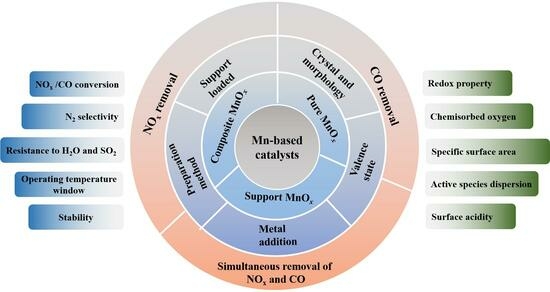
A Review of Mn-Based Catalysts for Abating NOx and CO in Low-Temperature Flue Gas: Performance and Mechanisms
Abstract
:1. Introduction
2. Mn-Based Catalysts for Abating NOx and CO
2.1. NOx Removal
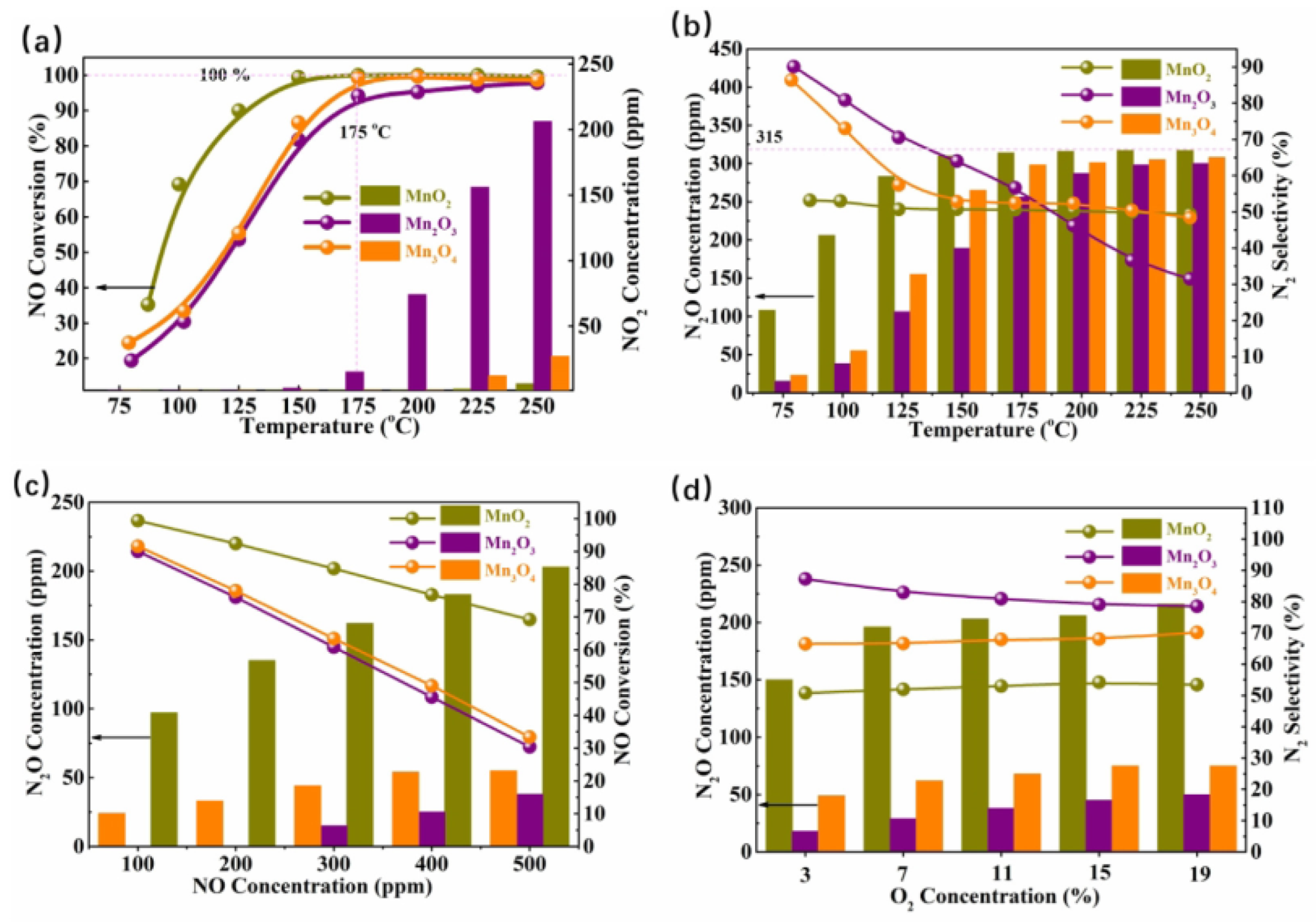
2.1.1. Single Mn Oxide Catalysts for NOx Removal
- (1)
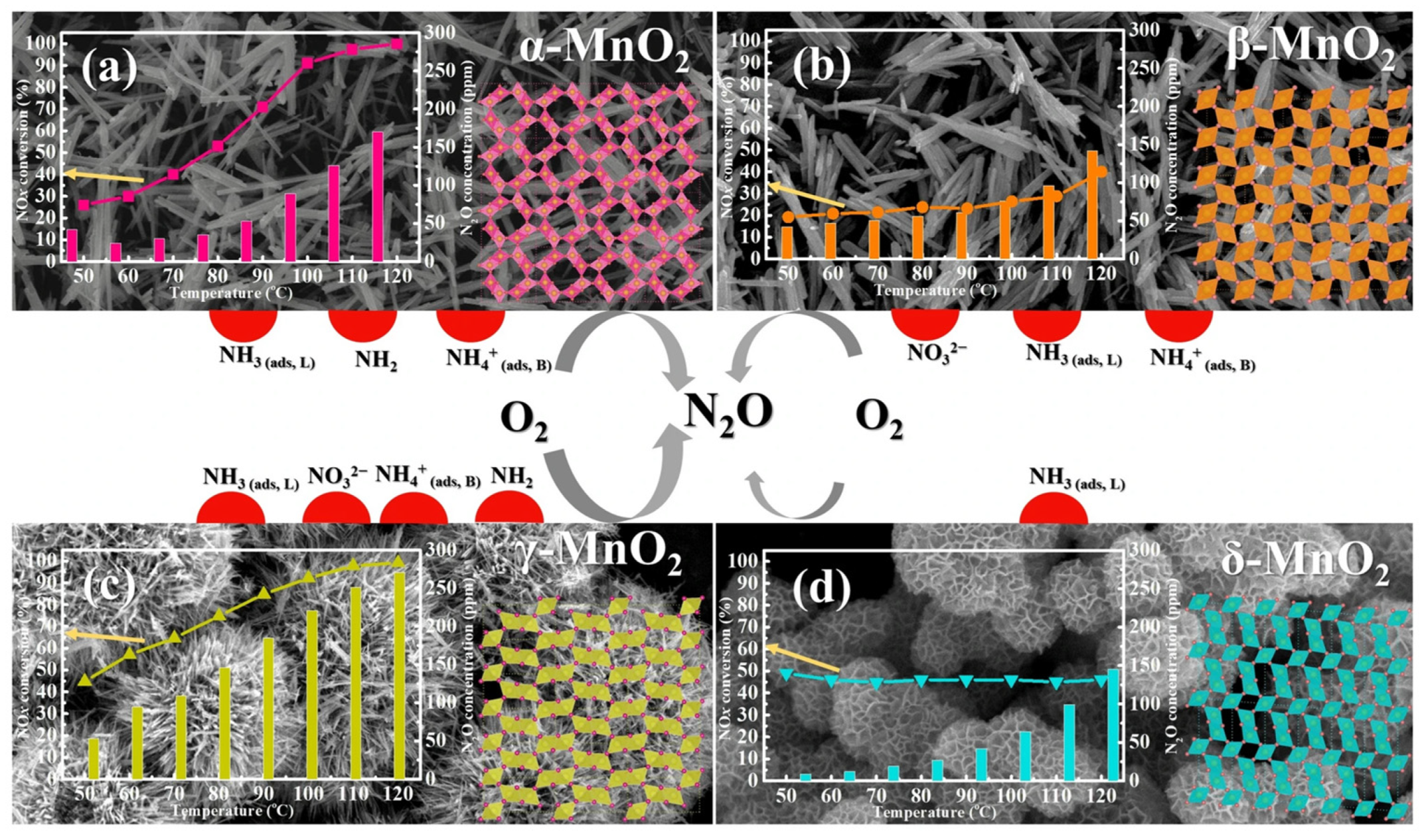
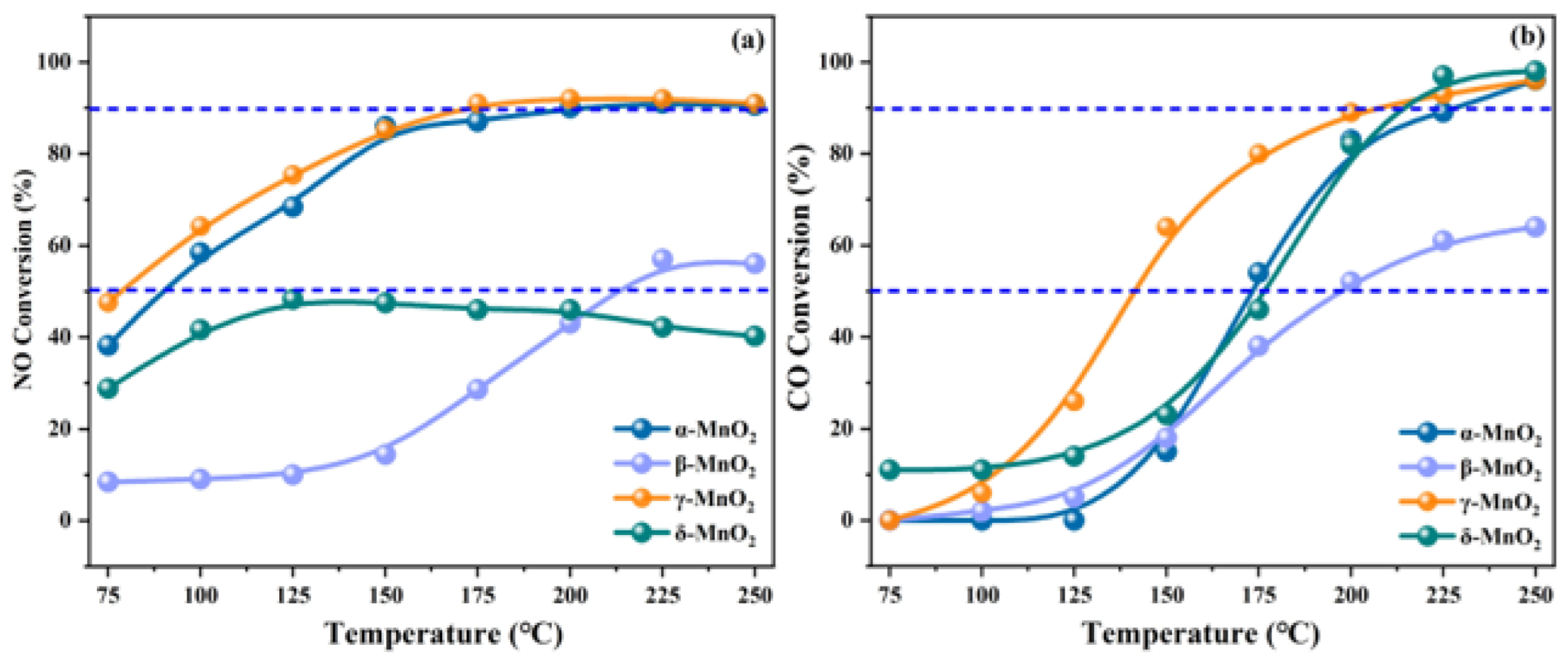
- Effect of crystal phase and morphology
- (2)
- Effect of valence state
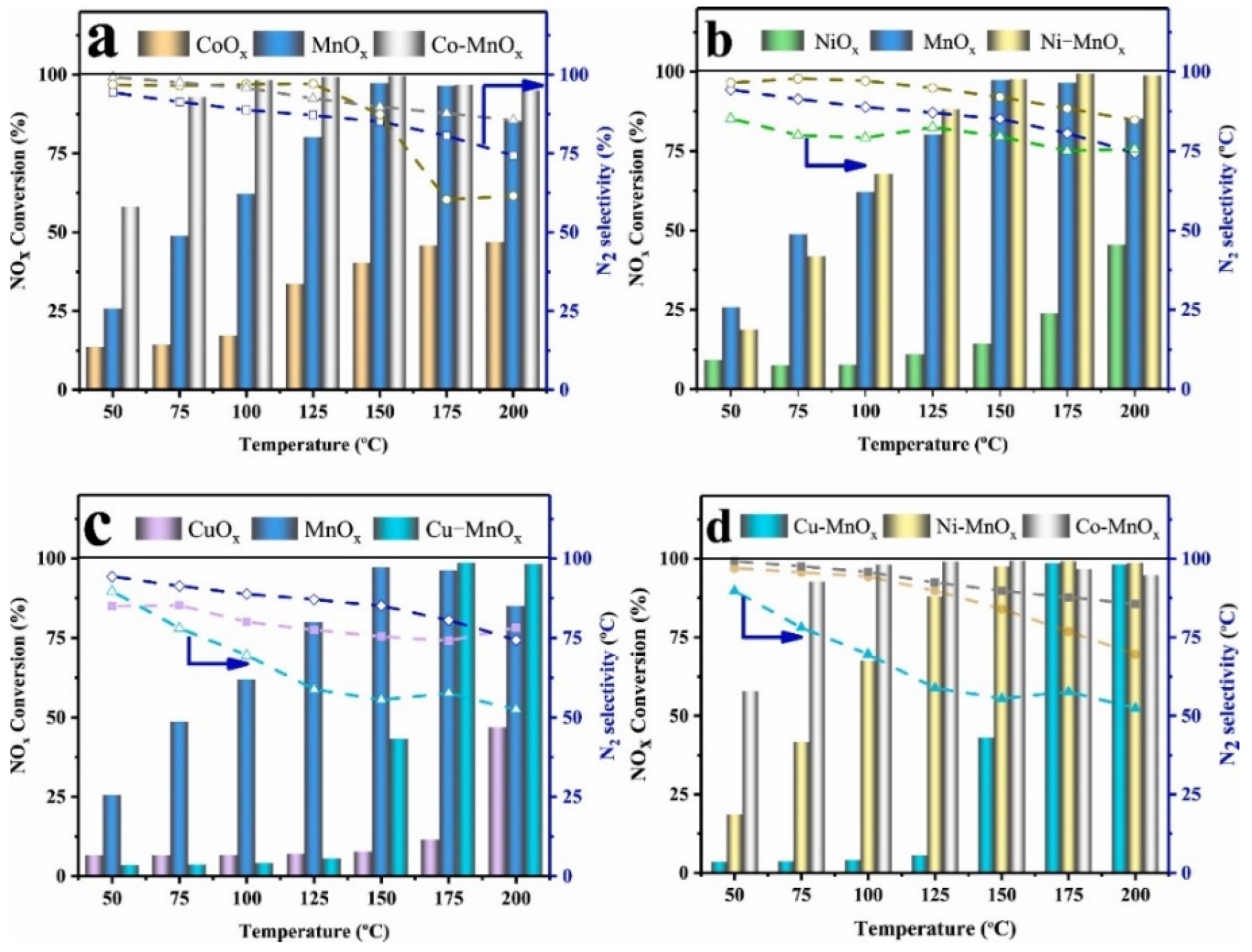
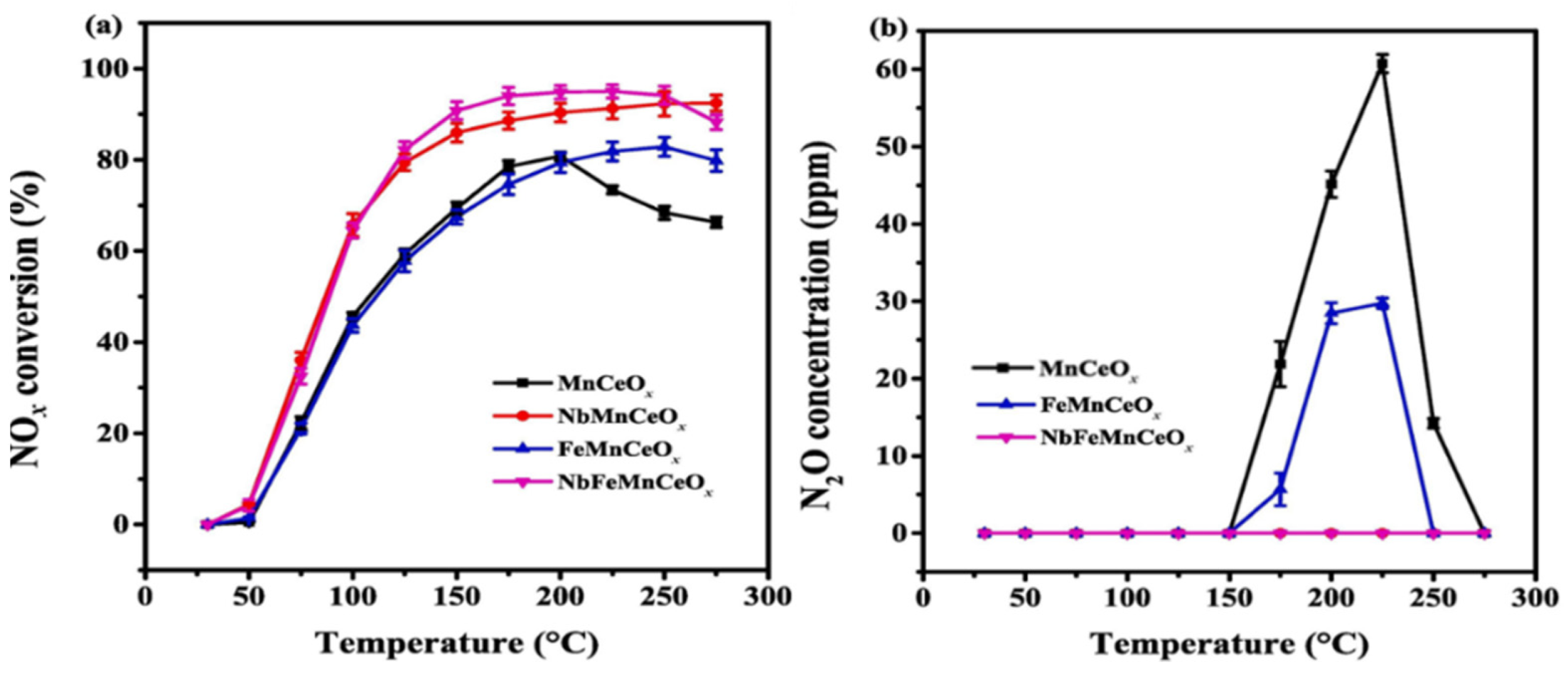
2.1.2. Composite MnOx Catalysts for NOx Removal
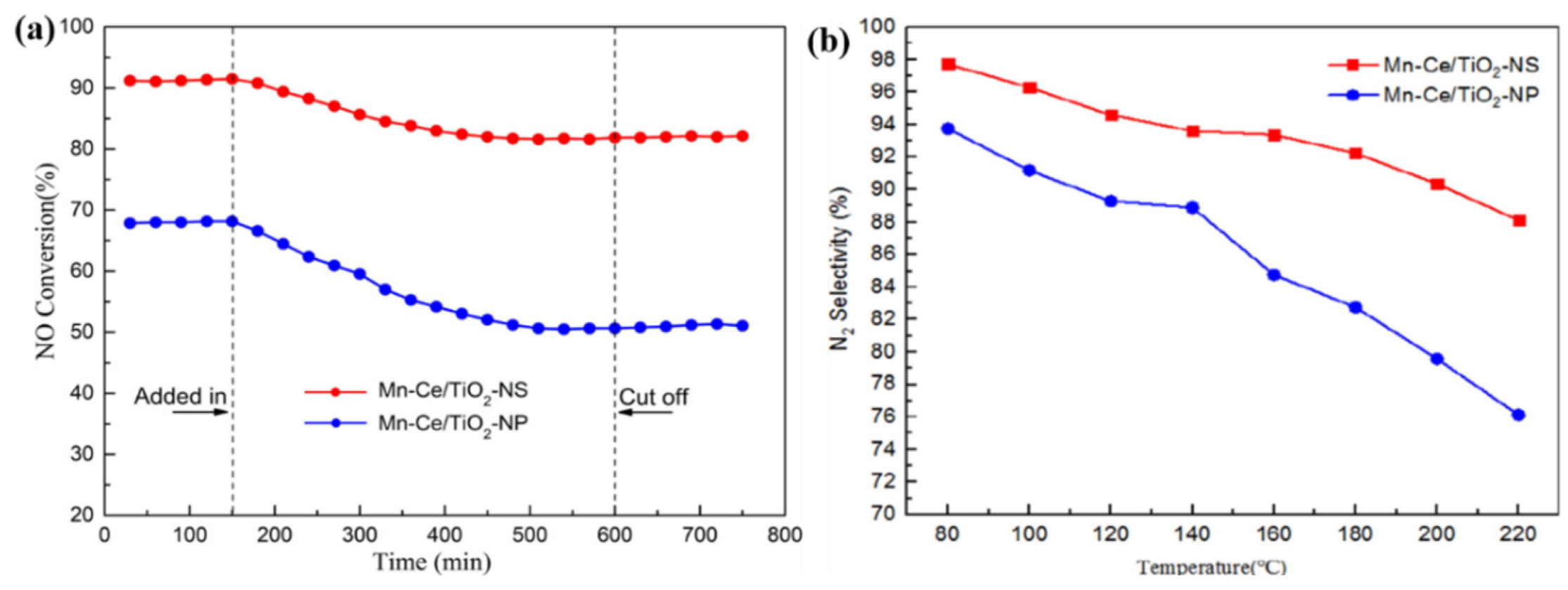
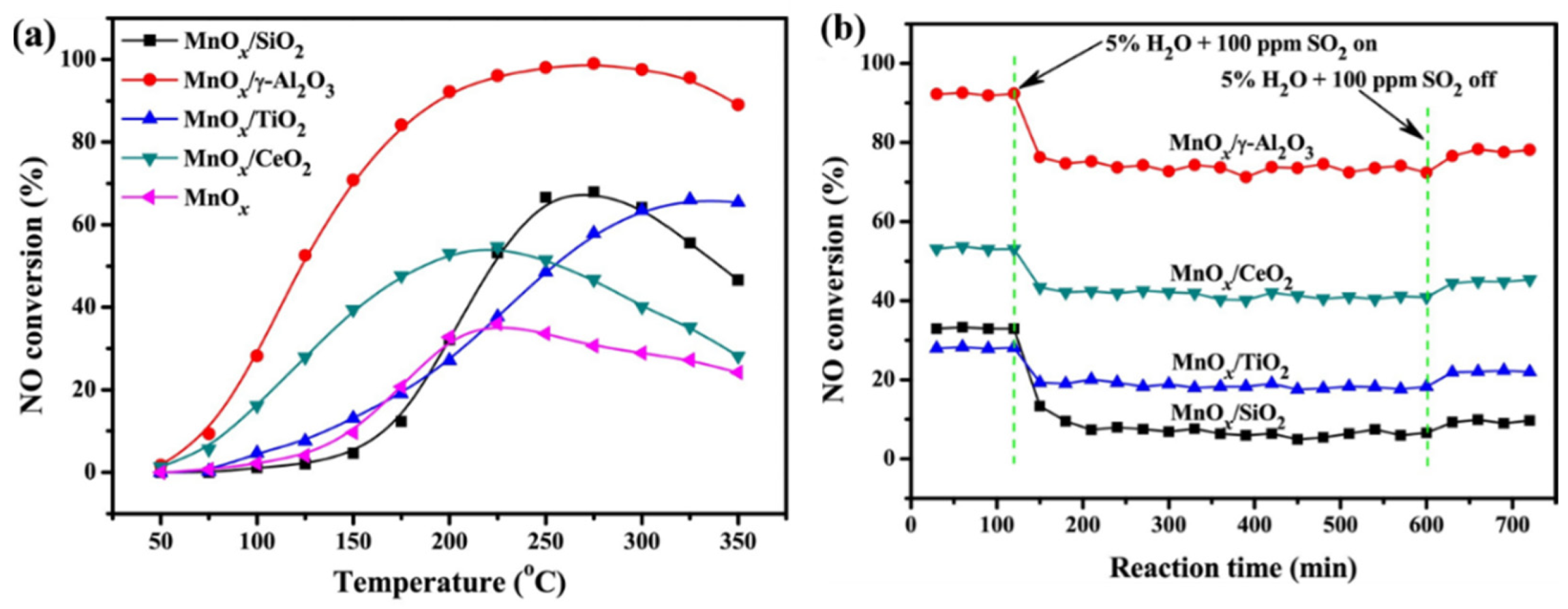
2.1.3. Supported MnOx Catalysts for NOx Removal
