Olive Yield and Physicochemical Properties of Olives and Oil in Response to Nutrient Application under Rainfed Conditions
Abstract
:1. Introduction
2. Results and Discussion
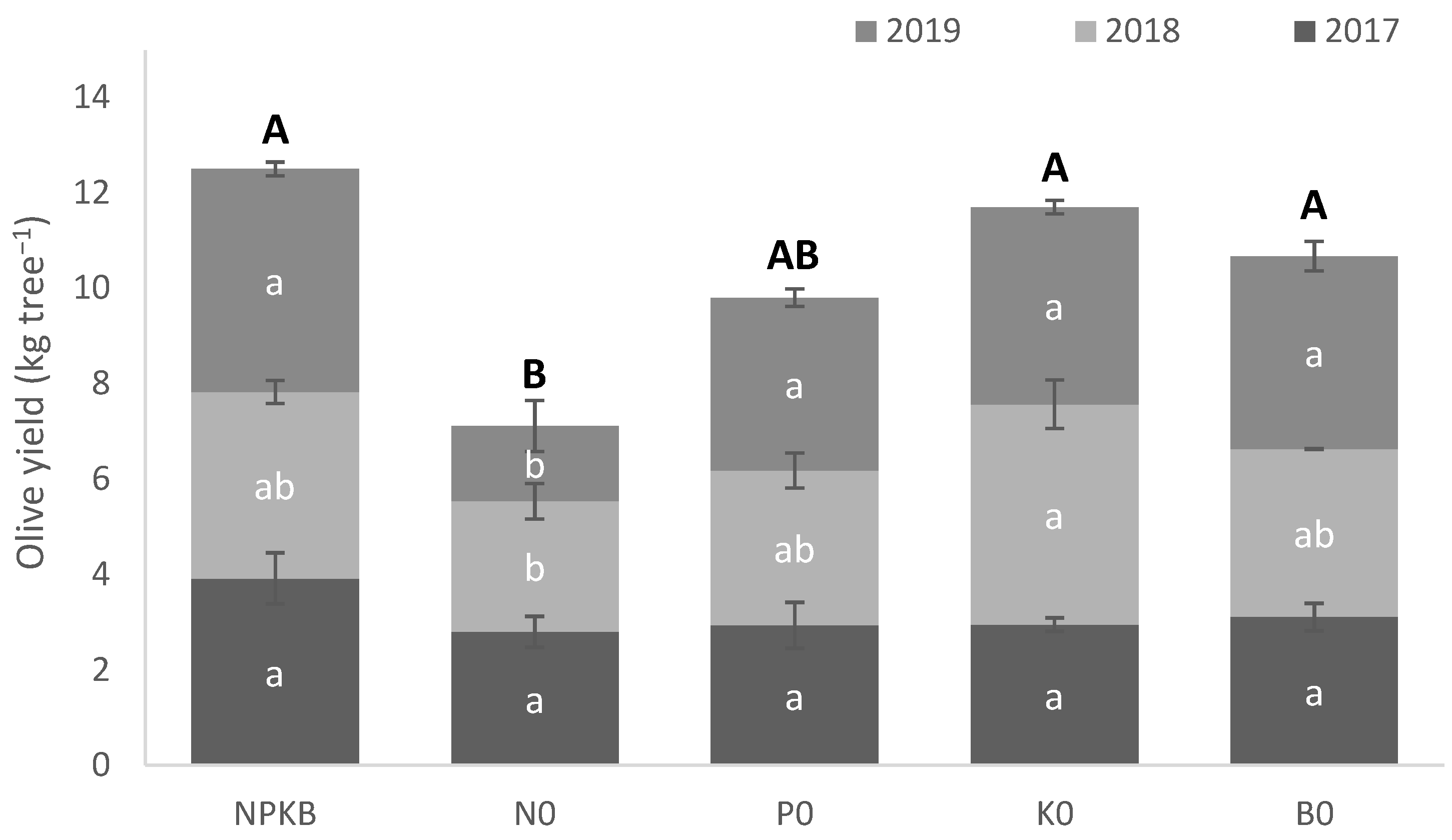
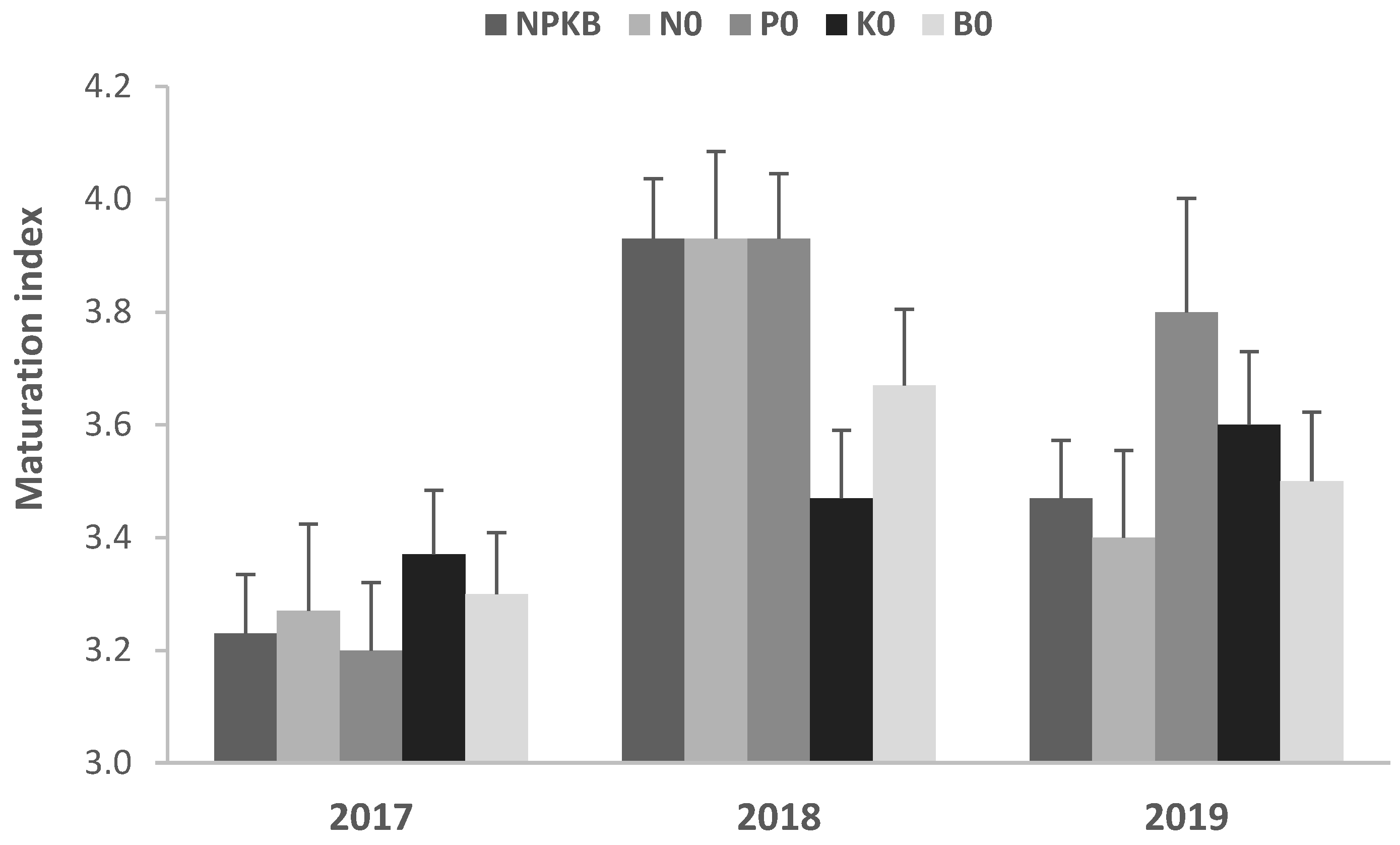
2.1. Crop Yield, Tree Nutritional and Water Status, Photosynthesis and Fruit Physicochemical Characterization
2.2. Nutritional Value of Olives and Oil
2.3. Phenolic Composition of Olives and Oil
2.4. Olive Oil Quality
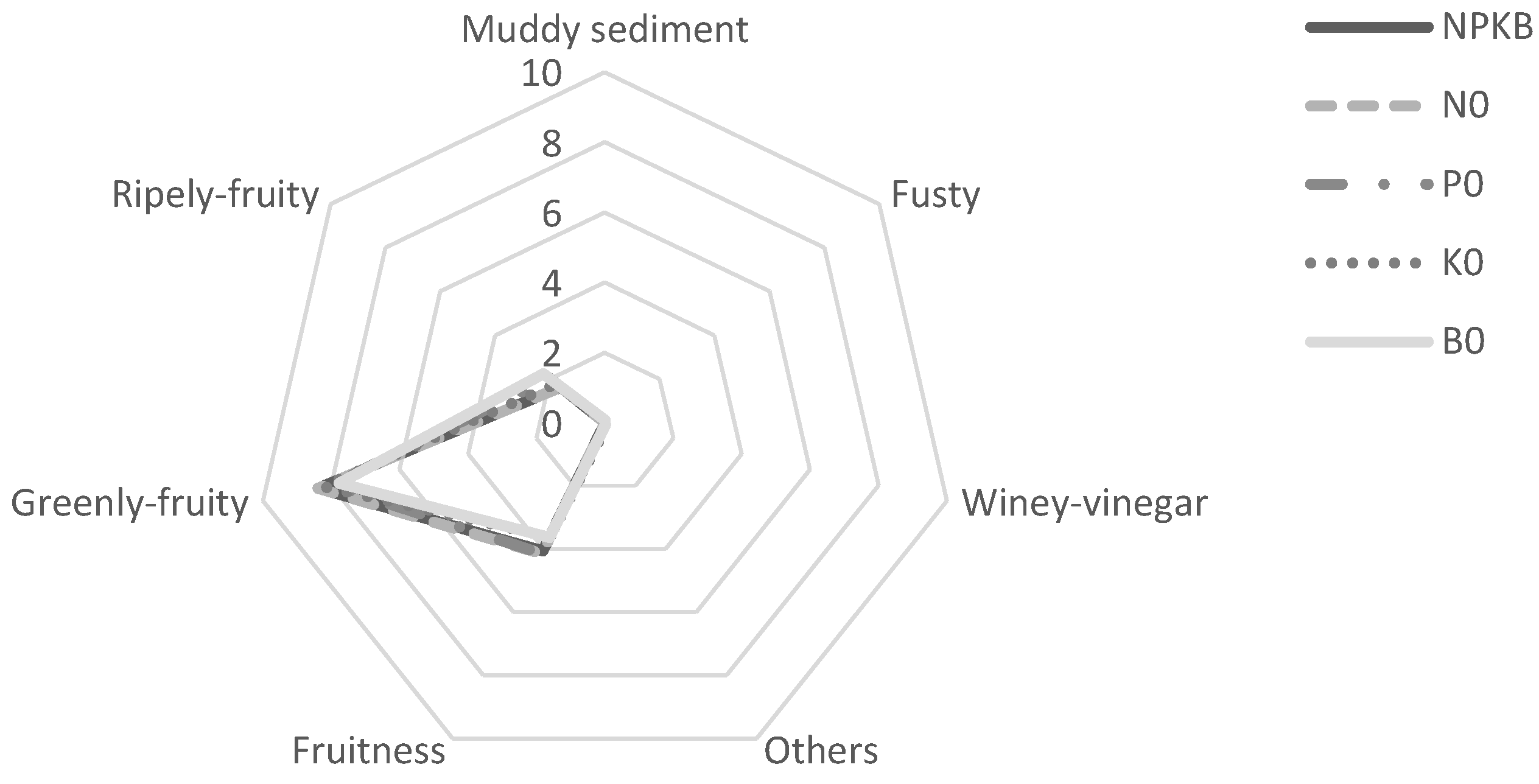
2.5. Olive Oil Storage and Sensorial Analysis
3. Materials and Methods
3.1. Plot Characterization and Experimental Design
3.2. Leaf Water Status and Net Photosynthetic Rate
3.3. Crop Harvest and Oil Extraction
3.4. Physical Characterization of Fruits and Maturation Index
3.5. Determination of Fruit Nutritional Variables
3.6. Extraction and Quantification of Polyphenolic Compounds from Olives and Oil
3.7. Free Acidity, Peroxide Value and UV Spectrophotometric Indices
3.8. Sensorial Analysis
3.9. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Crops and livestock products. Food and Agriculture Organization of the United Nations. FAOSTAT Statistical Database. Rome. 2022. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 4 April 2022).
- Cabrera-Bañegil, M.; Schaide, T.; Manzano, R.; Delgado-Adámez, J.; Durán-Merás, I.; Martín-Vertedor, D. Optimization and validation of a rapid liquid chromatography method for determination of the main polyphenolic compounds in table olives and in olive paste. Food Chem. 2017, 233, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Sordini, B.; Esposto, S.; Taticchi, A.; Urbani, S.; Sebastiani, L. Metabolomics of Olive Fruit: A Focus on the Secondary Metabolites. In The Olive Tree Genome; Rugini, E., Baldoni, L., Muleo, R., Sebastiani, L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 123–139. [Google Scholar]
- Rallo, L.; Díez, C.M.; Morales-Sillero, A.; Miho, H.; Priego-Capote, F.; Rallo, P. Quality of olives: A focus on agricultural preharvest factors. Sci. Hortic. 2018, 233, 491–509. [Google Scholar] [CrossRef]
- Martins, S.; Silva, E.; Brito, C.; Martins-Gomes, C.; Gonçalves, A.; Arrobas, M.; Rodrigues, M.Â.; Correia, C.M.; Nunes, F.M. Zeolites and biochar modulate olive fruit and oil polyphenolic profile. Antioxidants 2022, 11, 1332. [Google Scholar] [CrossRef]
- Gavahian, M.; Mousavi Khaneghah, A.; Lorenzo, J.M.; Munekata, P.E.S.; Garcia-Mantrana, I.; Collado, M.C.; Meléndez-Martínez, A.J.; Barba, F.J. Health benefits of olive oil and its components: Impacts on gut microbiota antioxidant activities, and prevention of noncommunicable diseases. Trends Food Sci. Technol. 2019, 88, 220–227. [Google Scholar] [CrossRef]
- Neves, B.; Pires, I.M. The Mediterranean Diet and the Increasing Demand of the Olive Oil Sector: Shifts and Environmental Consequences. Region 2018, 5, 101–112. [Google Scholar] [CrossRef]
- Caporaso, N. Virgin Olive Oils: Environmental Conditions, Agronomical Factors and Processing Technology Affecting the Chemistry of Flavor Profile. J. Food Chem. Nanotechnol. 2016, 2, 21–31. [Google Scholar] [CrossRef]
- Erel, R.; Ben-Gal, A.; Dag, A.; Schwartz, A.; Yermiyahu, U. Sodium replacement of potassium in physiological processes of olive trees (var. Barnea) as affected by drought. Tree Physiol. 2014, 34, 1102–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Qarnifa, S.; El Antari, A.; Hafidi, A. Effect of Maturity and Environmental Conditions on Chemical Composition of Olive Oils of Introduced Cultivars in Morocco. J. Food Qual. 2019, 2019, 1854539. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, I.Q.; Arrobas, M.; Moutinho-Pereira, J.; Correia, C.; Rodrigues, M.Â. Olive response to potassium applications under different water regimes and cultivars. Nutr. Cycl. Agroecosystems 2018, 112, 387–401. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, I.; Rodrigues, M.Â.; Arrobas, M. Soil and foliar applied boron in olive: Tree crop growth and yield, and boron remobilization within plant tissues. Span. J. Agric. Res. 2019, 17, e0901. [Google Scholar] [CrossRef]
