Synthesis and Characterization of Novel Thiosalicylate-based Solid-Supported Ionic Liquid for Removal of Pb(II) Ions from Aqueous Solution
Abstract
:1. Introduction
2. Results and Discussions
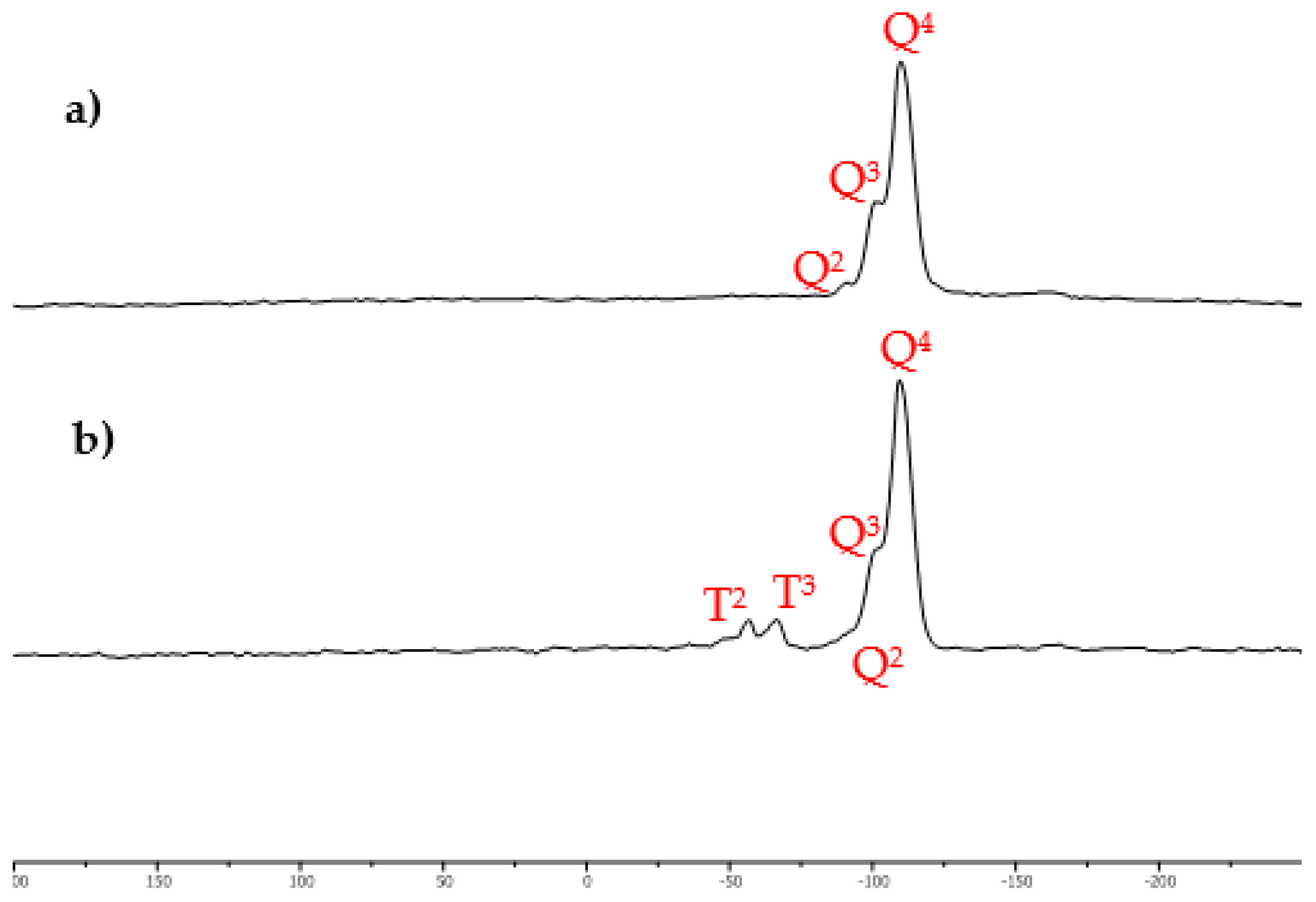
2.1. Characterization
2.2. Adsorption Studies
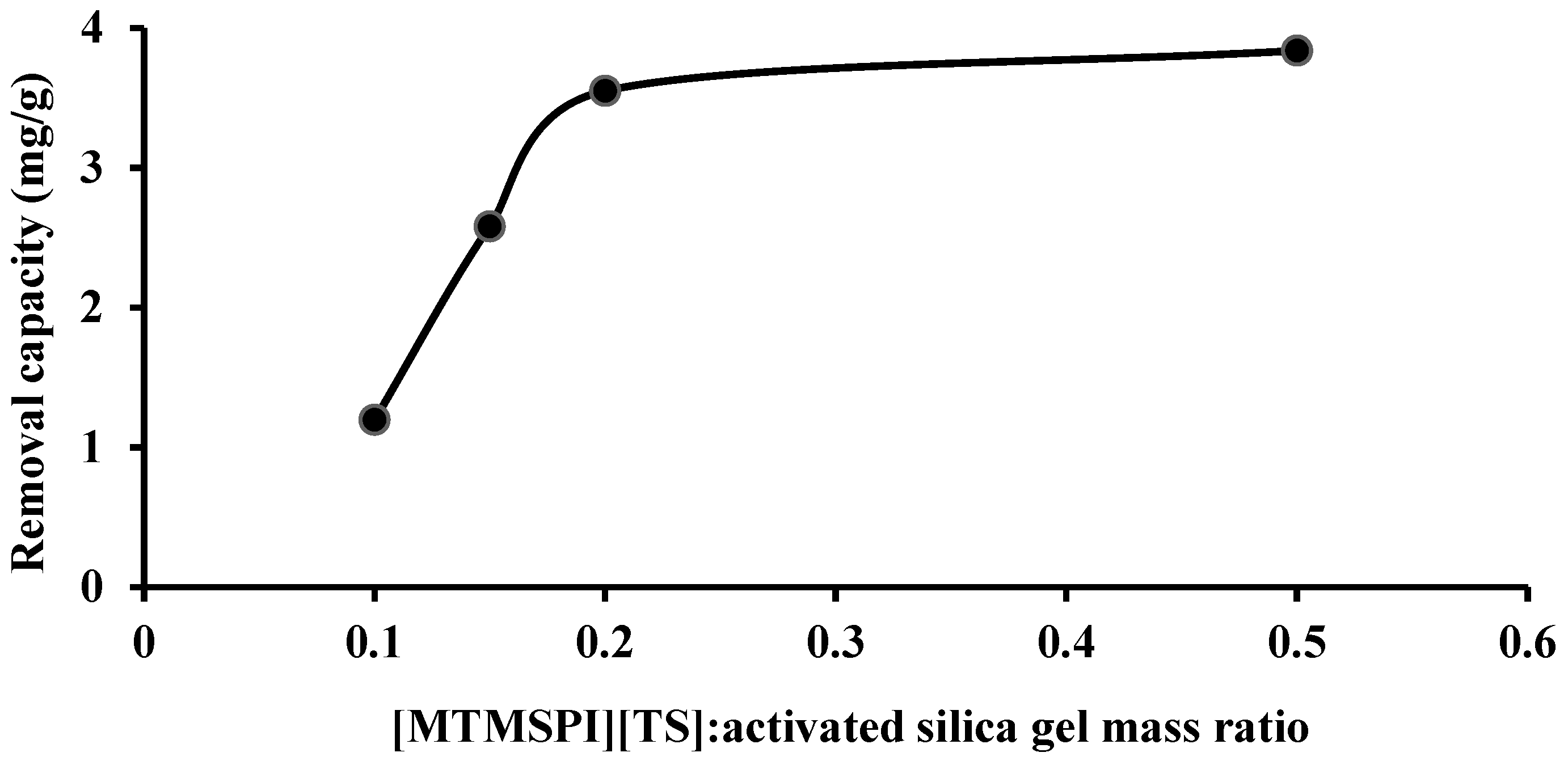
2.2.1. Effect of [MTMSPI][TS]: Activated Silica gel Mass Ratio in Si-TS-SSIL on the Removal Efficiency
2.2.2. Effect of Contact Time
2.2.3. Effect of pH
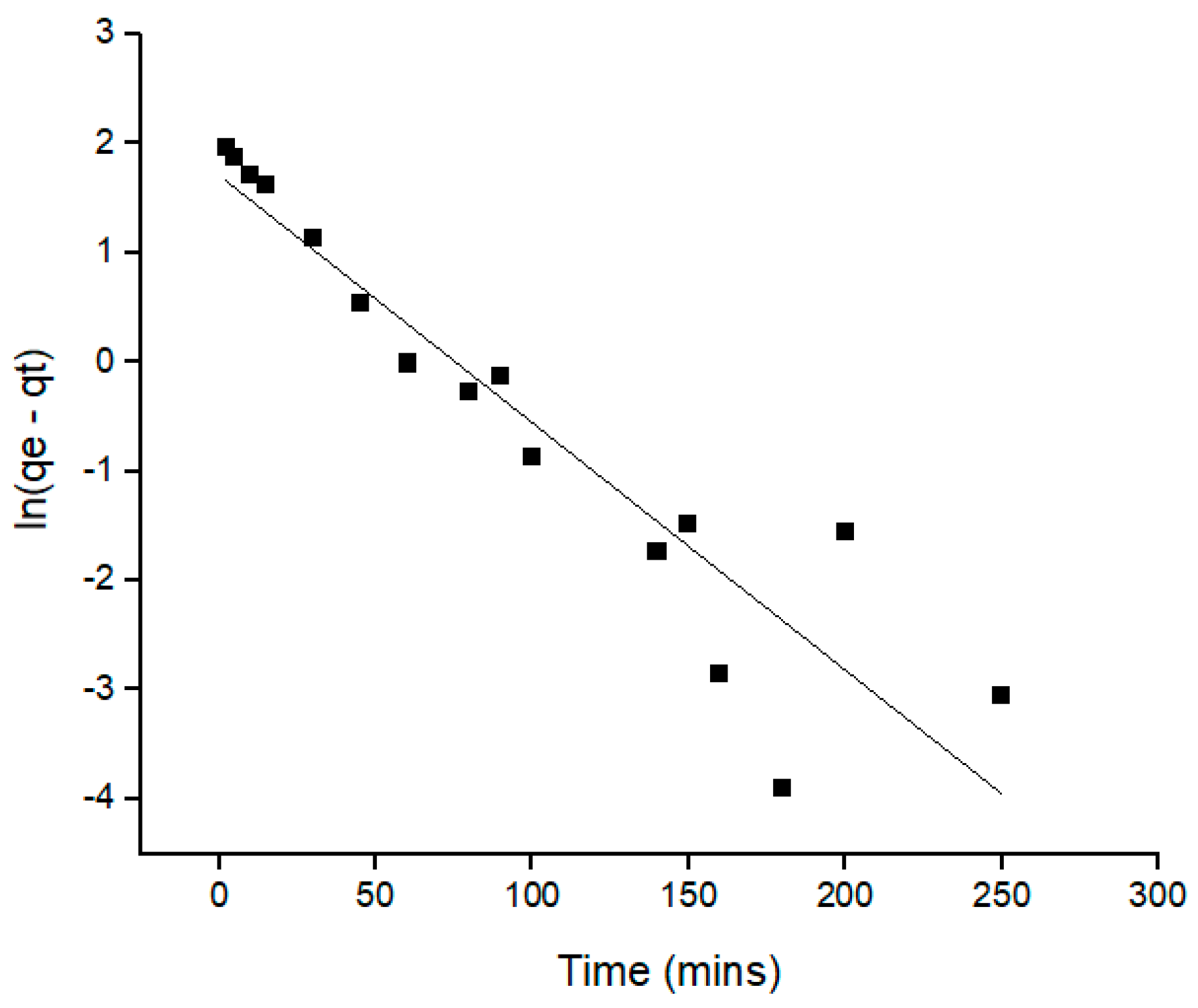
2.3. Adsorption Kinetics
| Extractant | Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|---|
| Si-TS-SSIL | qe exp (mg/g) | qe cal (mg/g) | R2 | qe exp (mg/g) | qe cal (mg/g) | R2 |
| 8.3970 | 5.5182 | 0.8705 | 8.3970 | 8.9718 | 0.9942 | |
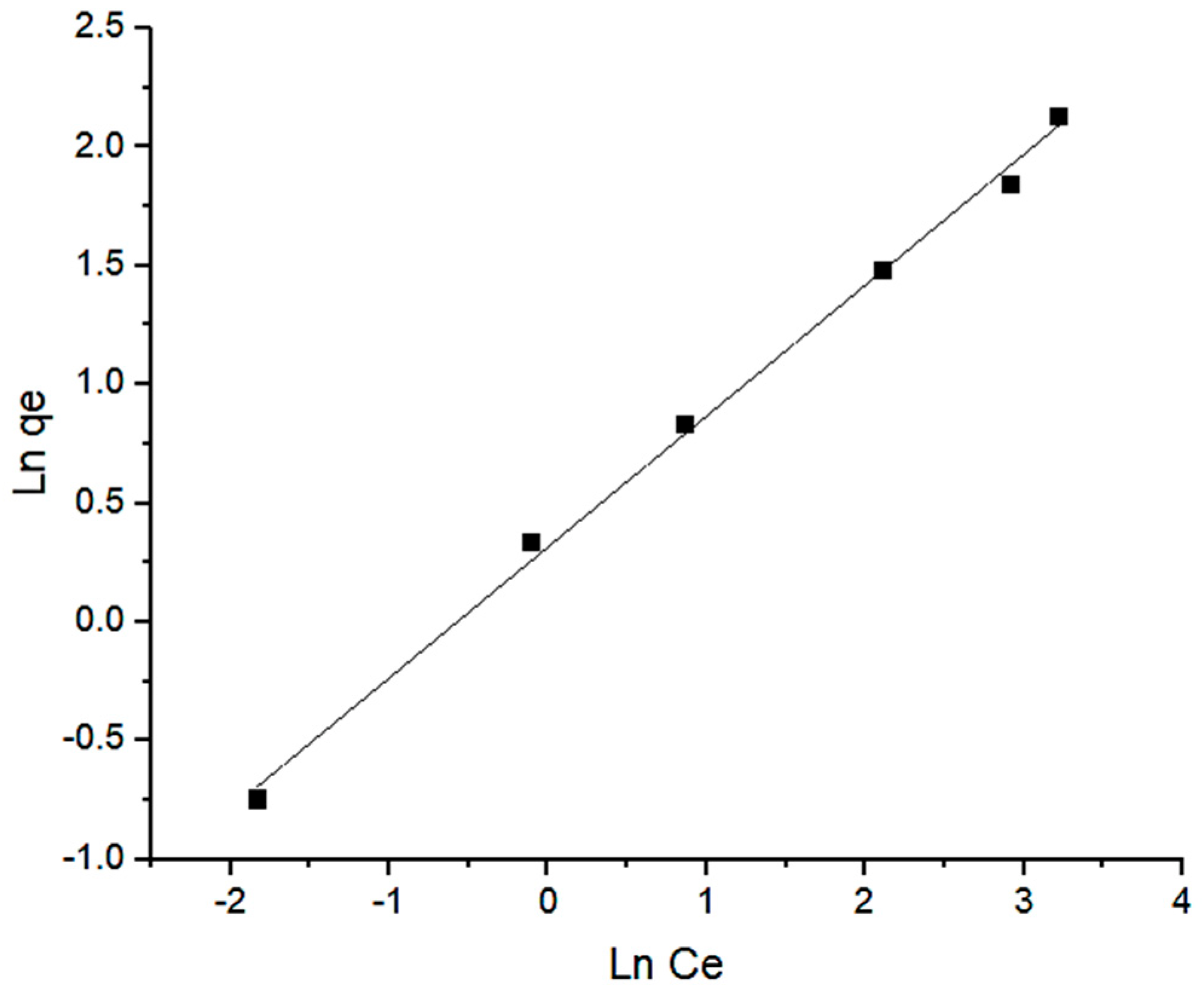
2.4. Adsorption Isotherm
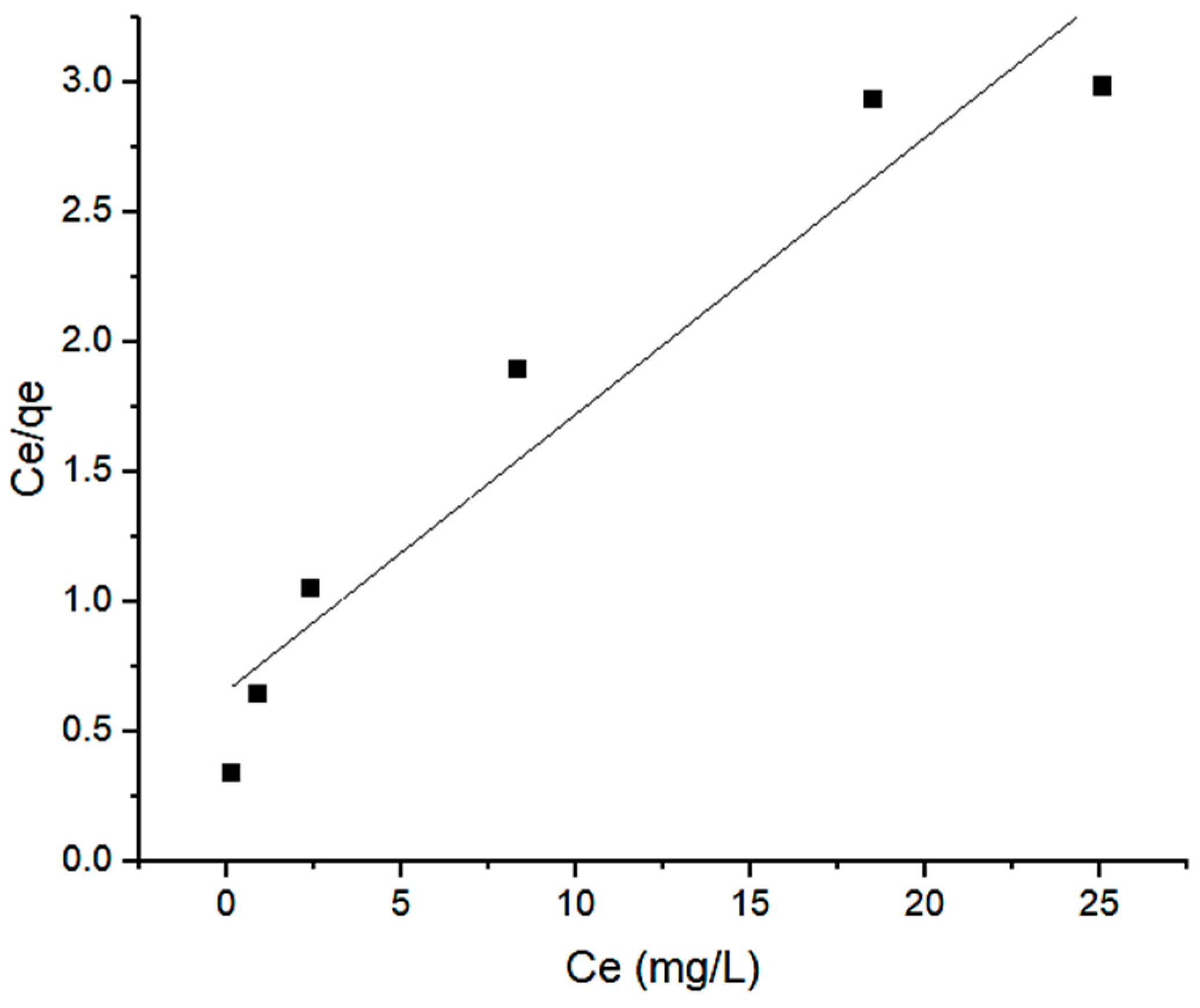
2.5. Adsorption Mechanism
2.6. Adsorption from Wastewater
3. Materials and Methods
3.1. Chemicals
3.2. Instrumentation
3.3. Procedures
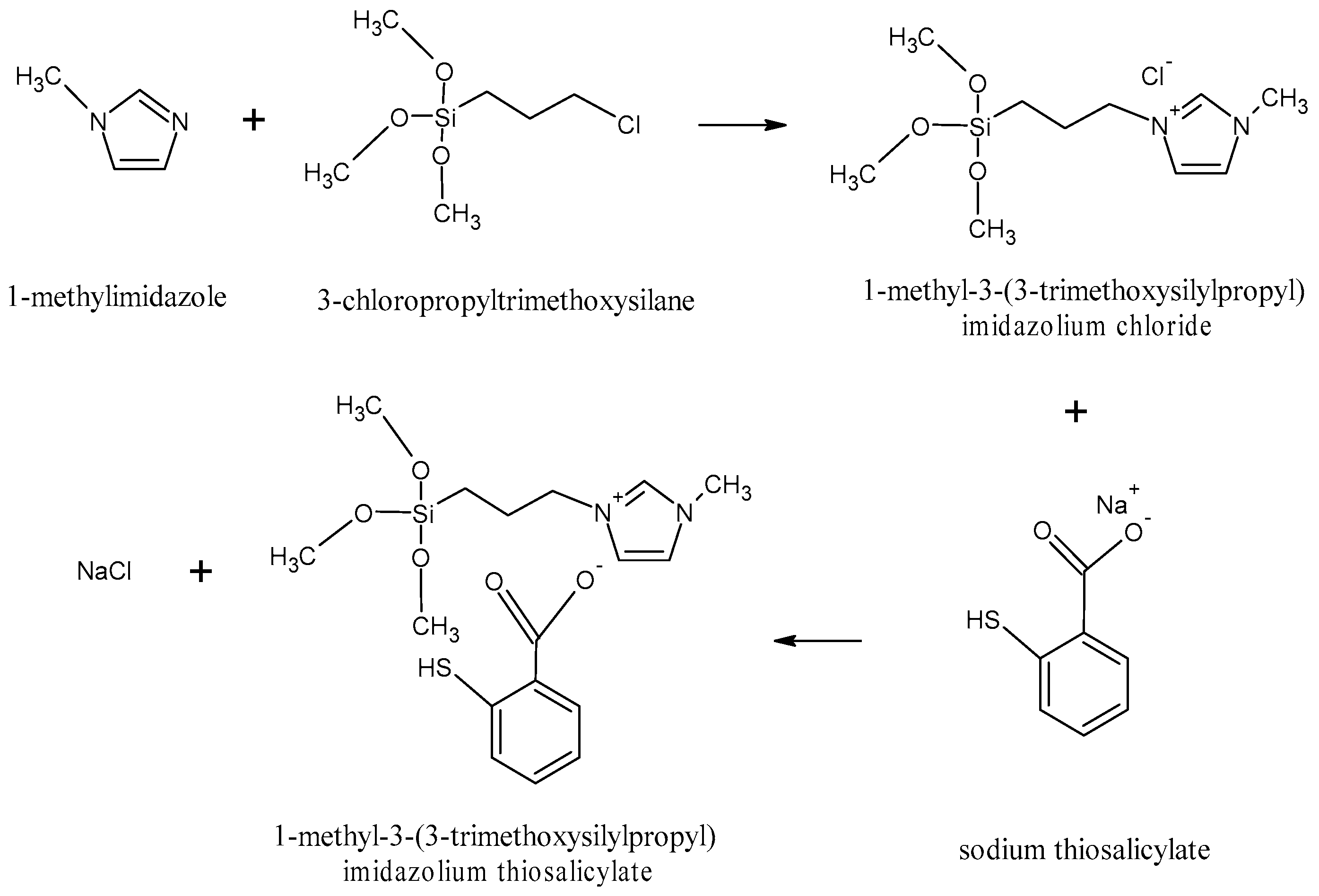
3.3.1. Synthesis of [MTMSPI][TS] Ionic Liquid
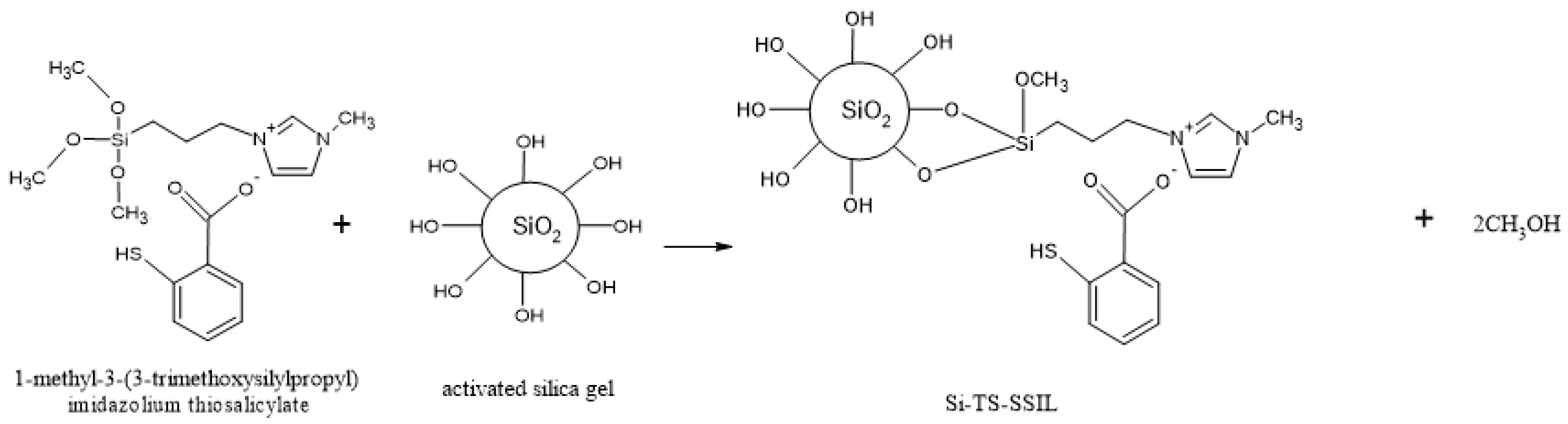
3.3.2. Preparation of Solid-Supported Ionic Liquid Containing the Thiosalicylate Functional Group (Si-TS-SSIL)
3.3.3. Removal Study
3.4. Adsorption Isotherm and Kinetics Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, W.; Zheng, J.; Ou, X.; Liu, X.; Song, Y.; Tian, C.; Rong, W.; Shi, Z.; Dang, Z.; Lin, Z. Effective Extraction of Cr(VI) from Hazardous Gypsum Sludge via Controlling the Phase Transformation and Chromium Species. Environ. Sci. Technol. 2018, 52, 13336–13342. [Google Scholar] [CrossRef] [PubMed]