- (1)
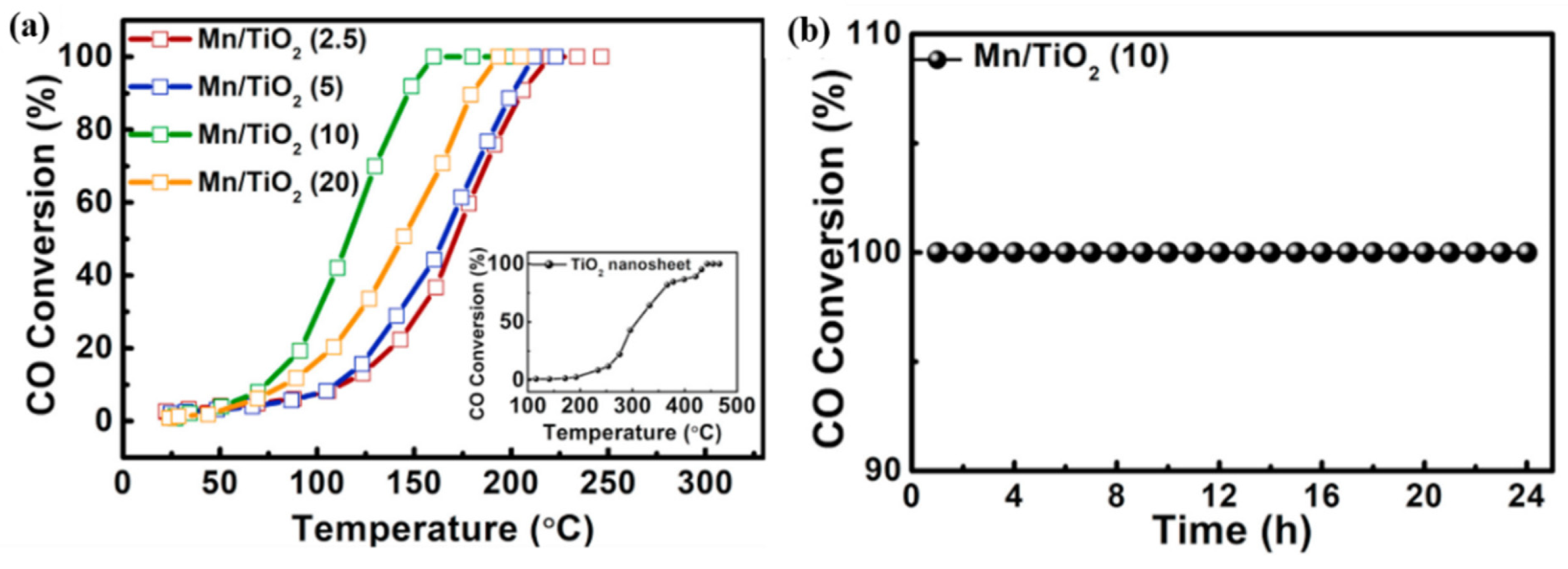
- TiO2 as support
- (2)
- Al2O3 as support
- (3)
- Carbon materials as support
2.2. CO Removal
2.2.1. Single MnOx Catalysts for CO Oxidation
2.2.2. Composite MnOx Catalysts for CO Oxidation
2.2.3. Supported MnOx Catalysts for CO Oxidation
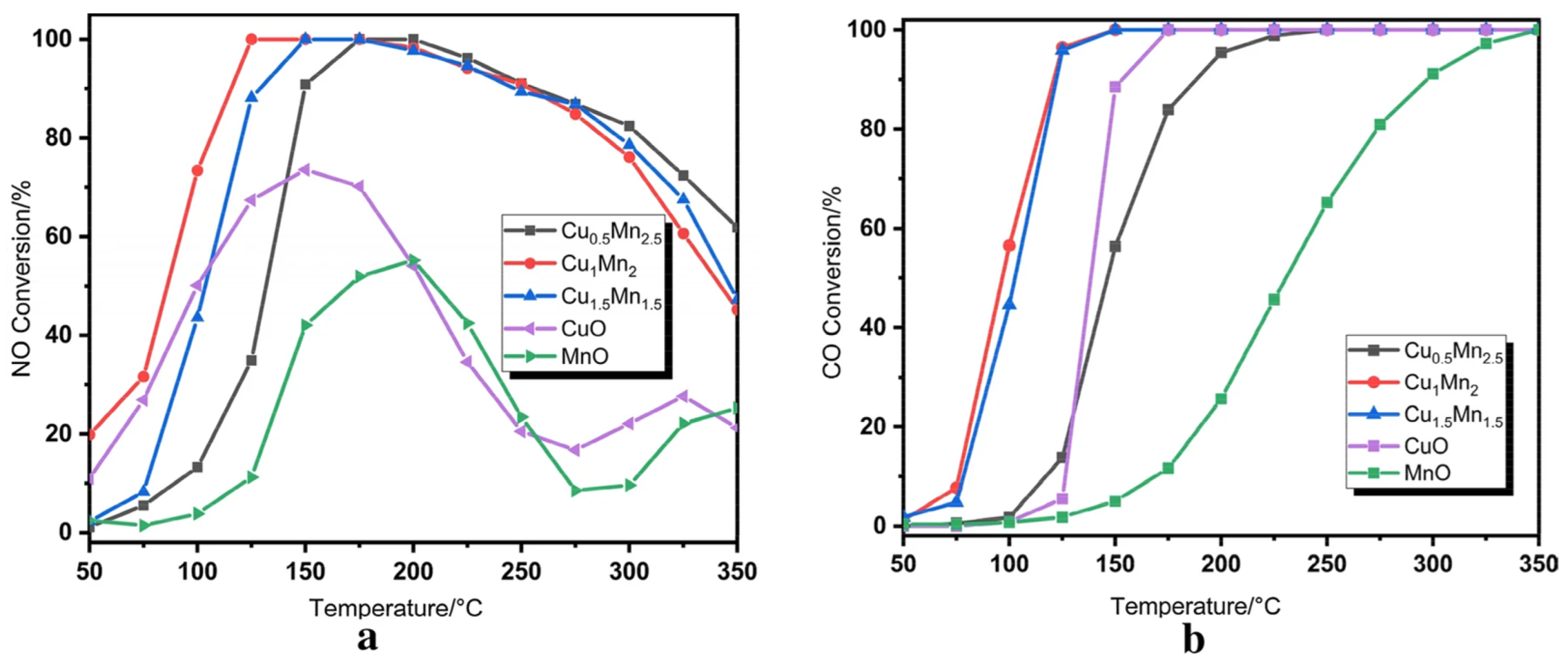
2.3. Simultaneous Removal of NOx and CO
3. Mechanisms and Interactions of NOx Catalytic Reduction and CO Catalytic Oxidation
3.1. Pathways and Mechanisms of NOx Catalytic Reduction on Mn-Based Catalysts
3.2. Mechanisms of CO Oxidation on Mn-Based Catalysts
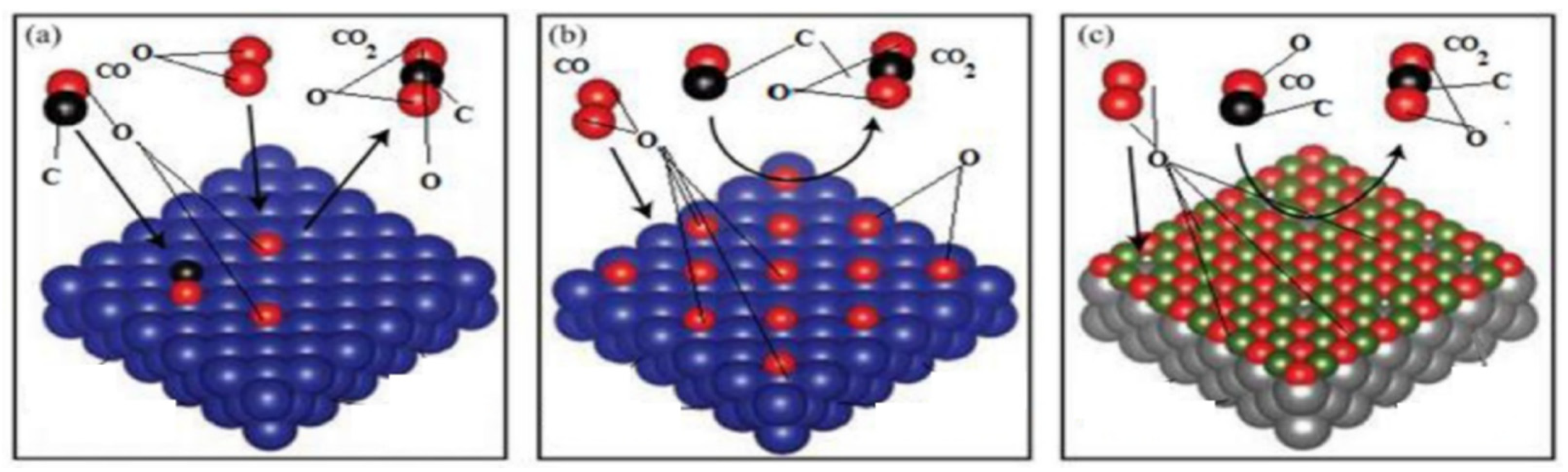
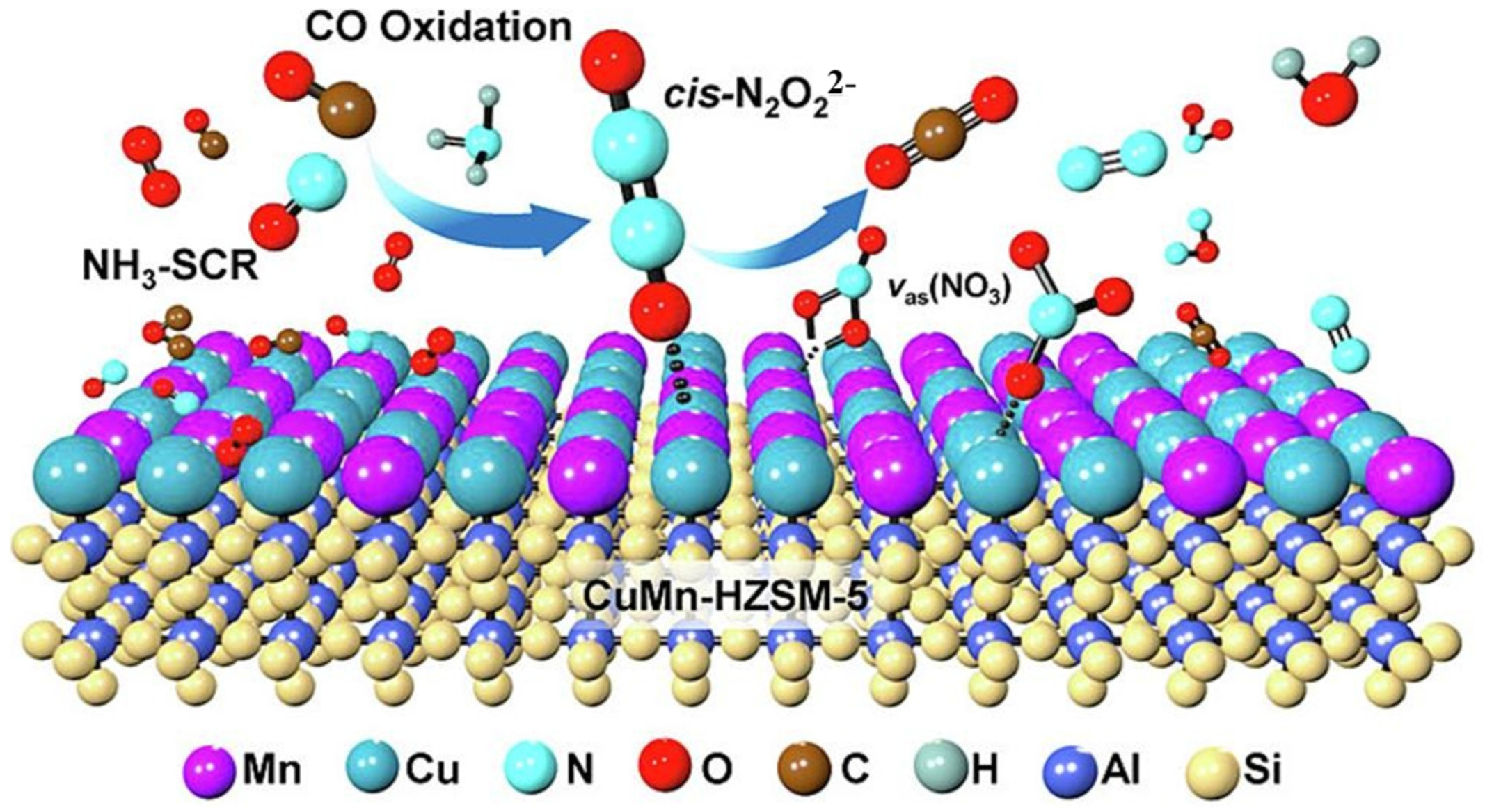
3.3. Interactions between Simultaneous NOx Catalytic Reduction and CO Catalytic Oxidation on Mn-Based Catalysts
3.3.1. Effect of CO Oxidation on NOx Reduction
3.3.2. Effect of NH3-SCR Atmosphere on CO Oxidation
4. Conclusions and Perspectives
- (1)
- Mn-based catalysts exhibit a poor N2 selectivity in the NH3-SCR reaction. This is primarily ascribed to the strong oxidizing property of Mn-based catalysts, resulting in the non-selective reduction of NH3 on the catalyst surface, thereby producing a large amount of the by-products, N2O. Further research should focus on improving the N2 selectivity. For enhancing the SCR catalytic properties, it is imperative to inhibit the non-selective catalytic reduction of NH3, thus enhancing the utilization rate of NH3.