- Ferreira, I.Q.; Arrobas, M.; Moutinho-Pereira, J.M.; Correia, C.M.; Rodrigues, M.Â. The effect of nitrogen applications on the growth of young olive trees and nitrogen use efficiency. Turk. J. Agric. For. 2020, 44, 278–289. [Google Scholar] [CrossRef]
- Lopes, J.I.; Gonçalves, A.; Brito, C.; Martins, S.; Pinto, L.; Moutinho-Pereira, J.; Raimundo, S.; Arrobas, M.; Rodrigues, M.Â.; Correia, C.M. Inorganic Fertilization at High N Rate Increased Olive Yield of a Rainfed Orchard but Reduced Soil Organic Matter in Comparison to Three Organic Amendments. Agronomy 2021, 11, 2172. [Google Scholar] [CrossRef]
- Erel, R.; Kerem, Z.; Ben-Gal, A.; Dag, A.; Schwartz, A.; Zipori, I.; Basheer, L.; Yermiyahu, U. Olive (Olea europaea L.) Tree Nitrogen Status Is a Key Factor for Olive Oil Quality. J. Agric. Food Chem. 2013, 61, 11261–11272. [Google Scholar] [CrossRef] [PubMed]
- Arbonés, A.; Rufat, J.; Pérez, M.A.; Pascual, M.; Benito, A.; De Lorenzo, C.; Villar, J.M.; Sastre, B. The influence of olive tree fertilization on the phenols in virgin olive oils. A review. Grasas Y Aceites 2022, 73, e470. [Google Scholar] [CrossRef]
- Zipori, I.; Erel, R.; Yermiyahu, U.; Ben-Gal, A.; Dag, A. Sustainable Management of Olive Orchard Nutrition: A Review. Agric. Agric. Sci. Procedia 2020, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Dag, A.; Ben-David, E.; Kerem, Z.; Ben-Gal, A.; Erel, R.; Basheer, L.; Yermiyahu, U. Olive oil composition as a function of nitrogen, phosphorus and potassium plant nutrition. J. Sci. Food Agric. 2009, 89, 1871–1878. [Google Scholar] [CrossRef]
- El-Motaium, R.A.; Hashim, M.E. Boron efficiency in increasing olive (cv. Frantoio) fruit productivity and oil yield and quality. J. Plant Nutr. 2020, 43, 2981–2989. [Google Scholar] [CrossRef]
- Toker, C.; Yavuz, N. The Effect of Boron Application on Chemical Characterization and Volatile Compounds of Virgin Olive Oil of Ayvalik Olive Cultivar. J. Geophys. Res. 2015, 92, 1421–1428. [Google Scholar] [CrossRef]
- Tekaya, M.; Mechri, B.; Bchir, A.; Attia, F.; Cheheb, H.; Daassa, M.; Hammami, M. Enhancement of Antioxidants in Olive Oil by Foliar Fertilization of Olive Trees. J. Am. Oil Chem. Soc. 2013, 90, 1377–1386. [Google Scholar] [CrossRef]
- LQARS. Manual de Fertilização das Culturas; Instituto Nacional de Investigação Agrária e Veterinária, I.P.—INIAV: Oeiras, Portugal, 2006. [Google Scholar]
- Erel, R.; Yermiyahu, U.; Van Opstal, J.; Ben-Gal, A.; Schwartz, A.; Dag, A. The importance of olive (Olea europaea L.) tree nutritional status on its productivity. Sci. Hortic. 2013, 159, 8–18. [Google Scholar] [CrossRef]
- Silva, E.; Arrobas, M.; Gonçalves, A.; Martins, S.; Raimundo, S.; Pinto, L.; Brito, C.; Moutinho-Pereira, J.; Correia, C.M.; Rodrigues, M.Â. A controlled-release fertilizer improved soil fertility but not olive tree performance. Nutr. Cycl. Agroecosystems 2021, 120, 1–15. [Google Scholar] [CrossRef]
- Erel, R.; Dag, A.; Ben-Gal, A.; Schwartz, A.; Yermiyahu, U. Flowering and fruit set of olive trees in response to nitrogen, phosphorus, and potassium. J. Am. Soc. Hortic. Sci. 2008, 133, 639–647. [Google Scholar] [CrossRef]
- Rodrigues, M.Â.; Pavão, F.; Lopes, J.I.; Gomes, V.; Arrobas, M.; Moutinho-Pereira, J.; Ruivo, S.; Cabanas, J.E.; Correia, C.M. Olive yields and tree nutritional status during a four-year period without nitrogen and boron fertilization. Commun. Soil Sci. Plant Anal. 2011, 42, 803–814. [Google Scholar] [CrossRef]
- Haberman, A.; Dag, A.; Shtern, N.; Zipori, I.; Erel, R.; Ben-Gal, A.; Yermiyahu, U. Significance of proper nitrogen fertilization for olive productivity in intensive cultivation. Sci. Hortic. 2019, 246, 710–717. [Google Scholar] [CrossRef]
- Ferreira, I.Q.; Rodrigues, M.Â.; Moutinho-Pereira, J.M.; Correia, C.M.; Arrobas, M. Olive tree response to applied phosphorus in field and pot experiments. Sci. Hortic. 2018, 234, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Soyergin, S. Effects of Soil and Leaf Treatments to Eliminate Boron Deficiency in Olives. Commun. Soil Sci. Plant Anal. 2010, 41, 2004–2010. [Google Scholar] [CrossRef]
