54 ± 0.06 | 0.45 ± 0.03 | 0.25 ± 0.00 | 59.25 ± 0.21 |
| W2 | 83.81 ± 1.48 | 1.41 ± 0.33 | 0.36 ± 0.02 | 7.30 ± 0.07 | 0.25 ± 0.01 | 2.43 ± 0.16 | 3.75 ± 0.09 | 0.34 ± 0.04 | 0.11 ± 0.00 | 99.75 ± 0.23 | |
| W3 | 24.97 ± 0.08 | 1.89 ± 0.3 | 0.11 ± 0.00 | 6.27 ± 0.07 | 0.17 ± 0.01 | 1.47 ± 0.09 | 3.30 ± 0.03 | 0.55 ± 0.02 | 0.38 ± 0.01 | 39.11 ± 0.04 | |
| W4 | 29.26 ± 0.89 | 3.02 ± 0.43 | 0.17 ± 0.01 | 4.12 ± 0.05 | 0.14 ± 0.01 | 0.65 ± 0.05 | 4.12 ± 0.04 | 0.59 ± 0.06 | 0.56 ± 0.02 | 42.64 ± 0.14 |
| V. vinifera cvs. | Medium Variant * | DPPH Assay (Inhibition %) | Fe2+ Chelating Activity Assay (Inhibition %) |
|---|---|---|---|
| Johanniter | W1 | 33.57 ± 4.16 | 21.94 ± 3.45 |
| W2 | 31.15 ± 2.82 | 10.00 ± 0.20 | |
| W3 | 19.99 ± 1.27 | 26.19 ± 1.44 | |
| W4 | 14.16 ± 1.27 | 21.37 ± 4.74 | |
| Chardonnay | W1 | 15.96 ± 3.16 | 20.47 ± 4.14 |
| W2 | 23.61 ± 2.32 | 33.26 ± 1.86 | |
| W3 | 6.33 ± 1.31 | 27.34 ± 3.25 | |
| W4 | 21.72 ± 5.68 | 13.79 ± 1.57 | |
| Riesling | W1 | 2.64 ± 1.50 | 7.36 ± 0.52 |
| W2 | 28.89 ± 0.82 | 10.29 ± 3.07 | |
| W3 | 17.63 ± 1.71 | 17.53 ± 3.06 | |
| W4 | 6.48 ± 1.32 | 35.18 ± 0.15 | |
| Cabernet Cortis | W1 | 6.93 ± 1.50 | 27.48 ± 3.23 |
| W2 | 8.47 ± 1.42 | 50.93 ± 2.69 | |
| W3 | 8.66 ± 1.03 | 32.75 ± 2.56 | |
| W4 | 9.42 ± 0.79 | 28.11 ± 2.99 | |
| Hibernal | W1 | 8.44 ± 1.50 | 11.07 ± 0.01 |
| W2 | 1.02 ± 0.59 | 8.21 ± 3.77 | |
| W3 | 11.49 ± 2.20 | 14.20 ± 3.32 | |
| W4 | 6.70 ± 1.44 | 14.79 ± 5.20 | |
| Regent | W1 | 1.23 ± 0.5 | 1.23 ± 0.52 |
| W2 | 9.40 ± 0.47 | 8,31 ± 0,42 | |
| W3 | 9.34 ± 0.40 | 15.39 ± 0.71 | |
| W4 | 9.49 ± 0.74 | 2.91 ± 0.52 | |
| Solaris | W1 | 4.63 ± 1.50 | 18.11 ± 1.77 |
| W2 | 5.46 ± 1.50 | 17.09 ± 1.70 | |
| W3 | 4.07 ± 0.41 | 14.83 ± 2.75 | |
| W4 | 2.07 ± 0.19 | 13.84 ± 0.99 | |
| Reference sample * | 73.73 ± 0.55 | 97.14 ± 0.15 |
| V. vinifera cvs. | Medium Variant * | Tyrosinase Inhibition Activity (Inhibition %) |
|---|---|---|
| Johanniter | W1 | 8.90 ± 0.36 |
| W2 | 5.89 ± 0.24 | |
| W3 | 16.29 ± 0.65 | |
| W4 | 5.38 ± 0.22 | |
| Chardonnay | W1 | 12.27 ± 0.49 |
| W2 | 4.54 ± 0.18 | |
| W3 | 11.64 ± 0.47 | |
| W4 | 9.91 ± 0.40 | |
| Riesling | W1 | 11.64 ± 0.47 |
| W2 | 8.78 ± 0.35 | |
| W3 | 9.87 ± 0.39 | |