- Akpoveta, O.V.; Osakwe, S.A. Determination of Heavy Metal Contents in Refined Petroleum Products. IOSR J. Appl. Chem. 2014, 7, 01–02. [Google Scholar] [CrossRef]
- Kinuthia, G.K.; Ngure, V.; Beti, D.; Lugalia, R.; Wangila, A.; Kamau, L. Levels of heavy metals in wastewater and soil samples from open drainage channels in Nairobi, Kenya: Community health implication. Sci. Rep. 2020, 10, 8434. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.S.; Hossain, M.M.; Jeong, H.M.; Kim, K. Heavy metal removal applications using adsorptive membranes. Nano Converg. 2020, 7, 36. [Google Scholar] [CrossRef]
- Gunatilake, S.K. Methods of Removing Heavy Metals from Industrial Wastewater. Methods 2015, 1, 12–18. [Google Scholar]
- Xiao, W.; Pan, D.; Niu, Z.; Fan, Y.; Wu, S.; Wu, W. Opportunities and challenges of high-pressure ion exchange chromatography for nuclide separation and enrichment. Chinese Chem. Lett. 2022, 33, 3413–3421. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Yahya, M.A.; Al-Shannag, M. On the performance of bioadsorption processes for heavy metal ions removal by low-cost agricultural and natural by-products bioadsorbent: A review. Desalin. Water Treat. 2017, 85, 339–357. [Google Scholar] [CrossRef]
- Naseem, K.; Farooqi, Z.H.; Begum, R.; Ur Rehman, M.Z.; Shahbaz, A.; Farooq, U.; Ali, M.; Ur Rahman, H.M.A.; Irfan, A.; Al-Sehemi, A.G. Removal of Cadmium (II) from Aqueous Medium Using Vigna radiata Leave Biomass: Equilibrium Isotherms, Kinetics and Thermodynamics. Zeitschrift fur Phys. Chemie 2019, 233, 669–690. [Google Scholar] [CrossRef]