- Dag, A.; Kerem, Z.; Yogev, N.; Zipori, I.; Lavee, S.; Ben-David, E. Influence of time of harvest and maturity index on olive oil yield and quality. Sci. Hortic. 2011, 127, 358–366. [Google Scholar] [CrossRef]
- Rosati, A.; Caporali, S.; Paoletti, A. Fertilization with N and K increases oil and water content in olive (Olea europaea L.) fruit via increased proportion of pulp. Sci. Hortic. 2015, 192, 381–386. [Google Scholar] [CrossRef]
- Mafrica, R.; Piscopo, A.; De Bruno, A.; Poiana, M. Effects of Climate on Fruit Growth and Development on Olive Oil Quality in Cultivar Carolea. Agriculture 2021, 11, 147. [Google Scholar] [CrossRef]
- Fernández, F.J.; Ladux, J.L.; Hammami, S.B.M.; Rapoport, H.F.; Searles, P.S. Fruit, mesocarp, and endocarp responses to crop load and to different estimates of source: Sink ratio in olive (cv. Arauco) at final harvest. Sci. Hortic. 2018, 234, 49–57. [Google Scholar] [CrossRef]
- Conte, P.; Squeo, G.; Difonzo, G.; Caponio, F.; Fadda, C.; Del Caro, A.; Urgeghe, P.; Montanari, L.; Montinaro, A.; Piga, A. Change in quality during ripening of olive fruits and related oils extracted from three minor autochthonous Sardinian cultivars. Emir. J. Food Agric. 2019, 31, 196–205. [Google Scholar] [CrossRef]
- Delıboran, A.; Cılgın, I.; Aydogdu, E.; Ataol Olmez, H.; Savran, K.; Dursun, O.; Eralp, O.; Pekcan, T.; Turan, H.S.; Savran, S.; et al. Response of Olive Trees to Different Boron Application in Izmir and Mugla Province of Turkey. Commun. Soil Sci. Plant Anal. 2022, 53, 1294–1307. [Google Scholar] [CrossRef]
- Heredia, A.; Ruiz-Gutierrez, V.; Felizón, B.; Guillén, R.; Jiménez, A.; Fernández-Bolaños, J. Apparent digestibility of dietary fibre and other components in table olives. Die Nahr. 1993, 37, 226–233. [Google Scholar] [CrossRef]
- Jiménez, A.; Rodríguez, R.; Fernández-Caro, I.; Guillén, R.; Fernández-Bolaños, J.; Heredia, A. Dietary fibre content of table olives processed under different European styles: Study of physico-chemical characteristics. J. Sci. Food Agric. 2000, 80, 1903–1908. [Google Scholar] [CrossRef]
- Lanza, B. Nutritional and Sensory Quality of Table Olives; In Tech: Rijeka, Croatia, 2012. [Google Scholar]
- Galanakis, C.M. Olive fruit dietary fiber: Components, recovery and applications. Trends Food Sci. Technol. 2011, 22, 175–184. [Google Scholar] [CrossRef]
- Toplu, C.; Önder, D.; Önder, S.; Yıldız, E. Determination of fruit and oil characteristics of olive (Olea europaea L. cv. ‘Gemlik’) in different irrigation and fertilization regimes. Afr. J. Agric. Res. 2009, 4, 649–658. [Google Scholar]
- Salas, J.J.; Sánchez, J.; Ramli, U.S.; Manaf, A.M.; Williams, M.; Harwood, J.L. Biochemistry of lipid metabolism in olive and other oil fruits. Prog. Lipid Res. 2000, 39, 151–180. [Google Scholar] [CrossRef]
- Migliorini, M.; Cherubini, C.; Mugelli, M.; Gianni, G.; Trapani, S.; Zanoni, B. Relationship between the oil and sugar content in olive oil fruits from Moraiolo and Leccino cultivars during ripening. Sci. Hortic. 2011, 129, 919–921. [Google Scholar] [CrossRef]
- Cecchi, L.; Breschi, C.; Migliorini, M.; Canuti, V.; Fia, G.; Mulinacci, N.; Zanoni, B. Moisture in Rehydrated Olive Paste Affects Oil Extraction Yield and Phenolic Compound Content and Profile of Extracted Olive Oil. Eur. J. Lipid Sci. Technol. 2019, 121, 1800449. [Google Scholar] [CrossRef]
- Charoenprasert, S.; Mitchell, A. Factors influencing phenolic compounds in table olives (Olea europaea). J. Agric. Food Chem. 2012, 60, 7081–7095. [Google Scholar] [CrossRef]
- Rotondi, A.; Bendini, A.; Cerretani, L.; Mari, M.; Lercker, G.; Toschi, T.G. Effect of olive ripening degree on the oxidative stability and organoleptic properties of cv. Nostrana di Brisighella extra virgin olive oil. J. Agric. Food Chem. 2004, 52, 3649–3654. [Google Scholar] [CrossRef] [PubMed]