| W4 | 4.80 ± 0.19 | |
| Cabernet Cortis | W1 | 1.98 ± 0.08 |
| W2 | 15.49 ± 0.62 | |
| W3 | 4.71 ± 0.19 | |
| W4 | 9.86 ± 0.39 | |
| Hibernal | W1 | 10.39 ± 0.42 |
| W2 | 9.78 ± 0.39 | |
| W3 | 8.78 ± 0.35 | |
| W4 | 17.50 ± 0.70 | |
| Regent | W1 | 10.04 ± 0.40 |
| W2 | 10.61 ± 0.42 | |
| W3 | 9.61 ± 0.38 | |
| W4 | 7.45 ± 0.30 | |
| Solaris | W1 | 6.25 ± 0.25 |
| W2 | 7.43 ± 0.30 | |
| W3 | 16.23 ± 0.65 | |
| W4 | 7.32 ± 0.29 | |
| Reference sample * | 11.05 ± 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharafan, M.; Malinowska, M.A.; Kubicz, M.; Kubica, P.; Gémin, M.-P.; Abdallah, C.; Ferrier, M.; Hano, C.; Giglioli-Guivarc’h, N.; Sikora, E.; et al. Shoot Cultures of Vitis vinifera (Vine Grape) Different Cultivars as a Promising Innovative Cosmetic Raw Material—Phytochemical Profiling, Antioxidant Potential, and Whitening Activity. Molecules 2023, 28, 6868. https://doi.org/10.3390/molecules28196868
Sharafan M, Malinowska MA, Kubicz M, Kubica P, Gémin M-P, Abdallah C, Ferrier M, Hano C, Giglioli-Guivarc’h N, Sikora E, et al. Shoot Cultures of Vitis vinifera (Vine Grape) Different Cultivars as a Promising Innovative Cosmetic Raw Material—Phytochemical Profiling, Antioxidant Potential, and Whitening Activity. Molecules. 2023; 28(19):6868. https://doi.org/10.3390/molecules28196868
Chicago/Turabian StyleSharafan, Marta, Magdalena Anna Malinowska, Marta Kubicz, Paweł Kubica, Marin-Pierre Gémin, Cécile Abdallah, Manon Ferrier, Christophe Hano, Nathalie Giglioli-Guivarc’h, Elżbieta Sikora, and et al. 2023. "Shoot Cultures of Vitis vinifera (Vine Grape) Different Cultivars as a Promising Innovative Cosmetic Raw Material—Phytochemical Profiling, Antioxidant Potential, and Whitening Activity" Molecules 28, no. 19: 6868. https://doi.org/10.3390/molecules28196868
APA StyleSharafan, M., Malinowska, M. A., Kubicz, M., Kubica, P., Gémin, M. -P., Abdallah, C., Ferrier, M., Hano, C., Giglioli-Guivarc’h, N., Sikora, E., Lanoue, A., & Szopa, A. (2023). Shoot Cultures of Vitis vinifera (Vine Grape) Different Cultivars as a Promising Innovative Cosmetic Raw Material—Phytochemical Profiling, Antioxidant Potential, and Whitening Activity. Molecules, 28(19), 6868. https://doi.org/10.3390/molecules28196868