- Hadj-kali, M.K.; El Blidi, L.; Mulyono, S.; Wazeer, I.; Ali, E. Deep Eutectic Solvents for the Separation of Toluene/1-Hexene via Liquid—Liquid Extraction. Separations 2022, 9, 369. [Google Scholar] [CrossRef]
- Phakoukaki, Y.V.; O’Shaughnessy, P.; Angeli, P. Intensified liquid-liquid extraction of biomolecules using ionic liquids in small channels. Sep. Purif. Technol. 2022, 282, 120063. [Google Scholar] [CrossRef]
- Chowdhury, I.R.; Chowdhury, S.; Mazumder, M.A.J.; Al-Ahmed, A. Removal of Lead Ions (Pb2+) from Water and Wastewater: A Review on the Low-Cost Adsorbents; Springer International Publishing: Cham, Switzerland, 2022; Volume 12, p. 8. ISBN 0123456789. [Google Scholar]
- Wang, Y.L.; Li, B.; Sarman, S.; Mocci, F.; Lu, Z.Y.; Yuan, J.; Laaksonen, A.; Fayer, M.D. Microstructural and Dynamical Heterogeneities in Ionic Liquids. Chem. Rev. 2020, 120, 5798–5877. [Google Scholar] [CrossRef] [PubMed]
- Angeli, P.; Ortega, E.G.; Tsaoulidis, D.; Earle, M. Intensified liquid-liquid extraction technologies in small channels: A review. Johnson Matthey Technol. Rev. 2019, 63, 299–310. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Silva, F.A.E.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Bi, W.; Tian, M.; Row, K.H. Application of ionic liquid for extraction and separation of bioactive compounds from plants. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 904, 1–21. [Google Scholar] [CrossRef]
- Ola, P.D.; Matsumoto, M. Metal Extraction with Ionic Liquids-Based Aqueous Two-Phase System. Recent Adv. Ion. Liq. 2018, 8, 146–158. [Google Scholar] [CrossRef] [Green Version]
- Friess, K.; Izák, P.; Kárászová, M.; Pasichnyk, M.; Lanč, M.; Nikolaeva, D.; Luis, P.; Jansen, J.C. A review on ionic liquid gas separation membranes. Membranes 2021, 11, 97. [Google Scholar] [CrossRef]