- El Riachy, M.; Bou-Mitri, C.; Youssef, A.; Andary, R.; Skaff, W. Chemical and sensorial characteristics of olive oil produced from the lebanese olive variety ‘baladi’. Sustainability 2018, 10, 4630. [Google Scholar] [CrossRef] [Green Version]
- Brito, C.; Dinis, L.-T.; Silva, E.; Gonçalves, A.; Matos, C.; Rodrigues, M.A.; Moutinho-Pereira, J.; Barros, A.; Correia, C. Kaolin and salicylic acid foliar application modulate yield, quality and phytochemical composition of olive pulp and oil from rainfed trees. Sci. Hortic. 2018, 237, 176–183. [Google Scholar] [CrossRef] [Green Version]
- Morelló, J.-R.; Motilva, M.-J.; Ramo, T.; Romero, M.-P. Effect of freeze injuries in olive fruit on virgin olive oil composition. Food Chem. 2003, 81, 547–553. [Google Scholar] [CrossRef]
- Charalampopoulos, I.; Polychroni, I.; Psomiadis, E.; Nastos, P. Spatiotemporal Estimation of the Olive and Vine Cultivations’ Growing Degree Days in the Balkans Region. Atmosphere 2021, 12, 148. [Google Scholar] [CrossRef]
- Baiano, A.; Terracone, C.; Viggiani, I.; Del Nobile, M.A. Changes produced in extra-virgin olive oils from cv. Coratina during a prolonged storage treatment. Czech J. Food Sci. 2014, 32, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Condelli, N.; Caruso, M.C.; Galgano, F.; Russo, D.; Milella, L.; Favati, F. Prediction of the antioxidant activity of extra virgin olive oils produced in the Mediterranean area. Food Chem. 2015, 177, 233–239. [Google Scholar] [CrossRef]
- Talhaoui, N.; Gómez-Caravaca, A.; León, L.; De La Rosa, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. From Olive Fruits to Olive Oil: Phenolic Compound Transfer in Six Different Olive Cultivars Grown under the Same Agronomical Conditions. Int. J. Mol. Sci. 2016, 17, 337. [Google Scholar] [CrossRef] [Green Version]
- Gouvinhas, I.; Machado, N.; Cunha, M.; Pereira, M.; Matos, C.; Gomes, S.; Lopes, J.; Martins-Lopes, P.; Barros, A.I. Trace Element Content of Monovarietal and Commercial Portuguese Olive Oils. J. Oleo Sci. 2015, 64, 1083–1093. [Google Scholar] [CrossRef]
- Regulation, E. Commission Implementing Regulation (EU) No 299/2013 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. Eur. Union L 2013, 90. [Google Scholar]
- Khaleghi, E.; Arzani, K.; Moallemi, N.; Barzegar, M. The efficacy of kaolin particle film on oil quality indices of olive trees (Olea europaea L.) cv ‘Zard’ grown under warm and semi-arid region of Iran. Food Chem. 2015, 166, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Salvador, M.D.; Aranda, F.; Fregapane, G. Influence of fruit ripening on ‘Cornicabra’ virgin olive oil quality A study of four successive crop seasons. Food Chem. 2001, 73, 45–53. [Google Scholar] [CrossRef]
- Hmida, R.B.; Gargouri, B.; Chtourou, F.; Abichou, M.; Sevim, D.; Bouaziz, M. Study on the effect of climate changes on the composition and quality parameters of virgin olive oil “Zalmati” harvested at three consecutive crop seasons: Chemometric discrimination. ACS Omega 2022, 7, 40078–40090. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive compounds and quality of extra virgin olive oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- Bendini, A.; Cerretani, L.; Salvador, M.D.; Fregapane, G.; Lercker, G. Stability of the sensory quality of virgin olive oil during storage: An overview. Ital. J. Food Sci. 2010, 21, 389–406. [Google Scholar]
- Lazzerini, C.; Cifelli, M.; Domenici, V. Pigments in Extra-Virgin Olive Oil: Authenticity and Quality; InTech: London, UK, 2016. [Google Scholar]
- Ghanbari Shendi, E.; Sivri Ozay, D.; Ozkaya, M.T.; Ustunel, N.F. Changes occurring in chemical composition and oxidative stability of virgin olive oil during storage. OCL 2018, 25, A602. [Google Scholar] [CrossRef] [Green Version]
- Cayuela, J.A.; Gómez-Coca, R.B.; Moreda, W.; Pérez-Camino, M.C. Sensory defects of virgin olive oil from a microbiological perspective. Trends Food Sci. Amp Technol. 2015, 43, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, G.D.; Ellis, A.C.; Gámbaro, A.; Barrera-Arellano, D. Sensory evaluation of high-quality virgin olive oil: Panel analysis versus consumer perception. Curr. Opin. Food Sci. 2018, 21, 66–71. [Google Scholar] [CrossRef]