- (2)
- The resistance to SO2 and H2O of Mn-based catalysts is insufficient in both the NH3-SCR and CO catalytic oxidation reactions. In future studies, scholars should concentrate their efforts on optimizing the active components and developing new structures and morphologies to avoid catalyst deactivation. Furthermore, a crucial focus should be placed on investigating the regeneration and recycling processes of the catalysts after deactivation.
- (3)
- The interaction mechanism between these two pollutants remains a controversial topic. In further studies, it is essential to employ other methods, such as DFT calculations and reaction kinetics, to gain a better understanding of the reaction processes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amoatey, P.; Omidvarborna, H.; Baawain, M.S.; Al-Mamun, A. Emissions and exposure assessments of SOx, NOx, PM10/2.5 and trace metals from oil industries: A review study (2000–2018). Process Saf. Environ. Prot. 2019, 123, 215–228. [Google Scholar] [CrossRef]
- Tamilselvan, P.; Nallusamy, N.; Rajkumar, S. A comprehensive review on performance, combustion and emission characteristics of biodiesel fuelled diesel engines. Renew. Sustain. Energy Rev. 2017, 79, 1134–1159. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, S.; Wang, M.; Yang, J.; Chen, L.; Liu, W.; Liu, Q.; Su, B. Insights into samarium doping effects on catalytic activity and SO2 tolerance of MnFeOx catalyst for low-temperature NH3-SCR reaction. Fuel 2022, 321, 124113. [Google Scholar] [CrossRef]
- Fan, Z.; Shi, J.W.; Gao, C.; Gao, G.; Wang, B.; Wang, Y.; He, C.; Niu, C. Gd-modified MnOx for the selective catalytic reduction of NO by NH3: The promoting effect of Gd on the catalytic performance and sulfur resistance. Chem. Eng. J. 2018, 348, 820–830. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, L.; Zhao, G.; Guo, L.; Tian, C.; Tao, X.; Du, M.; Nan, B.; Liu, X.; Li, L. Enhancing the low-temperature CO oxidation over Pt/Y2O3 catalyst: The effects of Pt dispersion and support basicity on the catalytic performance. Appl. Surf. Sci. 2023, 614, 156210. [Google Scholar] [CrossRef]
- Li, X.; Ren, S.; Chen, Z.; Chen, L.; Wang, M.; Wang, L.; Wang, A. Promoting mechanism of CuO on catalytic performance of CeFe catalyst for simultaneous removal of NOx and CO. Fuel 2023, 347, 128435. [Google Scholar] [CrossRef]
- Hu, Y.; Griffiths, K.; Norton, P.R. Surface science studies of selective catalytic reduction of NO: Progress in the last ten years. Surf. Sci. 2009, 603, 1740–1750. [Google Scholar] [CrossRef]
- Du, Y.; Gao, F.; Zhou, Y.; Yi, H.; Tang, X.; Qi, Z. Recent advance of CuO-CeO2 catalysts for catalytic elimination of CO and NO. J. Environ. Chem. Eng. 2021, 9, 106372. [Google Scholar] [CrossRef]
- Yahiro, H.; Iwamoto, M. Copper ion-exchanged zeotile catalysts in deNOx reaction. Appl. Catal. A-Gen. 2001, 222, 163–181. [Google Scholar] [CrossRef]
- Xue, J.; Wang, X.; Qi, G.; Wang, J.; Shen, M.; Li, W. Characterization of copper species over Cu/SAPO-34 in selective catalytic reduction of NOx with ammonia: Relationships between active Cu sites and de-NOx performance at low temperature. J. Catal. 2013, 297, 56–64. [Google Scholar] [CrossRef]
- Ma, J.; Chang, S.; Yu, F.; Lai, H.; Zhao, Y. Research progress on sulfur deactivation and regeneration over Cu-CHA zeolite catalyst. Catalysts 2022, 12, 1499. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Meng, C. Coadsorption interfered CO oxidation over atomically dispersed Au on h-BN. Molecules 2022, 27, 3627. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Liu, J. CO oxidation on metal oxide supported single Pt atoms: The role of the support. Ind. Eng. Chem. Res. 2017, 56, 6916–6925. [Google Scholar] [CrossRef]
- Krishnan, R.; Wu, S.Y.; Chen, H.T. Single Pt atom supported on penta-graphene as an efficient catalyst for CO oxidation. Phys. Chem. Chem. Phys. 2019, 21, 12201–12208. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, M.; Wan, L.; Zhu, H.; Yao, K.; Linguerri, R.; Chambaud, G.; Han, Y.; Meng, C. Superior catalytic performance of atomically dispersed palladium on graphene in CO oxidation. ACS Catal. 2020, 10, 3084–3093. [Google Scholar] [CrossRef]
- Prasad, R.; Singh, P. A review on CO oxidation over copper chromite catalyst. Catal. Rev. 2012, 54, 224–279. [Google Scholar] [CrossRef]
- Raphulu, M.; Mcpherson, J.; Pattrick, G.; Ntho, T.; Mokoena, L.; Moma, J. Co oxidation: Deactivation of Au/TiO2 catalysts during storage. Gold Bull. 2009, 42, 328–336. [Google Scholar] [CrossRef]
- Xanthopoulou, G.; Vekinis, G. Investigation of catalytic oxidation of carbon monoxide over a Cu-Cr-oxide catalyst made by self-propagating high-temperature synthesis. Appl. Catal. B-Environ. 1998, 19, 37–44. [Google Scholar] [CrossRef]
- Schlatter, J.; Klimisch, R.; Taylor, K. Exhaust catalysts: Appropriate conditions for comparing platinum and base metal. Science 1973, 179, 798–800. [Google Scholar] [CrossRef]
- Damma, D.; Boningari, T.; Ettireddy, P.R.; Reddy, B.M.; Smirniotis, P.G. Direct decomposition of NOx over TiO2 supported transition metal oxides at low temperatures. Ind. Eng. Chem. Res. 2018, 57, 16615–16621. [Google Scholar] [CrossRef]
- Chen, L.; Ren, S.; Liu, W.; Yang, J.; Chen, Z.; Wang, M.; Liu, Q. Low-temperature NH3-SCR activity of M (M = Zr, Ni and Co) doped MnOx supported biochar catalysts. J. Environ. Chem. Eng. 2021, 9, 106504. [Google Scholar] [CrossRef]
- Thirupathi, B.; Smirniotis, P.G. Co-doping a metal (Cr, Fe, Co, Ni, Cu, Zn, Ce, and Zr) on Mn/TiO2 catalyst and its effect on the selective reduction of NO with NH3 at low-temperatures. Appl. Catal. B-Environ. 2011, 110, 195–206. [Google Scholar] [CrossRef]
- Fan, L.; Shi, J.; Niu, C.; Wang, B.; He, C.; Cheng, Y. The insight into the role of Al2O3 in promoting the SO2 tolerance of MnOx for low-temperature selective catalytic reduction of NOx with NH3. Chem. Eng. J. 2020, 398, 125572. [Google Scholar] [CrossRef]
- Li, G.; Mao, D.; Chao, M.; Li, G.; Yu, J.; Guo, X. Low-temperature NH3-SCR of NOx over MnCeOx/TiO2 catalyst: Enhanced activity and SO2 tolerance by modifying TiO2 with Al2O3. J. Rare Earth. 2021, 39, 805–816. [Google Scholar] [CrossRef]
- Meng, D.; Zhan, W.; Guo, Y.; Wang, L.; Lu, G. A highly effective catalyst of Sm-MnOx for the NH3-SCR of NOx at low temperature: Promoting role of Sm and its catalytic performance. ACS Catal. 2015, 5, 5973–5983. [Google Scholar] [CrossRef]
- Huang, L.; Hu, X.; Yuan, S.; Li, H.; Yan, T.; Shi, L.; Zhang, D. Photocatalytic preparation of nanostructured MnO2-(Co3O4)/TiO2 hybrids: The formation mechanism and catalytic application in SCR deNOx reaction. Appl. Catal. B-Environ. 2017, 203, 778–788. [Google Scholar] [CrossRef]