- Lanaridi, O.; Sahoo, A.R.; Limbeck, A.; Naghdi, S.; Eder, D.; Eitenberger, E.; Csendes, Z.; Schnürch, M.; Bica-Schröder, K. Toward the Recovery of Platinum Group Metals from a Spent Automotive Catalyst with Supported Ionic Liquid Phases. ACS Sustain. Chem. Eng. 2021, 9, 375–386. [Google Scholar] [CrossRef]
- Wang, J.; Luo, J.; Feng, S.; Li, H.; Wan, Y.; Zhang, X. Recent development of ionic liquid membranes. Green Energy Environ. 2016, 1, 43–61. [Google Scholar] [CrossRef] [Green Version]
- Wolny, A.; Chrobok, A. Silica-Based Supported Ionic Liquid-like Phases as Heterogeneous Catalysts. Molecules 2022, 27, 5900. [Google Scholar] [CrossRef]
- Gawande, M.; Hosseinpour, R.; Luque, R. Silica Sulfuric Acid and Related Solid-supported Catalysts as Versatile Materials for Greener Organic Synthesis. Curr. Org. Synth. 2014, 11, 526–544. [Google Scholar] [CrossRef]
- Regina, K.; Bernhard, K. Novel thiosalicylate-based ionic liquids for heavy metal extractions. J. Hazard. Mater. 2016, 314, 164–171. [Google Scholar] [CrossRef]
- Ellerbrock, R.; Stein, M.; Schaller, J. Comparing amorphous silica, short-range-ordered silicates and silicic acid species by FTIR. Sci. Rep. 2022, 12, 1–8. [Google Scholar] [CrossRef]
- Fadavipoor, E.; Badri, R.; Kiasat, A.; Sanaeishoar, H. CuO supported 1-methyl-3-(3-(trimethoxysilyl) propyl) imidazolium chloride (MTMSP-Im/Cl) nanoparticles as an efficient simple heterogeneous catalysts for synthesis of β-azido alcohols. J. Iran. Chem. Soc. 2019, 16, 1451–1458. [Google Scholar] [CrossRef]