- von Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef]
- El Yamani, M.; Sakar, E.H.; Boussakouran, A.; Rharrabti, Y. Influence of ripening index and water regime on the yield and quality of “Moroccan Picholine” virgin olive oil. OCL 2020, 27, 19. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; AoOA Chemists: Washington, DC, USA, 2006. [Google Scholar]
- Irigoyen, J.J.; Einerich, D.W.; Sánchez-Díaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Sousa, C.; Gouvinhas, I.; Barreira, D.; Carvalho, M.T.; Vilela, A.; Lopes, J.; Martins-Lopes, P.; Barros, A.I. ‘Cobrançosa’ olive oil and drupe: Chemical composition at two ripening stages. J. Am. Oil Chem. Soc. 2014, 91, 599–611. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Mateos, R.; Espartero, J.L.; Trujillo, M.; Ríos, J.J.; León-Camacho, M.; Alcudia, F.; Cert, A. Determination of Phenols, Flavones, and Lignans in Virgin Olive Oils by Solid-Phase Extraction and High-Performance Liquid Chromatography with Diode Array Ultraviolet Detection. J. Agric. Food Chem. 2001, 49, 2185–2192. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant activities of the extracts from chestnut flower, leaf, skins and fruit. Food Chem. 2008, 107, 1106–1113. [Google Scholar] [CrossRef]
- Barros, L.; Cabrita, L.; Boas, M.V.; Carvalho, A.M.; Ferreira, I.C.F.R. Chemical, biochemical and electrochemical assays to evaluate phytochemicals and antioxidant activity of wild plants. Food Chem. 2011, 127, 1600–1608. [Google Scholar] [CrossRef]
- IOC. International Olive Concil. COI/T.20/Doc. no. 22. Method for the Organoleptic Assessment of Extra Virgin Olive Oil Applying to Use a Designation of Origin. 2005. Available online: http://www.internationaloliveoil.org (accessed on 3 September 2022).


| NPKB | N0 | P0 | K0 | B0 | p-Values | SR | |
|---|---|---|---|---|---|---|---|
| Macronutrients (g k−1) | |||||||
| 1 Nitrogen | 17.7 a | 15.7 b | 17.5 a | 18.4 a | 18.0 a | 0.0002 | 16–21 |
| 2 Phosphorus | 1.4 a | 1.4 a | 1.5 a | 1.2 a | 1.3 a | 0.1828 | 1–3 |
| 3 Potassium | 7.1 ab | 7.8 a | 6.5 ab | 5.7 b | 6.6 ab | 0.0350 | 6–9 |
| 3 Calcium | 4.7 a | 4.6 a | 4.7 a | 4.5 a | 4.8 a | 0.9613 | 10–20 |
| 3 Magnesium | 1.2 a | 1.3 a | 1.3 a | 1.4 a | 1.3 a | 0.2170 | 1–3 |
| Micronutrients (mg kg−1) | |||||||
| 2 Boron | 29.4 a | 25.8 a | 27.6 a | 27.9 a | 18.4 b | <0.0001 | 15–50 |
| 3 Iron | 78.5 a | 94.3 a | 80.7 a | 86.2 a | 92.2 a | 0.1172 | >40 |
| 3 Zinc | 15.9 a | 16.2 a | 19.9 a | 17.8 a | 16.9 a | 0.5497 | 12–35 |
| 3 Copper | 7.4 a | 7.0 a | 7.7 a | 7.7 a | 7.4 a | 0.8589 | 5–20 |
| 3 Manganese | 51.1 a | 38.8 a | 51.4 a | 53.6 a | 57.3 a | 0.1173 | 20–80 |
| NPKB | N0 | P0 | K0 | B0 | p-Values | ||
|---|---|---|---|---|---|---|---|
| Fruit FW (g) | 2017 | 2.97 ± 0.07 | 3.01 ± 0.06 | 3.12 ± 0.09 | 3.11 ± 0.10 | 2.97 ± 0.08 | 0.5266 |
| 2018 | 3.59 ± 0.09 a | 3.15 ± 0.06 bc | 3.28 ± 0.07 b | 2.93 ± 0.06 c | 3.14 ± 0.06 bc | <0.0001 | |
| 2019 | 2.96 ± 0.10 | 3.16 ± 0.08 | 2.99 ± 0.11 | 3.02 ± 0.01 | 2.95 ± 0.08 | 0.6585 | |
| Equat. Length (mm) | 2017 | 15.2 ± 0.1 | 15.4 ± 0.1 | 15.6 ± 0.2 | 21.6 ± 6.1 | 15.2 ± 0.2 | 0.3143 |
| 2018 | 16.1 ± 0.2 a | 15.3 ± 0.1 bc | 15.6 ± 0.1 ab | 14.9 ± 0.1 c | 15.3 ± 0.1 bc | <0.0001 | |
| 2019 | 14.5 ± 0.2 | 15.2 ± 0.1 | 14.8 ± 0.2 | 14.9 ± 0.2 | 14.8 ± 0.1 | 0.1355 | |
| Long. Length (mm) | 2017 | 21.7 ± 0.2 | 21.4 ± 0.2 | 22.1 ± 0.2 | 21.6 ± 0.3 | 21.5 ± 0.2 | 0.1972 |
| 2018 | 22.9 ± 0.2 a | 22.0 ± 0.1 b | 22.2 ± 0.2 ab | 21.2 ± 0.1 b | 22.2 ± 0.2 ab | 0.0014 | |
| 2019 | 20.9 ± 0.3 | 21.7 ± 0.2 | 21.0 ± 0.3 | 21.4 ± 0.3 | 21.1 ± 0.2 | 0.2705 | |
| Pulp FW (g) | 2017 | 2.18 ± 0.06 | 2.22 ± 0.05 | 2.35 ± 0.08 | 2.31 ± 0.09 | 2.18 ± 0.06 | 0.3358 |
| 2018 | 2.84 ± 0.08 a | 2.39 ± 0.08 bc | 2.48 ± 0.07 b | 2.20 ± 0.05 c | 2.38 ± 0.06 bc | <0.0001 | |
| 2019 | 2.27 ± 0.08 | 2.43 ± 0.07 | 2.25 ± 0.09 | 2.30 ± 0.09 | 2.23 ± 0.07 | 0.5349 | |