- Yang, S.; Qi, F.; Xiong, S.; Dang, H.; Liao, Y.; Wong, P.K.; Li, J. MnOx supported on Fe-Ti spinel: A novel Mn based low temperature SCR catalyst with a high N2 selectivity. Appl. Catal. B-Environ. 2016, 181, 570–580. [Google Scholar] [CrossRef]
- He, G.; Gao, M.; Peng, Y.; Yu, Y.; Shan, W.; He, H. Superior oxidative dehydrogenation performance toward NH3 determines the excellent low-temperature NH3-SCR activity of Mn-based catalysts. Environ. Sci. Technol. 2021, 55, 6995–7003. [Google Scholar] [CrossRef]
- Tang, X.; Hao, J.; Xu, W.; Li, J. Low temperature selective catalytic reduction of NOx with NH3 over amorphous MnOx catalysts prepared by three methods. Catal. Commun. 2007, 8, 329–334. [Google Scholar] [CrossRef]
- Kang, M.; Park, E.D.; Kim, J.M.; Yie, J.E. Manganese oxide catalysts for NOx reduction with NH3 at low temperatures. Appl. Catal. A-Gen. 2007, 327, 261–269. [Google Scholar] [CrossRef]
- Tang, X.; Li, J.; Sun, L.; Hao, J. Origination of N2O from NO reduction by NH3 over β-MnO2 and α-Mn2O3. Appl. Catal. B-Environ. 2010, 99, 156–162. [Google Scholar] [CrossRef]
- Hayashi, E.; Yamaguchi, Y.; Kamata, K.; Tsunoda, N.; Kumagai, Y.; Oba, F.; Hara, M. Effect of MnO2 crystal structure on aerobic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid. J. Am. Chem. Soc. 2019, 141, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.M.; Go, Y.B.; Mui, M.; Gardner, G.; Zhang, Z.; Mastrogiovanni, D.; Garfunkel, E.; Li, J.; Greenblatt, M.; Dismukes, G.C. Photochemical water oxidation by crystalline polymorphs of manganese oxides: Structural requirements for catalysis. J. Am. Chem. Soc. 2013, 135, 3494–3501. [Google Scholar] [CrossRef]
- Yang, R.; Fan, Y.; Ye, R.; Tang, Y.; Cao, X.; Yin, Z.; Zeng, Z. MnO2-based materials for environmental applications. Adv. Mater. 2021, 33, 2004862. [Google Scholar] [CrossRef]
- Li, Y.F.; Zhu, S.C.; Liu, Z.P. Reaction network of layer-to-tunnel transition of MnO2. J. Am. Chem. Soc. 2016, 138, 5371–5379. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, Y.; Song, S.; Xue, D. Tunnel-dependent supercapacitance of MnO2: Effects of crystal structure. J. Appl. Crystallogr. 2013, 46, 1128–1135. [Google Scholar] [CrossRef]
- Gong, P.; Xie, J.; Fang, D.; Han, D.; He, F.; Li, F.; Qi, K. Effects of surface physicochemical properties on NH3-SCR activity of MnO2 catalysts with different crystal structures. Chin. J. Catal. 2017, 38, 1925–1934. [Google Scholar] [CrossRef]
- Dai, Y.; Li, J.H.; Peng, Y.; Tang, X.F. Effects of MnO2 crystal structure and surface property on the NH3-SCR reaction at low temperature. Acta Phys.-Chim Sin. 2012, 28, 1771–1776. [Google Scholar]
- Yang, J.; Ren, S.; Su, B.; Zhou, Y.; Hu, G.; Jiang, L.; Cao, J.; Liu, W.; Yao, L.; Kong, M. Insight into N2O formation over different crystal phases of MnO2 during low-temperature NH3-SCR of NO. Catal. Lett. 2021, 151, 2964–2971. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, J.; Zhang, Y.; Zhang, M.; Zhou, Y.; Phoutthavong, T.; Liang, P.; Zhang, H. Simultaneous removal of NO and Hg0 in flue gas over Co-Ce oxide modified rod-like MnO2 catalyst: Promoting effect of Co doping on activity and SO2 resistance. Fuel 2020, 276, 118018. [Google Scholar] [CrossRef]
- Luo, S.; Zhou, W.; Xie, A.; Wu, F.; Yao, C.; Li, X.; Zuo, S.; Liu, T. Effect of MnO2 polymorphs structure on the selective catalytic reduction of NOx with NH3 over TiO2-Palygorskite. Chem. Eng. J. 2016, 286, 291–299. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, J.; Zhou, X.; Du, Y.; Huang, W.; Liu, J.; Zhang, W.; Shi, J.; Chen, H. Nanoflower-like weak crystallization manganese oxide for efficient removal of low-concentration NO at room temperature. J. Mater. Chem. A 2015, 3, 7631–7638. [Google Scholar] [CrossRef]
- Liu, J.; Wei, Y.; Li, P.Z.; Zhang, P.; Su, W.; Sun, Y.; Zou, R.; Zhao, Y. Experimental and theoretical investigation of mesoporous MnO2 nanosheets with oxygen vacancies for high-efficiency catalytic deNOx. ACS Catal. 2018, 8, 3865–3874. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Wan, Y.; Zhan, S.; Guan, Q.; Tian, Y. Structure-performance relationships of MnO2 nanocatalyst for the low-temperature SCR removal of NOx under ammonia. RSC Adv. 2016, 6, 54926–54937. [Google Scholar] [CrossRef]
- Gao, J.; Han, Y.; Mu, J.; Wu, S.; Tan, F.; Shi, Y.; Li, X. 2D, 3D mesostructured silicas templated mesoporous manganese dioxide for selective catalytic reduction of NOx with NH3. J. Colloid Interface Sci. 2018, 516, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yan, Q.; Zhang, C.; Wang, Q. A novel highly active and sulfur resistant catalyst from Mn-Fe-Al layered double hydroxide for low temperature NH3-SCR. Catal. Today 2019, 327, 81–89. [Google Scholar] [CrossRef]
- Kapteijn, F.; Singoredjo, L.; Andreini, A.; Moulijn, J.A. Activity and selectivity of pure manganese oxides in the selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B-Environ. 1994, 3, 173–189. [Google Scholar] [CrossRef]
- Yang, J.; Ren, S.; Zhou, Y.; Su, Z.; Liu, Q. In situ IR comparative study on N2O formation pathways over different valence states manganese oxides catalysts during NH3-SCR of NO. Chem. Eng. J. 2020, 397, 125446. [Google Scholar] [CrossRef]
- Wan, Y.; Zhao, W.; Tang, Y.; Li, L.; Wang, H.; Cui, Y.; Gu, J.; Li, Y.; Shi, J. Ni-Mn bi-metal oxide catalysts for the low temperature SCR removal of NO with NH3. Appl. Catal. B-Environ. 2014, 148, 114–122. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Shi, L.; Fang, C.; Li, H.; Gao, R.; Huang, L.; Zhang, J. In situ supported MnOx-CeOx on carbon nanotubes for the low-temperature selective catalytic reduction of NO with NH3. Nanoscale 2013, 5, 1127–1136. [Google Scholar] [CrossRef]
- Pappas, D.K.; Boningari, T.; Boolchand, P.; Smirniotis, P.G. Novel manganese oxide confined interweaved titania nanotubes for the low-temperature selective catalytic reduction (SCR) of NOx by NH3. J. Catal. 2016, 334, 1–13. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, S.; Xing, X.; Li, X.; Chen, L.; Wang, M. Unveiling the inductive strategy of different precipitants on MnFeOx catalyst for low-temperature NH3-SCR reaction. Fuel 2023, 335, 126986. [Google Scholar] [CrossRef]
- Guo, Y.; Zheng, Y.; Peng, Y.; Yue, T.; Zhu, T. Bifunctional catalyst of CuMn-HZSM-5 for selective catalytic reduction of NO and CO oxidation under oxygen atmosphere. Chem. Eng. J. 2023, 462, 142113. [Google Scholar] [CrossRef]
- Wang, C.; Gao, F.; Ko, S.; Liu, H.; Yi, H.; Tang, X. Structural control for inhibiting SO2 adsorption in porous MnCe nanowire aerogel catalysts for low-temperature NH3-SCR. Chem. Eng. J. 2022, 434, 134729. [Google Scholar] [CrossRef]