- Bilgiç, A.; Çimen, A. Removal of chromium(vi) from polluted wastewater by chemical modification of silica gel with 4-acetyl-3-hydroxyaniline. RSC Adv. 2019, 9, 37403–37414. [Google Scholar] [CrossRef] [Green Version]
- Kogelnig, D.; Stojanovic, A.; Galanski, M.; Groessl, M.; Jirsa, F.; Krachler, R.; Keppler, B.K. Greener synthesis of new ammonium ionic liquids and their potential as extracting agents. Tetrahedron Lett. 2008, 49, 2782–2785. [Google Scholar] [CrossRef]
- Konshina, D.N.; Lupanova, I.A.; Konshin, V. V Imidazolium-Modified Silica Gel for Highly Selective Preconcentration of Ag (I) from the Nitric Acid Medium. Chemistry 2022, 4, 1702–1713. [Google Scholar] [CrossRef]
- Cestari, A.R.; Airoldi, C. A new elemental analysis method based on thermogravimetric data and applied to alkoxysilane immobilized on silicas. J. Therm. Anal. 1995, 44, 79–87. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, S.; Zhou, R.; Wu, X.; Zhang, W.; Han, X.; Wang, J. Thermal Degradation Behavior of Thiol-ene Composites Loaded with a Novel Silicone Flame Retardant. Polymers 2022, 14, 20. [Google Scholar] [CrossRef]
- Akl, M.A.A.; Kenawy, I.M.M.; Lasheen, R.R. Organically modified silica gel and flame atomic absorption spectrometry: Employment for separation and preconcentration of nine trace heavy metals for their determination in natural aqueous systems. Microchem. J. 2004, 78, 143–156. [Google Scholar] [CrossRef]
- Protsak, I.S.; Morozov, Y.M.; Dong, W.; Le, Z.; Zhang, D.; Henderson, I.M. A 29Si, 1H, and 13C Solid-State NMR Study on the Surface Species of Various Depolymerized Organosiloxanes at Silica Surface. Nanoscale Res. Lett. 2019, 14, 160. [Google Scholar] [CrossRef] [Green Version]