| Pit FW (g) | 2017 | 0.782 ± 0.019 | 0.793 ± 0.023 | 0.768 ± 0.019 | 0.798 ± 0.021 | 0.785 ± 0.026 | 0.9095 |
| 2018 | 0.756 ± 0.016 | 0.759 ± 0.015 | 0.799 ± 0.026 | 0.730 ± 0.014 | 0.764 ± 0.014 | 0.0852 | |
| 2019 | 0.696 ± 0.02 | 0.735 ± 0.02 | 0.747 ± 0.03 | 0.714 ± 0.02 | 0.729 ± 0.01 | 0.5777 | |
| Pulp/Pit ratio | 2017 | 2.82 ± 0.09 | 2.85 ± 0.010 | 3.06 ± 0.08 | 2.92 ± 0.10 | 2.82 ± 0.09 | 0.321 |
| 2018 | 3.79 ± 0.12 a | 3.19 ± 0.11 b | 3.18 ± 0.14 b | 3.03 ± 0.08 b | 3.14 ± 0.11 b | <0.0001 | |
| 2019 | 3.27 ± 0.09 | 3.32 ± 0.09 | 3.04 ± 0.11 | 3.24 ± 0.08 | 3.06 ± 0.08 | 0.1183 |
| NPKB | N0 | P0 | K0 | B0 | p-Values | ||
|---|---|---|---|---|---|---|---|
| Dry matter (%) | 2017 | 45.2 ± 0.7 | 44.8 ± 1.4 | 49.0 ± 1.2 | 46.7 ± 2.0 | 49.4 ± 0.5 | 0.0590 |
| 2018 | 35.8 ± 1.5 a | 34.7 ± 1.7 a | 34.8 ± 2.8 a | 36.6 ± 0.6 a | 26.9 ± 1.3 b | 0.0080 | |
| Organic matter (%) | 2017 | 92.5 ± 0.6 | 93.3 ± 0.2 | 87.3 ± 5.4 | 93.9 ± 1.0 | 92.9 ± 0.6 | 0.3450 |
| 2018 | 90.8 ± 0.6 | 89.7 ± 0.9 | 91.0 ± 0.4 | 92.5 ± 0.4 | 89.9 ± 1.6 | 0.2250 | |
| Ash (%) | 2017 | 3.90 ± 0.26 | 2.84 ± 0.24 | 2.81 ± 0.39 | 2.91 ± 0.62 | 3.19 ± 0.24 | 0.9170 |
| 2018 | 1.70 ± 0.18 ab | 2.01 ± 0.27 ab | 2.33 ± 0.16 b | 1.30 ± 0.06 c | 3.28 ± 0.27 a | <0.0001 | |
| Total dietary fiber (%) | 2017 | 11.9 ± 0.1 ab | 12.8 ± 0.3 a | 11.5 ± 0.2 b | 12.3 ± 0.0.1 ab | 12.8 ± 0.2 a | 0.0030 |
| 2018 | 12.2 ± 0.2 ab | 12.1 ± 0.3 ab | 11.8 ± 0.2 b | 13.4 ± 0.4 a | 12.6 ± 0.4 ab | 0.0130 | |
| Crude protein (%) | 2017 | 0.99 ± 0.06 b | 2.38 ± 0.36 a | 0.94 ± 0.03 b | 0.92 ± 0.06 b | 2.38 ± 0.04 a | <0.0001 |
| 2018 | 3.31 ± 0.18 a | 2.62 ± 0.07 b | 3.19 ± 0.11 ab | 3.80 ± 0.19 a | 3.26 ± 0.12 a | 0.0010 | |
| Oil content (%) | 2017 | 55.3 ± 1.1 a | 50.1 ± 1.4 a | 54.3 ± 1.7 ab | 49.9 ± 2.2 b | 50.9 ± 1.6 ab | 0.0410 |
| 2018 | 46.7 ± 2.0 ab | 49.0 ± 2.0 ab | 50.0 ± 1.3 a | 43.4 ± 1.2 b | 50.7 ± 1.1 a | 0.0110 | |
| Soluble sugars (mg g−1) | 2017 | 98.2 ± 2.0 b | 108.9 ± 1.9 ab | 109.4 ± 1.7 a | 111.0 ± 4.4 a | 101.3 ± 1.4 ab | 0.0090 |
| 2018 | 157.2 ± 6.0 a | 141.2 ± 1.5 b | 135.3 ± 2.4 b | 158.9 ± 5.1 a | 134.4 ± 1.5 b | <0.0001 |
| Olive Fruit | Olive Oil | |||||||
|---|---|---|---|---|---|---|---|---|
| TP (mg g−1 DW) | o-DP (mg g−1 DW) | Fl (mg g−1 DW) | TAC (mmol g−1 DW) | TP (mg kg−1 DW) | o-DP (mg kg−1 DW) | Fl (mg kg−1 DW) | TAC (mmol kg−1 DW) | |
| 2017 | ||||||||
| NPKB | 26.6 ± 0.7 d | 24.3 ± 0.9 b | 24.9 ± 1.3 c | 91.8 ± 2.0 b | 116.2 ± 1.0 cd | 54.6 ± 0.5 d | 61.9 ± 2.4 c | 66.1 ± 1.5 c |
| N0 | 31.3 ± 0.9 c | 29.0 ± 0.8 ab | 27.2 ± 2.9 bc | 102.0 ± 3.0 b | 132.3 ± 1.4 b | 71.8 ± 0.8 a | 73.3 ± 2.1 bc | 73.9 ± 2.4 bc |
| P0 | 35.5 ± 0.7 ab | 32.7 ± 1.3 a | 38.3 ± 4.6 a | 120.1 ± 2.1 a | 125.1 ± 1.6 bc | 59.5 ± 0.8 c | 78.9 ± 2.8 b | 81.4 ± 2.4 b |
| K0 | 38.1 ± 1.5 a | 31.5 ± 2.4 a | 38.0 ± 3.6 a | 114.6 ± 3.9 a | 143.6 ± 3.7 a | 67.7 ± 1.5 b | 105.6 ± 4.6 a | 96.5 ± 3.1 a |
| B0 | 34.2 ± 0.9 bc | 28.1 ± 1.4 ab | 35.7 ± 4.4 ab | 102.4 ± 2.2 b | 115.4 ± 2.5 d | 57.1 ± 0.7 cd | 75.6 ± 3.2 b | 74.8 ± 1.3 bc |
| p-values | <0.0001 | 0.0030 | 0.0200 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| 2018 | ||||||||
| NPKB | 29.0 ± 1.0 b | 37.9 ± 2.2 ab | 25.7 ± 3.0 | 67.9 ± 4.0 b | 170.5 ± 1.2 c | 88.5 ± 1.4 b | 109.2 ± 2.0 c | 122.4 ± 3.1 c |
| N0 | 34.4 ± 0.3 a | 43.7 ± 2.5 a | 29.9 ± 4.3 | 90.5 ± 2.1 a | 229.4 ± 4.9 a | 102.8 ± 1.1 a | 143.5 ± 3.1 a | 179.5 ± 3.3 a |
| P0 | 28.1 ± 0.4 b | 45.4 ± 2.0 a | 23.4 ± 1.6 | 94.2 ± 3.2 a | 183.0 ± 7.2 bc | 84.9 ± 3.5 b | 102.1 ± 2.9 c | 125.2 ± 3.6 c |
| K0 | 24.9 ± 0.5 c | 32.9 ± 2.3 b | 20.5 ± 1.4 | 73.3 ± 1.7 b | 199.6 ± 4.4 b | 99.6 ± 1.3 a | 121.1 ± 2.4 b | 160.0 ± 4.3 b |
| B0 | 26.8 ± 0.5 bc | 43.6 ± 1.7 a | 25.3 ± 4.5 | 89.0 ± 1.5 a | 190.2 ± 2.5 b | 89.7 ± 1.1 b | 106.7 ± 1.7 c | 125.6 ± 1.8 c |
| p-values | <0.0001 | 0.0010 | 0.3620 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| ATX (°C) | ATN (°C) | ATE (°C) | TX (°C) | HD | TN (°C) | FD | Rainfall (mm) | Accumulated Rainfall (mm) | GDD (°C) | Accumulated GDD (°C) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | |||||||||||