- Han, L.; Gao, M.; Feng, C.; Shi, L.; Zhang, D. Fe2O3-CeO2@Al2O3 Nanoarrays on Al-mesh as SO2-tolerant monolith catalysts for NOx reduction by NH3. Environ. Sci. Technol. 2019, 53, 5946–5956. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ren, S.; Zhang, T.S.; Su, Z.H.; Long, H.M.; Kong, M.; Yao, L. Iron doped effects on active sites formation over activated carbon supported Mn-Ce oxide catalysts for low-temperature SCR of NO. Chem. Eng. J. 2020, 379, 122398. [Google Scholar] [CrossRef]
- Lia, H.K.; Liaw, B.J.; Chen, Y.Z. Removal of CO in excess hydrogen over CuO/Ce1−xMnxO2 catalysts. Chem. Eng. J. 2011, 172, 452–458. [Google Scholar]
- Shi, Y.; Yi, H.; Gao, F.; Zhao, S.; Xie, Z.; Tang, X. Evolution mechanism of transition metal in NH3-SCR reaction over Mn-based bimetallic oxide catalysts: Structure-activity relationships. J. Hazard. Mater. 2021, 413, 125361. [Google Scholar] [CrossRef]
- Kang, H.; Wang, J.; Zheng, J.; Chu, W.; Tang, C.; Ji, J.; Ren, R.; Wu, M.; Jing, F. Solvent-free elaboration of Ni-doped MnOx catalysts with high performance for NH3-SCR in low and medium temperature zones. Mol. Catal. 2021, 501, 111376. [Google Scholar] [CrossRef]
- Jiang, B.; Deng, B.; Zhang, Z.; Wu, Z.; Tang, X.; Yao, S.; Lu, H. Effect of Zr addition on the low-temperature SCR activity and SO2 tolerance of Fe-Mn/Ti catalysts. J. Phys. Chem. C 2014, 118, 14866–14875. [Google Scholar] [CrossRef]
- Long, L.; Tian, S.; Zhao, Y.; Zhang, X.; Luo, W.; Yao, X. Promotional effects of Nb5+ and Fe3+ co-doping on catalytic performance and SO2 resistance of MnOx-CeO2 low-temperature denitration catalyst. J. Colloid Interface Sci. 2023, 648, 876–888. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Lyu, F.; Wang, Y.; Zhang, S.; Zhong, Q.; Zhong, Z. New insight on N2O formation over MnOx/TiO2 catalysts for selective catalytic reduction of NOx with NH3. Mol. Catal. 2022, 525, 112356. [Google Scholar] [CrossRef]
- Liu, X.; Yu, Q.; Chen, H.; Jiang, P.; Shen, Z. The promoting effect of S-doping on the NH3-SCR performance of MnO/TiO2 catalyst. Appl. Surf. Sci. 2019, 508, 144694. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, H.; Luo, X.; Shi, K.; Zhao, H.; Wang, W.; Chen, Q.; Fan, H.; Wu, T. Promotion effect and mechanism of the addition of Mo on the enhanced low temperature SCR of NOx by NH3 over MnOx/γ-Al2O3 catalysts. Appl. Catal. B-Environ. 2019, 245, 743–752. [Google Scholar] [CrossRef]
- Kijlstra, W.S.; Brands, D.S.; Poels, E.K.; Bliek, A. Kinetics of the selective catalytic reduction of NO with NH3 over MnOx/Al2O3 catalysts at low temperature. Catal. Today 1999, 50, 133–140. [Google Scholar] [CrossRef]
- Kijlstra, W.S.; Brands, D.S.; Smit, H.I.; Poels, E.K.; Bliek, A. Mechanism of the selective catalytic reduction of NO with NH3 over MnOx/Al2O3. J.Catal. 1997, 171, 219–230. [Google Scholar] [CrossRef]
- Kong, T.; Chen, L.; Ding, S.; Yang, F.; Dong, L. Enhanced low-temperature NH3-SCR performance of MnOx/CeO2 catalysts by optimal solvent effect. Appl. Surf. Sci. 2017, 420, 407–415. [Google Scholar]
- Hsu, L.; Tsai, M.; Lu, Y.; Chen, H. Computional inverstigation of CO adsorption and oxidation on Mn/CeO2(111) surfface. J. Phys. Chem. C. 2013, 117, 433–441. [Google Scholar] [CrossRef]
- Li, J.; Zhu, P.; Zhou, R. Effect of the preparation method on the performance of CuO-MnOx-CeO2 catalysts for selective oxidation of CO in H2-rich streams. J. Power Sources 2011, 196, 9590–9598. [Google Scholar] [CrossRef]
- Vasilyeva, M.; Rudnev, V.; Ustinov, A.; Tsvetnov, M. Formation, composition, structure, and catalytic activity in CO oxidation of SiO2 + TiO2/Ti composite before and after modification by MnOx or CoOx. Surf. Coat. Technol. 2015, 275, 84–89. [Google Scholar] [CrossRef]
- Shen, B.; Zhang, X.; Ma, H.; Yao, Y.; Liu, T. A comparative study of Mn/CeO2, Mn/ZrO2 and Mn/Ce-ZrO2 for low temperature selective catalytic reduction of NO with NH3 in the presence of SO2 and H2O. J. Environ. Sci. 2013, 25, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Guo, J.; Luo, H.; Shu, S.; Fang, N.; Li, J. Study of NO removal and resistance to SO2 and H2O of MnOx/TiO2, MnOx/ZrO2 and MnOx/ZrO2-TiO2. Appl. Catal. A-Gen. 2018, 553, 82–90. [Google Scholar] [CrossRef]
- Yao, L.; Liu, Q.; Mossin, S.; Nielsen, D.; Kong, M.; Jiang, L.; Yang, J.; Ren, S.; Wen, J. Promotional effects of nitrogen doping on catalytic performance over manganese-containing semi-coke catalysts for the NH3-SCR at low temperatures. J. Hazard. Mater. 2020, 387, 121704. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, C.; Li, Q.; Gao, T.; Yu, S.; Tan, P.; Fang, Q.; Chen, G. Promoting mechanism of SO2 resistance performance by anatase TiO2 {001} facets on Mn-Ce/TiO2 catalysts during NH3-SCR reaction. Chem. Eng. Sci. 2022, 251, 117438. [Google Scholar] [CrossRef]
- Ettireddy, P.R.; Ettireddy, N.; Mamedov, S.; Boolchand, P.; Smirniotis, P.G. Surface characterization studies of TiO2 supported manganese oxide catalysts for low temperature SCR of NO with NH3. Appl. Catal. B-Environ. 2007, 76, 123–134. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kwon, H.J.; Nam, I.S.; Choung, J.W.; Kil, J.K.; Kim, H.J.; Cha, M.S.; Yeo, G.K. High deNOx performance of Mn/TiO2 catalyst by NH3. Catal. Today 2010, 151, 244–250. [Google Scholar] [CrossRef]
- Smirniotis, P.G.; Peña, D.A.; Uphade, B.S. Low-temperature selective catalytic reduction (SCR) of NO with NH3 by using Mn, Cr, and Cu oxides supported on Hombikat TiO2. Angew. Chem. Int. Ed. 2001, 40, 2479–2482. [Google Scholar] [CrossRef]
- Yao, X.; Kong, T.; Yu, S.; Li, L.; Yang, F.; Dong, L. Influence of different supports on the physicochemical properties and denitration performance of the supported Mn-based catalysts for NH3-SCR at low temperature. Appl. Surf. Sci. 2017, 402, 208–217. [Google Scholar] [CrossRef]
- Jin, R.; Liu, Y.; Wu, Z.; Wang, H.; Gu, T. Low-temperature selective catalytic reduction of NO with NH3 over MnCe oxides supported on TiO2 and Al2O3: A comparative study. Chemosphere 2010, 78, 1160–1166. [Google Scholar] [CrossRef]
- Li, L.; Ji, J.; Tan, W.; Song, W.; Wang, X.; Wei, X.; Guo, K.; Zhang, W.; Tang, C.; Dong, L. Enhancing low-temperature NH3-SCR performance of Fe-Mn/CeO2 catalyst by Al2O3 modification. J. Rare Earth. 2022, 40, 1454–1461. [Google Scholar] [CrossRef]
- Gao, L.; Li, C.; Li, S.; Zhang, W.; Du, X.; Huang, L.; Zhu, Y.; Zhai, Y.; Zeng, G. Superior performance and resistance to SO2 and H2O over CoOx-modified MnOx/biomass activated carbons for simultaneous Hg0 and NO removal. Chem. Eng. J. 2019, 371, 781–795. [Google Scholar] [CrossRef]
- Su, Z.; Ren, S.; Zhang, T.; Yang, J.; Zhou, Y.; Yao, L. Effects of PbO poisoning on Ce-Mn/AC catalyst for low-temperature selective catalytic reduction of NO with NH3. J. Iron Steel Res. Int. 2021, 28, 133–139. [Google Scholar] [CrossRef]