- Duan, Y.; Chen, Y.; Lei, M.; Hou, C.; Li, X.; Chen, S.; Fang, K.; Wang, T. Hybrid silica material as a mixed-mode sorbent for solid-phase extraction of hydrophobic and hydrophilic illegal additives from food samples. J. Chromatogr. A 2022, 1672, 463049. [Google Scholar] [CrossRef]
- Chrobok, A.; Baj, S.; Pudło, W.; Jarzebski, A. Supported hydrogensulfate ionic liquid catalysis in Baeyer-Villiger reaction. Appl. Catal. A Gen. 2009, 366, 22–28. [Google Scholar] [CrossRef]
- Sharifah, T.; Tengku, M.; Yarmo, M.A.; Hakim, A.; Najiha, M.; Tahari, A.B.U.; Hin, T.Y.U.N. Immobilization and Characterizations of Imidazolium-Based Ionic Liquid on Silica for CO 2 Adsorption/Desorption Studies. Mater. Sci. Forum 2016, 840, 404–409. [Google Scholar] [CrossRef]
- Saleem, S.; Nauman, A.; Saqib, S.; Mujahid, A.; Hanif, M. Extraction of Pb (II) from water samples by ionic liquid-modified silica sorbents Extraction of Pb (II) from water samples by ionic liquid-modified silica sorbents. Desalination Water Treat. 2014, 52, 7915–7924. [Google Scholar] [CrossRef]
- Lutfullah; Rashid, M.; Haseen, U.; Rahman, N. An advanced Cr(III) selective nano-composite cation exchanger: Synthesis, characterization and sorption characteristics. J. Ind. Eng. Chem. 2014, 20, 809–817. [Google Scholar] [CrossRef]
- Panda, H.; Tiadi, N.; Mohanty, M.; Mohanty, C.R. Studies on adsorption behavior of an industrial waste for removal of chromium from aqueous solution. South African J. Chem. Eng. 2017, 23, 132–138. [Google Scholar] [CrossRef]