| January | 9.6 | −0.8 | 4.4 | 17.7 | −7.7 | 18 | 51.8 | 51.8 | 10.1 | 10.1 | |
| February | 12.5 | 3.3 | 7.9 | 19.6 | −2.0 | 2 | 163.8 | 215.6 | 39.3 | 49.3 | |
| March | 15.4 | 4.2 | 9.8 | 24.0 | −1.1 | 3 | 47.2 | 262.9 | 107.3 | 156.6 | |
| April | 21.0 | 5.7 | 13.4 | 26.6 | −0.7 | 1 | 13.0 | 275.8 | 191.8 | 348.4 | |
| May | 22.7 | 10.4 | 16.5 | 31.2 | 3.5 | 51.8 | 327.6 | 295.7 | 644.1 | ||
| June | 29.0 | 14.3 | 21.7 | 38.1 | 4 | 6.9 | 4.6 | 332.2 | 439.6 | 1083.7 | |
| July | 30.7 | 14.0 | 22.3 | 36.2 | 4 | 9.7 | 7.1 | 339.3 | 474.6 | 1558.3 | |
| August | 30.1 | 14.1 | 22.1 | 36.6 | 1 | 7.1 | 5.1 | 344.4 | 468.7 | 2027.0 | |
| September | 25.8 | 10.0 | 17.9 | 31.6 | 2.6 | 0.0 | 344.4 | 326.5 | 2353.5 | ||
| October | 24.2 | 8.0 | 16.1 | 31.6 | 2.0 | 14.0 | 358.4 | 280.9 | 2634.3 | ||
| November a | 16.1 | 2.2 | 9.1 | 18.5 | −2.7 | 4 | 24.1 | 382.5 | 38.6 | 2672.9 | |
| 2017 a | 21.6 | 7.8 | 14.6 | 38.1 | 9 | −7.7 | 28 | ||||
| November | 14.7 | 1.5 | 8.0 | 18.5 | −4.9 | 11 | 37.3 | 419.8 | 51.4 | 2724.2 | |
| December | 10.3 | 0.7 | 5.5 | 14.5 | −5.7 | 16 | 110.0 | 529.8 | 21.0 | 2745.2 | |
| 2017 | 13.5 | 38.1 | 9 | −7.7 | 51 | ||||||
| 2018 | |||||||||||
| January | 10.2 | 1.7 | 6.0 | 15.5 | −4 | 9 | 50.8 | 50.8 | 21.9 | 21.9 | |
| February | 10.4 | −0.7 | 4.8 | 16.2 | −7.2 | 18 | 92.7 | 143.5 | 36.5 | 58.4 | |
| March | 10.1 | 2.5 | 6.3 | 19.5 | −3.7 | 3 | 193.8 | 337.3 | 14.0 | 72.4 | |
| April | 16.2 | 5.8 | 11.0 | 27.1 | 0.2 | 271.0 | 608.3 | 143.3 | 215.7 | ||
| May | 20.9 | 8.4 | 14.7 | 27.7 | 1.8 | 38.6 | 646.9 | 238.2 | 453.8 | ||
| June | 24.5 | 12.8 | 18.7 | 32.5 | 6.5 | 124.2 | 771.1 | 353.5 | 807.3 | ||
| July | 27.3 | 14.2 | 20.8 | 31.7 | 11.2 | 26.16 | 797.2 | 427.1 | 1234.4 | ||
| August | 31.7 | 15.0 | 23.3 | 39.2 | 5 | 10.7 | 0 | 797.2 | 387.0 | 1621.4 | |
| September | 28.9 | 14.3 | 21.6 | 31.6 | 10.4 | 2.0 | 799.3 | 387.0 | 2008.3 | ||
| October | 19.3 | 7.5 | 13.4 | 28.6 | 2.1 | 49.3 | 848.5 | 200.9 | 2209.2 | ||
| November | 9.4 | 3.2 | 6.3 | 19.0 | −1.2 | 3 | 137.9 | 986.4 | 29.1 | 2238.2 | |
| December a | 10.7 | 5.4 | 8.1 | 12.2 | 2.8 | 0 | 986.4 | 3.1 | 2241.3 | ||
| 2018 a | 18.3 | 7.5 | 12.9 | 39.2 | 5 | −7.2 | 33 | ||||
| December | 10.8 | 3.3 | 7.1 | 16.5 | −1.2 | 5 | 52.3 | 1038.8 | 29.8 | 2271.1 | |
| 2018 | 12.5 | 39.2 | 5 | −7.2 | 38 | ||||||
| 2019 | |||||||||||
| January | 10.4 | −0.4 | 5.0 | 15.2 | −4.9 | 20 | 32.5 | 32.5 | 7.8 | 7.8 | |
| February | 13.6 | 1.1 | 7.3 | 20.5 | −4 | 8 | 72.4 | 104.9 | 32.5 | 40.2 | |
| March | 16.7 | 3.1 | 9.9 | 21.1 | −1.7 | 2 | 21.1 | 126.0 | 91.6 | 131.8 | |
| April | 15.4 | 5.0 | 10.2 | 25 | −0.2 | 1 | 114.8 | 240.8 | 110.1 | 241.9 | |
| May | 23.7 | 8.3 | 16.0 | 31.2 | 5.1 | 9.2 | 249.9 | 241.7 | 483.6 | ||
| June | 23.8 | 9.8 | 16.8 | 33.5 | 2.2 | 39.6 | 289.6 | 296.0 | 779.5 | ||
| July | 30.1 | 14.8 | 22.5 | 36.5 | 1 | 9.4 | 22.11 | 311.7 | 479.5 | 1259.0 | |
| August | 28.9 | 14.2 | 21.6 | 33.2 | 6.7 | 23.37 | 335.0 | 451.4 | 1710.4 | ||
| September | 25.5 | 11.7 | 18.6 | 32 | 5.3 | 41.9 | 377.0 | 344.6 | 2054.9 | ||
| October | 19.4 | 8.7 | 14.1 | 28.7 | 1.1 | 47.8 | 424.7 | 219.0 | 2273.9 | ||
| November a | 11.5 | 5.8 | 8.7 | 18.5 | 1.8 | 52.32 | 477.0 | 36.2 | 2310.0 | ||
| 2019 a | 19.9 | 7.5 | 13.7 | 36.5 | −4.9 | 31 | |||||
| November | 11.6 | 5.6 | 8.6 | 18.5 | −1.9 | 1 | 172.0 | 649.0 | 97.4 | 2407.4 | |
| December | 10.5 | 2.7 | 6.6 | 15.5 | −3.4 | 10 | 235.5 | 884.4 | 25.3 | 2432.7 | |
| 2019 | 12.8 | 36.5 | −4.9 | 42 |
| NPKB | N0 | P0 | K0 | B0 | p-Values | ||
|---|---|---|---|---|---|---|---|
| Free acidity (%) | 2017 | 0.046 ± 0.023 | 0.117 ± 0.022 | 0.136 ± 0.031 | 0.116 ± 0.022 | 0.048 ± 0.024 | 0.0750 |
| 2018 | 0.140 ± 0.012 | 0.158 ± 0.012 | 0.127 ± 0.007 | 0.160 ± 0.001 | 0.151 ± 0.006 | 0.0950 | |
| Peroxide index (mEq O2 kg−1) | 2017 | 8.09 ± 0.30 a | 5.42 ± 0.09 b | 6.80 ± 1.40 ab | 5.03 ± 0.22 b | 5.62 ± 0.10 b | 0.0450 |