- Su, Y.; Fan, B.; Wang, L.; Liu, Y.; Huang, B.; Fu, M.; Chen, L.; Ye, D. MnOx supported on carbon nanotubes by different methods for the SCR of NO with NH3. Catal. Today 2013, 201, 115–121. [Google Scholar] [CrossRef]
- Xiao, X.; Sheng, Z.; Yang, L.; Dong, F. Low-temperature selective catalytic reduction of NOx with NH3 over a manganese and cerium oxide/graphene composite prepared by a hydrothermal method. Catal. Sci. Technol. 2016, 6, 1507–1514. [Google Scholar] [CrossRef]
- Jiang, L.; Xu, Y.; Jiang, W.; Pu, Y.; Yang, L.; Dai, Z.; Yao, L. The promotion of NH3-SCR performance by AC addition on Mn-Fe/Z catalyst. Sep. Purif. Technol. 2023, 322, 124274. [Google Scholar] [CrossRef]
- Liang, S.; Teng, F.; Bulgan, G.; Zong, R.; Zhu, Y. Effect of phase structure of MnO2 nanorod catalyst on the activity for CO oxidation. J. Phys. Chem. C 2008, 112, 5307–5315. [Google Scholar] [CrossRef]
- Xu, R.; Wang, X.; Wang, D.; Zhou, K.; Li, Y. Surface structure effects in nanocrystal MnO2 and Ag/MnO2 catalytic oxidation of CO. J. Catal. 2006, 237, 426–430. [Google Scholar] [CrossRef]
- Frey, K.; Iablokov, V.; Sáfrán, G.; Osán, J.; Sajó, I.; Szukiewicz, R.; Chenakin, S.; Kruse, N. Nanostructured MnOx as highly active catalyst for CO oxidation. J. Catal. 2012, 287, 30–36. [Google Scholar] [CrossRef]
- Wang, X.; Huo, W.; Xu, Y.; Guo, Y.; Jia, Y. Modified hierarchical birnessite-type manganese oxide nanomaterials for CO catalytic oxidation. New J. Chem. 2018, 42, 13803–13812. [Google Scholar] [CrossRef]
- Ramesh, K.; Chen, L.; Chen, F.; Liu, Y.; Wang, Z.; Han, Y.F. Re-investigating the CO oxidation mechanism over unsupported MnO, Mn2O3 and MnO2 catalysts. Catal. Today 2008, 131, 477–482. [Google Scholar] [CrossRef]
- Cai, L.N.; Guo, Y.; Lu, A.H.; Branton, P.; Li, W.C. The choice of precipitant and precursor in the co-precipitation synthesis of copper manganese oxide for maximizing carbon monoxide oxidation. J. Mol. Catal. A-Chem. 2012, 360, 35–41. [Google Scholar] [CrossRef]
- Pan, H.; Chen, X.; López-Cartes, C.; Martínez-López, J.; Bu, E.; Delgado, J.J. Hydrothermal synthesis and characterization of Cu-MnOx catalysts for CO oxidation: Effect of Cu: Mn molar ratio on their structure and catalytic activity. Catal. Today 2023, 418, 114085. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, Y.; Tian, P.; Shang, H.; Xu, J.; Han, Y. Dynamic active sites over binary oxide catalysts: In situ/operando spectroscopic study of low-temperature CO oxidation over MnOx-CeO2 catalysts. Appl. Catal. B-Environ. 2016, 191, 179–191. [Google Scholar] [CrossRef]
- Dong, S.; Wang, J.; Tang, X.; Li, J.; Zhang, X.; Liu, B. Low-temperature and stable CO oxidation of in-situ grown monolithic Mn3O4/TiO2 catalysts. J. Alloys Compd. 2021, 855, 157444. [Google Scholar] [CrossRef]
- Camposeco, R.; Castillo, S.; Nava, N.; Medina, J.C.; Zanella, R. Effect of gold nanoparticles on MnOx/TiO2 nanostructures for improving the CO oxidation at low temperature. Top. Catal. 2020, 63, 492–503. [Google Scholar] [CrossRef]
- Dey, S.; Dhal, G.C.; Mohan, D.; Prasad, R. Characterization and activity of CuMnOx/γ-Al2O3 catalyst for oxidation of carbon monoxide. Mater. Discov. 2017, 8, 26–34. [Google Scholar] [CrossRef]
- Li, L.; Han, W.; Dong, F.; Zong, L.; Tang, Z.; Zhang, J. Controlled pore size of ordered mesoporous Al2O3-supported Mn/Cu catalysts for CO oxidation. Microporous Mesoporous Mater. 2017, 249, 1–9. [Google Scholar] [CrossRef]
- Xu, J.; Deng, Y.Q.; Luo, Y.; Mao, W.; Yang, X.J.; Han, Y.F. Operando Raman spectroscopy and kinetic study of low-temperature CO oxidation on an α-Mn2O3 nanocatalyst. J. Catal. 2013, 300, 225–234. [Google Scholar] [CrossRef]
- Xu, G.; Guo, X.; Cheng, X.; Yu, J.; Fang, B. A review of Mn-based catalysts for low-temperature NH3-SCR: NOx removal and H2O/SO2 resistance. Nanoscale 2021, 23, 7052–7080. [Google Scholar] [CrossRef]
- Li, X.; Ren, S.; Chen, Z.; Jiang, Y.; Wang, M.; Wang, L.; Liu, M. Unraveling the morphology and crystal plane dependence of bifunctional MnO2 catalyst for simultaneous removal of NO and CO at low temperature. Sep. Purif. Technol. 2023, 325, 124760. [Google Scholar] [CrossRef]
- Zheng, Y.; Guo, Y.; Xu, X.; Zhu, T. Study of Cu/Mn catalysts for coreactions of NH3-SCR and CO oxidation. Catal. Lett. 2022, 152, 1752–1759. [Google Scholar] [CrossRef]
- Gui, R.; Xiao, J.; Gao, Y.; Li, Y.; Zhu, T.; Wang, Q. Simultaneously achieving selective catalytic reduction of NOx with NH3 and catalytic oxidation of CO with O2 over one finely optimized bifunctional catalyst Mn2Cu1Al1Ox at low temperatures. Appl. Catal. B-Environ. 2022, 306, 121104. [Google Scholar] [CrossRef]
- Wang, J.; Xing, Y.; Su, W.; Li, K.; Zhang, W. Bifunctional Mn-Cu-CeOx/γ-Al2O3 catalysts for low-temperature simultaneous removal of NOx and CO. Fuel 2022, 321, 124050. [Google Scholar] [CrossRef]
- Guo, J.; Xiao, J.; Gui, R.; Gao, Y.; Wang, Q. Efficient simultaneous removal of NOx and CO at low temperatures over integrated Mn2Co1Ox/iron mesh monolithic catalyst via NH3-SCR coupling with CO oxidation reactions. Chem. Eng. J. 2023, 465, 142611. [Google Scholar] [CrossRef]
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective catalytic reduction of NOx with NH3 by using novel catalysts: State of the art and future prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef] [PubMed]
- Marbán, G.; Valdés-Solís, T.; Fuertes, A.B. Mechanism of low-temperature selective catalytic reduction of NO with NH3 over carbon-supported Mn3O4: Role of surface NH3 species: SCR mechanism. J. Catal. 2004, 226, 138–155. [Google Scholar] [CrossRef]
- Xu, S.; Chen, J.; Li, Z.; Liu, Z. Highly ordered mesoporous MnOx catalyst for the NH3-SCR of NOx at low temperatures. Appl. Catal. A-Gen. 2023, 649, 118966. [Google Scholar] [CrossRef]
- Liu, C.; Shi, J.W.; Gao, C.; Niu, C. Manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3: A review. Appl.Catal. A-Gen. 2016, 522, 54–69. [Google Scholar] [CrossRef]
- Wei, L.; Cui, S.; Guo, H.; Ma, X. Study on the role of Mn species in low temperature SCR on MnOx/TiO2 through experiment and DFT calculation. Mol. Catal. 2018, 445, 102–110. [Google Scholar] [CrossRef]
- Freund, H.J.; Meijer, G.; Scheffler, M.; Schlögl, R.; Wolf, M. CO oxidation as a prototypical reaction for heterogeneous processes. Angew. Chem. Int. Ed. 2011, 50, 10064–10094. [Google Scholar] [CrossRef]
- Stampfl, C.; Scheffler, M. Density-functional theory study of the catalytic oxidation of CO over transition metal surfaces. Surf. Sci. 1999, 433, 119–126. [Google Scholar] [CrossRef]
- Morgan, K.; Cole, K.J.; Goguet, A.; Hardacre, C.; Hutchings, G.J.; Maguire, N.; Shekhtman, S.O.; Taylor, S.H. TAP studies of CO oxidation over CuMnOx and Au/CuMnOx catalysts. J.Catal. 2010, 276, 38–48. [Google Scholar] [CrossRef]