- Adina, N.; Lupa, L.; Mihaela, C.; Negrea, P. Characterization of Strontium Adsorption from Aqueous Solutions Using Inorganic Materials Impregnated with Ionic Liquid Characterization of strontium adsorption from aqueous solutions using inorganic materials impregnated with ionic liquid. Int. J. Chem. Eng. Appl. 2013, 4, 326. [Google Scholar] [CrossRef] [Green Version]
- Rahman, N.; Nasir, M. Facile synthesis of thiosalicylic acid functionalized silica gel for effective removal of Cr(III): Equilibrium modeling, kinetic and thermodynamic studies. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100353. [Google Scholar] [CrossRef]
- Safari, J.; Zarnegar, Z. A highly efficient magnetic solid acid catalyst for synthesis of 2,4,5-trisubstituted imidazoles under ultrasound irradiation. Ultrason. Sonochem. 2013, 20, 740–746. [Google Scholar] [CrossRef]
- Mohammad, N.; Atassi, Y. Enhancement of removal efficiency of heavy metal ions by polyaniline deposition on electrospun polyacrylonitrile membranes. Water Sci. Eng. 2021, 14, 129–138. [Google Scholar] [CrossRef]
- Tian, M.; Fang, L.; Yan, X.; Xiao, W.; Row, K.H. Determination of Heavy Metal Ions and Organic Pollutants in Water Samples Using Ionic Liquids and Ionic Liquid-Modified Sorbents. J. Anal. Methods Chem. 2019, 2019, 1948965. [Google Scholar] [CrossRef]
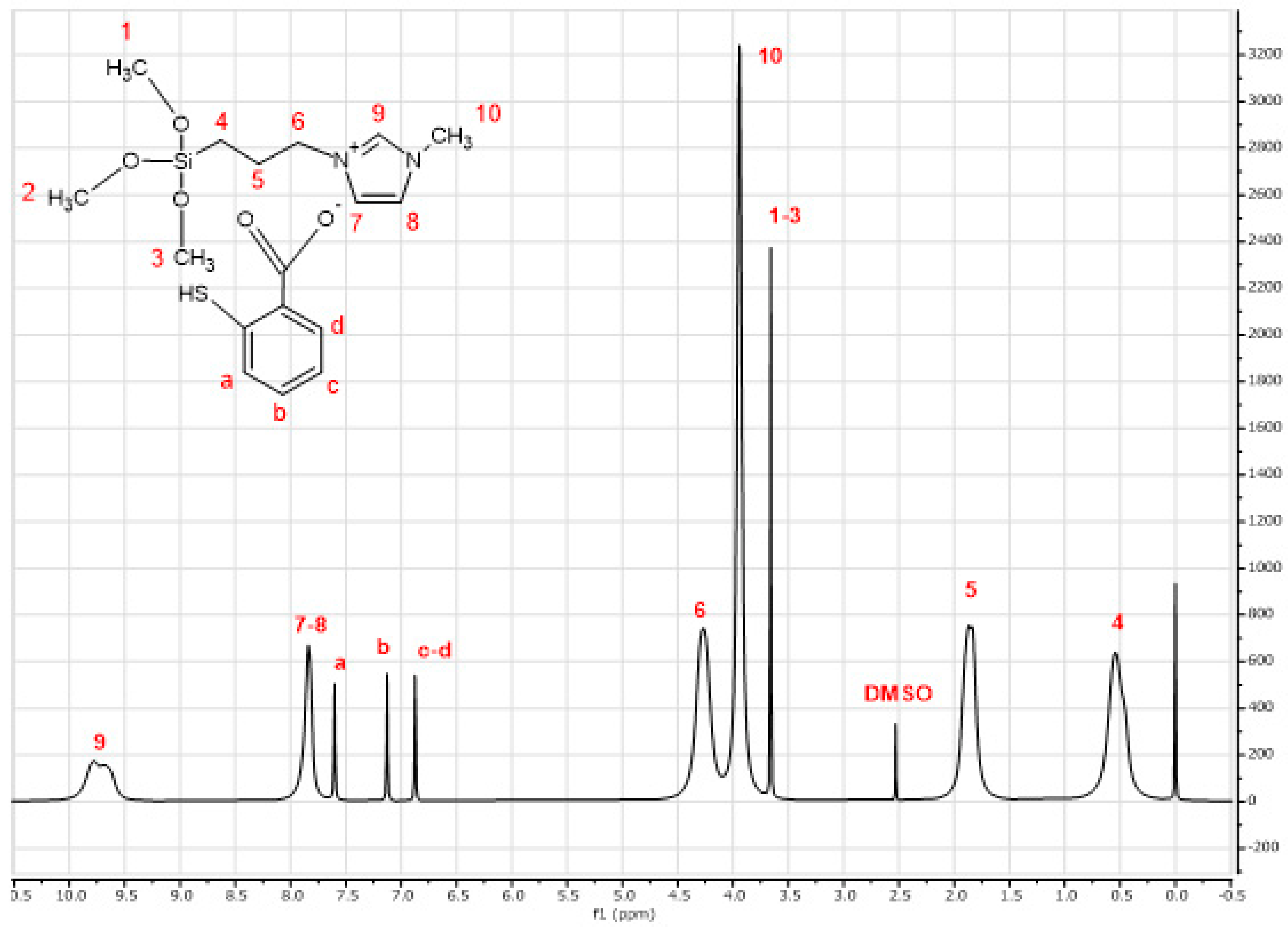
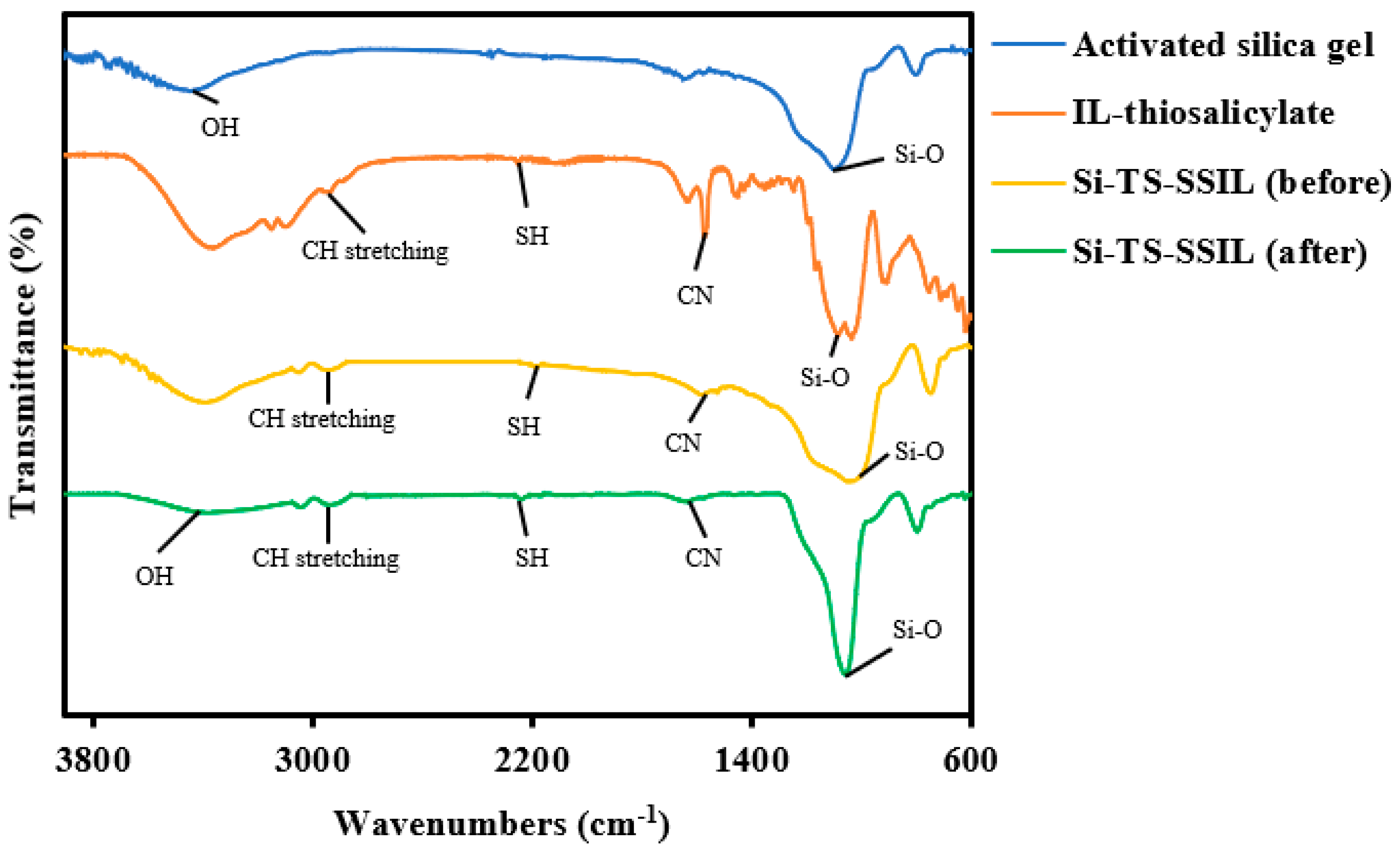
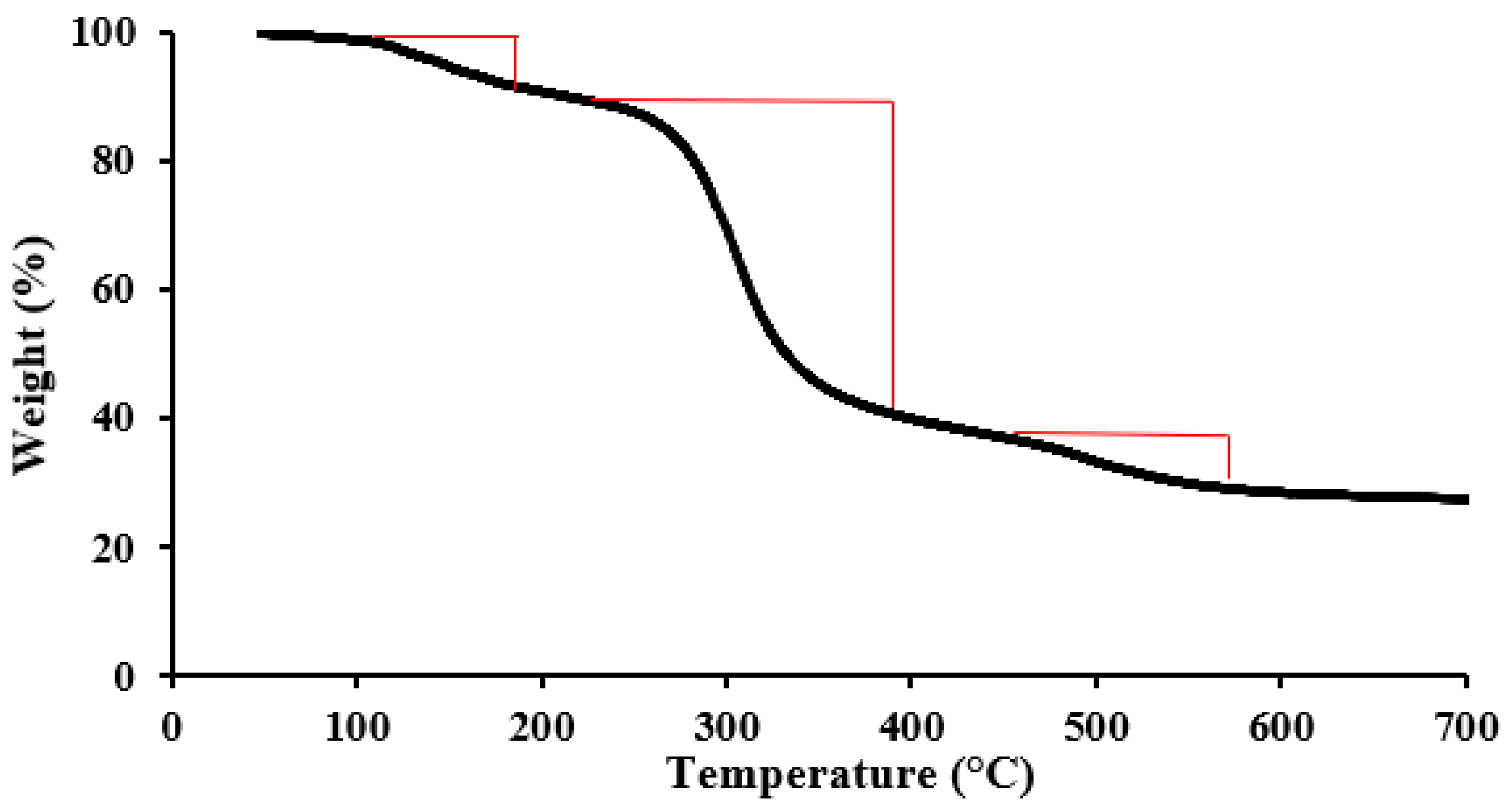



| Chloride Content (ppm) | Concentration (%) | Purity (%) |
|---|---|---|
| 9.08 | 0.0908 | 99.91 |
| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| Activated silica gel | 275.8132 | 0.7021 | 11.5176 |
| Si-TS-SSIL (before washing with dichloromethane) | 225.6244 | 0.5758 | 9.9599 |