| 2018 | 7.99 ± 0.81 | 10.30 ± 0.20 | 10.90 ± 0.30 | 9.19 ± 1.58 | 9.24 ± 1.07 | 0.3000 | |
| K232 | 2017 | 1.12 ± 0.14 | 0.94 ± 0.05 | 1.14 ± 0.126 | 0.99 ± 0.08 | 1.09 ± 0.202 | 0.7690 |
| 2018 | 2.18 ± 0.14 | 2.39 ± 0.20 | 2.38 ± 0.20 | 2.37 ± 0.05 | 2.28 ± 0.07 | 0.8140 | |
| K270 | 2017 | 0.079 ± 0.010 b | 0.066 ± 0.004 b | 0.085 ± 0.007 ab | 0.116 ± 0.002 a | 0.079 ± 0.010 b | 0.0080 |
| 2018 | 0.186 ± 0.006 a | 0.164 ± 0.0003 b | 0.171 ± 0.0003 ab | 0.185 ± 0.001 a | 0.183 ± 0.0003 a | 0.0090 | |
| ΔK | 2017 | 0.004 ± 0.001 ab | 0.003 ± 0.000 b | 0.004 ± 0.001 ab | 0.006 ± 0.001 a | 0.003 ± 0.001 b | 0.0040 |
| 2018 | 0.031 ± 0.023 | 0.006 ± 0.000 | 0.006 ± 0.0003 | 0.006 ± 0.0007 | 0.006 ± 0.0003 | 0.3860 |
| Month | NPKB | N0 | P0 | K0 | B0 | p-Values | |
|---|---|---|---|---|---|---|---|
| Peroxide index (meq O2 kg−1) | 3 | 7.99 ± 0.81 | 10.27 ± 0.2 | 10.91 ± 0.31 | 9.19 ± 1.58 | 9.24 ± 1.07 | 0.3001 |
| 15 | 5.94 ± 0.55 | 7.50 ± 0.25 | 7.28 ± 0.86 | 7.41 ± 0.38 | 7.09 ± 0.66 | 0.3679 | |
| K232 | 3 | 2.18 ± 0.14 b | 2.39 ± 0.20 b | 2.38 ± 0.19 a | 2.37 ± 0.05 b | 2.28 ± 0.07 b | 0.8140 |
| 15 | 4.29 ± 0.19 b | 4.41 ± 0.24 b | 4.82 ± 0.64 a | 5.54 ± 0.55 b | 5.26 ± 0.56 c | <0.0001 | |
| K270 | 3 | 0.186 ± 0.009 a | 0.165 ± 0.003 b | 0.171 ± 0.004 b | 0.184 ± 0.0009 a | 0.183 ± 0.003 a | <0.0001 |
| 15 | 0.266 ± 0.001 b | 0.299 ± 0.006 b | 0.312 ± 0.004 a | 0.302 ± 0.012 b | 0.289 ± 0.001 c | <0.0001 | |
| ΔK | 3 | 0.032 ± 0.023 b | 0.005 ± 0.00005 b | 0.006 ± 0.0002 a | 0.006 ± 0.0005 b | 0.006 ± 0.0002 b | <0.0001 |
| 15 | 0.0019 ± 0.0001 b | 0.0032 ± 0.00002 b | 0.0005 ± 0.00003 a | 0.0017 ± 0.0001 b | 0.0015 ± 0.0001 b | <0.0001 |
| Soil Properties | |
|---|---|
| 1 Organic carbon (g kg−1) | 5.7 ± 0.10 |
| 2 pH(H2O) (1:2.5) | 8.9 ± 0.46 |
| 3 Extractable P (mg kg−1, P2O5) | 63.2 ± 7.02 |
| 3 Extractable K (mg kg−1, K2O) | 90.0 ± 7.41 |
| 4 Exchangeable Ca (cmolc kg−1) | 6.7 ± 0.68 |
| 4 Exchangeable Mg (cmolc kg−1) | 2.2 ± 0.16 |
| 4 Exchangeable K (cmolc kg−1) | 0.2 ± 0.02 |
| 4 Exchangeable Na (cmolc kg−1) | 0.4 ± 0.04 |
| 4 Exchangeable acidity (cmolc kg−1) | 0.15 |
| Cation exchange capacity (cmolc kg−1) | 10.5 ± 0.85 |
| Macronutrients (g kg−1) | SR | Micronutrients (mg kg−1) | SR | ||
|---|---|---|---|---|---|
| 1 Nitrogen | 19.4 ± 0.88 | 16–21 | 2 Boron | 34.8 ± 2.38 | 15–50 |
| 2 Phosphorus | 1.3 ± 0.23 | 1–3 | 3 Iron | 86.1 ± 10.98 | >40 |
| 3 Potassium | 6.4 ± 1.11 | 6–9 | 3 Zinc | 11.1 ± 1.51 | 12–35 |
| 3 Calcium | 5.2 ± 0.62 | 10–20 | 3 Copper | 5.9 ± 0.91 | 5–20 |
| 3 Magnesium | 1.1 ± 0.20 | 1–3 | 3 Manganese | 47.3 ± 8.02 | 20–80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, E.; Gonçalves, A.; Martins, S.; Brito, C.; Ferreira, H.; Ferreira, L.M.M.; Moutinho-Pereira, J.; Rodrigues, M.Â.; Correia, C.M. Olive Yield and Physicochemical Properties of Olives and Oil in Response to Nutrient Application under Rainfed Conditions. Molecules 2023, 28, 831. https://doi.org/10.3390/molecules28020831
Silva E, Gonçalves A, Martins S, Brito C, Ferreira H, Ferreira LMM, Moutinho-Pereira J, Rodrigues MÂ, Correia CM. Olive Yield and Physicochemical Properties of Olives and Oil in Response to Nutrient Application under Rainfed Conditions. Molecules. 2023; 28(2):831. https://doi.org/10.3390/molecules28020831
Chicago/Turabian StyleSilva, Ermelinda, Alexandre Gonçalves, Sandra Martins, Cátia Brito, Helena Ferreira, Luís M. M. Ferreira, José Moutinho-Pereira, Manuel Ângelo Rodrigues, and Carlos M. Correia. 2023. "Olive Yield and Physicochemical Properties of Olives and Oil in Response to Nutrient Application under Rainfed Conditions" Molecules 28, no. 2: 831. https://doi.org/10.3390/molecules28020831
APA StyleSilva, E., Gonçalves, A., Martins, S., Brito, C., Ferreira, H., Ferreira, L. M. M., Moutinho-Pereira, J., Rodrigues, M. Â., & Correia, C. M. (2023). Olive Yield and Physicochemical Properties of Olives and Oil in Response to Nutrient Application under Rainfed Conditions. Molecules, 28(2), 831. https://doi.org/10.3390/molecules28020831