- Cacciatore, M.; Rutigliano, M.; Billing, G. Eley-Rideal and Langmuir-Hinshelwood recombination coefficients for oxygen on silica surfaces. J. Thermophys. Heat Transf. 1999, 13, 195–203. [Google Scholar] [CrossRef]
- Dey, S.; Dhal, G.C.; Mohan, D.; Prasad, R. Effect of preparation conditions on the catalytic activity of CuMnOx catalysts for CO oxidation. Bull. Chem. React. Eng. Catal. 2017, 12, 437–451. [Google Scholar] [CrossRef]
- Prasad, R.; Singh, P. A novel route of single step reactive calcination of copper salts far below their decomposition temperatures for synthesis of highly active catalysts. Catal. Sci. Technol. 2013, 3, 3326–3334. [Google Scholar] [CrossRef]
- Dey, S.; Dhal, G.C.; Mohan, D.; Prasad, R. Effect of various metal oxides phases present in CuMnOx catalyst for selective CO oxidation. Mater. Discov. 2018, 12, 63–71. [Google Scholar] [CrossRef]
- Zeng, Y.; Rong, W.; Zhang, S.; Wang, Y.; Zhong, Q. Promoting NH3-SCR denitration via CO oxidation over CuO promoted V2O5-WO3/TiO2 catalysts under oxygen-rich conditions. Fuel 2022, 323, 124357. [Google Scholar] [CrossRef]
- Liu, L.; Liu, T.; Zhou, Y.; Zheng, X.; Su, S.; Yu, J.; Xiang, J. Inhibitory effect of CO on NH3-SCR of NO over Mn/TiO2 catalyst at low temperature: Inhibitory mechanism investigated by in situ DRIFTS. Appl. Surf. Sci. 2023, 638, 158003. [Google Scholar] [CrossRef]



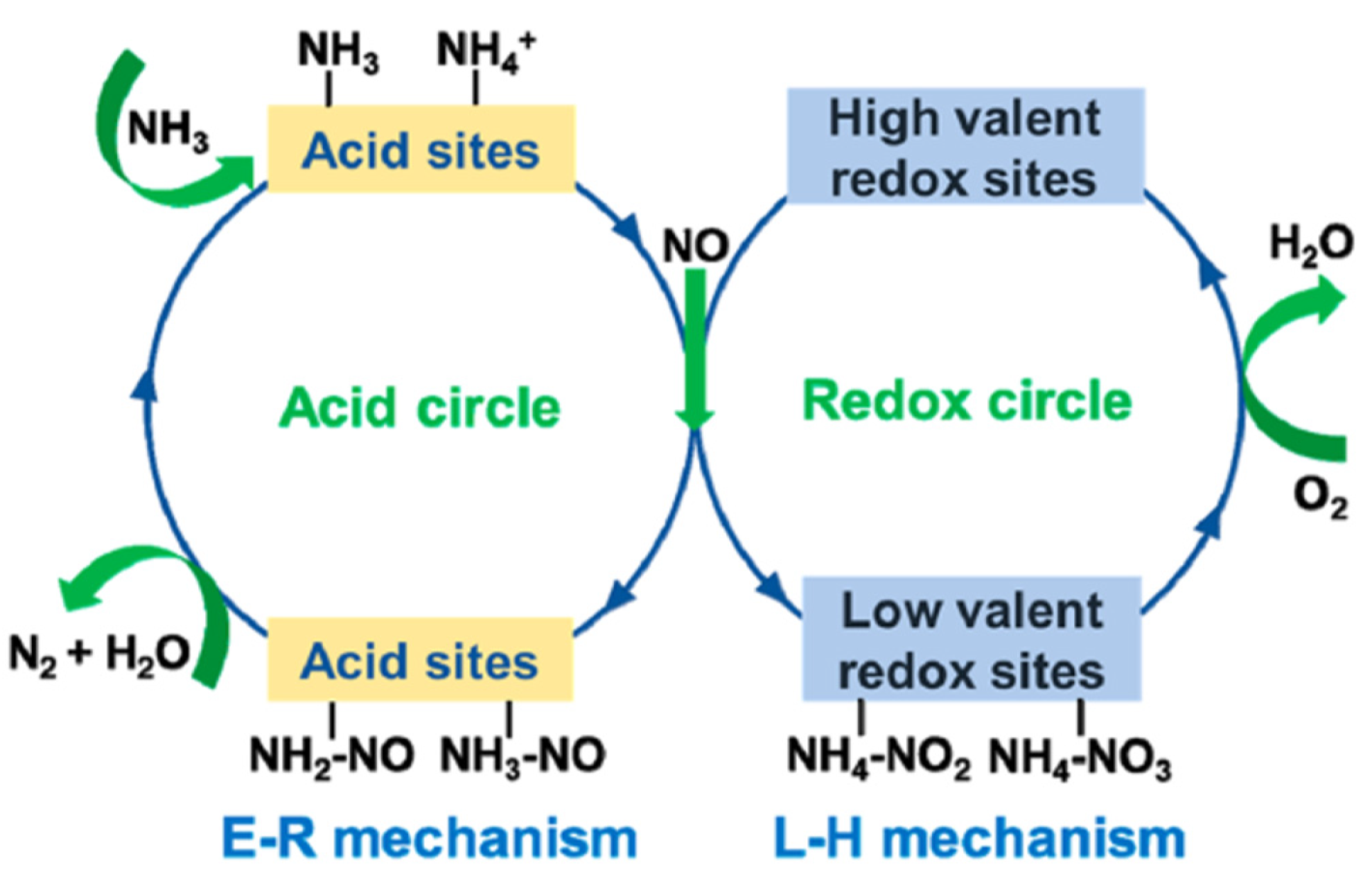
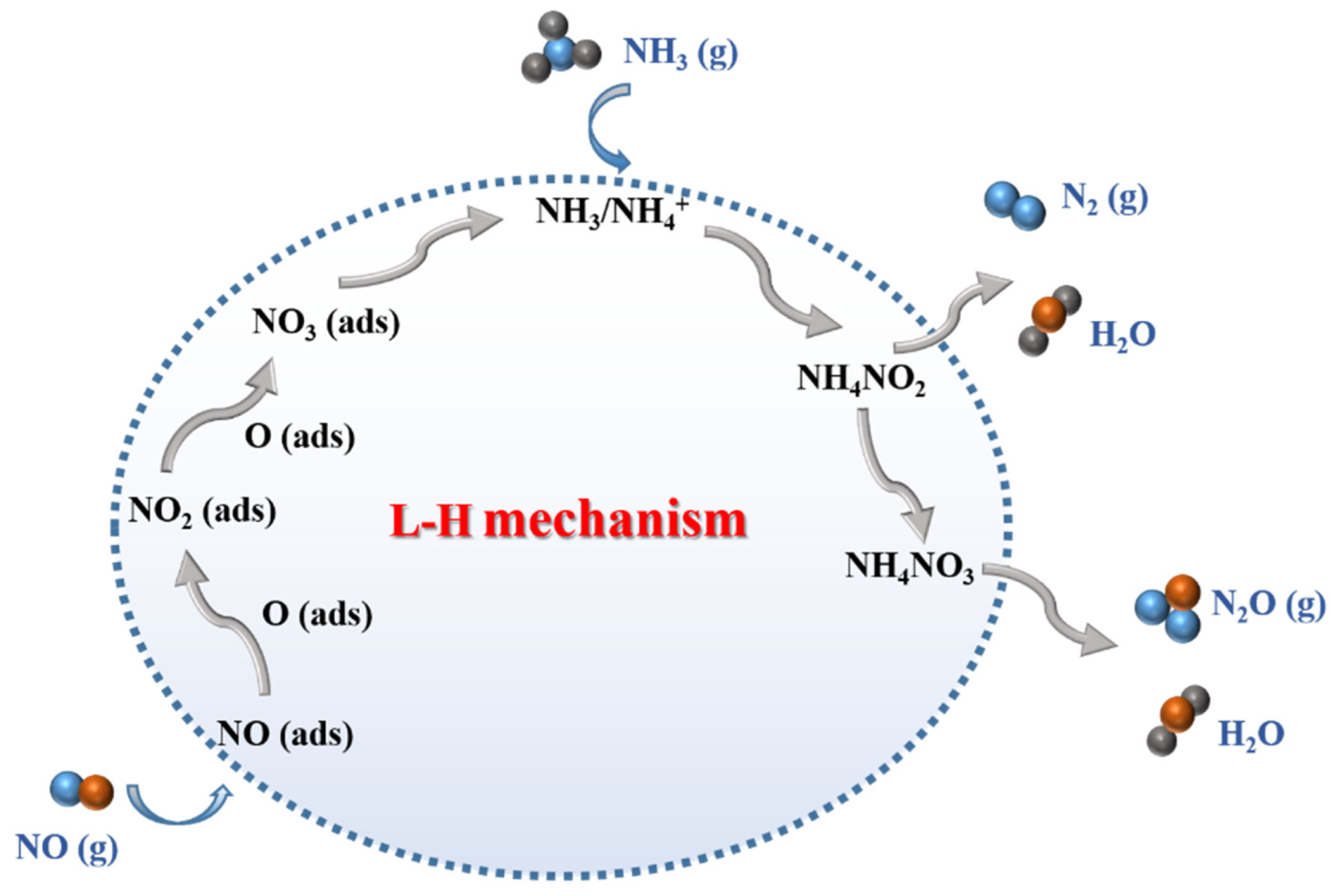

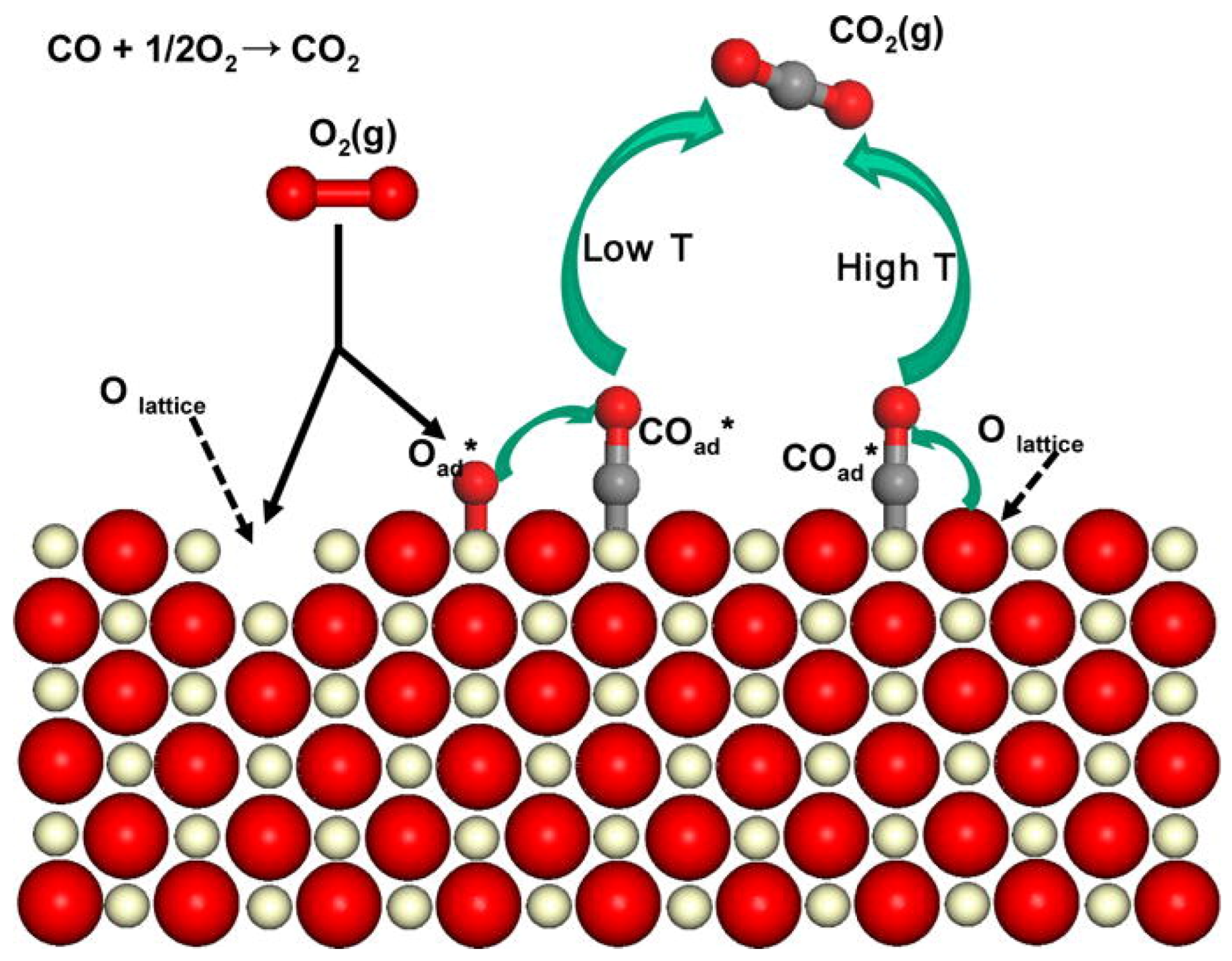
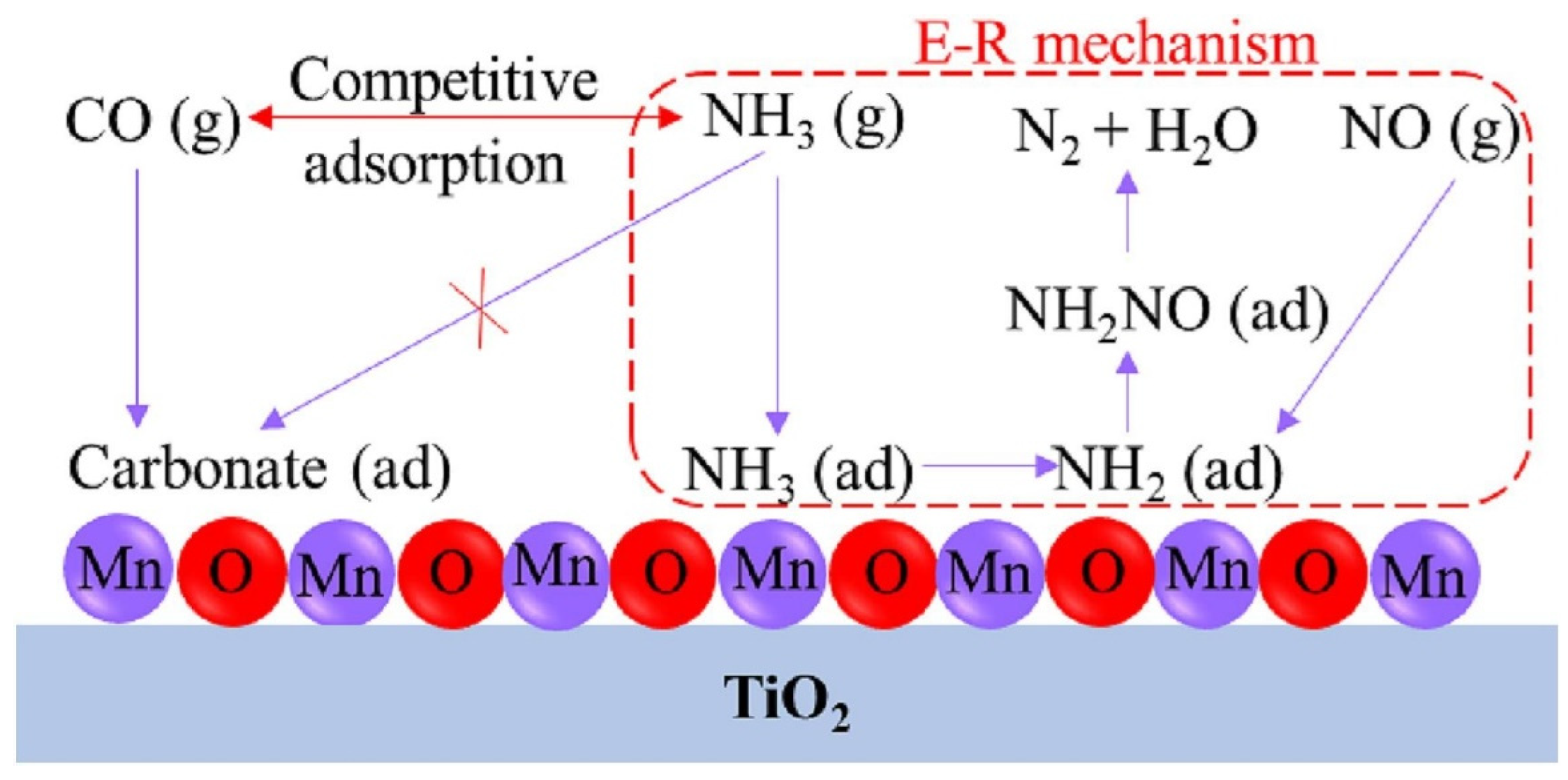
| Catalysts | Preparation Method | Reaction Conditions | NOx/NO Conversion (%) | T (°C) | References |
|---|---|---|---|---|---|
| α-MnO2 | Hydrothermal | 0.1% NO, 0.1% NH3, 2% O2, N2 as balance, 38,000 h−1 | 90% | 125 °C | [38] |
| γ-MnO2 | Hydrothermal | 500 ppm NO, 500 ppm NH3, 19% O2, N2 as balance, 36,000 h−1 | 90% | 100 °C | [39] |
| MnO2 nanosphere | Hydrothermal | 500 ppm NO, 500 ppm NH3, 3% O2, N2 as balance, 28,000 h−1 | 95% | 150 °C | [44] |
| MnO2-KIT-6 | Impregnation | 1000 ppm NO, 1000 ppm NH3, 5% O2, Ar as balance, 30,000 h−1 | 98% | 100 °C | [45] |
| MnO2 | Hydrothermal | 500 ppm NO, 500 ppm NH3, 19% O2, N2 as balance, 36,000 h−1 | 100% | 150 °C | [48] |
| Mn3O4 | Hydrothermal | 500 ppm NO, 500 ppm NH3, 19% O2, N2 as balance, 36,000 h−1 | 100% | 175 °C | [48] |
| Mn0.25/TNT-H | Hydrothermal | 900 ppm NO, 100 ppm NO2, 1000 ppm NH3, 10% O2, He as balance, 50,000 h−1 | 100% | 100 °C | [51] |
| MnFeOx | Co-precipitation | 500 ppm NO, 500 ppm NH3, 5% O2, N2 as balance, 75,000 h−1 | 100% | 100 °C | [52] |
| MnCe nanowire | Hydrothermal+ co-precipitation | 500 ppm NO, 500 ppm NH3, 5% O2, N2 as balance, 32,000 h−1 | 100% | 150 °C | [44] |
| Co-MnOx | Solvothermal | 2000 ppm NO, 2000 ppm NH3, 8% O2, N2 as balance, 128,000 h−1 | 100% | 100 °C | [58] |
| NbFeMnCeOx | Co-precipitation | 500 ppm NO, 500 ppm NH3, 11% O2, N2 as balance, 60,000 h−1 | 95% | 175 °C | [61] |
| Mn/γ-Al2O3 | Sol-gel | 500 ppm NO, 500 ppm NH3, 5% O2, N2 as balance, 60,000 h−1 | 95% | 200 °C | [78] |
| Mn-Ce/Al2O3 | Impregnation | 800 ppm NO, 800 ppm NH3, 3% O2, N2 as balance, 120,000 h−1 | 90% | 180 °C | [79] |
| FeMn/CeAl | Impregnation | 500 ppm NO, 500 ppm NH3, 5% O2, N2 as balance, 30,000 h−1 | 100% | 100 °C | [80] |
| Ce-Mn/AC | Impregnation | 500 ppm NO, 500 ppm NH3, 5% O2, N2 as balance, 30,000 h−1 | 95% | 175 °C | [81] |
| Mn/CNT | Impregnation | 0.08% NO, 0.08% ppm NH3, 5% O2, A2 as balance, 35,000 h−1 | 95% | 200 °C | [83] |
| MnOx-CeO2/GR | Hydrothermal | 500 ppm NO, 500 ppm NH3, 5% O2, N2 as balance, 24,000 h−1 | 100% | 200 °C | [84] |
| Mn-Fe/Z-AC | Hydrothermal | 450 ppm NO, 450 ppm NH3, 5% O2, N2 as balance, 2,000 h−1 | 98% | 125 °C | [85] |
| Catalysts | Preparation Method | Reaction Condition | Best CO Conversion (%) | T (°C) | Reference |
|---|---|---|---|---|---|
| MnOx-CeO2 | Co-precipitation | 1% CO, 20% O2, Ar as balance, 75,000 h−1 | 100% | 175 °C | [68] |
| α-MnO2 | Hydrothermal | 2% CO, 98% air, 12,000 h−1 | 100% | 120 °C | [86] |
| β-MnO2 | Hydrothermal | 1% CO, 16% O2, N2 as balance, 60,000 h−1 | 90% | 169 °C | [87] |
| Ce-MnO2 | Hydrothermal | 1% CO, 10% O2, N2 as balance, 30,000 h−1 | 100% | 175 °C | [89] |
| Cu-MnOx | Hydrothermal | 1% CO, 0.6% O2, He as balance, 150,000 h−1 | 100% | 150 °C | [92] |
| Mn3O4/TiO2 | Urea-assisted deposition | 1% CO, 20% O2, He as balance, 7200 h−1 | 100% | 150 °C | [94] |
| CuMnOx/γ-Al2O3 | Sol-gel + co-precipitation | 2.5% CO, air as balance, 30,000 h−1 | 100% | 120 °C | [96] |
| CuMn-Al2O3 | Co-precipitation | 1% CO, air as balance, 10,000 h−1 | 100% | 120 °C | [97] |
| Catalysts | Preparation Method | Reaction Conditions | NOx Conversion (%) | CO Conversion (%) | T (°C) | Reference |
|---|---|---|---|---|---|---|
| CuMn-HZSM-5 | Impregnation | 500 ppm NO, 500 ppm NH3, 5000 ppm CO, 5% O2, N2 as balance, 120,000 h−1 | 90% | 100% | 200 °C | [53] |
| γ-MnO2 | Hydrothermal | 500 ppm NO, 500 ppm NH3, 1000 ppm CO, 11% O2, N2 as balance, 90,000 h−1 | 91% | 80% | 175 °C | [100] |
| Cu1Mn2 | Co-precipitation | 500 ppm NO, 500 ppm NH3, 2000 ppm CO, 5% O2, N2 as balance, 100,000 h−1 | 96% | 100% | 125 °C | [101] |
| Mn2Cu2Al1Ox | Aqueous miscible organic solvent treatment | 500 ppm NO, 500 ppm NH3, 5000 ppm CO, 5% O2, Ar as balance, 80,000 h−1 | 97% | 100% | 200 °C | [102] |
| MnCuCeOx/γ-Al2O3 | Impregnation | 300 ppm NO, 300 ppm NH3, 3000 ppm CO, 16% O2, N2 as balance, 25,000 h−1 | 100% | 100% | 200 °C | [103] |
| Mn2Co1Ox/IM | Hydrothermal | 500 ppm NO, 500 ppm NH3, 5% O2, 5000 ppm CO, A2 as balance, | 98% | 100% | 200 °C | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Ren, S.; Chen, Z.; Wang, M.; Chen, L.; Chen, H.; Yin, X. A Review of Mn-Based Catalysts for Abating NOx and CO in Low-Temperature Flue Gas: Performance and Mechanisms. Molecules 2023, 28, 6885. https://doi.org/10.3390/molecules28196885
Li X, Ren S, Chen Z, Wang M, Chen L, Chen H, Yin X. A Review of Mn-Based Catalysts for Abating NOx and CO in Low-Temperature Flue Gas: Performance and Mechanisms. Molecules. 2023; 28(19):6885. https://doi.org/10.3390/molecules28196885
Chicago/Turabian StyleLi, Xiaodi, Shan Ren, Zhichao Chen, Mingming Wang, Lin Chen, Hongsheng Chen, and Xitao Yin. 2023. "A Review of Mn-Based Catalysts for Abating NOx and CO in Low-Temperature Flue Gas: Performance and Mechanisms" Molecules 28, no. 19: 6885. https://doi.org/10.3390/molecules28196885
APA StyleLi, X., Ren, S., Chen, Z., Wang, M., Chen, L., Chen, H., & Yin, X. (2023). A Review of Mn-Based Catalysts for Abating NOx and CO in Low-Temperature Flue Gas: Performance and Mechanisms. Molecules, 28(19), 6885. https://doi.org/10.3390/molecules28196885