| Si-TS-SSIL (after washing with dichloromethane) | 232.6600 | 0.5793 | 10.2075 |
| Extractant | Removal Capacity (mg/g) |
|---|---|
| Activated silica gel | 1.370 |
| [MTMSPI][TS] | 5.801 |
| Si-TS-SSIL | 5.285 |
| Extractant | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| Si-TS-SSIL | KL (L/mg) | qm (mg/g) | R2 | KF (mg/g) | n | R2 |
| 0.1626 | 9.3729 | 0.9102 | 0.8592 | 1.8149 | 0.9961 | |
| Extractant | Source | Removal Efficiency (%) |
|---|---|---|
| Si-TS-SSIL | Aqueous solution | 99.33 |
| Wastewater | 80.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamaruddin, N.A.L.; Taha, M.F.; Wilfred, C.D. Synthesis and Characterization of Novel Thiosalicylate-based Solid-Supported Ionic Liquid for Removal of Pb(II) Ions from Aqueous Solution. Molecules 2023, 28, 830. https://doi.org/10.3390/molecules28020830
Kamaruddin NAL, Taha MF, Wilfred CD. Synthesis and Characterization of Novel Thiosalicylate-based Solid-Supported Ionic Liquid for Removal of Pb(II) Ions from Aqueous Solution. Molecules. 2023; 28(2):830. https://doi.org/10.3390/molecules28020830
Chicago/Turabian StyleKamaruddin, Nur Anis Liyana, Mohd Faisal Taha, and Cecilia Devi Wilfred. 2023. "Synthesis and Characterization of Novel Thiosalicylate-based Solid-Supported Ionic Liquid for Removal of Pb(II) Ions from Aqueous Solution" Molecules 28, no. 2: 830. https://doi.org/10.3390/molecules28020830
APA StyleKamaruddin, N. A. L., Taha, M. F., & Wilfred, C. D. (2023). Synthesis and Characterization of Novel Thiosalicylate-based Solid-Supported Ionic Liquid for Removal of Pb(II) Ions from Aqueous Solution. Molecules, 28(2), 830. https://doi.org/10.3390/molecules28020830






